Abstract
The focal intent of this study was to find out an alternative strategy for the antibiotic usage against bacterial infections. The quorum sensing inhibitory (QSI) activity of marine sponges collected from Palk Bay, India was evaluated against acyl homoserine lactone (AHL) mediated violacein production in Chromobacteriumviolaceum (ATCC 12472), CV026 and virulence gene expressions in clinical isolate Serratiamarcescens PS1. Out of 29 marine sponges tested, the methanol extracts of Aphrocallistesbocagei (TS 8), Haliclona (Gellius) megastoma (TS 25) and Clathriaatrasanguinea (TS 27) inhibited the AHL mediated violacein production in C. violaceum (ATCC 12472) and CV026. Further, these sponge extracts inhibited the AHL dependent prodigiosin pigment, virulence enzymes such as protease, hemolysin production and biofilm formation in S. marcescens PS1. However, these sponge extracts were not inhibitory to bacterial growth, which reveals the fact that the QSI activity of these extracts was not related to static or killing effects on bacteria. Based on the obtained results, it is envisaged that the marine sponges could pave the way to prevent quorum sensing (QS) mediated bacterial infections.
Keywords: Marine sponges, Quorum sensing inhibition, Acyl homoserine lactone, Serratia marcescens PS1, Biofilm, Bacterial pathogenesis
Introduction
Serratia marcescens is an opportunistic human pathogen causing infections including respiratory tract, urinary tract, meningitis, septicemia, pneumonia and wound infections by secreting a number of virulence factors capable of damaging human cells and tissues [6]. It shows high resistance against a wide range of β-lactam, aminoglycoside and fluoroquinolone antibiotics and thereby makes the antibiotic treatment become ineffective [25, 28]. Thus, the emergence of antibiotic resistance among bacterial pathogen necessitate the findings of alternative strategies to antibiotic treatment. The recent discoveries in the field of bacterial cell–cell communication system suggest an alternative treatment to conventional chemotherapy [1, 8]. It has been known well that in S. marcescens the quorum sensing (QS) regulates the expression of genes responsible for the production of prodigiosin pigment, virulence factors like hemolysin, proteases, chitinase, chloroperoxidase, multiple isozymes of alkaline phosphatase, the ability to swim, swarm and biofilm maturation [20, 21].
Marine sponges are considered to be a rich depository of bioactive compounds with antibacterial, antifungal, antiviral, antifouling, anti-HIV, immunosuppressant and cytotoxic activities. Sponges like Haliclona, Petrosia and Discodermia are known to produce powerful anti-cancer and anti-inflammatory compounds [13, 18]. Similarly, Manoalide derivatives in the extract of marine sponge Luffariella variabilis have shown a strong quorum sensing inhibitory (QSI) activity and acted as a good antagonist against the QS systems of P. aeruginosa [23]. Although, the marine sponges are known for their bioactive potential, studies on its QSI properties remain scanty. Hence, the present investigation is aimed to study the effect of QSI activity of marine sponge extracts against the QS systems of C. violaceum and S. marcescens PS1.
Materials and Methods
Preparation of Sponge Extracts
A total of 29 different sponges found lying on the shore line of fisherman harbor, Palk Bay coastal area (latitude 9°45′N and longitude 79°3′E), Bay of Bengal, India were collected. The collected samples were washed thoroughly with sterile distilled water, shade dried and powdered. 10 g of powdered samples were suspended in 100 ml of methanol and the suspensions were kept on a shaker for overnight (O/N) at 150 rpm. The obtained crude extracts were weighed and stored at 4 °C until further use. The sponges which showed potential QSI activity were identified by Zoological Survey of India, Port Blair, Andaman, India.
Bacterial Strains and Their Culture Conditions
In the present study C. violaceum (ATCC 12472), CV026 a mini-Tn5 mutant derived from C. violaceum (ATCC 31532) and clinical isolate S. marcescens PS1 were used. C. violaceum(ATCC 12472) and CV026 were used as marker strains. C. violaceum (ATCC 12472) is a wild type strain and has the ability to synthesize QS mediated violacein pigment by its own autoinducer known as N-hexanoyl homoserine lactone (C6-AHL); whereas, CV026 is unable to synthesis C6-AHL but respond to external source of AHL. This mutant strain was obtained as generous gift from Prof. Jo Handelsman, (University of Wisconsin, USA). S. marcescens PS1 used in this study is a clinical strain isolated from a patient with urinary tract infection and identified through 16S rRNA gene sequences (GenBank accession number: FJ584421). All the bacterial strains were allowed to grow aerobically in Luria–Bertani (LB) broth (Hi-Media, India) at their optimum temperature (30 °C) to an OD600nm = 1.2 and used for the further analysis. In each experiment, 1 % of O/N culture of respective test bacterial strains were added to the fresh LB medium (OD adjusted to 0.4 at 600 nm) and cultivated in the presence and absence of crude sponge extracts. After 18 h incubation, the cell free supernatants were obtained by centrifugation at 10,000×g for 10 min [1] and were subjected to further analysis.
Evaluation of QSI Efficiency of Sponge Extracts
Violacein Inhibition Assay
Totally, 29 different sponge extracts were screened against violacein production in C. violaceum (ATCC 12472) and CV026. In qualitative analysis, 1 % (10 μl) of O/N culture (OD adjusted to 0.4 at 600 nm) of C. violaceum (ATCC 12472) was added to the individual wells of 24 well micro titre plates (MTP) (Greiner Bio-One) containing 1 ml of LB broth without and with the test extracts at the final concentration of 7 mg/ml [4]. Based on QSI efficiency in qualitative analysis, three sponges such as TS8, TS25 and TS27 were selected to assess their inhibitory potential against AHL mediated violacein production in C. violaceum (ATCC 12472) using agar diffusion assay by following the method of McLean et al. [15] with slight modification. The agar plates were swabbed with C. violaceum (ATCC 12472) and the pieces of washed sponges were placed over the swabbed agar plates. The plates were incubated at room temperature for 24 h and observed for the presence of colourless zone around sponges. In quantitative analysis, CV026 along with pure C6-AHL (Sigma, USA) at the concentration of 1 μmol/ml was added in LB broth treated with selected sponge extracts (1.75, 3.5, 5.25 and 7 mg/ml) and the control set was maintained without addition of sponge extracts. The violacein pigment production in CV026 supernatant was quantified at OD585nm [4] in UV–Vis spectrophotometer (HITACHI U-2800, Japan).
Biofilm Prevention Assay
In biofilm prevention assay, 10 μl of O/N culture of S. marcescens PS1 in above mentioned cell density was added to 1 ml of LB broth in the wells of 24 well MTP containing glass slides (1 × 1 cm) and supplemented with and without sponge extracts (7 mg/ml). Culture set up was incubated without agitation at 30 °C for 18 h [27]. After the incubation, planktonic cells and spent media were discarded; adherent cells in glass slides were gently rinsed twice with deionized water (Millipore–Milli-Q). The biofilm was stained with 0.1 % acridine orange and 0.4 % crystal violet solutions for Confocal Laser Scanning Microscope (CLSM) and light microscopic analyses, respectively for 2 min. Further, the glass slides were rinsed twice with deionized water to remove the excess stain. The stained glass slides were observed at magnification of 20× under the CLSM (LSM 710, Carl Zesis, Germany) and 400× under light microscope with an attached digital camera (Nikon Eclipse Ti 100).
Prodigiosin Inhibition Assay
The S. marcescens cells were harvested from 2 ml of both sponge extract treated (1.75–7 mg/ml) and untreated cultures by centrifugation at 10,000×g for 10 min. The prodigiosin pigment from the harvested cells was extracted in 1 ml of acidified ethanol (4 ml of 1 M HCl in 96 ml of ethanol). The amount of prodigiosin production was measured at OD534nm [24].
Azocasein Assay
Azocasein assay was carried out to estimate the total proteolytic activity of the S. marcescens PS1. 100 μl of sponge extracts treated (1.75–7 mg/ml) and untreated culture supernatant was added to 1 ml of 0.3 % azocasein (Sigma, USA) in 0.05 M Tris–HC1, 0.5 mM CaCl2 (pH 7.5) and incubated at 30 °C for 15 min. Reactions were stopped by adding 500 μl of trichloroacetic acid (l0 %), the precipitate was removed by centrifugation and absorbance of the clear supernatants was measured at OD400nm [17].
Hemolysin Assay
In hemolysin assay, the cell free supernatants (100 μl each) of both sponge extracts treated (1.75 and 7 mg/ml) and untreated cultures were supplemented with 900 μl of 2 % washed sheep erythrocytes containing 20 mM CaCl2, 10 mM Tris, and 160 mM NaCl at pH 7.4. The mixture was kept for 20 min in ice, centrifuged and the released hemoglobin in the supernatant was measured at OD530nm. The results were expressed as percent lysis compared with the lysis of erythrocytes in distilled water [22].
Growth Curve Assay
The effect of sponge extracts on cell proliferation was determined by monitoring the growth of C. violaceum (ATCC 12472) and S. marcescens PS1. Briefly, OD of O/N culture was adjusted as mentioned above and allowed to grow in 50 ml of LB medium in the presence and absence of sponge extracts (7 mg/ml). The cell growth was measured up to 24 h with 1 h interval at OD600nm [1].
Statistical Analysis
All the experiments were performed in triplicates to validate reproducibility and the p values were calculated statistically by Student’s t test. Comparison analysis was performed for the tested sponge extracts used at every concentration.
Results
Interference of Violacein Production by Sponge Extracts
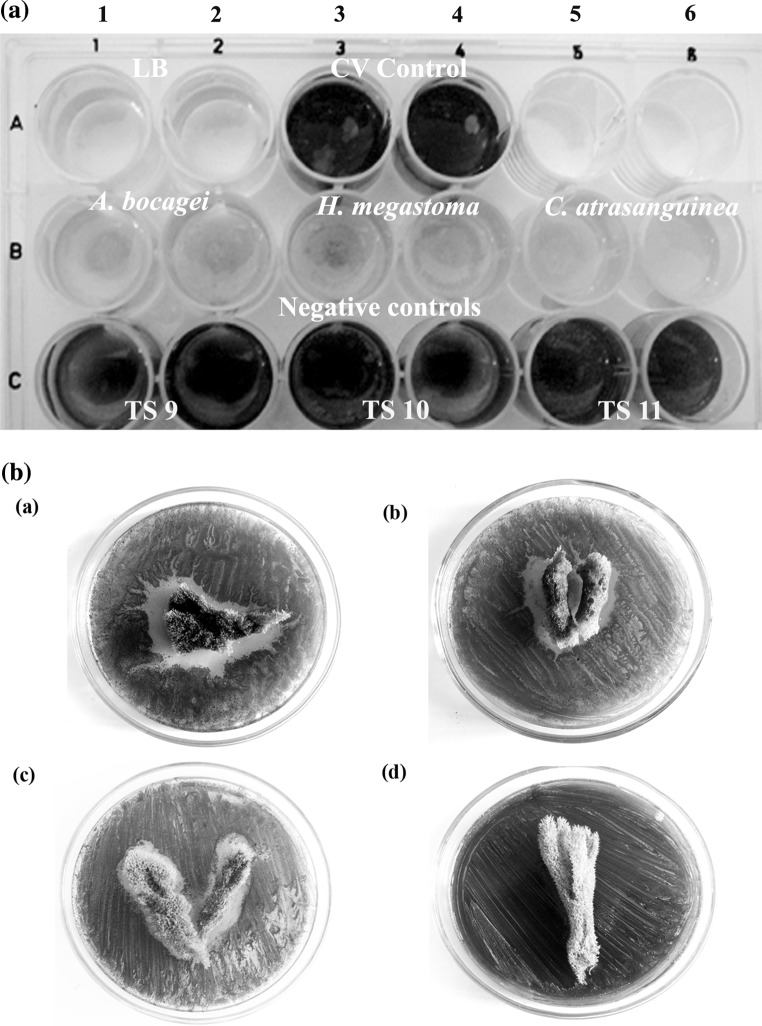
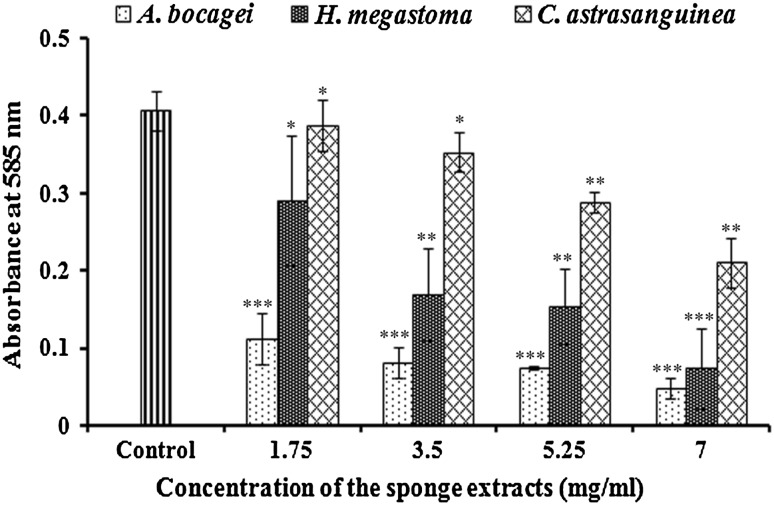
In violacein inhibition assay, out of 29 different sponges screened, only the extracts of TS8, TS25 and TS27 exhibited reduction in violacein production in C. violaceum (ATCC 12472) (Fig. 1A). Further, in agar diffusion assay, the lack of violacein pigment of C. violaceum (ATCC 12472) around the above mentioned sponges indicated the inhibition of violacein production (Fig. 1B, a–c), while in the negative control no such activity was observed (Fig. 1B, d). Based on the morphological characters these potent sponges were identified as Aphrocallistes bocagei (TS8), Haliclona (Gellius) megastoma (TS25) and Clathria atrasanguinea (TS27). In quantitative analysis with CV026, these sponge extracts exhibited a concentration dependent decrease in violacein production with that of control. The extract of A. bocagei (1.75–7 mg/ml) showed a maximum of 72–87 % (p < 0.005) decrease in violacein production. A substantial decrease was observed with H. megastoma and C. atrasanguinea extracts to the level of 29–75 % (p < 0.005) and 5–46 % (p < 0.025), respectively (Fig. 2).
Fig. 1.
Effect of sponge extracts on C6-AHL dependent violacein production in C. violaceum. A Qualitative inhibition of violacein production in C. violaceum (ATCC 12472) by methanol extracts of marine sponges at a concentration of 7 mg/ml. (RowA: Well 1 and 2 Media control, Well 3 and 4––C. violaceum (ATCC 12472) control, RowB: C. violaceum (ATCC 12472) treated: Well 1 and 2––A. bocagei (TS 8), Well 3 and 4––H. megastoma (TS 25), Well 5 and 6––C.atrasanguinea (TS 27), RowC: Negative controls such as TS 9, TS 10 and TS 11. There is no growth inhibition evident in all the wells, B Agar diffusion assay for the inhibition of violacein production in C. violaceum (ATCC 12472) by marine sponges: (a) A. bocagei, (b) H. megastoma, (c) C. atrasanguinea, (d) Negative control (TS 10)
Fig. 2.
Dose dependent inhibition of violacein production in CV026 by marine sponge extracts. The data represents the mean values of three independent experiments. ***p ≤ 0.0005, **p ≤ 0.01, *p ≤ 0.10 indicates the statistical value of treated compared to control
Effect of Sponge Extracts on Prodigiosin Production
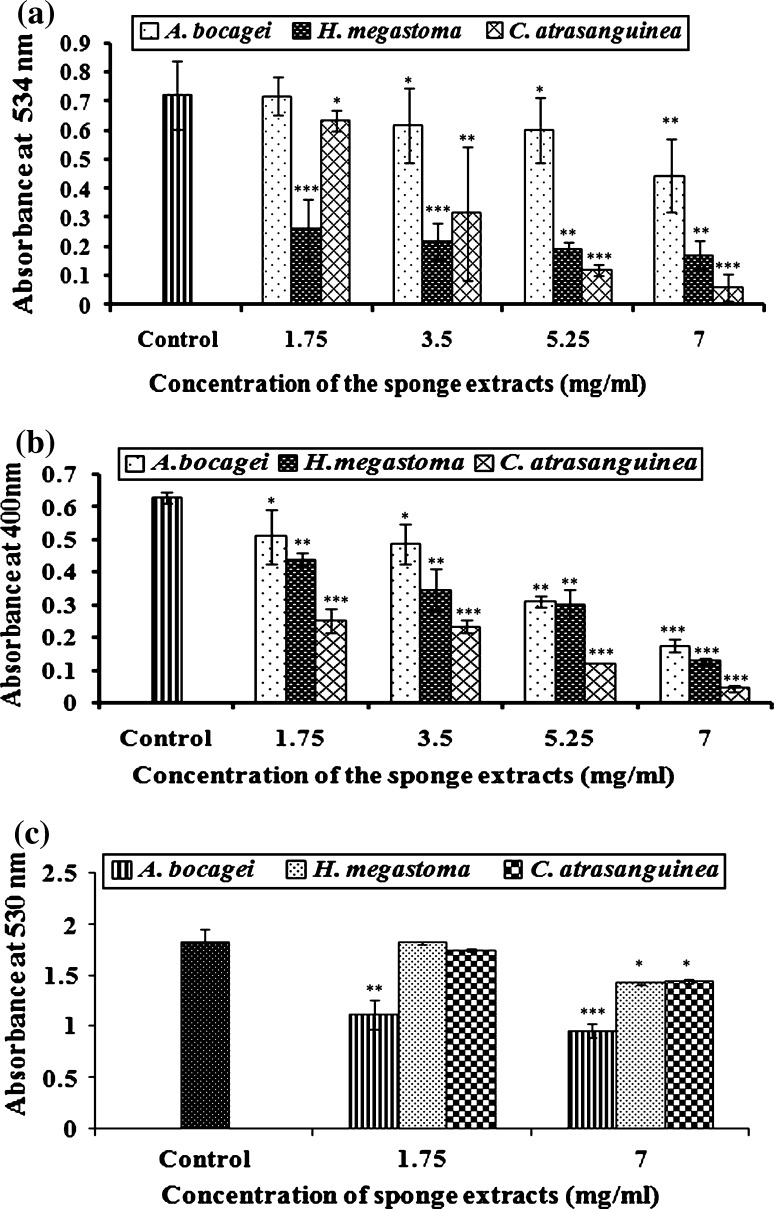
In prodigiosin assay, the extract of C.atrasanguinea (1.75–7 mg/ml) effectively interfered with the QS system of S. marcescens PS1 and showed significant reduction in prodigiosin production to the level of 12–93 % (p < 0.0005). Similarly, at the same concentrations the extracts of H. megastoma and A. bocagei also inhibited the pigment production to the level of 64–76 % (p < 0.005) and 0–41 % (p < 0.05), respectively (Fig. 3a).
Fig. 3.
Effect of sponge extracts on C4-AHL mediated phenotypic characters such as prodigiosin, protease and C6-AHL dependent hemolysin production of S. marcescens PS1 a Prodigiosin production is expressed as OD at 534 nm after incubation with acidified ethanol solution, b Inhibition of protease––total proteolytic activity is expressed as the decrease in OD at 400 nm per microgram of protein, c Inhibition of hemolysis is expressed as percent lysis compared with the lysis of erythrocytes in distilled water. All the assays are performed in triplicates. The data represent the mean value of three independent experiments. *** p ≤ 0.0005, ** p ≤ 0.01, * p ≤ 0.10 indicates the statistical value of treated compared to control
Inhibition of Protease Production
In protease assay, the extract of C. atrasanguinea (1.75–7 mg/ml) showed inhibition in protease production (p < 0.0005) to the level of 60–93 %. Similarly, H. megastoma and A. bocagei showed gradual reduction in protease production to the level of 30–80 % (p < 0.0005) and 19–72 % (p < 0.005), respectively (Fig. 3b).
Secreted Hemolysin Inhibitory Activity
A gradual increase in the viability of sheep erythrocytes was observed with sponge extracts at the concentrations of 1.75 and 7 mg/ml. Reduction in the sheep erythrocyte lysis in the presence of sponge extracts were compared with that of 100 % distilled water lysis. A significant (p < 0.005) reduction of 39–47 % was observed in S. marcescens PS1 with the sponge extract A. bocagei. Further, a considerable reduction in hemolysin production was observed with the extracts of C. atrasanguinea and H. megastoma to the level of 3–21 % (p < 0.10) and 0–22 % (p < 0.10), respectively (Fig. 3c).
Biofilm Inhibitory Activity
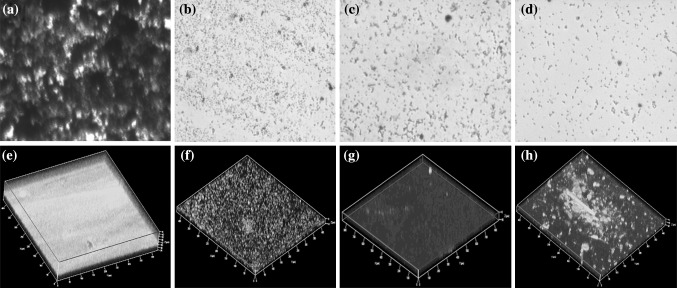
The impact of sponge extracts against the biofilm forming ability of S. marcescens PS1 was assessed through light microscopic and CLSM image analyses. In light microscopic analysis, a visible reduction in the biofilm formation of S. marcescens was observed as shown in Fig. 4b–d when treated with sponge extracts such as A. bocagei,H. megastoma and C. atrasanguinea with that of untreated control (Fig. 4a). Further, in CLSM analysis all the three sponge extracts showed reduction in the thickness of the biofilm as shown in Fig. 4f–h with that of untreated control (Fig. 4e).
Fig. 4.
Effect of sponge extracts (7 mg/ml) on biofilm formation of S. marcescens. Light microscopic analysis of a untreated control and b–dA. bocagei, H. megastoma and C. atrasanguinea extracts treated biofilm of S. marcescens, respectively. CLSM analysis of e untreated and f–hA. bocagei,H. megastoma and C. atrasanguinea extracts treated biofilm of S. marcescens, respectively
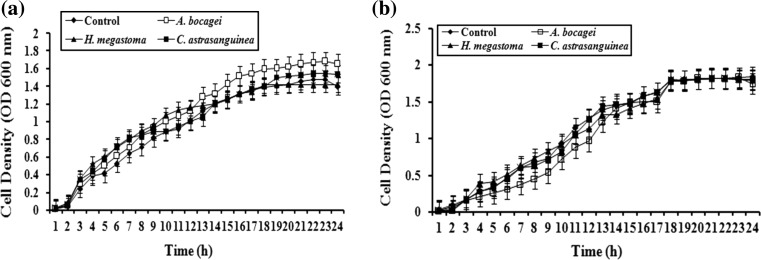
Growth Inhibition Analysis
In growth inhibition analysis, the bacterial cultures treated with the sponge extracts at the tested concentration of 7 mg/ml did not show any significant variation in the cell density of C. violaceum and S. marcescens with that of controls (Fig. 5a, b). These results confirmed that the inhibition of QS dependent phenotypic expressions in C. violaceum and S. marcescens by sponge extracts were not due to antibiotic activity, but due to QSI activity.
Fig. 5.
Effect of sponge extracts (7 mg/ml) on growth of aC. violaceum (ATCC 12472) and bS. marcescens PS1. Extracts were added at 0 h. The data represent the mean values of experiments performed in triplicates. Data are presented as the absorbance of mean ± SD
Discussion
QS signals play an important role in the interaction between bacteria and their eukaryotic hosts. Eukaryotes can detect and respond to the bacterial QS signals [2, 26]. Several potential QS inhibitor compounds have been discovered from eukaryotic and prokaryotic origin, which includes metabolites like halogenated furanones from Australian macroalgae Delisea pulchra [14], AHL degrading enzymes such as lactonase from Bacillus sp. [7], lactonase and acylase from actinobacteria, α, β and γ proteobacteria [9] and other unknown compounds from edible plants and fruits [16], garlic [5], Laminaria digitata [3], Ahnfeltiopsis flabelliformis [12], Coral associated bacterial extracts [27] and Bacillus sp. SS4 [17] that are known to interfere with AHL molecules mediated QS.
Out of 29 sponge extracts tested, three sponges such as A. bocagei,H. megastoma and C. atrasanguinea showed potential QSI activity against violacein production in both C. violaceum (ATCC 12472) and CV026 (Figs. 1, 2). Results obtained in the present study are comparable with the previous work performed with the extracts of Vanilla planifolia [4] and unknown compounds obtained from essential oils [11]. Hence, these three sponge extracts such as A. bocagei,H. megastoma and C. atrasanguinea alone were further subjected to evaluate their QSI activity against QS mediated phenomena in clinical strain S. marcescens PS1.
Earlier studies with S. marcescens reveals that C4-AHL (N-butanoyl homoserine lactone) regulates the expression of phenotypic characters such as prodigiosin, protease, biofilm formation, while, C6-AHL regulates the hemolysin production [21]. Prodigiosin is a reddish pigment otherwise known as 2-methyl-3-pentyl-6-methoxyprodiginine [24]. The protease is believed to play a major role in human infections [21]. Similarly, hemolysin is a well characterized virulence factor and has the ability to lyse the red blood cells [22]. All the three tested sponge extracts significantly reduced the above mentioned QS mediated phenomena in S. marcescens PS1 as shown in Fig. 3a–c. The inhibition of prodigiosin, protease enzyme and hemolysin production by the sponge extracts observed in the present study falls in line with the previous studies with bacterial extracts [10, 19].
Biofilms are high density, matrix-encapsulated populations attached to the surfaces [27]. The biofilm formation in S. marcescens is a major virulence factor which is controlled by QS system [20]. Biofilm has the ability to resist host immune response as well as conventional antibiotics. Hence, the control measures are required to prevent the biofilm formation [27]. In the present study, biofilm images revealed that the three sponge extracts effectively disturb the biofilm formation as shown in light microscopic (Fig. 4a–d) and CLSM (Fig. 4e–h) analyses.
Pertaining to the nature of QSI compounds, mostly it may be of either AHL degrading enzymes which degrade AHL molecules or the non-protein based compounds, which has the structural similarity with AHL and thereby acts as competitor. In the present study, since the test sponge extracts were made with methanol, the possibility of enzymatic degradation has been ruled out. Therefore, it is envisaged that the active principle exhibiting QSI activity might be an analogue of AHL molecule. However, it requires further purification and characterization to find out the active principle with the QS inhibitor activity. In conclusion, the reduction in QS controlled gene expressions and the end effect on virulence factors production without affecting the bacterial growth provided some insight into the potential of marine sponges as QS inhibitors. It is also envisaged that the QSI potential of these sponges could be used as anti-pathogenic drugs to combat with S. marcescens and other bacterial infections.
Acknowledgments
We gratefully acknowledge the computational and bioinformatics facility provided by the Alagappa University Bioinformatics Infrastructure Facility (funded by the Department of Biotechnology, Government of India; Grant No. BT/BI/25/001/2006). A. Annapoorani gratefully acknowledges the Council of Scientific and Industrial Research (CSIR), New Delhi for the financial assistance rendered [CSIR SRF No. 9/688 (0014)/2011]. We also extend our thanks to Dr. C. Ragunathan, Zoological Survey of India, Port Blair, Andaman, India for the identification of sponges.
References
- 1.Adonizio A, Kong KF, Mathee K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by south florida plant extracts. Antimicrob Agents Chemother. 2008;52:98–203. doi: 10.1128/AAC.00612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhargava N, Sharma P, Capalash N. Quorum sensing in Acinetobacter: an emerging pathogen. Crit Rev Microbiol. 2010;36:349–360. doi: 10.3109/1040841X.2010.512269. [DOI] [PubMed] [Google Scholar]
- 3.Borchardt SA, Allain EJ, Michels JJ, Stearns GW, Kelly RF, McCoy WF. Reaction of acylated homoserine lactone bacterial signaling molecules with oxidized halogen antimicrobials. Appl Environ Microbiol. 2001;67:3174–3179. doi: 10.1128/AEM.67.7.3174-3179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choo JH, Rukayadi Y, Hwang JK. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol. 2006;42:637–641. doi: 10.1111/j.1472-765X.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 5.Harjai K, Kumar R, Sukhvinder S. Garlic blocks quorum sensing and attenuates the virulence of Pseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2010;58:161–168. doi: 10.1111/j.1574-695X.2009.00614.x. [DOI] [PubMed] [Google Scholar]
- 6.Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol. 1997;46:903–912. doi: 10.1099/00222615-46-11-903. [DOI] [PubMed] [Google Scholar]
- 7.Huma N, Shankar P, Kushwah J, Bhushan A, Joshi J, Mukherjee T, Raju SC, Purohit HJ, Kalia VC. Diversity and polymorphism in AHL-lactonase gene (aiiA) of Bacillus. J Microbiol Biotechnol. 2011;21:1001–1011. doi: 10.4014/jmb.1105.05056. [DOI] [PubMed] [Google Scholar]
- 8.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 9.Kalia VC, Raju SC, Purohit HJ. Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-homoserine lactone-acylase and lactonase. Open Microbiol J. 2011;5:1–13. doi: 10.2174/1874285801105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khadar SM, Shunmugiah KP, Arumugam VR. Inhibition of quorum-sensing-dependent phenotypic expression in Serratia marcescens by marine sediment Bacillus spp. SS4. Ann Microbiol. 2012;62:443–447. doi: 10.1007/s13213-011-0262-1. [DOI] [Google Scholar]
- 11.Khan MSA, Zahin M, Hasan S, Husain FM, Ahmad I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett Appl Microbiol. 2009;49:354–360. doi: 10.1111/j.1472-765X.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Kim YH, Seo YW, Park S. Quorum sensing inhibitors from the red alga, Ahnfeltiopsis flabelliformis. Biotechnol Bioprocess Eng. 2007;12:308–311. doi: 10.1007/BF02931109. [DOI] [Google Scholar]
- 13.Kumar RJ, Zi-rong X. Biomedical compounds from marine organisms. Mar Drugs. 2004;2:123–146. doi: 10.3390/md203123. [DOI] [Google Scholar]
- 14.Manefield M, Rocky DN, Kumar N, Read R, Givskov M, Steinberg P, Kjelleberg S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999;145:283–291. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- 15.McLean RJC, Pierson LS, Fuqua C. A simple screening protocol for the identification of quorum signal antagonists. J Microbiol Methods. 2004;58:351–360. doi: 10.1016/j.mimet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Musthafa KS, Ravi AV, Annapoorani A, Packiavathy ISV, Pandian SK. Evaluation of anti-quorum-sensing activity of edible plants and fruits through inhibition of N-acyl-homoserine-lactone system in Chromobacterium violaceum and Pseudomonas aeruginosa. Chemotherapy. 2010;56:333–339. doi: 10.1159/000320185. [DOI] [PubMed] [Google Scholar]
- 17.Musthafa KS, Saroja V, Pandian SK, Ravi AV. Antipathogenic potential of marine Bacillus sp. SS4 on N-acyl-homoserine-lactone-mediated virulence factors production in Pseudomonas aeruginosa (PAO1) J Biosci. 2011;36:55–67. doi: 10.1007/s12038-011-9011-7. [DOI] [PubMed] [Google Scholar]
- 18.Narsinh LT, Werner EGM. Biotechnological potential of marine sponges. Curr Sci. 2004;86:10-11. [Google Scholar]
- 19.Nithya C, Aravindraja C, Pandian SK. Bacillus pumilus of Palk Bay origin inhibits quorum-sensing-mediated virulence factors in Gram-negative bacteria. Res Microbiol. 2010;161:293–304. doi: 10.1016/j.resmic.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Rice SA, Koh KS, Queck SY, Labbate M, Lam KW, Kjelleberg S. Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. J Bacteriol. 2005;187:3477–3485. doi: 10.1128/JB.187.10.3477-3485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarah JC, Neil RW, Abigail KPH, David RS, George PCS. Metabolic and regulatory engineering of Serratiamarcescens: mimicking phage-mediated horizontal acquisition of antibiotic biosynthesis and quorum-sensing capacities. Microbiology. 2006;152:1899–1911. doi: 10.1099/mic.0.28803-0. [DOI] [PubMed] [Google Scholar]
- 22.Scheffer J, Konig W, Braun V, Goebel W. Comparison of four hemolysin-producing organisms (Escherichia coli, Serratia marcescens, Aeromonas hydrophila and Listeria monocytogenes) for release of inflammatory mediators from various cells. J Clin Microbiol. 1998;26:544–551. doi: 10.1128/jcm.26.3.544-551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skindersoe M, Epstein EP, Rasmussen TB, Bjarnsholt T, Rocky Nys, Givskov M. Quorum sensing antagonism from marine organisms. Mar Biotechnol. 2008;10:56–63. doi: 10.1007/s10126-007-9036-y. [DOI] [PubMed] [Google Scholar]
- 24.Slater H, Crow M, Everson L, Salmond GP. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and-independent pathways. Mol Microbiol. 2003;47:303–320. doi: 10.1046/j.1365-2958.2003.03295.x. [DOI] [PubMed] [Google Scholar]
- 25.Stock I, Burak S, Sherwood KJ, Gruger T, Wiedemann B. Natural antimicrobial susceptibilities of strains of ‘unusual’ Serratia species: S. ficaria, S. fonticola, S. odorifera, S. plymuthica and S. rubidaea. J Antimicrob Chemother. 2003;51:865–885. doi: 10.1093/jac/dkg156. [DOI] [PubMed] [Google Scholar]
- 26.Tait K, Joint I, Daykin M, Milton DL, Williams P, Camara M. Disruption of quorum sensing in seawater abolishes attraction of zoospores of the green alga Ulva to bacterial biofilms. Environ Microbiol. 2005;7:229–240. doi: 10.1111/j.1462-2920.2004.00706.x. [DOI] [PubMed] [Google Scholar]
- 27.Thenmozhi R, Nithyanand P, Rathna J, Pandian SK. Antibiofilm activity of coral-associated bacteria against different clinical M serotypes of Streptococcus pyogenes. FEMS Immunol Med Microbiol. 2009;57:284–294. doi: 10.1111/j.1574-695X.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 28.Traub WH. Antibiotic susceptibility of Serratia marcescens and Serratia liquefaciens. Chemotherapy. 2000;46:315–321. doi: 10.1159/000007304. [DOI] [PubMed] [Google Scholar]