Abstract
Staphylococcal enterotoxins (SEs) are the second most common causal agents of food poisoning throughout the world. Staphylococcal enterotoxin B (SEB) is one of the most potent and a listed biological warfare agent. Therefore, its quick, accurate and sensitive detection is of paramount importance. But availability of sensitive and specific antibodies against SEB is the major bottleneck in the development of an immunodetection system. Therefore, in the present study seb gene was cloned and expressed in a heterologous host resulting in a yield of 92 mg pure toxin per litre of culture broth after Ni–NTA affinity purification. Antibodies raised against the recombinant toxin did not cross react with related enterotoxins and organisms that can gain access in the food. Further, a sandwich ELISA was developed to detect SEB after extraction from artificially spiked food samples like milk, orange juice, skim milk and khoya. The sandwich ELISA was able to detect SEB in the range of 0.25 to 0.49 ng/ml or g of food. The detection system developed in the present study is at least as specific and sensitive as other commercially available kits which use monoclonal antibodies.
Keywords: Recombinant SEB, Cloning, Detection, ELISA, Food
Introduction
Staphylococcus aureus is one of the major causes of food borne diseases (FBD) throughout the world. This toxigenic disease is primarily caused by staphylococcal enterotoxins (SEs). Among SEs, staphylococcal enterotoxin B is one of the most common causative agents of FBD worldwide [1]. SEB, a 28.4 kDa protein, is also defined as superantigen that binds directly to invariant regions of MHC class II and Vβ region of T cell receptor and hyperstimulate T lymphocytes coupled with the release of cytokines causing clinical symptoms including fever, hypotension, and death in severe cases [2]. The physiological effect of SEB is emesis and diarrhoea that is generally defined as food poisoning [1, 3]. The ingestion of 100–200 ng of enterotoxin can induce symptoms of food poisoning [4] and foods implicated in staphylococcal food poisoning typically contain about 0.5–10 μg of toxin per 100 g [5]. SEB also has the potential for use as biological warfare (BW) agent and its toxic dose for aerosol inhalation is less as compared to food poisoning dose [6]. Thus, the sensitivity of any detection method needs to be below this level, preferably in the region of 0.1–0.2 μg per 100 g [7].
Different immunological methods have been proposed for detection of staphylococcal enterotoxins in food samples including immunodiffusion assays [8], radioimmunoassay [9] and enzyme linked immunosorbent assays [10–12]. But the important bottleneck is the availability of high titre and specific antibodies for the development of sensitive and specific detection system against staphylococcal enterotoxins. Production of such specific and high titre antibodies require large amount of pure protein because of the superantigenic nature of SEB. Various techniques are being used to purify SEB like HPLC, multistep ion-exchange chromatography, gel filtration, chromatofocussing, dye ligand chromatography, etc. [1, 13]. But these techniques are very cumbersome, expensive and time consuming, and in most cases do not result in the desired purity of the enterotoxin for specific and high titre antibody production leading to poor detection potential. Therefore, the present investigation is focused to get large amount of purified SEB by heterologous expression for high titre and specific antibody generation. Antibodies generated in the present investigation were also tested for their suitability as diagnostic reagents in sandwich ELISA format for SEB detection from food.
Materials and Methods
Staphylococcal Enterotoxin B (seb) Gene Amplification
SEB producing S. aureus strain ATCC14458 was used to amplify the toxin gene for cloning and expression. Genomic DNA was isolated using GenElute bacterial genomic DNA kit. The nucleotide sequences of forward and reverse primers were: 5′-AGAGAGTCAACCAGATCCTAA-3′ (21 NT) and 5′-TCACTTTTTCTTTGTCGTAACAT-3′ (23 NT), respectively [14]. The optimized PCR conditions were: initial denaturation at 94°C for 2 min followed by 25 cycles of denaturation (94°C/30 s), annealing (52°C/60 s), extension (72°C/90 s); and final extension at 72°C for 5 min.
Cloning and Expression of seb into E. coli
The seb amplicon was gel purified and ligated into pQE-30UA vector (ampicillin resistant), containing 6× His tag as per manufacturer’s instructions (Qiagen, Germany). The ligated product was transformed in the expression host, E. coli SG13009 (kanamycin resistant). Recombinant clones were screened by PCR for the presence of toxin gene. PCR positive clones were checked for the expression of SEB after IPTG (isopropyl-β-D-thiogalactopyranoside) induction by SDS–PAGE followed by western blot using anti His HRP conjugate (Qiagen, Germany).
Optimization of Expression Conditions and Localization of Recombinant SEB
One of the SEB positive recombinant clones, rBUS6, was used to optimize the growth and induction conditions for expression of recombinant SEB (rSEB). Induction point (OD600 0.2–0.7), induction duration (2–6 h) and inducer (IPTG) concentration (0.5–1.5 mmol/l) were optimized. The presence of recombinant protein was tested in different subcellular compartments, viz., extracellular, periplasm and cytoplasm. The formation of inclusion bodies was also checked at different induction durations as described by Sambrook and Russell [15].
Affinity Purification of Recombinant SEB
Recombinant SEB was purified from cell lysate using Ni–NTA superflow resin as per manufacturer’s instructions (Qiagen, Germany) and the purity was tested on SDS–PAGE by silver staining. After purification, recombinant protein was dialysed gradually against decreasing concentration of urea followed by final resuspension in PBS. Protein yield was calculated by estimating the total protein and the purified rSEB using BCA kit [16].
Immunization of Animals
Female Balb/c mice were immunized intraperitonially at an interval of 7 days. Doses of 20 ng, 100 ng, 500 ng, 1 μg, 10 μg, 100 μg and 150 μg were used for mice immunization while doses of 100 ng, 1 μg, 100 μg, 1 mg at 14 days interval were used subcutaneously for female New Zealand white rabbit. Two additional boosters of last dose were also administered prior to final bleed.
Evaluation of Antibodies Raised against rSEB
Mice and rabbit were bled through retro-orbital route and cardiac puncture, respectively after 14 days of final booster. Antisera titre and their cross reactivity with SEA, SEC, SED producing S. aureus strains, E. coli SG13009, non-enterotoxigenic S. aureus strain ATCC6538P and other microorganisms that can gain access in the food, viz., Bacillus suitilis, Bacillus cereus, Enterococcus faecalis and Vibrio cholarae were determined by western blotting.
Development of Sandwich ELISA for SEB Detection
Dilutions of capture (1:500–1:16000 dilution) and revealing antibodies (1:500–1:16000) for detection of SEB (250 ng/well) were standardized using checkerboard titration. Colour was developed using OPD as substrate and plate was read at 492 nm in an ELISA plate reader (Bio-Tek Instruments Inc., USA). Results were noted and interpreted for the best dilution combinations of capturing and revealing antibodies. Sandwich plate ELISA was carried out for detection of the minimum amount of SEB (600 ng to 73 pg/ml) after optimization of capture and revealing antibody dilutions. ELISA Results were compared using the formula of subtraction [17].
Detection of Spiked SEB from Culture Supernatant and Food Samples
Known amount of SEB was spiked in the culture supernatant (600 ng/ml) of non-enterotoxigenic S. aureus ATCC6538P and food samples (1 μg/ml or g), i.e., milk, orange juice, skim milk powder and khoya. Unspiked Culture supernatant of ATCC6538P was used as negative control. Extraction of SEB from food samples was done by adapting the method described by Vernozy-Rozand and Coworkers [18]. Extracted toxin was subjected to ELISA as described above.
Results and Discussion
SEs rank second among the causal agents of food poisoning all over the world [19–21]. As little as 100 ng of SEs may make a person ill with symptoms of classic food poisoning (nausea, vomiting and/or diarrhoea) [4]. The most common SEs implicated in food poisoning includes SEA, SEB, SEC and SED [19, 22]. Among these, SEB is the most potent and a listed BW agent [23, 24]. Therefore, it is of utmost importance to have an immunological system for detection of SEB. But the important bottleneck in the development of an immunological detection system of SEB includes the limited availability of high affinity and specific antibodies due to its superantigenic nature. A slight contamination of SEB with other proteins leads to non-specific and low titre antibody response against it. Therefore, all possible efforts should be made to obtain large amount of pure SEB for raising specific and high titre antibodies. Conventionally, SEB is purified by size exclusion chromatography, ion-exchange chromatography, chromatofocussing, etc. which are not only cumbersome and time consuming but also do not guarantee the purity of the protein apart from the poor yield [1, 13, 25]. Alternatively, high level of protein purity can be obtained by heterologous expression of the desired gene into a suitable vector containing a fusion partner that can be used as affinity tag for single step purification [26].
In this study, seb gene of S. aureus ATCC14458 was amplified by PCR that resulted in 720 bp amplicon. The PCR amplified gene was ligated into pQE-30UA vector having 6× His tag. PCR positive clones were analysed for expression and a thick band of rSEB toxin was observed at approximately 30.6 kDa position on SDS–PAGE gel (Fig. 1A). The position of rSEB toxin was also confirmed by western blot developed with anti His HRP conjugated antibodies (Fig. 1B). The 6× His affinity tag facilitates purification using Ni–NTA matrix. The 6× His tag is poorly immunogenic and, generally, does not affect secretion, compartmentalization, or folding of the fusion protein. In addition, anti-His antibodies can be used for detection. Among the inducible clones, one clone (rBUS6) was selected for further studies of gene expression and protein purification. Optimum optical density (OD) for inducer (IPTG) addition was 0.4–0.5 which was achieved at 1.5 h of inoculation. The maximum yield of rSEB was obtained when rBUS6 was induced by IPTG at a concentration of 0.75 mmol/l for 5 h. rSEB was localized in the cytoplasm and maximum protein was found as loosely bound inclusion bodies. The purified rSEB showed single band as revealed by silver stained gel (Fig. 2). The yield of purified rSEB obtained in the present study was 92 mg/l (w/v) of culture broth which corresponds to 33% (w/w) of total protein. The estimated yield of rSEB reported by Ignatov and Coworkers [27] was only 1.7% whereas Yang and Coworkers [28] reported a yield of 33.3% in E. coli strain JM109. The major limitation of both these investigations was that they did not use affinity tag for purification of the toxin. Moreover, none of these workers indicated the specific applications of rSEB. In our earlier studies, seb gene was expressed in E. coli [14] but the yield was very less which is a limiting factor for its use in further studies of superantigenicity and detection system development.
Fig. 1.
SDS–PAGE profile and western blot showing inducible expression of SEB. A Molecular weight markers (66, 45, 29, 20 kDa) (lane 1); E. coli host SG13009 (lane 2); Uninduced recombinant clone (lane 3); Induced recombinant clone showing SEB band at 30.6 kDa (lane 4); B rSEB band at 30.6 kDa (lane 1); Pre-stained molecular weight markers (113, 91, 49.9, 35.1, 28.4, 20.8 kDa) (lane 2)
Fig. 2.
SDS–PAGE of affinity purified rSEB. Lane 1 rBUS6 Cell lysate after induction, Lane 2 Flow-through after binding of protein with Ni–NTA resin, Lane 3–4 Wash, Lane 5–7 Eluates, Lane 8 Molecular weight markers (66, 45, 29 kDa)
In the present study, purified rSEB was used to raise polyclonal antibodies in mice and rabbit models. The titer of antisera as revealed by western blot was 1:32000 in both mice and rabbit. These antibodies were non-cross reactive to other staphylococcal enterotoxins (SEA, SEC and SED), non-enterotoxigenic S. aureus as well as other bacterial species, i.e., Bacillus subtilis, B. cereus, Enterococcus faecalis and Vibrio cholera which may find entry into food. These results encouraged us to develop sandwich ELISA for detection of SEB. The detection sensitivity of the sandwich ELISA method was found to be 0.15 ng/ml with toxin alone and toxin spiked in non-enterotoxigenic S. aureus culture supernatant (Fig. 3). A number of reports have been published on the detection of staphylococcal enterotoxins in foods by immunodiffusion [8], latex agglutination [20], radioimmunoassay [9], competitive ELISA [29] as well as double antibody sandwich ELISA [11, 12, 30]. Among these, the sandwich ELISA was shown to be the most satisfactory method for SEB detection [10, 12, 31]. The double antibody sandwich ELISA allowed the detection of toxin at around 1 ng/g of sample in several types of foods [10, 11, 30, 32, 33]. However, in many of the cases, antibodies used in the immunodetection system of SEs were monoclonal obtained from hybridoma clones [30, 32]. The low detection limit of the sandwich ELISA developed in the present study encouraged us to evaluate it further for SEB detection after extraction from artificially spiked food samples. The TCA precipitation method [18, 34] was used for extraction of SEB as it is more user friendly, fast and efficient. The detection limit of toxin was 0.25 ng/ml for milk (Fig. 4), 0.49 ng/ml for orange juice (Fig. 5), 0.25 ng/g for skim milk (Fig. 6) and 0.49 ng/g for khoya (Fig. 7). This detection limit is well below the microslide immunodiffusion method approved by the AOAC [35]. The limit of SEB detection is at par or even better than many of the methods employing monoclonal antibodies. Moreover, time, skill and the cost involved in raising polyclonal antibodies are far less than that of monoclonal antibodies.
Fig. 3.
Detection of SEB in spiked non-enterotoxigenic S. aureus culture supernatant by sandwich ELISA. Data points and error bars show mean and standard deviation of triplicates. Cut off value for negative control was 0.34. Sensitivity of detection was 0.15 ng/ml
Fig. 4.
Detection of SEB in spiked milk by sandwich ELISA. Data points and error bars show mean and standard deviation of triplicates. Cut off value for negative control was 0.37. Limit of detection was 0.25 ng/ml
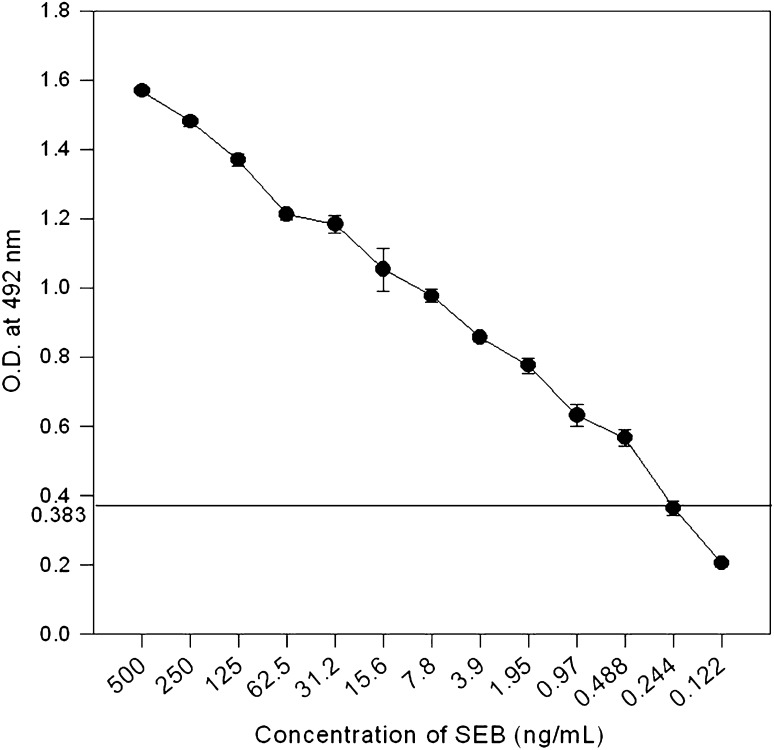
Fig. 5.
Detection of SEB in spiked orange juice by sandwich ELISA. Data points and error bars show mean and standard deviation of triplicates. Cut off value for negative control was 0.383. Limit of detection was 0.49 ng/ml
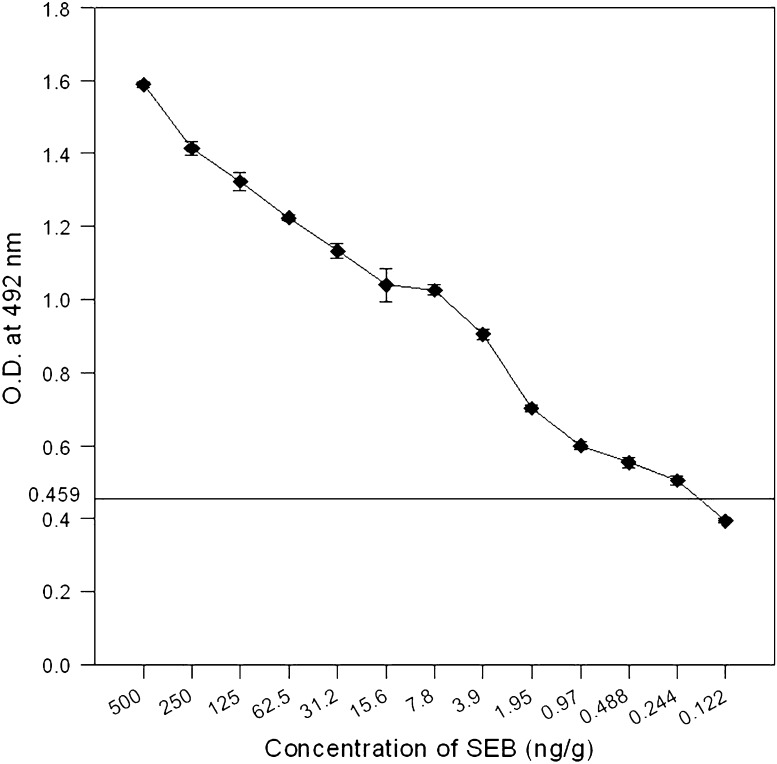
Fig. 6.
Detection of SEB in spiked skim milk by sandwich ELISA. Data points and error bars show mean and standard deviation of triplicates. Cut off value for negative control was 0.459. Limit of detection was 0.25 ng/g
Fig. 7.
Detection of SEB in spiked khoya by sandwich ELISA. Data points and error bars show mean and standard deviation of triplicates. Cut off value for negative control was 0.544. Limit of detection was 0.49 ng/g
Therefore, monospecific polyclonal antibodies generated by means of cloning and hyperexpression of SEB may serve as useful reagents to develop sensitive and specific detection system without generating monoclonal antibodies, the facility of which is not available in most of the laboratories especially in the developing world. The reported detection system can also be used for QA/QC of food products as well as retrospective investigations of SEB used by miscreants for biological terrorism.
Acknowledgments
Authors are thankful to Director, DRDE, Gwalior for facilitating the study.
References
- 1.Dainiak M, Hedstrom M, Galaev IY, Mattiasson B. Improved methods for prepurification and detection of staphylococcal enterotoxin B from cell-free culture filtrate. Biotechnol Prog. 2005;21:1347–1351. doi: 10.1021/bp050099j. [DOI] [PubMed] [Google Scholar]
- 2.Baker MD, Acharya KR. Superantigens: structure-function relationships. Int J Med Microbiol. 2004;293:529–537. doi: 10.1078/1438-4221-00298. [DOI] [PubMed] [Google Scholar]
- 3.Le Loir Y, Baron F, Gautier M. Staphylococcus aureus and food poisoning. Genet Mol Res. 2003;2:63–76. [PubMed] [Google Scholar]
- 4.Evenson ML, Hinds MW, Bernstein RS, Bergdoll MS. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food poisoning involving chocolate milk. Int J Food Microbiol. 1988;7:311–316. doi: 10.1016/0168-1605(88)90057-8. [DOI] [PubMed] [Google Scholar]
- 5.Bergdoll MS. Symposium on microbiology update: old friends and new enemies. Staphylococcus aureus. J Assoc Off Anal Chem. 1991;74:706–710. [PubMed] [Google Scholar]
- 6.Sapsford KE, Taitt CR, Loo N, Ligler FS. Biosensor detection of botulinum toxoid A and staphylococcal enterotoxin B in food. Appl Environ Microbiol. 2005;71:5590–5592. doi: 10.1128/AEM.71.9.5590-5592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pimbley DW, Patel PD. A review of analytical methods for the detection of bacterial toxins. Symp Ser Soc Appl Microbiol. 1998;27:98S–109S. doi: 10.1046/j.1365-2672.1998.0840s198s.x. [DOI] [PubMed] [Google Scholar]
- 8.Casman EP, Bennett RW, Dorsey AE, Stone JE. The micro-slide gel double diffusion test for the detection and assay of staphylococcal enterotoxins. Health Lab Sci. 1969;6:185–198. [PubMed] [Google Scholar]
- 9.Janin F, Buyser M-L, Lapeyre C, Feinberg M. Radioimmunological quantitative determination of Staphylococcus aureus enterotoxin A in various foods. Sci Aliments. 1983;3:397–412. [Google Scholar]
- 10.Morissette C, Goulet J, Lamoureux G. Rapid and sensitive sandwich enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxin B in cheese. Appl Environ Microbiol. 1991;57:836–842. doi: 10.1128/aem.57.3.836-842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park CE, Warburton D, Laffey PJ. A collaborative study on the detection of staphylococcal enterotoxins in foods by an enzyme immunoassay kit (RIDASCREEN) Int J Food Microbiol. 1996;29:281–295. doi: 10.1016/0168-1605(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 12.Wieneke AA, Gilbert RJ. The use of a sandwich ELISA for the detection of staphylococcal enterotoxin A in foods from outbreaks of food poisoning. J Hyg. 1985;95:131–138. doi: 10.1017/S0022172400062367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balaban N, Rasooly A. Analytical chromatography for recovery of small amounts of staphylococcal enterotoxins from food. Int J Food Microbiol. 2001;64:33–40. doi: 10.1016/S0168-1605(00)00439-6. [DOI] [PubMed] [Google Scholar]
- 14.Kamboj DV, Nema V, Pandey AK, Goel AK and Singh L (2006) Heterologous expression of staphylococcal enterotoxin B (seb) gene for antibody production. Elect J Biotechnol vol 9 no 5 [online]. Available from www.ejbiotechnology.info/content/vol9/issue5/full/3/index.html
- 15.Sambrook J, Russel DW. Molecular cloning: a laboratory manual. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 16.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 17.Snyder DB, Marquardt WW, Mallinson ET, Russek E. Rapid serological profiling by enzyme-linked immunosorbent assay. I. Measurement of antibody activity titer against Newcastle disease virus in a single serum dilution. Avian Dis. 1983;27:161–170. doi: 10.2307/1590381. [DOI] [PubMed] [Google Scholar]
- 18.Vernozy-Rozand CM, Mazuy-Cruchaudet C, Bavari C, Richard Y. Improvement of a concentration protocol based on trichloroacetic acid for extracting staphylococcal enterotoxins in dairy products. Rev Méd Vet. 2004;155:533–537. [Google Scholar]
- 19.Balaban N, Rasooly A. Staphylococcal enterotoxins. Int J Food Microbiol. 2000;61:1–10. doi: 10.1016/S0168-1605(00)00377-9. [DOI] [PubMed] [Google Scholar]
- 20.Di Pinto A, Forte VT, Ciccarese G, Conversano MC, Tantillo GM. Comparison of reverse passive latex agglutination test and immunoblotting for detection of staphylococcal enterotoxin A and B. J Food Saf. 2005;24:231–238. doi: 10.1111/j.1745-4565.2004.00533.x. [DOI] [Google Scholar]
- 21.Nema V, Agrawal R, Kamboj DV, Goel AK, Singh L. Isolation and characterization of heat resistant enterotoxigenic Staphylococcus aureus from a food poisoning outbreak in Indian subcontinent. Int J Food Microbiol. 2007;117:29–35. doi: 10.1016/j.ijfoodmicro.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Smyth DS, Hartigan PJ, Meaney WJ, Fitzgerald JR, Deobald CF, Bohach GA, Smyth CJ. Superantigen genes encoded by the egc cluster and SaPIbov are predominant among Staphylococcus aureus isolates from cows, goats, sheep, rabbits and poultry. J Med Microbiol. 2005;54:401–411. doi: 10.1099/jmm.0.45863-0. [DOI] [PubMed] [Google Scholar]
- 23.Kaempfer R, Arad G, Levy R, Hillman D. Defense against biologic warfare with superantigen toxins. Isr Med Assoc J. 2002;4:520–523. [PubMed] [Google Scholar]
- 24.Kamboj DV, Goel AK, Singh L. Biological warfare agents. Def Sci J. 2006;56:495–506. [Google Scholar]
- 25.Tranter HS, Brehm RD. Production, purification and identification of the staphylococcal enterotoxins. Soc Appl Bacteriol Symp Ser. 1990;19:109S–122S. doi: 10.1111/j.1365-2672.1990.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee S, Shukla A, Guptasarma P. Single-step purification of a protein-folding catalyst, the SlyD peptidyl prolyl isomerase (PPI), from cytoplasmic extracts of Escherichia coli. Biotechnol Appl Biochem. 2003;37:183–186. doi: 10.1042/BA20020044. [DOI] [PubMed] [Google Scholar]
- 27.Ignatov KB, Chistiakova LG, Shemchukova OB, Gorodetskaia SB, Kiselev VI. Cloning the Staphylococcus aureus enterotoxin B gene, obtained by polymerase chain reaction, and its expression in Escherichia coli cells. Bioorg Khim. 1993;19:75–80. [PubMed] [Google Scholar]
- 28.Yang LQ, Wu WF, Shi CB, Lu AG, Feng JX, Bai XL. Cloning of staphylococcal enterotoxin B gene and its highly expression in Escherichia coli. Sheng Wu Gong Cheng Xue Bao. 2002;18:597–600. [PubMed] [Google Scholar]
- 29.Kuo JS, Silverman GJ. Application of enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxins in food. J Food Prot. 1980;43:404–407. doi: 10.4315/0362-028X-43.5.404. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki T, Terano Y, Shibata T, Kawamoto H, Kuzuguchi T, Kohyama E, Watanabe T, Ohyama T, Gemba M. Establishment of highly specific and quantitative immunoassay systems for staphylococcal enterotoxin A, B, and C using newly-developed monoclonal antibodies. Microbiol Immunol. 2005;49:589–597. doi: 10.1111/j.1348-0421.2005.tb03650.x. [DOI] [PubMed] [Google Scholar]
- 31.Bennett RW. Staphylococcal enterotoxin and its rapid identification in foods by enzyme-linked immunosorbent assay-based methodology. J Food Prot. 2005;68:1264–1270. doi: 10.4315/0362-028x-68.6.1264. [DOI] [PubMed] [Google Scholar]
- 32.Kijek TM, Rossi CA, Moss D, Parker RW, Henchal EA. Rapid and sensitive immunomagnetic-electrochemiluminescent detection of staphyloccocal enterotoxin B. J Immunol Methods. 2000;236:9–17. doi: 10.1016/S0022-1759(99)00234-3. [DOI] [PubMed] [Google Scholar]
- 33.Sapsford KE, Francis J, Sun S, Kostov Y, Rasooly A. Miniaturized 96-well ELISA chips for staphylococcal enterotoxin B detection using portable colorimetric detector. Anal Bioanal Chem. 2009;394:499–505. doi: 10.1007/s00216-009-2730-z. [DOI] [PubMed] [Google Scholar]
- 34.Meyrand A, Atrache V, Bavai C, Montet MP, Vernozy-Rozand C. Evaluation of an alternative extraction procedure for enterotoxin determination in dairy products. Lett Appl Microbiol. 1999;28:411–415. doi: 10.1046/j.1365-2672.1999.00562.x. [DOI] [PubMed] [Google Scholar]
- 35.McLandsborough L, Tatini SR. A 6 h microslide immunodiffusion assay for confirmed detection of staphylococcal enterotoxins. Lett Appl Microbiol. 1991;12:81–84. doi: 10.1111/j.1472-765X.1991.tb00510.x. [DOI] [PubMed] [Google Scholar]