Abstract
Cyanobacterial populations introduced into crop fields as biofertilizer become non-target organisms for the pesticides and fungicides applied in the field. Effect of four commonly used pesticides viz. Bagalol, Mancozeb (fungicides), Thiodan and Phorate (insecticides) was studied on growth and different enzymes of four cyanobacterial species viz. Nostoc ellipsosporum, Scytonema simplex, Tolypothrix tenuis, and Westiellopsis prolifica. EC 50 concentration of each pesticide was determined for all cyanobacteria. Bagalol and Thiodan were found to be the most toxic. Both the fungicides and insecticides inhibited the activity of nitrogenase and glutamine synthetase (GS) at EC 50 concentration in all the four species studied. Bagalol incurred maximum inhibition of nitrogenase and GS activity on N. ellipsosporum and S. simplex while Thiodan and Phorate had maximum effect on T. tenuis, and W. prolifica. Mancozeb had lesser effect on all the above enzymes. One catabolic enzyme of carbohydrate metabolism, isocitrate dehydrogenase (ICDH) and one anabolic enzyme isocitrate lyase (ICL), which is related to glyoxylate pathway as well as gluconeogenesis, were also assayed. Cell free extracts of cyanobacteria treated with pesticides for 7 days show a drastic reduction of ICDH activity. ICL activity was induced in the organisms when treated with pesticides.
Keywords: Cyanobacteria, Pesticides, Growth, Nitrogenase, GS, ICDH, ICL
Introduction
Cyanobacteria play an important role in soil fertility and crop productivity. Application of cyanobacteria in rice fields as biofertilizer for better yield of paddy is an age-old practice [1–4]. Thus, an investigation into the effect of pesticides on nitrogen fixing cyanobacteria is an important issue. The effects of insecticides and fungicides on cyanobacterial growth and nitrogen fixation in paddy field ecosystems have received attention in India and it has been shown that the nature of pesticides is related to the effect on cyanobacteria [5–10].
Nitrogen fixation and its incorporation in cyanobacterial cell (ammonium assimilation) greatly depend on cellular energy and is a complex interrelated metabolic cycle. Nitrogen is reduced by nitrogenase complex and assimilation of ammonium in photosynthetic organisms occurs via the glutamine synthetase (GS)-glutamate synthase pathway, which requires energy, reducing power and 2-oxoglutarate to synthesize glutamate [11]. NADP dependent isocitrate dehydrogenase (ICDH) catalyzes the synthesis of 2-oxoglutarate from isocitrate, thus, involvement of this enzyme in the supply of 2-oxoglutarate for ammonium assimilation has been proposed [12–15]. Induction of isocitrate lyase (ICL), an important enzyme in glyoxylate pathway was found under in vitro nutrient stress or environmental stress in some eukaryotic algae [16] and bacteria [17–19]. In cyanobacterium Spirulina platensis the activity level and some physico-chemical properties of enzymes of the tricarboxylic acid cycle (TCA cycle) and the associated enzyme ICL have also been studied under in vitro conditions [20]. In each study ICL activity was induced to combat stress by generating anaplerotic metabolites via glyoxylate cycle. In the present investigation effects of Bagalol, Mancozeb (fungicide), Phorate and Thiodan (insecticide) on growth and activities of nitrogenase, GS, ICDH and ICL of four nitrogen fixing cyanobacteria viz. Nostoc ellipsosporum (VBCCC006), Scytonema simplex (VBCCC036) Tolypothrixtenuis (VBCCC018), and Westiellopsisprolifica (VBCCC022) have been studied.
Materials and Methods
Organisms and Culture Conditions
Four cyanobacterial isolates viz., Nostoc ellipsosporum, Scytonemasimplex, Tolypothrixtenuis, and Westiellopsis prolifica isolated from rice field soil sample of Bolpur (lateritic–alluvial soil) were used for present investigation. Pure cultures were maintained in liquid BG11 medium (–N) under 28 ± 1°C temperature and 25–30 μmol photons m−2 s−1 illumination in 12 h light/dark cycles.
Pesticides Used
The fungicides chosen and concentration used for the present study were Bagalol-6 (methoxy ethyl mercuric chloride, 0.001–1.0 ppm) and mancozeb-35 (sulphar-dithiocarbamate, 1–100 ppm). The insecticides used were Thiodan (endosulphan-hexachlorohexahydromethano-2,4,3-benzodioxathiepin 3-oxide, 0.001–5.0 ppm), and phortax-10G or phorate (organophosphate-O,O-diethyl S-(ethylthio) methyl phosphorodithioate, 0.001–5.0 ppm).
Determination of Effective Concentration 50 (EC 50)
EC 50 of each pesticide was determined as expressed in terms of concentration of pesticides, which reduces growth by 50% as compared to the control (Table 1). This was done following Regression line equation, based on survivality response in terms of chlorophyll a (Chl a) content.
Table 1.
Effective concentrations (EC 50) of four studied pesticides determined on the basis of survivability response of cyanobacterial strains
| Pesticides | Effective concentration in ppm (EC 50) | |||
|---|---|---|---|---|
| N. ellipsosporum | W. prolifica | S. simplex | T. tenuis | |
| Bagalol | 0.025 ± 0.001 | 0.03 ± 0.001 | 0.04 ± 0.002 | 0.042 ± 0.002 |
| Mancozeb | 40.0 ± 1 | 25.5 ± 0.5 | 50.5 ± 0.5 | 72.2 ± 2.2 |
| Thiodan | 0.025 ± 0.001 | 0.029 ± 0.001 | 0.048 ± 0.002 | 0.05 ± 0.002 |
| Phorate | 0.40 ± 0.016 | 0.50 ± 0.025 | 0.52 ± 0.02 | 0.80 ± 0.03 |
Inoculation
The absorbance of inoculum (homogenized biomass) during inoculation in the liquid medium containing pesticides at 750 nm was 0.2. Inoculation was done aseptically in a laminar-airflow.
Growth and Heterocyst Frequency (H%)
Growth was determined in terms of Chl a content following Mackinney [21]. Heterocyst frequency was calculated by counting the number of heterocysts present per hundred vegetative cells under light microscope.
Acetylene Reduction Assay
The nitrogenase activity was estimated by acetylene reduction assay (ARA) following Turner and Gibson [22]. The cyanobacterial biomass was incubated with freshly prepared acetylene for 24 h. Ethylene formation from acetylene and its detection was done by a gas chromatograph (Hewlett-Packard) fitted with Porapack N (80–100 mesh) column and ionizing detector. Column temperature, detector temperature and port temperature ware 100, 170 and 140°C respectively. The amount of ethylene produced was calculated by integration of the peak and converted to nmol of ethylene formed per μg Chl a by comparison to a standard curve developed from injected standard amount of ethylene.
Preparation of Cyanobacterial Cell Free Extracts
Cyanobacterial populations in culture were harvested in a centrifuge at 10,000×g for 10 min at 4°C. The cells were washed thrice by 0.05 M phosphate buffer at pH 7.0. The washed cells were finally suspended at 20% in 0.05 M phosphate buffer, pH 7.0, containing 5 mM β-mercaptoethanol. Washed cell suspension thus obtained was subjected to sonication at 4°C with an ultrasonic needle probe at 100 W for 5 min. After centrifugation at 15,000×g for 10 min, supernatant which is the crude cell free extract (CFE), was kept in an ice bath and used as source of enzymes. Enzyme assays were carried out at room temperature (25–27°C) and optical densities (O.D.) of the assay mixtures were recorded in Simadzu 1700 UV–visible spectrophotometer.
Assay of GS (EC 6.3.1.2)
Activity of GS was assayed following Shapiro and Stadtman [23]. The reaction mixture contained, in a final volume of 1 ml, 50 μmol 4-(2-hydroxyethyl)-l-piperazineethane sulfonic acid (HEPES)–KOH buffer (pH 7.5), 30 μmol l-glutamine, 3 μmol MnCl2, 60 μmol NH2OH, 0.4 μmol ADP, 20 μmol Na2HAsO4, and cell free extract. Reaction was started by the addition of sodium arsenate and the amount of 7-glutamylhydroxamate (7-GH) formed after 15 min of incubation at 30°C was measured spectrophotometrically at 540 nm.
The protein content of the crude cell free extract was estimated by the method of Lowry et al. [24] using bovine serum albumin as the standard.
Assay of ICDH (EC. 1.1.1.42)
This enzyme was assayed by measuring the rate of conversion of NADP+ to NADPH at 340 nm and taking the molar extinction coefficient value of reduced NADP+ into consideration following Khouw and Mc Curdy [25]. The change in O.D./min was calculated from the linear portion of enzyme activity curve and was expressed as ΔO.D./min. The reaction mixture contained 30 μmol Tris HCl-buffer (pH 7.6), 10 μmol MgCl2, 0.2 μmol NADP+, 10 μmol dl-isocitrate and CFE containing approximately 300–500 μl protein to a total volume of 1 ml.
Assay of ICL (EC. 4.1.3.1)
ICL was assayed by determining the amount of glyoxylate formed from isocitrate following Mc Fadden [26]. A reaction mixture of 1.5 ml Tris–Mg2+ buffer; 0.125 M reduced glutathione, 0.2 ml; and CFE, 0.1 ml, was pre incubated at 30°C. The reaction was initiated with the addition of 0.2 ml of 40 mM trisodium dl-isocitrate solution and thorough mixing. After 10 min incubation the reaction was stopped with the addition of 1 ml 10% TCA. Then to 1 ml of reaction mixture 6 ml of oxalic acid–phenyl hydrazine hydrochloride mixture was added and heated until just boiling. The mixture was chilled on ice. 4 ml of concentrated HCl was added to it, followed by 1 ml 5% potassium ferricyanide and the preparation was mixed thoroughly. Optical density of the mixture was read at 520 nm against a water blank, 7 min after the addition of ferricyanide. Specific activity is defined as nmol of glyoxylate formed min−1 mg−1 of protein.
Results and Discussion
Inhibitory effect of the pesticides on the growth of cyanobacteria was observed as Chl a content was reduced after treatment. Bagalol and Thiodan appeared to be the most toxic pesticides as they reduced growth by 50% at concentration ranging from 0.025 to 0.05 ppm in all the four organisms. The concentration of Phorate in effecting 50% inhibition ranged from 0.40 to 0.80 ppm. Mancozeb was the least toxic as compared to other pesticides for the tested cyanobacteria and the range of concentration was 40.0–72.2 ppm. T. tenuis has maximum tolerance to the pesticides followed by S. simplex, W. prolifica and N. ellipsosporum (Table 1). Earlier reports [27, 28] of effects of mercury fungicide and endosulfan supports the present findings. However, in each organism an increase of Chl a synthesis was observed up to 20 ppm concentrations for Mancozeb (<EC 50), but beyond this concentration (30–100 ppm) Chl a content declined. There are earlier reports of such effect of pesticides on growth of cyanobacteria [29–33]. The increased pigmentation could be due to mutagenesis to detoxification or even metabolizing the pesticide [34, 35]. Inhibition of chlorophyll synthesis by pesticides in Anabaena flos-aquae, T. scytonemoides and T. ceylonica has been reported earlier [36, 37].
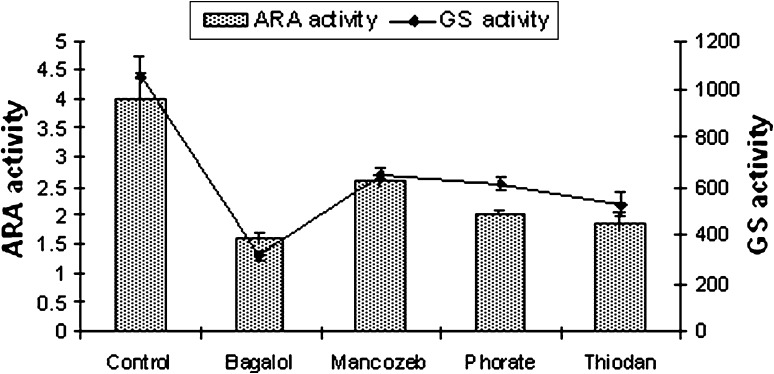
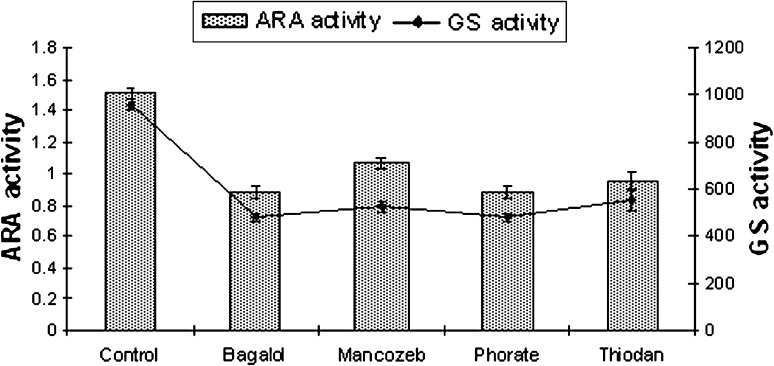
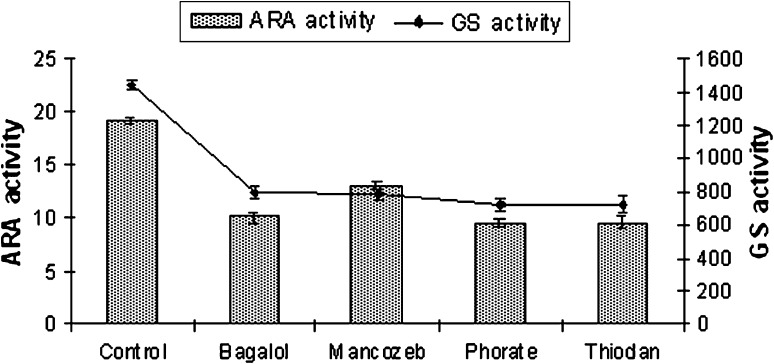
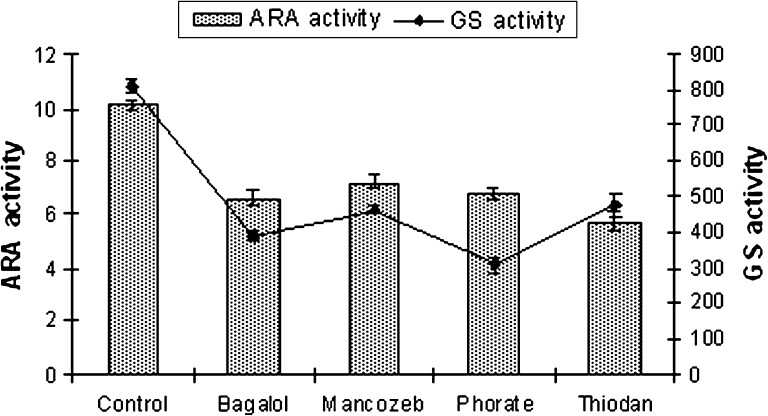
The results recorded from the present study indicate no alteration in heterocyst frequency per filament in N. ellipsosporum (6–7%), S. simplex (5%), T. tenuis (10–12%) and W. prolifica (20–22%) compared to their untreated culture sets. Bagalol inhibited nitrogenase activity by 60% and GS activity by 70% in N. ellipsosporum (Fig. 1). Phorate inhibited nitrogenase activity by 52.2% in W. prolifica and GS activity by 62% in T. tenuis. Thiodan inhibited nitrogenase activity by 51.2% in W. prolifica and GS activity by 50% in both N. ellipsosporum and W. prolifica. Mancozeb inhibited nitrogenase activity by 32% and GS activity by 46% in W. prolifica. Many investigators have reported the inhibition of nitrogenase by pesticides [38–42]. The present finding supports the earlier observations [43, 44] that cyanobacteria with out sheath like N. ellipsosporum are much susceptible to pesticide stress comparison to sheathed members S. simplex, W. prolifica and T. tenuis (Figs. 1, 2, 3, 4). Variability in an organism’s response to the pesticides is reflected in earlier reports. In N. calcicola, nitrogenase activity was reduced to 70 and 35% by Phosphomidon and Dithane respectively [31] but Quinolphos and Monocrotophos totally inhibited nitrogenase activity [45]. In T. scytonemoides, Monocrotophos decreased the expression of nitrogenase to a greater extent [37].
Fig. 1.
ARA (nmol C2H2 μgChl-a−1h−1) and GS (nmol substrate mg−1 protein min−1) activity of N. ellipsosporum at EC 50 dose of pesticides
Fig. 2.
ARA (nmol C2H2 μgChl-a−1h−1) and GS (nmol substrate mg−1 protein min−1) activity of S. simplex at EC 50 dose of pesticides
Fig. 3.
ARA (nmol C2H2 μgChl-a−1h−1) and GS (nmol substrate mg−1 protein min−1) activity of W prolifica at EC 50 dose of pesticides
Fig. 4.
ARA (nmol C2H2 μgChl-a−1h−1) and GS (nmol substrate mg−1 protein min−1) activity T tenuis at EC 50 dose of pesticides
Enhancement or reduction of heterocyst frequency is of interest as it has direct relevance to nitrogen fixation. Though heterocysts number was not affected by exposure to the EC 50 concentrations of Bagalol, Thiodan, Phorate and Mancozeb but the level of nitrogenase activity was reduced. Nitrogenase activity of A. doliolum was reduced by 38% by carbofuran treatment with no observed change in heterocyst frequency [33]. On the other hand, heterocysts were not found in N. muscorum in the presence of the pesticide [46]. This could be attributed to the interference of these four pesticides with heterocyst’s envelope and maturation rather than their differentiation process and affects their efficiency in N2-fixation. Orus and Marco [47], reported that the destabilization of the heterocyst envelope is the first target of insecticidal action causing inhibition of dinitrogen fixation. The reduced nitrogenase activity under the increasing concentration of pesticides in cyanobacteria (Figs. 1, 2, 3, 4) may possibly be related to the reduction in photosynthetic activity related to the lack of reducing agent pool [48] under pesticide stress. Thus, ARA activity might be hampered in two ways, either by heterocyst non-functionality or deficiency in energy supply by inhibition of photosynthesis or by inhibition of energy metabolism and reduction of supply of reducing molecule such as NADH or NADPH.
GS activity was found to decline in response to all the pesticide treatments in the present study (Figs. 1, 2, 3, 4). Bagalol inhibited GS activity by 70% at EC 50 concentration in N. ellipsosporum. In earlier studies [37, 40], GS activity was suppressed by Propanil in N. muscorum and by Monochrotophos, Bavistin and Nimbicidin in T. scytonemoides.
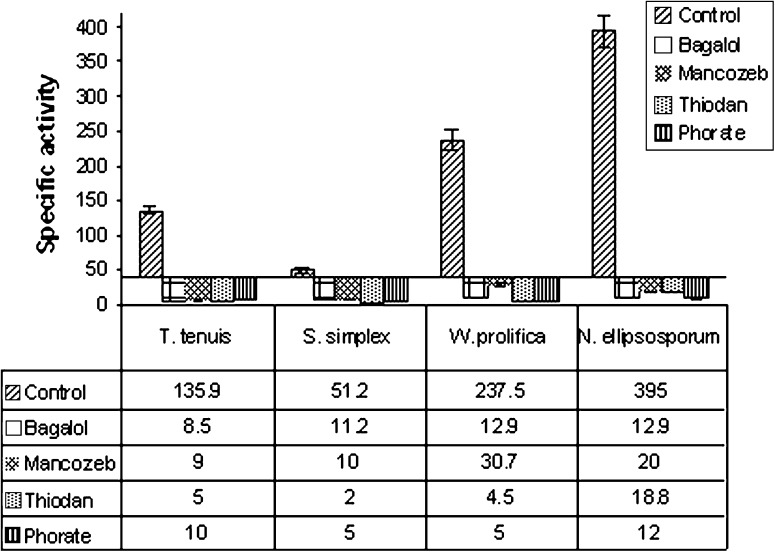
ICDH is considered to be an important enzyme in the TCA cycle. Results indicated a drastic reduction of activity of this enzyme at EC 50 concentration of the pesticides (Fig. 5). Maximum inhibition of ICDH activity was observed in W. prolifica (98%) by Thiodan followed by S. simplex (96.1%) and T. tenuis (96.3%), respectively (Fig. 5). Marked decrease of ICDH activity (Fig. 5) was exhibited by Phorate in N. ellipsosporum (97%).
Fig. 5.
Specific activity of ICDH (nmol NADP min−1 mg protein−1) in four pesticides treated cyanobacteria
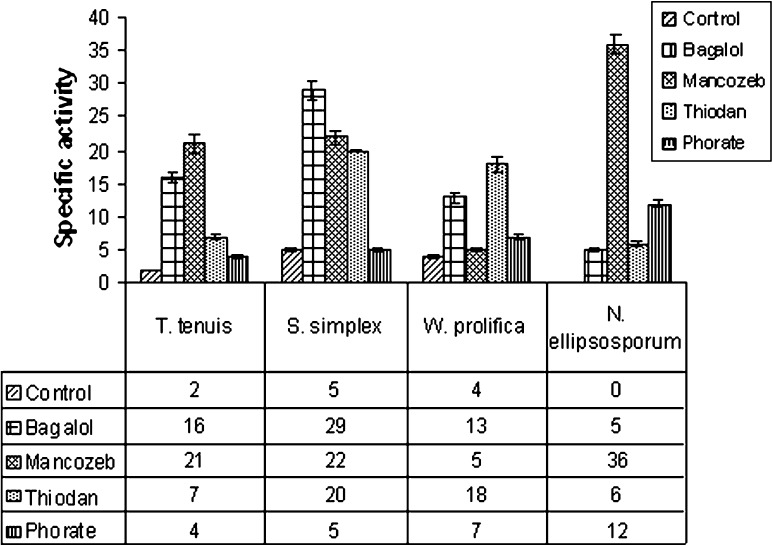
In the cyanobacterial isolates, treated with pesticides, glyoxylate pathway is induced as indicated by the induction of ICL in all four isolates (Fig. 6). However, in S. simplex ICL activity was not induced by Phorate treatment. Under control condition, ICL activity of cyanobacteria was found to be absent or very low (2–5 nmol substrate min−1 mg−1 protein). Greatest induction of ICL was by Mancozeb (36 mol substrate min−1mg−1 protein) in N. ellipsosporum (Fig. 6). In T. tenuis 10.5, 8, 3.5 and 2 fold increase of ICL activity were observed under Mancozeb, Bagalol, Thiodan and Phorate treatment respectively (Fig. 6). About 6, 4.5 and 4 fold increase were observed in S. simplex under Bagalol, Mancozeb and Thiodan respectively (Fig. 6). Comparatively less ICL activity was observed in W. prolifica (3.25, 0.25, 4.5 and 1.7 fold) for all four pesticides (Bagalol, Mancozeb, Thiodan and Phorate respectively) treatment. As ICDH and ICL compete for the same substrate isocitrate, thus, with the increase of activity of ICL, ICDH activity is decreased. Induction of ICL activity in pesticide treated culture at EC 50 concentration might be an adaptive strategy for survival under stressed environment which might be analogical to an earlier observation of induction of ICL activity in carbon starved cells of Rhizobiaum as an adaptive strategy [19].
Fig. 6.
Specific activity of ICL (nmol substrate min−1 mg protein−1) in four pesticide treated cyanobacteria
It is fact that with the lowering of ICDH activity energy required for nitrogen fixation is declined [16, 17]. Thus, to maintain cellular energy budget for survival, cyanobacteria slowed down the highly energy consuming nitrogen fixation process and as a consequence of this GS activity was also reduced. On the other hand, marked enhance of ICL activity under similar pesticides treatment cultures observed. As a result TCA cycle is modified or by passed to the glyoxylate cycle to combat pesticides stress.
Among the pesticides, Bagalol incurred maximum toxicity at EC 50 on investigated cyanobacteria in the present study. Its high toxicity to all four isolates possibly due to the presence of mercury in it. Presently use of this fungicide has been stopped to avoid long term (half life 30–45 days) mercury toxicity in agricultural field.
Acknowledgments
The financial assistance by UGC, India for the project and fellowship to MD is thankfully acknowledged. We are also thankful to Prof. K.C. Saha, BCKV, India, for providing facility for ARA.
Contributor Information
Manojit Debnath, Email: mandeb@rediffmail.com.
Samit Ray, Email: ray_samit@rediffmail.com.
References
- 1.De PK. The role of blue-green algae in nitrogen fixation in rice fields. Proc Roy Soc London, B. 1939;127:121–139. doi: 10.1098/rspb.1939.0014. [DOI] [Google Scholar]
- 2.Relwani LL. Role of blue green algae on paddy field. Curr Sci. 1963;32:417–418. [Google Scholar]
- 3.McCann AE, Cullimore DR. Influence of pesticides on the soil algal flora. Residue Rev. 1979;72:1–31. [Google Scholar]
- 4.Mian MH, Stewart WDP. Fate of nitrogen applied as Azolla and blue-green algae (cyanobacteria) in waterlogged rice soils: A 15N tracer study. Plant Soil. 1985;83:363–370. doi: 10.1007/BF02184448. [DOI] [Google Scholar]
- 5.Singh PK. Effect of pesticides on blue green algae. Arch Mikrobiol. 1973;89:317–320. doi: 10.1007/BF00408898. [DOI] [PubMed] [Google Scholar]
- 6.Nayak DN, Rao RV. Pesticides and nitrogen fixation in paddy soils. Soil Biol Biochem. 1982;14:207–210. doi: 10.1016/0038-0717(82)90025-6. [DOI] [Google Scholar]
- 7.Adhikary SP, Dash B, Pattanaik H. Effect of carbamet insecticide Sevin on Anabaena sp. and Westiellopsis prolifica. Acta Microbiol. 1984;31:335–338. [PubMed] [Google Scholar]
- 8.Stratton GW. The effects of pesticides and heavy metals on phototrophic micro-organisms. Rev Environ Toxicol. 1987;3:71–112. [Google Scholar]
- 9.Sahu J, Das MK, Adhikary SP. Reaction of blue-green algae of rice-field soils to pesticide application. Trop Agric (Trinidad) 1992;69:362–364. [Google Scholar]
- 10.Kolte SO, Goyal SK (1990) Inhibition of growth and nitrogen fixation in Calothrix marchica by herbicide in vitro. In: Kaushik BD (ed) Proceedings of National Symposium on Cyanobacterial Nitrogen Fixation. Pub Associated Co., New Delhi, pp 507–510
- 11.Wallsgrove RM, Keys AJ, Lea PJ, Miflin BJ. Photosynthesis, photorespiration and nitrogen metabolism. Plant Cell Environ. 1983;6:301–309. [Google Scholar]
- 12.Elias BA, Givan CV. Alpha-ketoglutarate supply for amino acid synthesis in higher plants chloroplasts. Plant Physiol. 1977;59:738–740. doi: 10.1104/pp.59.4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen RD, Le MP, Vidal J, Jacquot JP, Gadal R. Purification and comparative properties of the cytosolic isocitrate dehydrogenase (NADP) from pea (Pisumsativum) roots and green leaves. Eur J Biochem. 1988;175:565–572. doi: 10.1111/j.1432-1033.1988.tb14229.x. [DOI] [PubMed] [Google Scholar]
- 14.Muro-Pastor MI, Florencio FJ. Purification and properties of NADP-isocitrate dehydrogenase from the unicellular cyanobacterium Synechocystis sp., PCC 6803. Eur J Biochem. 1991;203:99–105. doi: 10.1111/j.1432-1033.1992.tb19833.x. [DOI] [PubMed] [Google Scholar]
- 15.El-Mansi M, Cozzone AJ, Shiloach J, Eikmanns BJ. Control of carbon flux through enzymes of central and intermediary metabolism during growth of Escherichia coli on acetate. Curr Opin Microbiol. 2006;9:173–179. doi: 10.1016/j.mib.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Rivas JM, Vega JM. Effect of culture conditions on the isocitrate dehydrogenase and isocitrate lyase activities in Chlamydomonas reinhardtii. Physiol Plant. 1993;88:599–603. doi: 10.1111/j.1399-3054.1993.tb01377.x. [DOI] [PubMed] [Google Scholar]
- 17.Hamel RD, Appanna VD. Modulation of TCA cycle enzymes and aluminum stress in Pseudomonas fluorescens. J Inorg Biochem. 2001;87:1–8. doi: 10.1016/S0162-0134(01)00307-5. [DOI] [PubMed] [Google Scholar]
- 18.Maharjan RP, Yu PL, Seeto S, Ferenci T. The role of isocitrate lyase and the glyoxylate cycle in Escherichia coli growing under glucose limitation. Res Microbiol. 2005;156:178–183. doi: 10.1016/j.resmic.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Bakshi D, Sinhbabu A, Mandal V, Mandal NC. Changes of carbon metabolic activity of Rhizobium under carbon starvation. J Plant Biochem Biotech. 2006;15:67–69. [Google Scholar]
- 20.Mendzhul MI, Lysenko TG, Shainskaia OA, Busakhina IV. Activity of tricarboxylic acid cycle enzymes in cyanobacteria Spirulina platensis. Mikrobiol Z. 2000;62(1):3–10. [PubMed] [Google Scholar]
- 21.Mackinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;40:315–322. [Google Scholar]
- 22.Turner GL, Gibson AH. Measurement of nitrogen fixation by indirect means. In: Bergerson FJ, editor. Methods for evaluating biological nitrogen fixation. UK: Wiley; 1980. pp. 111–138. [Google Scholar]
- 23.Shapiro BM, Stadtman ER (1970) Glutamine synthetase (Escherichia coli). Methods Enzymol 17A:910–922 [DOI] [PubMed]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Khouw BT, Mc Curdy HD. Tricarboxylic acid cycle enzymes and morphogenesis of Blastocladiella emersonii. J Bacteriol. 1969;99:197–205. doi: 10.1128/jb.99.1.197-205.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFadden BA. Isocitrate lyase. In: Lowenstein JM, editor. Methods of enzymology, vol. 13. New York: Academic Press; 1969. pp. 163–170. [Google Scholar]
- 27.Prasad AB, Samanta R, Vishwakarma ML, Vaishampayan A. Biological effects of a Mercury fungicide on a N2-fixing blue green alga Nostoc muscorum: isolation and preliminary characterization of a Hg resistant mutant. New Phytol. 1986;102:45–49. doi: 10.1111/j.1469-8137.1986.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 28.Prasad SM, Kumar D, Zeeshan M. Growth, photosynthesis, active oxygen species and antioxidants responses of paddy Weld cyanobacterium Plectonema boryanum to endosulfan stress. J Gen Appl Microbiol. 2005;51:115–124. doi: 10.2323/jgam.51.115. [DOI] [PubMed] [Google Scholar]
- 29.Anand N, Veerappan B. Effect of pesticides and fungicides on blue green algae. Phykos. 1980;19:210–212. [Google Scholar]
- 30.Goyal SK. Stress compatibility in Cyanobacteria. Phykos. 1989;28:267–273. [Google Scholar]
- 31.Anand N, Subramanian TD. Effect of certain pesticides on the physiology of Nostoc calcicola. Phykos. 1997;36:15–20. [Google Scholar]
- 32.Panigrahi S, Padhy S, Padhy RN. Toxicity of parathion-methyl to cells, akinetes and heterocysts of the cyanobacterium Cylindrospermum sp. and the probit analysis of toxicity. Annu Appl Biol. 2003;143:195–199. doi: 10.1111/j.1744-7348.2003.tb00286.x. [DOI] [Google Scholar]
- 33.Hammouda O. Response of the paddy field Cyanobacterium Anabaena doliolum to Carbofuran. Ecotoxicol Environ Saf. 1999;44:215–219. doi: 10.1006/eesa.1999.1823. [DOI] [PubMed] [Google Scholar]
- 34.Megharaj M, Venkateswarlu K, Rao AS. Effects of carbofuran on the growth of green algae and two cyanobacteria isolated from a rice soil. Agric Ecosyst Environ. 1989;25:329–336. doi: 10.1016/0167-8809(89)90129-1. [DOI] [Google Scholar]
- 35.Jha MN, Mishra SK. Biological responses of cyanobacteria to insecticides and their insecticide degrading potential. Bull Environ Contam Toxicol. 2005;75:374–381. doi: 10.1007/s00128-005-0764-2. [DOI] [PubMed] [Google Scholar]
- 36.Chinnaswamy R, Patel RJ. Effect of pesticide mixtures on the blue green alga Anabaena flos-aquae. Microb Lett. 1983;24:141–143. [Google Scholar]
- 37.Rajendran UM, Elango K, Anand N. Effects of a fungicide, an insecticide, and a biopesticide on Tolypothrix scytonemoides. Pesticide Biochem Physiol. 2007;87:164–171. doi: 10.1016/j.pestbp.2006.07.006. [DOI] [Google Scholar]
- 38.DaSilva EJ, Henriksson LE, Henriksson E. Effect of pesticides on blue-green algae and nitrogen-fixation. Arch Environ Contam Toxicol. 1975;3:193–204. doi: 10.1007/BF02220788. [DOI] [PubMed] [Google Scholar]
- 39.Lal R, Saxena DM. Cytological and biochemical effects of pesticides on microorganisms. Residue Rev. 1980;73:49–86. doi: 10.1007/978-1-4612-6068-4_4. [DOI] [PubMed] [Google Scholar]
- 40.Singh LJ, Tiwari DN. Effect of selected rice Weld herbicides on photosynthesis, respiration and nitrogen assimilating enzyme systems of paddy soil diazotrophic Cyanobacteria. Pestic Biochem Physiol. 1988;31:120–128. doi: 10.1016/0048-3575(88)90014-4. [DOI] [Google Scholar]
- 41.Padhy RN, Mohapatra K. Anabaena PCC 7120 and computations of partial lethal concentrations by the probit method. Microbios. 2001;106:81–95. [PubMed] [Google Scholar]
- 42.Pandey V, Rai LC. Interactive effects of UV-B and pesticides on photosynthesis and nitrogen fixation of Anabaena doliolum. J Microbiol Biotechnol. 2002;12:423–430. [Google Scholar]
- 43.Kaushik BD, Venkataraman GS. Response of cyanobacterial nitrogen fixation to insecticides. Curr Sci. 1983;52:321–323. [Google Scholar]
- 44.Rath B, Adhikary SP. Relative tolerance of several nitrogen fixing cyanobacteria to commercial grade furadan (carbofuran, 3%) Indian J Exp Biol. 1994;32:213–215. [Google Scholar]
- 45.Megharaj M, Venkateswaralu K, Rao AS. Effect of insecticides and phenolics on nitrogen fixation by Nostoc linckia. Bull Environ Contam Toxicol. 1988;41:277–281. doi: 10.1007/BF01705442. [DOI] [PubMed] [Google Scholar]
- 46.Vaishampayan A. Biological effects of rice-field herbicide Monuron on a nitrogen-fixing cyanobacterium Nostoc muscorum. Microbios Lett. 1984;28:105–111. [Google Scholar]
- 47.Orus MI, Marco E. Heterocysts structure alteration and oxygen-mediated inhibition of dinitrogen fixation by trichlorfon in Anabaena 7119. J Exp Botany. 1991;95:1595–1600. doi: 10.1093/jxb/42.12.1595. [DOI] [Google Scholar]
- 48.Legane′s F, Ferna′ndez-Valiente E. Effects of phenoxyacetic herbicides on growth, photosynthesis and nitrogenase activity in the cyanobacteria from rice fields. Arch Environ Contam Toxicol. 1992;22:130–134. doi: 10.1007/BF00213311. [DOI] [PubMed] [Google Scholar]