Abstract
Purpose
This study is an attempt to evaluate the use of autologous platelet rich plasma (PRP) to promote wound healing and osseous regeneration in human third molar extraction sockets.
Materials and method
PRP was prepared after two centrifugation and the gelling agent used was freshly prepared 10% calcium chloride.PRP gel was placed in one of the extracted sockets of bilateral impacted mandibular third molars. IOPA Xrays were used to evaluate the wound dehiscence, probing depth, bone density & alveolar bone level after 1st, 2nd and 7th day and 3rd & 6th month respectively.
Results
On evaluation, it was found that PRP grafted sockets showed dehiscence in 8% cases. The decrease in alveolar bone level was highly significant in PRP grafted sockets in 3rd and 6th month post operatively. There was significant difference between pre-operative density of adjacent bone and bone formed in extraction sockets at 3rd and 6th month in PRP grafted sockets. There was significant reduction in probing depth from initial period to 3 and 6 months in both the groups, but PRP grafted sockets showed greater decrease in probing depth.
Conclusion
PRP is an inexpensive and widely available modality to minimize postoperative complication and enhance both hard and soft tissue healing potentials. This autologous product eliminates concern about immunogenic reaction and disease transmission. Its beneficial outcomes in dental clinic, including decrease in bleeding and rapid wound healing hold promise for further procedures.PRP is thus a new application in tissue engineering and developing area for clinician and researchers.
Keywords: Autologous, Growth factors, Platelet-rich plasma, Bone density, Soft tissue healing
Introduction
In recent years, our knowledge of wound healing has been greatly enhanced by the identification and understanding of growth factors and technologic means to use them. [1].
Platelet-rich plasma (PRP), an autologous concentrate of platelets in a relatively small volume of plasma, enables delivery of growth factors in increased amounts to surgical sites to promote wound healing. Platelets contain growth factors such as platelet derived growth factor (PDGF) (including αα, αβ and ββ isomers), transforming growth factor-β (TGF-β) (including β1 and β2 isomers), fibroblast growth factor, insulin-like growth factor-I (IGF-I), epithelial growth factor, vascular endothelial growth factor, and numerous other secretory proteins. PDGF and TGF-β improve soft tissue and bone healing, stimulate collagen production, improve wound strength, and initiate callus formation [2, 3].
PRP was first introduced to the oral surgery community by Whitman et al. in their 1997 article entitled “Platelet Gel: An Autologous Alternative to Fibrin Glue with Applications in Oral and Maxillofacial Surgery.” PRP enjoyed a great increase in popularity in the oral and maxillofacial surgery community after the publication of a landmark article by Marx et al. in 1998. As suggested by Marx, PRP is not osteoinductive and the bone regeneration process starts with release of PDGF and TGF-β through degranulation of platelet [2].
The overall rate of Alveolar Osteites in PRP treated sites was 3.4% versus the untreated sites which was 12.8%, representing an almost fourfold increase [4].
PRP in combination with tri-calcium phosphate has also been used in the treatment of periapical inflammatory lesion. It is also being used to deliver growth factors in high concentration to sites requiring osseous grafting [2].
Periodontal healing after impacted third molar surgeries, ridge augmentation and sinus grafting with freeze-dried bone allograft in combination with PRP provide a viable therapeutic alternative for implant placements [3, 5–8].
The extraction of impacted third molars may cause multiple periodontal defects at the distal root of the second molar. These complications are even more frequent in older patients and when there are preoperative periodontal defects on the distal surface of the second molar before extraction of the impacted third molar [5, 9].
This study was carried out to evaluate the efficacy of PRP on bone regeneration after surgical removal of mandibular third molars. The results were evaluated both clinically and radiographically [3, 6, 10].
Materials and method [11]
This study has been conducted in the Department of Oral and Maxillofacial Surgery and Implantology, I.T.S. Centre for Dental Studies and Research, Muradnagar, Ghaziabad. A sample size of 25 patients signed an informed consent before the study.
PRP is produced by sequential centrifugation of fresh autologous blood, producing plasma with an approximate threefold increase in the concentration of intact platelets. Calcium chloride (10%) is added to PRP to initiate the clotting process and activation of the alpha granules of the platelets [2, 9, 10].
All healthy people between 18 and 70 years, including male and female with bilaterally mandibular third molar impactions were included in the study. All patients signed an informed consent before participating in the study, which was reviewed and approved by the ethical committee of our institute.
Two groups were divided:
GROUP I: socket in which Platelet rich plasma is placed after surgical removal of impacted tooth.
GROUP II: socket without Platelet rich plasma.
In both the groups the patients were recalled on first, second and seventh day postoperatively to assess the healing of the wound.
The clinical evaluation included the measurement of the probing depth distal to the second molar preoperatively, 3rd and 6th month post operatively.
IOPA radiographs were taken preoperatively, 3rd and 6th month post operatively to assess the alveolar bone height distal to second molar and the bone density. Bone Density was measured by mean gray level histogram values of scanned IOPA images of the extraction sockets obtained through Adobe Photoshop 7.0 software.
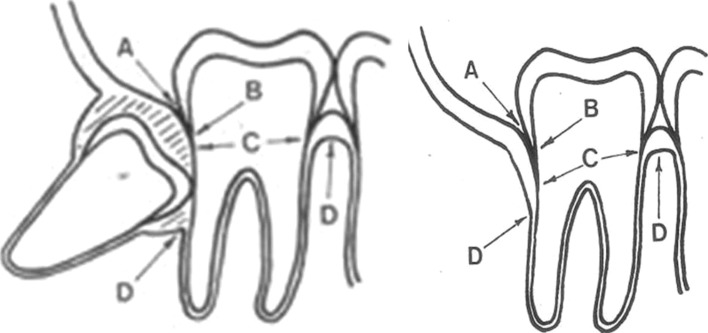
Measurement of Various Parameters (Fig. 1)
Fig. 1.
Landmarks for clinical and radiographic evaluation A Free gingival margin B Cementoenamel junction C Depth of pocket D Alveolar crest
For clinical and radiographic evaluation certain landmarks were used:
-
Measurement of wound dehiscence:
In both the groups, the extraction socket was evaluated on 1st, 2nd and 7th post operative day for any wound dehiscence.
The observation for wound dehiscence was recorded as present/absent.
-
Probing depth (PD): Probing depth was measured using Michigan “O” probe with William’s markings from “fgm” to the bottom of the pocket in nearest mm.
The probe tip was inserted into gingival sulcus parallel to the long axis of the tooth until slight resistence was met.
Average of PD at the three different positions i.e. DB, D, DL on the distal surface of 2nd molar was taken preoperatively, 3rd and 6th month post operatively (Fig. 1).
Alveolar bone level (ABL): IOPA radiographs were taken preoperatively, 3rd and 6th post operative month in every case. The radiographs were taken by paralleling technique and the ABL distal to second molar was measured with a calliper from CEJ to the alveolar crest.
Bone density: Bone density was measured using mean gray scale measurement of the extraction sockets of scanned IOPA images. IOPA radiographs were taken preoperatively, 3rd and 6th post operative month in every case.
Pre-Operative Density = Average density from A to A′ + Average Density from B to B′.
Where, Point A = Point at alveolar crest just mesial to third molar.
Point A′ = Point just mesial to 3rd molar at the level of apical third.
Point B and B′ = Points in similar fashion but distal to mandibular third molar.
Post-operative Density = Average density from C to C′ + Average Density from D to D′.
Where, Point C = Mesial Point in the 3rd molar socket at the alveolar crest level.
Point C′ = Mesial Point in the 3rd molar socket at the apical third level.
Point D and D′ = Points in similar fashion but distal to mandibular third molar extraction site.
Method of Preparation of PRP gel [1, 11, 13]
First step: Collection of Blood
Under all aseptic conditions, 16 ml of blood was withdrawn from the anticubital region of the patients forearm using vaccutainer needle and vaccutainers containing anti-coagulant 4 ml each. The vaccutainers were thoroughly shaken to ensure mixing of anti-coagulant with the drawn blood.
Second step: Preparation of cPRP
15–16 ml of blood collected in vaccutainer containing anti-coagulant. The whole blood is then centrifuged at 1000 rpm for 10 min. The supernatant formed is PRP.
PRP, Buffy coat and upper 1–2 mm of RBC layer is collected in a fresh vaccutainer and again centrifuged at 1,000 rpm for 10 min. The upper half of the supernatant is discarded and the lower half is mixed thoroughly to yield cPRP.
Third step: Preparation of Autologous Thrombin
2.5 ml of PRP is thoroughly mixed with 0.08 ml of CaCl2. This resulted in a clot formation and thrombin formation. The clot is discarded and the thrombin is used for the preparation of cPRP.
Fourth step: PRP gel Preparation
6 ml cPRP + 1 ml thrombin at 370°C for 2–3 min leads to formation of PRP gel.
Statistical Analysis
All the results were calculated using the mean value and standard deviation for each of the parameters considered and checked for statistical significance using student paired-t test.
The differences in the results of PD, ABH and Bone Density at 3 and 6 months were compared between each group using student unpaired-t test.
Z test for proportion was used to evaluate the results of wound status (wound dehiscence).
Results
The present study was conducted in the Department of Oral and Maxillofacial surgery and Oral Implantology, I.T.S Centre for Dental Studies and Research, Muradnagar, Ghaziabad.The objective was to evaluate the ability of platelet rich plasma to enhance the rate of wound healing and to assess whether PRP is a more predictable method of tissue regeneration in third molar extraction sockets and to evolve a technique that may help in obtaining the efficacious results.
A total of 25 patients requiring bilateral extraction of third molar were included in the present study. The wound status and bone density of the extraction sockets, probing depth, alveolar bone level distal to second molar were evaluated in post operative follow up visits on 1st, 2nd, 7th day, 3rd and 6th month post operatively.
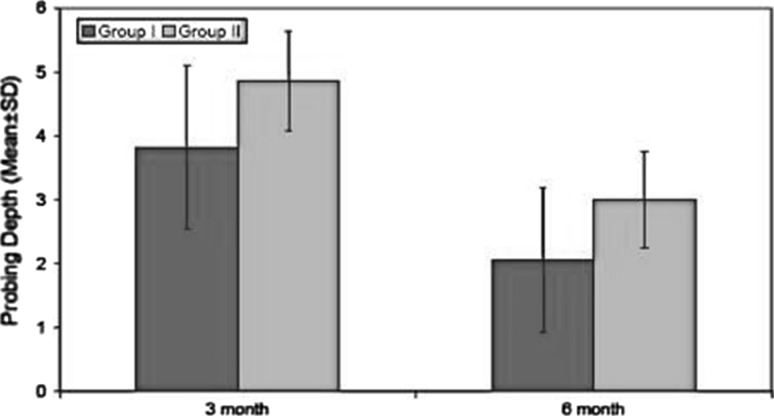
On evaluation of wound dehiscence, in extraction socket areas, it was found that group I (PRP grafted sockets) showed dehiscence in 8% cases while group II (No PRP) showed dehiscence in 92% cases. There was significant reduction in probing depth from initial period to 3 and 6 months in both the groups, but group I showed greater decrease in probing depth (Graph 1; Table 1).
Graph 1.
Assesment of probing depth at different time intervals
Table 1.
Assessment of probing depth at different time intervals
| S.No. | Time interval (months) | Group I (n = 25) | Group II (n = 25) | Statistical significance | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | “T” | “P” | ||
| 1. | 3 | 3.82 | 1.27 | 4.86 | 1.13 | 6.279 | <0.001 |
| 2. | 6 | 2.06 | 0.77 | 3.00 | 0.76 | 7.224 | <0.001 |
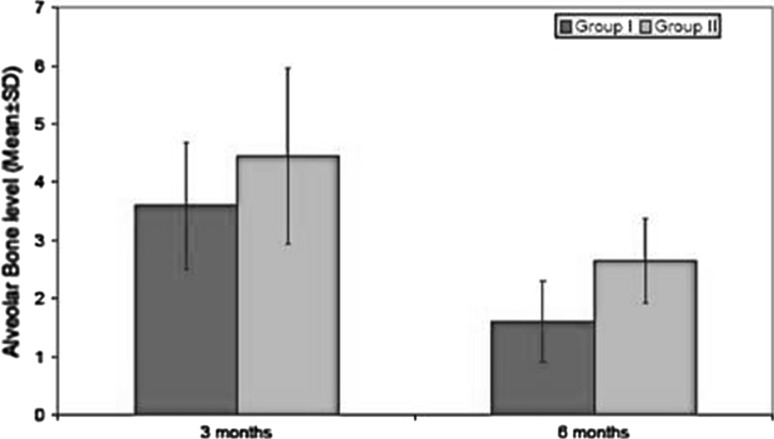
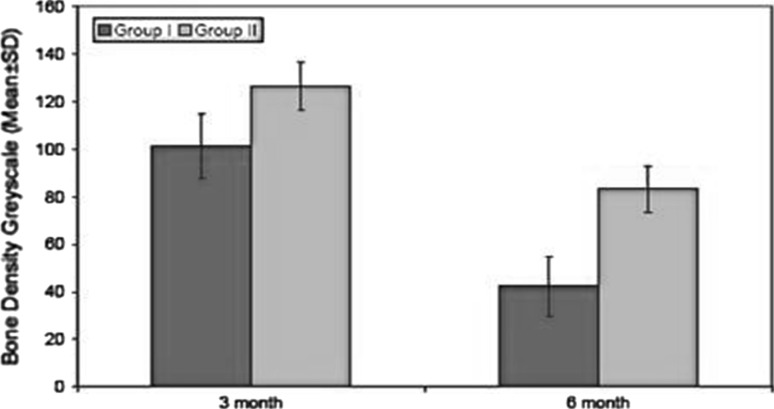
The decrease in alveolar bone level (Graph 2; Table 2) was highly significant in group I as compared to group II in 3rd and 6th month post operatively. There was significant difference between pre-operative density of adjacent bone and bone formed in extraction sockets at 3rd and 6th month (Graph 3; Table 3). But this difference in bone density between group I at 3rd and 6th month and was not statistically significant in group II at 3rd and 6th month postoperatively. The results of this study demonstrate that PRP contributed to better healing of soft tissue and bone as compared to the extracted socket without its use.
Graph 2.
Assesment of alveolar bone level at different time intervals
Table 2.
Assesment of alveolar bone level at different time intervals
| S.No. | Time interval (months) | Group I (n = 24) | Group II (n = 24) | Statistical significance | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | “T” | “P” | ||
| 1. | 3 | 3.60 | 1.09 | 4.44 | 1.51 | 3.142 | 0.005 |
| 2. | 6 | 1.60 | 0.71 | 2.65 | 0.73 | 9.630 | <0.001 |
Graph 3.
Assesment of bone density at different time intervals
Table 3.
Assessment of bone density at different time intervals
| S.No. | Time interval (months) | Group I (n = 25) | Group II (n = 25) | Statistical significance | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | “T” | “P” | ||
| 1. | 3 | 101.28 | 13.50 | 126.56 | 12.45 | 11.659 | <0.001 |
| 2. | 6 | 42.28 | 10.30 | 83.24 | 9.81 | 13.361 | <0.001 |
Discussion
Periodontal complications distal to the root of the 2nd mandibular molar after extraction of adjacent 3rd molar are very common, Kugelberg’s thesis also summarized that extraction of impacted 3rd mandibular molar resulted in bony periodontal defects on distal surface of adjacent second molar in 44.4% cases [10, 12].
There are various methods for preparing PRP. In this study PRP was prepared from 16 cc of patients blood twice in a clinical centrifuge as advocated by Landesberg et al. [13], Kim et al. [7] PRP when activated to form gel causes degranulation of α-granules in the platelets, releasing number of known growth factors.PRP can be activated with CaCl2 alone, CaCl2 along with bovine thrombin, human thrombin, TRAP (thrombin receptor activator peptide) or ITA gelling agent [3, 6].
Landesberg et al. [13] and Marx [21] discussed the potential of bovine thrombin to cross react with human clotting factor-V and produce postoperative coagulopathies. In the present technique, autologous thrombin was prepared and mixed with PRP to form autologous platelet gel.
Landesberg et al. observed that the use of EDTA as anticoagulant caused fragmentation of platelets. Our study used CPDA (citrate phosphate dextrose adenin) due to its compatibility with platelet membranes [13, 14].
Juan et al. elaborated characteristics of PRP as: It provides adhesiveness and tensile strength for clot stabilization, is biologically acceptable to the root surface, contains important growth factors such as PDGF and TGF-β released by platelets, promotes angiogenesis, contains a dense fibrin network that is highly osteoconductive, has haemostatic properties, improves wound healing and is affordable treatment modality [10, 14, 17–19].
In this study we used PRP in mandibular 3rd molar sockets to enhance soft tissue healing and bone regeneration. No graft material was used. We evaluated soft tissue healing and gingival attachment by measuring the wound dehiscence and probing depth, bone regeneration was measured by evaluating the alveolar bone level and bone density distal to mandibular second molar.
In the present study, wound dehiscence was evaluated for both groups (with PRP and without PRP) on 1st, 2nd and 7th post operative day. On day 1, all subjects of both the groups had wound dehiscence. Statistically significant intergroup difference was seen on 2nd post operative day (P < 0.001%).Group I (extraction socket with PRP) showed no sign of dehiscence on 2nd postoperative day while group II showed positive sign of dehiscence. This signifies a better soft tissue healing of extracted socket with PRP as compared to the socket without PRP. On 7th day none of the subjects had wound dehiscence.
Mancuso [4] in his study also reported decrease in alveolar osteitis; objectively faster soft tissue flap healing and less oedema 7 days post surgically in PRP treated sockets. Marx [21] also supported the presence of GF’s in high concentration in PRP which is responsible for its effect in accelerating both soft and hard tissue healing.
In this study, probing depth was assessed for both the Groups. Group I showed significant (P < 0.001) decrease in probing depth than group II. Sammartina et al. [10, 15] also observed notable reduction in probing depth, improvement in probing attachment level distal to 2nd molar in 3rd and 6th month after extraction of impacted mandibular third molar in PRP grafted sockets. Measurement of alveolar bone level and density was performed in 3rd and 6th month postoperatively. Bone level was measured using caliper and Bone density was measured using gray scale value in 7.0 adobe photoshop software. As gray scale value increases the density decreases. Group I showed decrease in gray scale reading during both time intervals as compared to Group II.Thus, statistical significance being P < 0.001.
There was significant difference in the mean value of gray scale in histogram in 3rd and 6th month post operatively. However, our findings were supported by Mancuso et al. [4] who reported more dense radiographic bone healing. Anitua [16] also reported improved epithelisation and bone density when platelet rich plasma was placed in extraction socket.
The present study may requires more longer post operative follow-up to comment on significance of periodontal breakdown distal to 2nd molar and to arrive at definitive conclusion on efficacy of PRP in initiating and enhancing both hard and soft tissue healing [10].
Summary and conclusion
This study was performed to evaluate efficacy of PRP in soft tissue and osseous regeneration distal to mandibular second molar after removal of impacted third molar. The results of this study demonstrate that PRP contributed to better healing of soft tissue and bone as compared to the extracted socket without its use. It offers surgeons access to various growth factors with a simple, safe, cost effective and available technology.
This study attempted to use autologous PRP to promote wound healing and osseous regeneration in human third molar extraction sites. It clearly indicates definite improvement in soft tissue healing and faster regeneration of bone after 3rd molar surgery in cases treated with PRP as compared to control group post operatively.
PRP is an inexpensive and widely available modality to minimize postoperative complication and enhance both hard and soft tissue healing potentials. However, this autologous product eliminates concern about immunogenic reaction and disease transmission. The beneficial outcomes of PRP in dental clinic, including decrease in bleeding and rapid wound healing hold promise for further procedures.
PRP is thus a new application in tissue engineering and developing area for clinician and researchers. The present study was done for follow up of 6 month; further clinical trials with longer duration follow up should be done to get more informative and conclusive results.
Contributor Information
Ruchi Pathak Kaul, Phone: +91-9310-831-980, Email: ruchi8380@gmail.com.
Suhas S. Godhi, Phone: +91-9899-450-488, Email: drgodhi@yahoo.com
Anurag Singh, Phone: +91-9818-916-969, Email: dranurag_in@yahoo.com.
References
- 1.Gonshor A. Technique for producing platelet rich plasma and of platelet concentrate: background and process. J Periodontics Restorative Dent. 2002;22:547–557. [PubMed] [Google Scholar]
- 2.Gurbuzer B, Pikdoken L, Urhan M, Suer T, Narin Y. Scintigraphic evaluation of early osteoblastic activity in extraction sockets treated with platelet rich plasma. J Oral Maxillofac Surg. 2008;66:2454–2460. doi: 10.1016/j.joms.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Vivek GK, Sripathi Rao BH. Potential for osseous regeneration of platelet rich plasma: a comparative study in mandibular third molar sockets. J Oral Maxillofac Surg. 2009;8(4):308–311. doi: 10.1007/s12663-009-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancuso JD. Platelet rich plasma: a preliminary report in routine impacted mandibular third molar surgery and prevention of alveolar osteitis. Am Assoc Oral Maxillofac Surg. 2003;40:276–279. [Google Scholar]
- 5.Sammartino G, Tia M, Marenzi G, Lauro AE, Agostino E, Claudio PP. Use of autologous platelet rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg. 2005;63:766–770. doi: 10.1016/j.joms.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Gawande PD, Halli R. Efficacy of platelet rich plasma in bone regeneration after surgical removal of impacted bilateral third molars: pilot study. J Oral Maxillofac Surg. 2009;8(4):301–307. doi: 10.1007/s12663-009-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SG, Chung CH, Kim YK, Park JC, Lim SC. Use of particulate dentin-plaster of paris combination with/without platelet rich plasma in the treatment of bone defects around implants. Int Oral Maxillofac Implants. 2002;17:86–94. [PubMed] [Google Scholar]
- 8.Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol. 2000;71:1654–1661. doi: 10.1902/jop.2000.71.10.1654. [DOI] [PubMed] [Google Scholar]
- 9.Efeoglu C, Ackay YD, Erturk SA. Modified method for preparing platelet rich plasma: an experimental study. J Oral Maxillofac Surg. 2004;62:1403–1407. doi: 10.1016/j.joms.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 10.Freymiller EG, Aghaloo TL. Platelet rich plasma: Ready or not? J Oral Maxillofac Surg. 2004;62:484–488. doi: 10.1016/j.joms.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Schliephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31:469–484. doi: 10.1054/ijom.2002.0244. [DOI] [PubMed] [Google Scholar]
- 12.Hoppenreijs VPT, Pels E, Vrensen GFJM, Treffers WF. Effects of platelet-derived growth factor on endothelial wound healing of human corneas. Invest Ophthalmol Vis Sci. 1994;35:150–161. [PubMed] [Google Scholar]
- 13.Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297–300. doi: 10.1016/S0278-2391(00)90058-2. [DOI] [PubMed] [Google Scholar]
- 14.Dodson TB. Reconstruction of alveolar bone defects after extraction of mandibular third molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:241–247. doi: 10.1016/S1079-2104(96)80346-5. [DOI] [PubMed] [Google Scholar]
- 15.Osborne WH, Snyder AJ, Tempel TR. Attachment levels and crevicular depths at the distal of mandibular second molars following removal of adjacent third molars. J Periodontol. 1982;53:93–95. doi: 10.1902/jop.1982.53.2.93. [DOI] [PubMed] [Google Scholar]
- 16.Whitman DH, Berry RL, Green DM. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294–1299. doi: 10.1016/S0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 17.Tozum TF, Demiralp B. Platelet rich plasma: a promising innovation in dentistry. J Can Dent Assoc. 2003;69:664. [PubMed] [Google Scholar]
- 18.Demiralp B, Keceli HG, Muhtarogullari M, Serper A, Demiralp A, Eratalay K. Treatment of periapical inflammatory lesion with the combination of platelet rich plasma and tricalcium phosphate: a case report . J Endo. 2004;30:796–800. doi: 10.1097/01.don.0000136211.98434.66. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez AR, Sheridan PJ, Kupp LI. Is platelet rich plasma perfect enhancement factor? A current review. Int Oral Maxillofac Implants. 2003;18:93–103. [PubMed] [Google Scholar]
- 20.Kugelberg CF, Ahlstrom U, Ericson S, Hugoson A. Periodontal healing after impacted lower third molar surgery. Int J Oral Surg. 1985;14:29–40. doi: 10.1016/S0300-9785(85)80007-7. [DOI] [PubMed] [Google Scholar]
- 21.Marx RE, et al. Platelet rich-plasma: a source of multiple autologous growth factors for bone grafts. Oral Med Oral Pathol Oral Radio Endodl. 1998;85:638–646. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 22.Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–535. [PubMed] [Google Scholar]