Abstract
Desizing of cotton and micropoly fabrics was done using thermostable xylanase from Bacillus pumilus ASH. Micropoly fabric showed better desizing than cotton under same conditions. Violet scale readings from the TEGEWA test after enzymatic desizing for 90 min at pH 7.0 and at 60°C showed the readings falling in the range of 4–5, indicating good desizing efficiency. During bioscouring the weight loss values and liberation of reducing sugars were highest when EDTA was used along with xylanase. The weight loss value of 1.5% was observed for dry cotton fabric after 1 h in case of agitated system at pH 7.0 and at an optimal enzyme dosage of 5 IU/g. The weight loss values and the liberation of reducing sugars were higher in case of cotton fabrics. Wetting time of fabrics was lowered significantly after 60 min of bioscouring using xylanase. Increase in temperature or concentration of surfactant led to further reduction in the wetting time. The whiteness values of fabrics after bioscouring were 0.9% higher than the chemically scoured fabrics indicating good efficacy of xylanase during the scouring process.
Keywords: Bacillus pumilus, Scouring, Desizing, Whiteness index, Wettability
Introduction
The use of microorganisms and their enzymes to replace or supplement older conventional chemical methods in the textile industry is gaining interest. Xylanases have been reported mainly from bacteria [1–3], fungi [1], actinomycetes [4] and yeast [5, 6]. Enzyme technology in the textile processing area is intended to make manufacturing processes friendlier to the environment [7]. Cotton fabric must be thoroughly prepared for subsequent wet-processing treatments such as dyeing, printing, or finishing. These pretreatment processes for cotton preparation include desizing to remove the size, scouring to make it hydrophilic and bleaching to reach a standard level of whiteness [8, 9].
Desizing involves removal of adhesive substance known as size from the warp threads which was coated to prevent the thread breaking during weaving and the process must be carried out by treating the fabric with chemicals such as acids, alkali or oxidizing agents. In scouring, non-cellulosic components such as fats, waxes, proteins, pectins, natural colorants, minerals, noncellulosic polysaccharides and water-soluble compounds largely found in the primary cell wall are completely or partially removed from native cotton. Scouring gives a fabric with a high and even wettability so that it can be bleached and dyed successfully. Highly alkaline chemicals like sodium hydroxide are used for scouring in industries. These chemicals not only remove the non-cellulosic impurities from the cotton, but also attack the cellulose leading to heavy strength loss and weight loss in the fabric. Moreover, the application of these hazardous chemicals results in high COD (Chemical Oxygen Demand), BOD (Biological Oxygen Demand) and TDS (Total Dissolved Solid) in the waste water. Much research has been directed to replace this chemical process by a more environmental friendly enzyme-based one [10–19]. The mild reaction conditions offered by enzymatic treatment provide an environment friendly alternative [10, 20, 21]. Most of the researchers have used pectinase and cellulase for desizing and scouring processes but there have been only a few reports so far utilizing commercial xylanase for scouring purpose [15, 22].
The commercialization of biotreatment processes in textile industry faces the problem of removal of seed coat fragments that are usually black or dark brown and may or may not have fibres and linters attached [23]. These are the most resistant impurities of cotton that are not removed completely even after using more concentrated solutions and the residual part is only bleached during cotton preparation. Xylanase treatment could lead to hydrolysis of the tiny fibres that attached the seed-coat fragments to the fabric. This could make the residual seed-coat fragments more accessible to chemicals, thereby reducing the consumption of hydrogen peroxide in the consecutive chemical bleaching step [24].
Important criteria for industrial implementation of any technology include the existence of inexpensive and highly active enzyme preparations which can be obtained in bulk quantities. Therefore, a very high level xylanase producing Bacillus pumilus ASH has been isolated and various nutritional and fermentation parameters have been optimized for maximum xylanase yield using wheat bran for cost-effective production under submerged fermentation [25]. Here we are reporting utilization of xylanase in textile processes and its effect on desizing and scouring. Weight loss, reducing sugar liberation, whiteness and other physical properties of the fabric were measured after the enzymatic treatments.
Materials and Methods
Microorganism
The organism was isolated from soil samples collected from sanitary landfill using xylan agar medium (pH 7.0) at a temperature of 37°C. The organism was identified as Bacillus pumilus ASH by the Institute of Microbial Technology (IMTECH), Chandigarh, India and has been given accession no. 7411.
Production of Xylanase Under Submerged Fermentation
The xylanase production was studied in Erlenmeyer flask (250 ml) containing 50 ml of medium having (g/l): Yeast extract, 2.5; Peptone, 2.5; KNO3, 2.5; Wheat bran, 20.0 and Olive oil, 0.2%(w/v); pH 8.0. The flasks were inoculated with 2.5% (O.D. ~0.5) of the overnight grown inoculum and incubated at 37°C under shaking conditions (200 rpm) for 26 h. The extracellular enzyme was harvested by centrifuging at 10,000×g for 15 min and assayed for xylanase activity [25].
Desizing of Fabric
Sized grey Cotton and Micropoly fabrics (122 g−2) of salt no. 0701DR007 and 5012117 respectively were desized using xylanase in the presence 3% of wetting agent (MRZ) and 2% of chelating agent (EDTA) added in distilled water with a liquor ratio of 1:100; with varying enzyme doses ranging from 5–7.5 IU/g of dry fabric. The fabrics were treated with enzyme in agitated and non-agitated systems, at various temperatures ranging from 55 to 70°C and at pH 6–8 of the solution for 0.5, 1, 1.5 and 2 h. In the agitation system, agitation speed was set to 50 rpm during the biotreatment. After enzymatic treatment, the fabrics were washed twice in boiling distilled water to deactivate the enzyme and to remove the sizing chemicals. Thereafter the fabrics were air-dried at room temperature. Reference treatments were carried out similarly without enzyme addition. The effluent so released after the desizing step was quantitatively assayed for the reducing sugars by DNS method [26].
Determination of Fabric Properties
The whiteness index (WI) value is a measure of whiteness achieved after the treatment. Whiteness of the fabric was measured through Datacolour International Spectraflash SF 600 plus – CT spectrophotometer. For accurate measurement of degree of desizing, TEGEWA test was performed and from Violet Scale Reading values, Desizing Efficiency was calculated. Percentage Desizing Efficiency (DE) was measured by calculating the ratio of difference of total sizing material used initially and sizing material left with initial value of the sized material used. Violet Scale Reading (VSR) corresponds to DE as the value on violet scale was in correlation with the percentage of the sizing material removed after the enzymatic desizing.
TEGEWA Test
To 100 ml of distilled water, 10 g of KI and 0.6358 g of I2 were added, stirred and agitated. When I2 was completely dissolved, filled up to 800 ml with distilled water and then final volume was made to 1 l by addition of ethanol. Solution was spotted drop wise onto the fabric and was gently rubbed. The change in color of the fabric was assessed by referring to violet scale reading. The test was carried out on fabric cooled to room temperature and the residual alkalinity was neutralized prior to test.
Fabric Bioscouring
To match the standard industrial scouring conditions and to obtain the similar level of percentage removal of hemicellulosic compounds besides achieving the similar level of water absorbing property of the fabric as it was with the conventional chemical process, the fabrics were subjected to bioscouring. The desized cotton and micropoly fabrics obtained after the enzymatic desizing treatment were subsequently suspended in buffers of pH ranging from 6 to 8 containing 4% MRZ as wetting agent, 1.5% EDTA as chelating agent and 2% Sirrix 2-UDI as stabilizer to aid the bioscouring process and the processing liquor ratio was set as 1:20. Different dosage of xylanase in the range of 5 to 7.5 IU/g were added to the solution for treatment times of 0.5, 1, 2 and 3 h at different incubation temperatures ranging from 55 to 70°C. The experiments were performed in both the non-agitated and agitated systems where the agitation speed was set to 50 rpm. Different sets of experiments were set up as demonstrated in Fig. 1. After the reaction period, the fabrics were then rinsed with buffer solution of pH 7.0, finally washed with water and air dried. The fabrics were then equilibrated in a constant temperature humidity chamber for at least 24 h before any subsequent measurements. Caustic scouring was performed at 80°C for 20 min in a solution containing 50 g/l sodium hydroxide and 1 g/l surfactant.
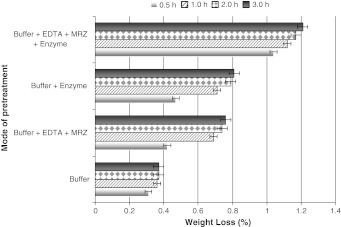
Fig. 1.
Effect of enzyme, chelating and wetting agent on the weight loss and reducing sugar liberation in non agitated solutions. Fabrics were treated in phosphate buffer; pH 7.0 at 60°C for 3 h
The whiteness, amount of reducing sugars released and weight loss of the fabric were determined and expressed as the difference before and after the bioscouring treatment. Bioscoured fabrics were compared with alkaline scoured fabrics and their whiteness, tensile strength and tearness were studied. Tensile strength was measured through tensile strength meter TITAN and Tearness was measured through ELMATEAR instrument and both are represented in g/f (gram per force). Scanning electron micrographs were taken to study the surface effects of the enzymatic treatments on the cotton fabrics.
In order to study the wetting time of the bioscoured fabrics, the fabrics were treated with the surfactants such as Tween-20 and Triton X-100 and water absorbing property of the fabrics was studied. Determination of the fabric wetting time was used for monitoring bioscouring performance and was measured by simple detection of the time required to absorb one drop (10 μl) of water. Average values of three determinations were taken.
Results
Our enzyme is active over a broad range of pH ranging from 5 to 10 and is thermostable up to 70°C that makes it suitable for application in textile industry.
Enzymatic Desizing
Several combinations of different parameters were used to optimize important physical conditions for enzymatic desizing. Maximum desizing efficiency was observed after 90 min treatment time using 5.0 IU/g enzyme concentration at 60°C and pH 7.0, where the maximum liberation of reducing sugars was also observed.
Some increase in whiteness value was noted upon the removal of the non-cellulosic substances from the fiber. Fabric type had an effect on the WI properties as the micropoly fabric showed more desizing than cotton under same conditions. Release of reducing sugars was more at pH 7.0 for both cotton and micropoly fabrics and whiteness of micropoly fabrics also increased at this pH. VSR from the TEGEWA test showed that at pH 7.0 and at 60°C the readings fall in the range of 4–5 indicating better desizing efficiency at these parameters (Table 1).
Table 1.
Optimized conditions for desizing by our enzyme
| pH | T (°C) | RT (in min) | ED (IU/g) | Cotton fabric (0701DR001) | DE (%) | VSR | Micropoly fabric (5012117) | DE (%) | VSR | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SL | Whiteness | SL | Whiteness | ||||||||
| 6.0 | 65 | 120 | 05 | 38.75 | 15.90 | 0.35 | 3–4 | 17.92 | 21.04 | 0.2 | 4–5 |
| 7.0 | 60 | 90 | 05 | 43.16 | 16.88 | 0.2 | 4–5 | 21.65 | 34.77 | 0.2 | 4–5 |
| 8.0 | 60 | 90 | 05 | 36.4 | 18.89 | 0.35 | 3–4 | 14.24 | 29.29 | 0.35 | 3–4 |
ED enzyme dosage, RT reaction time, DE desizing efficiency, SL sugar liberated expressed in mg/ml
Bioscouring
Enzymatic degradation of cotton is generally characterized by weight loss. The treatment of fabrics in phosphate buffer; pH 7.0 at 60°C for 3 h resulted in weight loss of the fabric. The water extractable impurities came into the buffer solution. The weight loss was increased by the addition of enzyme to the buffer. For simultaneous treatment with enzyme and EDTA, the weight loss values are higher compared to the individual use of enzyme or EDTA (Fig. 1). While weight loss data characterized the overall degradation effect of these treatments, reducing sugars in the treatment filtrates reveal the effectiveness of the enzyme action. The pH of the solution had a significant effect on the weight loss and the liberation of reducing sugars from the fabric (Table 2). Maximum weight loss was observed when the fabrics were suspended in phosphate buffer of pH 7.0.
Table 2.
Effect of different pH and treatment time on bioscouring of desized fabrics with xylanase
| pH | Ta | Cotton | Micropoly | ||
|---|---|---|---|---|---|
| WLb (%) | SL | WLb (%) | SL | ||
| 6.0 | 30 | 0.617 | 1.3 | 0.608 | 1.26 |
| 60 | 0.665 | 3.15 | 0.657 | 3.13 | |
| 120 | 0.74 | 5.9 | 0.738 | 5.88 | |
| 180 | 0.76 | 6.12 | 0.764 | 6.15 | |
| 7.0 | 30 | 1.04 | 3.51 | 0.82 | 3.48 |
| 60 | 1.12 | 4.16 | 0.91 | 4.15 | |
| 120 | 1.17 | 7.03 | 1.04 | 6.98 | |
| 180 | 1.21 | 8.13 | 1.06 | 7.91 | |
| 8.0 | 30 | 0.51 | 1.9 | 0.49 | 1.05 |
| 60 | 0.58 | 2.94 | 0.56 | 2.93 | |
| 120 | 0.59 | 3.62 | 0.59 | 3.62 | |
| 180 | 0.60 | 3.87 | 0.62 | 3.94 | |
These experiments have been performed at enzyme dosage of 5 IU/g under non-agitated conditions. Bold signifies optimum values
SL Sugar liberated expressed in mg/ml
aTime in minutes
bWeight Loss of desized fabric with respect to control
The weight loss value of the fabrics and liberation of reducing sugars seemed to increase with an increase in the treatment time of the fabrics in presence of the enzyme. Treatment for 30 min caused much lower degradation than for 3 h at an optimal enzyme dosage of 5 IU/g. The degrading ability of the enzyme could be slightly enhanced by agitation, thus the weight loss value of 1.5% was observed for cotton fabric after 1 h in case of agitated systems (Table 3). The results suggest that there is a direct relationship between the weight loss of the fabrics and the amount of reducing sugars liberated in the bath. Increase in reaction time and agitation also results in increase in reducing sugar production. Also, the weight loss values and the liberation of reducing sugars were estimated to be higher in case of cotton as compared to the micropoly fabrics.
Table 3.
Effect of different treatment time, enzyme dosage and agitation on bioscouring of Desized fabrics with xylanase
| Ta | EDb | Agitation | Cotton | Micropoly | ||
|---|---|---|---|---|---|---|
| WLc (%) | SL | WLc (%) | SL | |||
| 30 | 05 | + | 1.18 | 3.52 | 0.98 | 3.84 |
| 60 | 05 | + | 1.50 | 5.07 | 1.16 | 4.22 |
| 120 | 05 | + | 1.53 | 7.61 | 1.28 | 7.13 |
| 180 | 05 | + | 1.55 | 8.49 | 1.34 | 8.18 |
| 30 | 7.5 | – | 0.57 | 1.97 | 0.45 | 1.69 |
| 60 | 7.5 | – | 0.76 | 2.84 | 0.55 | 2.52 |
| 120 | 7.5 | – | 0.79 | 3.14 | 0.57 | 3.14 |
| 180 | 7.5 | – | 0.81 | 3.67 | 0.60 | 3.84 |
| 30 | 7.5 | + | 0.59 | 2.32 | 0.57 | 1.53 |
| 60 | 7.5 | + | 0.77 | 2.85 | 0.57 | 2.57 |
| 120 | 7.5 | + | 0.80 | 3.79 | 0.62 | 3.15 |
| 180 | 7.5 | + | 0.82 | 3.81 | 0.63 | 3.88 |
These experiments have been performed at pH 7.0. Bold signifies optimum values
SL sugar liberated expressed in mg/ml
aTime in minutes
bEnzyme Dose in IU/g
cWeight Loss of desized fabric with respect to control
The bioscouring of fabrics with xylanase resulted in enhancement of various physical properties of the fabrics viz. whiteness, tensile strength and tearness by 0.9, 1.12 and 1.1% respectively, as compared to the conventionally alkaline scoured fabrics.
Wettability and Absorbance Properties
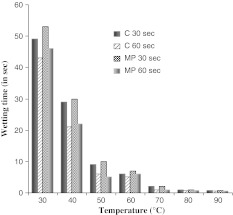
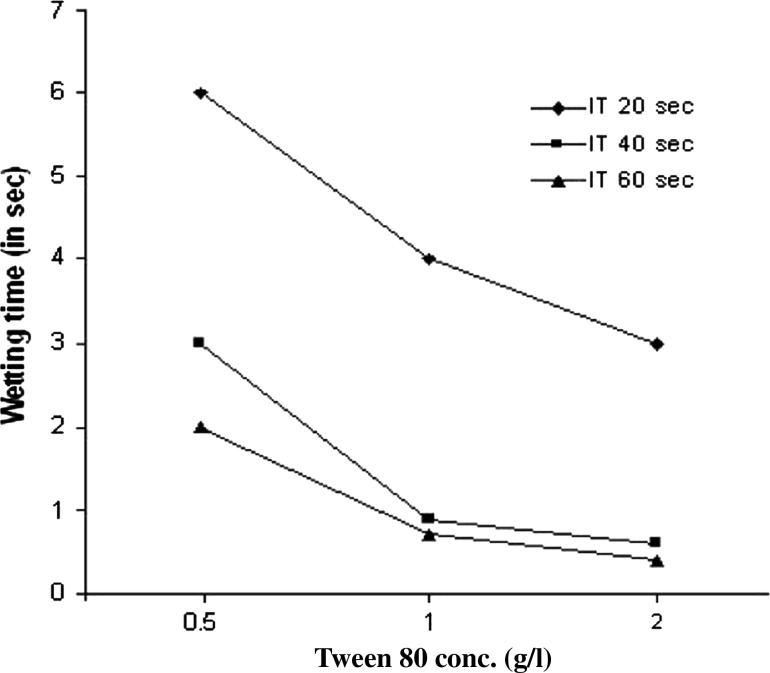
It was found that the wetting time values of fabrics obtained after 60 min of treatment during bioscouring were significantly lower to that of fabrics obtained after 30 min of treatment whereas further increasing the treatment time from 60 to 120 min did not result in much lowering of the wetting time. This clearly indicates the efficiency of xylanase in the scouring process as better the scouring; the lower would be the wetting time of fabric. Different parameters like surfactant concentration and temperature were examined for their impact on the wetting velocity. Full wetting was realized rapidly at temperatures of 80°C or above after treatment with surfactants in case of bioscoured (Fig. 2) and grey fabrics (Fig. 3). The results obtained after treating cotton with different concentrations of Triton X-100 (Fig. 4) and grey fabrics with different concentrations of Tween-20 (Fig. 5) indicate that with a surfactant dosage of 0.5 g/l and above wetting time decreased significantly. There was no appreciable effect on treating cotton and micropoly fabrics with Tween-20 and grey fabrics with Triton X-100. Wetting time for fabrics treated with Triton X-100 or Tween-20 for 30 and 60 s had value less than 01 s at 80 and 90°C. On increasing the concentration of either surfactant, the fabrics showed reduced wetting time values. It was also apparent from the results that at same temperatures and substrate concentration, the micropoly fabrics took longer time for wetting than cotton (Fig. 2).
Fig. 2.
Effect of varying temperature on the wetting time of bioscoured cotton and micropoly fabrics after Triton X-100 treatments. These experiments were performed at surfactant concentration of 0.5 g/l. Temperature of reaction mixture in °C. Temperature was varied from 30 to 90°C. C Cotton, MP Micropoly, C 30 s Effect on cotton after incubating for 30 s with Triton X-100, C 60 s Cotton incubated for 60 s, MP 30 s Micropoly fabric incubated for 30 s, MP 60 s Micropoly fabric incubated for 60 s
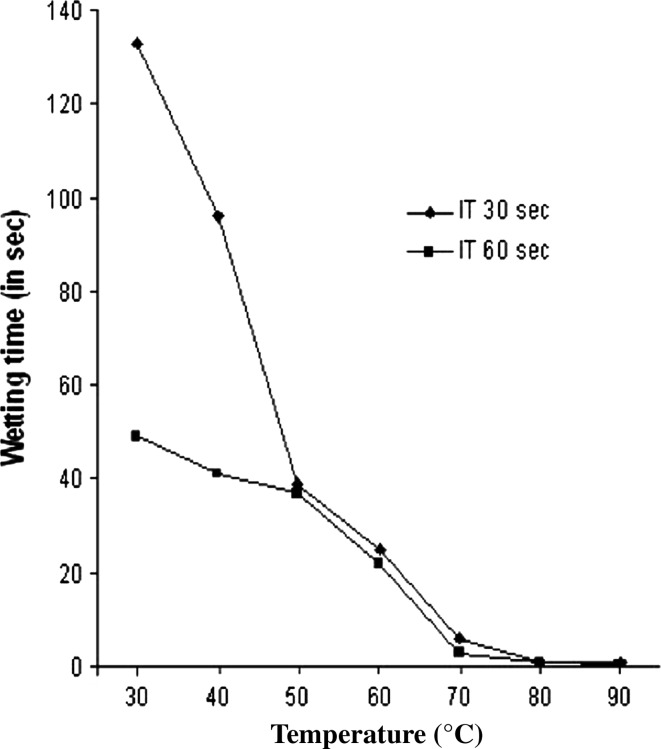
Fig. 3.
Effect of varying incubation temperature on the wetting time of Grey fabric (5012117) after treatment with Tween-20 and xylanase. Experiments were performed at surfactant concentration of 0.5 g/l. Temperature of reaction mixture in °C, IT Incubation time in seconds
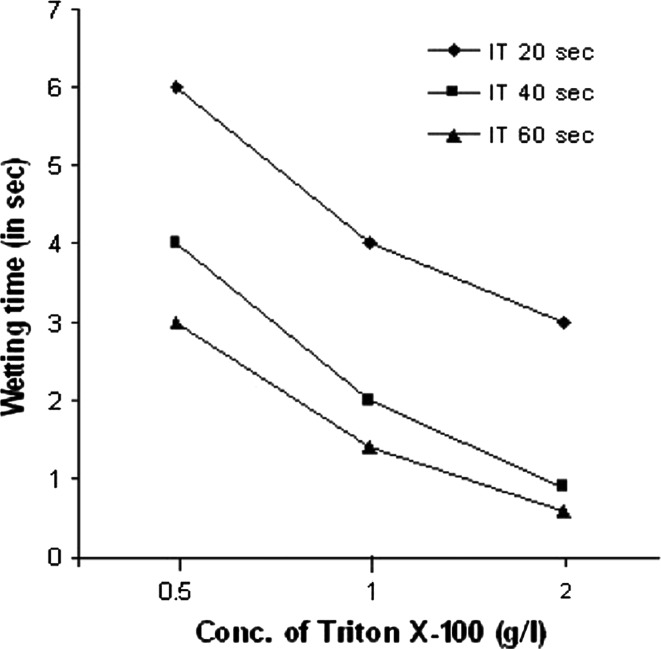
Fig. 4.
Effect of different concentrations of Triton X-100 on the wetting time of Cotton fabric after bioscouring. Experiments were performed at temperature 70°C, IT Incubation time in seconds
Fig. 5.
Effect of varying Tween-20 concentrations on the wetting time of Grey fabric (5012117) after xylanase treatment. Experiments were performed at temperature 70°C, IT Incubation time in seconds
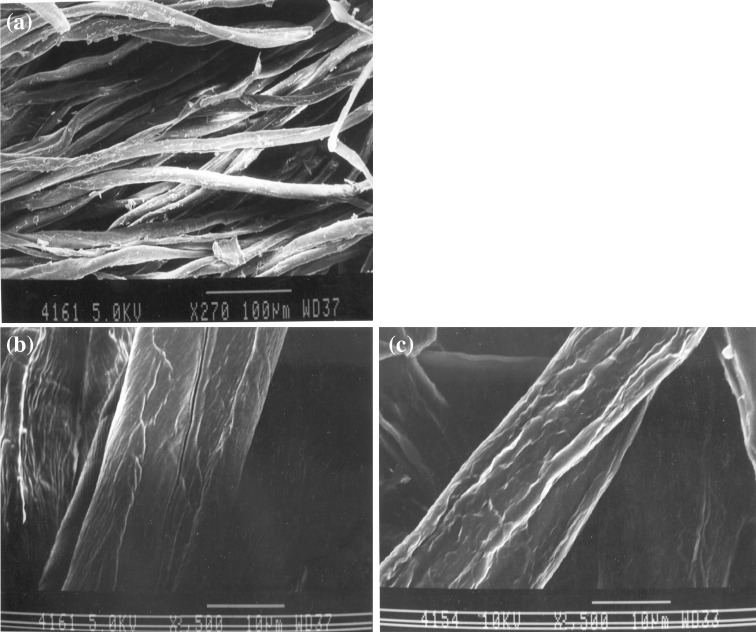
Electron micrographs of the cotton fabric clearly depict the enzymatic action during desizing and bioscouring. The xylanase treated fabrics were much softer and showed strength and weight loss as compared to the untreated fabric, because of the added strength of the pectins, hemicelluloses, and gums in the untreated fabric and removal of these non-cellulosic impurities from the fabric surface by the enzymatic action (Fig. 6a, b, c).
Fig. 6.
Scanning electron micrographs of a untreated control fabric at low magnification, b enzymatic desized fabric at high magnification c bioscoured fabric using xylanase at high magnification
Discussion
To integrate the desizing with the bioscouring process, the availability of an enzyme with broad operational windows is desirable. The operational pH for most enzymes available for these processes are in the acidic (4–7) or alkaline (7–9.5) range, while they all inactivated at temperatures above approximately 50–55°C [19]. The stability of B. pumilus enzyme at a broad pH and temperature range prompted us to apply it in fabric pretreatment processes.
Enzymatic desizing of both cotton and micropoly fabrics was most efficient at 60°C and at pH 7.0. During bioscouring, the significant weight loss was observed when chelating and wetting agent were combined with xylanase. Addition of EDTA to the enzyme solution resulted in higher weight loses of both the cotton and micropoly fabrics. Adding chelating and wetting agent to the enzyme solution can accelerate the hydrolysis degree enormously, indicating the synergistic effect of the enzyme and EDTA applied together in one treatment bath. EDTA modifies the substrate structure by removing the calcium ions from the cross bridges that link the macromolecules in pectin to one another or pectin to other polysaccharides., therefore, when applied simultaneously with the enzyme seems to assist in the creation of free and accessible areas for the enzyme present [22]. Our results support earlier observations that EDTA has a positive effect on the scouring process of the fabric [24]. Weight loss values obtained by using our enzyme are higher as compared to the values obtained by using commercial xylanase under similar conditions. Csiszar et al. [24] have reported weight loss of 1.4% during treatment of cotton fabric for 1 h using commercial xylanase with chelating agent in agitated system. The small weight loss values caused by the enzyme shows that the noncellulosic constituents were removed without significant cotton cellulose degradation. It is likely that mainly the surface fibrils, small protruding fibers, seed coat fragments and other natural impurities of cotton fibers are degraded significantly.
The surfactant adsorption influences the wettability of a cotton fabric and temperature is of major importance in this procedure, because at 80°C or above, the majority of the hydrophobic constituents are above their melting temperatures [27, 28], facilitating the hydrophobic constituent-surfactant interaction. The wetting time for enzymatically scoured fabrics is significantly lower to that of chemically scoured fabrics which indicates the efficiency of using enzyme in the scouring process.
After this bioscouring process, the cotton had an intact cellulose structure, with lower weight loss and strength loss. The fabric showed better wetting and penetration properties, making subsequent bleach process easy and resultantly giving much better dye uptake. The xylanase scouring also allowed the reduction of the hydrogen peroxide consumption in the consecutive chemical bleaching step. Thus the use of this enzyme in textile industry could lead to reactions that can reduce the negative environmental impact apart from improving the physical properties of the fabric.
Acknowledgments
The authors gratefully acknowledge Nahar Group of Industries, Punjab, India for providing cotton and micropoly fabrics and the laboratory facilities. Bindu Battan greatly acknowledges the financial assistance from Council of Scientific and Industrial Research, India in the form of Senior Research Fellowship during the course of investigation. Saurabh Sudha Dhiman and Sonia Ahlawat wish to thank Kurukshetra University, Kurukshetra for University Research Scholarship.
References
- 1.Sunna A, Antranikian G. Xylanolytic enzymes from fungi and bacteria. Crit Rev Biotechnol. 1997;17:39–67. doi: 10.3109/07388559709146606. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert HJ, Hazlewood GP. Bacterial cellulases and xylanase. J Gen Microbiol. 1999;139:187–194. [Google Scholar]
- 3.Battan B, Sharma JK, Kuhad RC. High-level xylanase production by alkalophilic Bacillus pumilus ASH under solid-state fermentation. World J Microbiol Biotechnol. 2006;22:1281–1287. doi: 10.1007/s11274-006-9173-x. [DOI] [Google Scholar]
- 4.Ball AS, McCarthy AJ. Saccharification of straw by actinomycete enzyme. J Appl Bacteriol. 1989;66:439–444. doi: 10.1111/j.1365-2672.1989.tb05113.x. [DOI] [Google Scholar]
- 5.Hrmova M, Biely P, Vrzanka M, Petrakova E. Induction of cellulose and xylan degrading enzyme complex in yeast Trichoderma cutaneum. Arch Microbiol. 1984;161:371–376. doi: 10.1007/BF00410906. [DOI] [Google Scholar]
- 6.Liu W, Zhu W, Lu Y, Kong Y, Ma G. Production, partial purification and characterization of xylanase from Trichosporon cutaneum SL409. Process Biochem. 1998;33:331–336. doi: 10.1016/S0032-9592(97)00071-X. [DOI] [Google Scholar]
- 7.Lipp-Symonowicz B, Tanska B, Wolukanis A, Wrzosek H. Influence of enzymatic treatment on the flax fibre morphological structure, physico-chemical properties and metrological parameters of yarn. Fibres Text. 2004;12:61–65. [Google Scholar]
- 8.Rouette HK (2001) Encyclopedia of textile finishing. Springer, Berlin, pp 1–3, ISBN 3-540-65031-8
- 9.Karmakar SR. Textile science and technology series. 1. Amsterdam: Elsevier Science B.V.; 1999. Chemical technology in the pretreatment processes of textiles; p. 12. [Google Scholar]
- 10.Hartzell MM, Hsieh YL. Enzymatic scouring to improve cotton fabric wettability. Text Res J. 1998;68(4):233–241. doi: 10.1177/004051759806800401. [DOI] [Google Scholar]
- 11.Li Y, Hardin IR. Enzymatic scouring of cotton-surfactants, agitation and selection of enzymes. Text Chem Color. 1998;30:23–29. [Google Scholar]
- 12.Etters JN. Cotton preparation with alkaline pectinase: an environmental advance. Text Chem Color Am Dyestuff Rep. 1999;1(3):33–36. [Google Scholar]
- 13.Traore MK, Buschle-Diller G (1999) Environmentally friendly scouring processes. In: Book of papers of the international conference and exhibition of the AATCC, Charlotte, pp 183–189
- 14.Buchert J, Pere J, Puolakka A, Nousiainen P. Scouring cotton with pectinases, proteases and lipases. Text Chem Color Am Dyestuff Rep. 2000;32(5):48–52. [Google Scholar]
- 15.Csiszar E, Urbanskzi K, Szakaes G. Biotreatment of desized cotton fabric by commercial cellulase and xylanase enzymes. J Mol Catal B. 2001;11:1065–1072. doi: 10.1016/S1381-1177(00)00149-1. [DOI] [Google Scholar]
- 16.Yachmenev VG, Bertoniere NR, Blanchard EJ. Effect of sonification on cotton preparation with alkaline pectinase. Text Res J. 2001;71(6):527–533. doi: 10.1177/004051750107100610. [DOI] [Google Scholar]
- 17.Lenting HBM, Zwier E, Nierstrasz VA. Identifying Important Parameters for a Continuous Bioscouring Process. Text Res J. 2002;72(9):825–831. doi: 10.1177/004051750207200912. [DOI] [Google Scholar]
- 18.Agrawal PB, Nierstrasz VA, Warmoeskerken MMCG (2004) Enhanced Bioscouring Performance. In: Proceedings of the 4th Autex conference, Roubaix, France, 22–24 June, pp 165–173
- 19.Lenting HBM, Warmoeskerken MMCG. A fast, continuous enzyme-based pretreatment process concept for cotton containing textiles. Biocatal Biotransform. 2004;22(5/6):361–368. doi: 10.1080/10242420400024557. [DOI] [Google Scholar]
- 20.Li Y, Hardin IR. Enzymatic scouring of cotton: effects on structure and properties. Text Chem Color. 1997;29:71–76. [Google Scholar]
- 21.Buchert J, Pere J, Puolakka A, Nousiainen P (1998) Enzymatic scouring of cotton. In: Book of papers, AATCC international conference and exhibition 1998, Philadelphia. Am Assoc Text Chem Color, 493–499
- 22.Losonczi A, Csiszar E, Szakacs G, Bezur L. Role of the EDTA chelating agent in bioscouring of cotton. Text Res J. 2005;75(5):411–417. doi: 10.1177/0040517505053812. [DOI] [Google Scholar]
- 23.Verschraege L (1989) Cotton fibre impurities. Neps, motes and seed coat fragments. ICAC review articles on cotton production research No 1. CAB International, Wallingford
- 24.Csiszar E, Losonczi A, Szakacs G, Rusznak I, Bezur L, Reicher J. Enzymes and chelating agents in cotton pretreatment. J Biotechnol. 2001;89(2–3):271–279. doi: 10.1016/S0168-1656(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 25.Battan B, Sharma J, Dhiman SS, Kuhad RC. Enhanced production of cellulase-free thermostable xylanase by Bacillus pumilus ASH and its potential application in paper industry. Enzyme Microb Technol. 2007;41:733–739. doi: 10.1016/j.enzmictec.2007.06.006. [DOI] [Google Scholar]
- 26.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 27.Hartzell-Lawson MM, Hsieh Y-L. Characterizing the non cellulosics in developing cotton fibres. Text Res J. 2000;70(9):810–819. doi: 10.1177/004051750007000909. [DOI] [Google Scholar]
- 28.Sindhu I, Chhibber S, Capalash N, Sharma P. Production of cellulase-free xylanase from Bacillus megaterium by solid state fermentation for biobleaching of pulp. Curr Microbiol. 2006;53:167–172. doi: 10.1007/s00284-006-0051-4. [DOI] [PubMed] [Google Scholar]