Abstract
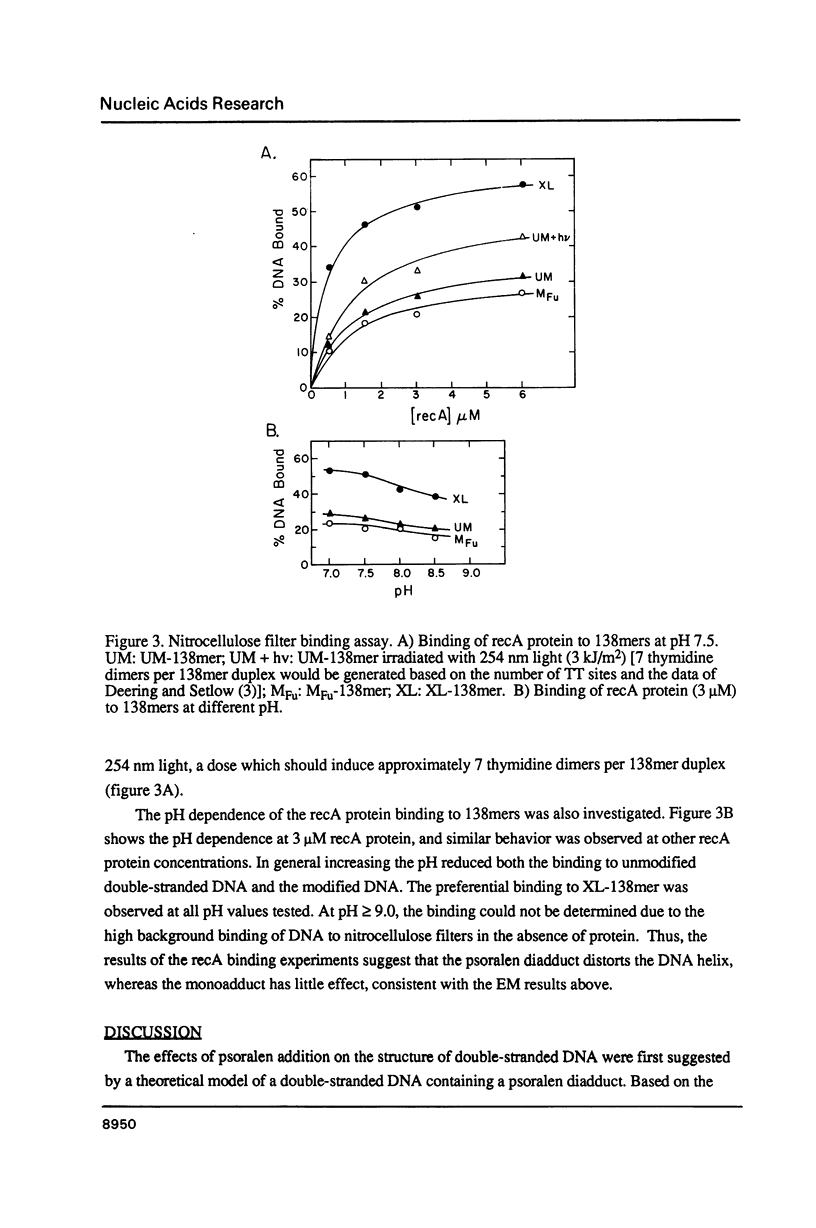
We have investigated the structural change in a double-stranded DNA helix caused by covalent addition of a psoralen. A synthetic double-stranded DNA was constructed to contain either a psoralen furan-side monoadduct or an interstrand diadduct at a specific site. When the unmodified and psoralen modified DNAs were examined by electron microscopy in the presence of distamycin, which stiffens the DNA helix, the DNA containing the psoralen interstrand diadduct appeared bent (or kinked), whereas the furan-side monoadducted DNA appeared similar to the unmodified DNA. RecA protein from E. coli has been shown to preferentially bind UV (ultra violet) irradiated DNA presumably due to alterations in the normal DNA helical structure. Using a nitrocellulose filter binding assay, we have found that the psoralen interstrand diadduct enhances the binding of recA protein to the double-stranded DNA, whereas a furan-side monoadduct has little effect. Thus both the recA protein binding and the electron microscopic data suggest that a psoralen diadduct causes deformation of a DNA helix, most likely by kinking the helix, and that a monoadduct has little effect on the DNA helix structure.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Shi Y. B., Hearst J. E. Wavelength dependence for the photoreversal of a psoralen-DNA cross-link. Biochemistry. 1986 May 20;25(10):3013–3020. doi: 10.1021/bi00358a042. [DOI] [PubMed] [Google Scholar]
- DEERING R. A., SETLOW R. B. Effects of ultraviolet light on thymidine dinucleotide and polynucleotide. Biochim Biophys Acta. 1963 Apr 30;68:526–534. doi: 10.1016/0006-3002(63)90181-1. [DOI] [PubMed] [Google Scholar]
- Ennis D. G., Fisher B., Edmiston S., Mount D. W. Dual role for Escherichia coli RecA protein in SOS mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3325–3329. doi: 10.1073/pnas.82.10.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D., Christiansen G. Electron microscope visualization of chromatin and other DNA-protein complexes. Annu Rev Biophys Bioeng. 1978;7:19–35. doi: 10.1146/annurev.bb.07.060178.000315. [DOI] [PubMed] [Google Scholar]
- Griffith J., Bleyman M., Rauch C. A., Kitchin P. A., Englund P. T. Visualization of the bent helix in kinetoplast DNA by electron microscopy. Cell. 1986 Aug 29;46(5):717–724. doi: 10.1016/0092-8674(86)90347-8. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Peckler S., Graves B., Kanne D., Rapoport H., Hearst J. E. Sharp kink of DNA at psoralen-cross-link site deduced from crystal structure of psoralen-thymine monoadduct. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):361–365. doi: 10.1101/sqb.1983.047.01.042. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lu C., Scheuermann R. H., Echols H. Capacity of RecA protein to bind preferentially to UV lesions and inhibit the editing subunit (epsilon) of DNA polymerase III: a possible mechanism for SOS-induced targeted mutagenesis. Proc Natl Acad Sci U S A. 1986 Feb;83(3):619–623. doi: 10.1073/pnas.83.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons B. J. Psoralen photochemistry. Photochem Photobiol. 1980 Dec;32(6):813–821. doi: 10.1111/j.1751-1097.1980.tb04061.x. [DOI] [PubMed] [Google Scholar]
- Pearlman D. A., Holbrook S. R., Pirkle D. H., Kim S. H. Molecular models for DNA damaged by photoreaction. Science. 1985 Mar 15;227(4692):1304–1308. doi: 10.1126/science.3975615. [DOI] [PubMed] [Google Scholar]
- Peckler S., Graves B., Kanne D., Rapoport H., Hearst J. E., Kim S. H. Structure of a psoralen-thymine monoadduct formed in photoreaction with DNA. J Mol Biol. 1982 Nov 25;162(1):157–172. doi: 10.1016/0022-2836(82)90166-8. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Gamper H., Van Houten B., Hearst J. E. Interaction of Escherichia coli RNA polymerase with DNA in an elongation complex arrested at a specific psoralen crosslink site. J Mol Biol. 1988 Jan 20;199(2):277–293. doi: 10.1016/0022-2836(88)90314-2. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Hearst J. E. Wavelength dependence for the photoreactions of DNA-psoralen monoadducts. 1. Photoreversal of monoadducts. Biochemistry. 1987 Jun 30;26(13):3786–3792. doi: 10.1021/bi00387a008. [DOI] [PubMed] [Google Scholar]
- Shi Y., Hearst J. E. Thermostability of double-stranded deoxyribonucleic acids: effects of covalent additions of a psoralen. Biochemistry. 1986 Oct 7;25(20):5895–5902. doi: 10.1021/bi00368a009. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Hagerman P. J. Interstrand psoralen cross-links do not introduce appreciable bends in DNA. Biochemistry. 1984 Dec 18;23(26):6299–6303. doi: 10.1021/bi00321a002. [DOI] [PubMed] [Google Scholar]
- Song P. S., Tapley K. J., Jr Photochemistry and photobiology of psoralens. Photochem Photobiol. 1979 Jun;29(6):1177–1197. doi: 10.1111/j.1751-1097.1979.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Tessman J. W., Isaacs S. T., Hearst J. E. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. Conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985 Mar 26;24(7):1669–1676. doi: 10.1021/bi00328a015. [DOI] [PubMed] [Google Scholar]
- Tomic M. T., Wemmer D. E., Kim S. H. Structure of a psoralen cross-linked DNA in solution by nuclear magnetic resonance. Science. 1987 Dec 18;238(4834):1722–1725. doi: 10.1126/science.3686011. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Construction of DNA substrates modified with psoralen at a unique site and study of the action mechanism of ABC excinuclease on these uniformly modified substrates. J Biol Chem. 1986 Oct 25;261(30):14135–14141. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigny P., Blais J., Ibanez V., Geacintov N. E. A flow linear dichroism study of the orientation of 4',5'-psoralen-DNA photoadducts. Photochem Photobiol. 1987 May;45(5):601–607. doi: 10.1111/j.1751-1097.1987.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Witkin E. M., Kogoma T. Involvement of the activated form of RecA protein in SOS mutagenesis and stable DNA replication in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7539–7543. doi: 10.1073/pnas.81.23.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]




