Abstract
We examined the intramolecular effect of altered cap structures on translation efficiency of artificial beta-globin mRNAs. For these studies, synthetic dinucleotides of the form X(5')ppp(5')G [X = 7-methyl guanosine (m7G), 2,7-dimethyl guanosine (m2(2,7)G) or 2,2,7-trimethyl guanosine (m3(2,2,7)G)], were transcriptionally incorporated into mRNAs, containing rabbit beta-globin coding sequences, using T7 RNA polymerase and a beta-globin cDNA template. These synthetic mRNAs were assayed in reticulocyte lysate for activity relative to m7G-capped mRNA. m2(2,7)G-Capped mRNA was found to be 1.5-fold more active than m7G-capped mRNA. Messenger RNA capped with m3(2,2,7)G was less active with activity of 0.24 relative to its m7G-capped counterpart (activity = 1.0). These data suggest that m7G-capped mRNAs become more active as translation templates after addition of a single N2 methyl moiety, which is especially pertinent to gene expression in togaviridae. The latter are observed to synthesize m2(2,7)G and m3(2,2,7)G-capped mRNAs in addition to m7G-capped templates during the course of infection in animal cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
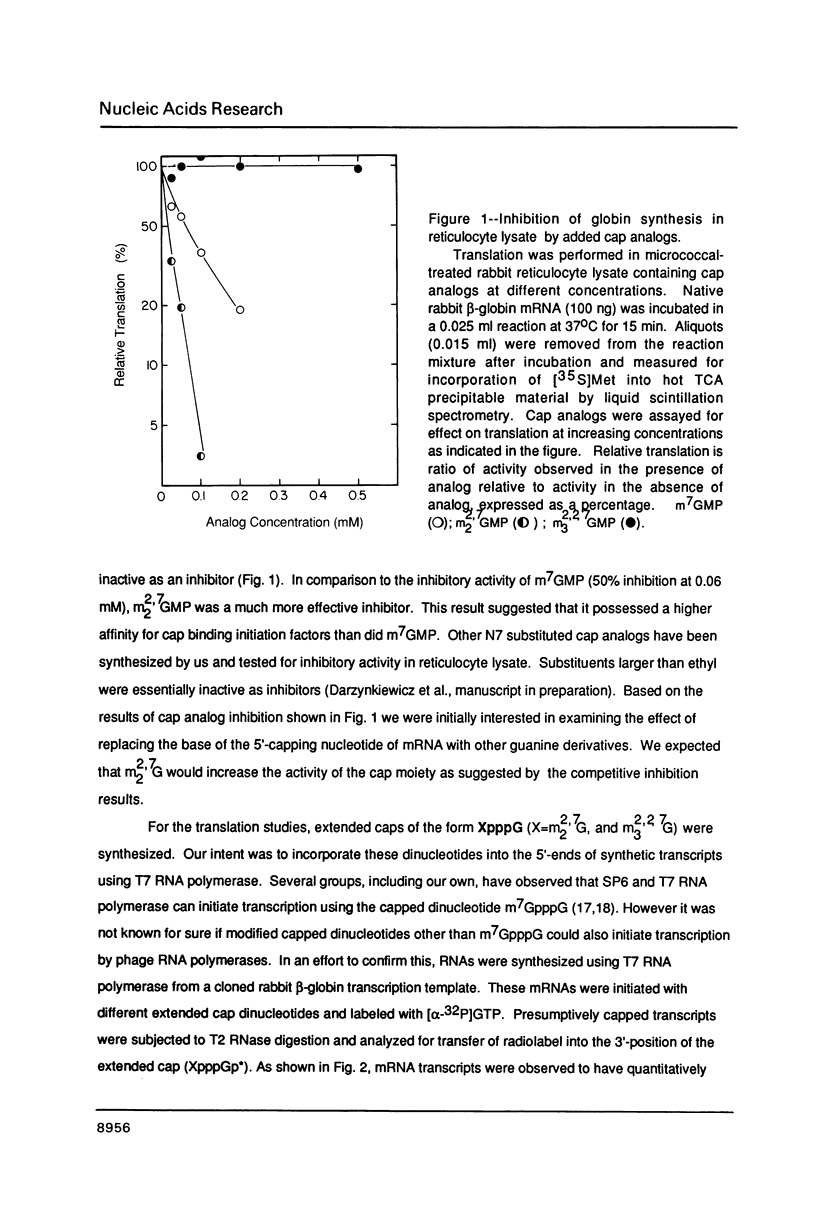
- Adams B. L., Morgan M., Muthukrishnan S., Hecht S. M., Shatkin A. J. The effect of "cap" analogs on reovirus mRNA binding to wheat germ ribosomes. Evidence for enhancement of ribosomal binding via a preferred cap conformation. J Biol Chem. 1978 Apr 25;253(8):2589–2595. [PubMed] [Google Scholar]
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton A. R., Krug R. M. Transcription antitermination during influenza viral template RNA synthesis requires the nucleocapsid protein and the absence of a 5' capped end. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6282–6286. doi: 10.1073/pnas.83.17.6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch H., Reddy R., Rothblum L., Choi Y. C. SnRNAs, SnRNPs, and RNA processing. Annu Rev Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz E., Antosiewicz J., Ekiel I., Morgan M. A., Tahara S. M., Shatkin A. J. Methyl esterification of m7G5'p reversibly blocks its activity as an analog of eukaryotic mRNA 5'-caps. J Mol Biol. 1981 Dec 5;153(2):451–458. doi: 10.1016/0022-2836(81)90289-8. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz E., Ekiel I., Lassota P., Tahara S. M. Inhibition of eukaryotic translation by analogues of messenger RNA 5'-cap: chemical and biological consequences of 5'-phosphate modifications of 7-methylguanosine 5'-monophosphate. Biochemistry. 1987 Jul 14;26(14):4372–4380. doi: 10.1021/bi00388a028. [DOI] [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- Edery I., Sonenberg N. Cap-dependent RNA splicing in a HeLa nuclear extract. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7590–7594. doi: 10.1073/pnas.82.22.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M. A., Shatkin A. J. Synthesis and translation of mRNA containing 5'-terminal 7-ethylguanosine cap. J Biol Chem. 1979 Jul 25;254(14):6732–6738. [PubMed] [Google Scholar]
- Georgiev O., Mous J., Birnstiel M. L. Processing and nucleo-cytoplasmic transport of histone gene transcripts. Nucleic Acids Res. 1984 Nov 26;12(22):8539–8551. doi: 10.1093/nar/12.22.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Leis J. P., Morgan M. A., Shatkin A. J., Merrick W. C. Characterization of eukaryotic initiation factor 4A, a protein involved in ATP-dependent binding of globin mRNA. J Biol Chem. 1982 May 10;257(9):5246–5252. [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Dubin D. T. Di-and trimethylated congeners of 7-methylguanine in Sindbis virus mRNA. Nature. 1976 Nov 11;264(5582):190–191. doi: 10.1038/264190a0. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984 Oct;38(3):731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986 Sep 12;46(6):905–911. doi: 10.1016/0092-8674(86)90072-3. [DOI] [PubMed] [Google Scholar]
- Miura K. I., Kodama Y., Shimotohna K., Fukui T., Ikehara M., Nakagawa I., Hata T. Inhibitory effect of methylated derivatives of guanylic acid for protein synthesis with reference to the functional structure of the 5'-'cap' in viral messenger RNA. Biochim Biophys Acta. 1979 Sep 27;564(2):264–274. doi: 10.1016/0005-2787(79)90224-7. [DOI] [PubMed] [Google Scholar]
- Morgan M. A., Shatkin A. J. Initiation of reovirus transcription by inosine 5'-triphosphate and properties of 7-methylinosine-capped, inosine-substituted messenger ribonucleic acids. Biochemistry. 1980 Dec 23;19(26):5960–5966. doi: 10.1021/bi00567a003. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Anagnou N. P., Ley T. J. Advances in thalassemia research. Blood. 1984 Apr;63(4):738–758. [PubMed] [Google Scholar]
- Pain V. M. Initiation of protein synthesis in mammalian cells. Biochem J. 1986 May 1;235(3):625–637. doi: 10.1042/bj2350625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Photochemical cross-linking of cap binding proteins to eucaryotic mRNAs: effect of mRNA 5' secondary structure. Mol Cell Biol. 1985 Nov;5(11):3222–3230. doi: 10.1128/mcb.5.11.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. A., 3rd, Snyder P. G., Kazazian H. H., Jr Ratios of alpha-to beta-globin mRNA and regulation of globin synthesis in reticulocytes. Nature. 1977 Sep 29;269(5627):442–445. doi: 10.1038/269442a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Lodish H. F. Translation in vitro of vesicular stomatitis virus mRNA lacking 5'-terminal 7-methylguanosine. Nature. 1976 Jul 1;262(5563):32–37. doi: 10.1038/262032a0. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985 Feb;40(2):223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara S. M., Morgan M. A., Shatkin A. J. Two forms of purified m7G-cap binding protein with different effects on capped mRNA translation in extracts of uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1981 Aug 10;256(15):7691–7694. [PubMed] [Google Scholar]
- Yamaguchi K., Nakagawa I., Sekine M., Hata T., Shimotohno K., Hiruta M., Miura K. Chemical synthesis of the 5'-terminal part bearing cap structure of messenger RNA of cytoplasmic polyhedrosis virus (CPV): m7G5'pppAmpG and m7G5'pppAmpGpU. Nucleic Acids Res. 1984 Mar 26;12(6):2939–2954. doi: 10.1093/nar/12.6.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki A., Kumashiro I., Takenishi T. Synthesis of guanosine and its derivatives from 5-amino-1-beta-D-ribofuranosyl-4-imidazolecarboxamide. II. Ring closure with sodium methylxanthate. J Org Chem. 1967 Oct;32(10):3032–3038. doi: 10.1021/jo01285a021. [DOI] [PubMed] [Google Scholar]
- van Duijn L. P., Kasperaitis M., Ameling C., Voorma H. O. Additional methylation at the N(2)-position of the cap of 26S Semliki Forest virus late mRNA and initiation of translation. Virus Res. 1986 Jul;5(1):61–66. doi: 10.1016/0168-1702(86)90065-1. [DOI] [PubMed] [Google Scholar]