Abstract
The role of prebiotics in improving human health has attracted global attention and the research is mostly focused on the strains belonging to the genera Bifidobacterium and Lactobacillus. Non-digestible oligosaccharides hold significant role in recent research due to their prebiotic nature. Soluble polysaccharides (SP, 14.4%), isolated from ragi bran consisted mainly of arabinose and xylose with minor quantities of rhamnose, mannose, galactose and glucose. Ragi bran SP subjected to purified endoxylanase (from 96 h ragi malt) treatment to obtain xylo-oligosaccharides which were further purified on Biogel P-2 followed by HPLC. The purified oligosaccharide yielded (RO-1; 17.9%) was identified as xylobiose by electrospray ionization mass spectrometry (282 + 23 = 305) and 1HNMR. In vitro studies carried out using Bifidobacterium and Lactobacillus sp. proved the prebiotic nature of the crude xylo-oligosaccharides (XOs) and RO-1. Acetate was found to be the chief short chain fatty acid released during fermentation of both crude XOs and purified xylobiose and 24 h bacterial culture showed high xylanase activity (1020–1690 μU min−1).
Keywords: Bifidogenic bacteria, Prebiotic activity, Ragi, Xylanase, Xylo-oligosacccharides
Introduction
Functional food/functional food ingredients, that can enhance the health of the consumer is having good market in today’s global food industry. A prebiotic is defined as ‘a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health’ [1]. Of the currently known functional foods, non-digestible oligosaccharides (NDOs) hold an important position with respect to their prebiotic activity.
Cereal brans (mainly consisting of pericarp + seed coat) in addition to cellulose are rich in non-cellulosic polysaccharides such as arabinoxylans, 1,3/1,4-β-d-glucans and lignocellulose complexes [2]. Xylans are polymers of 1,4-β-D xylopyranose residues mainly substituted with α-l-arabinofuranose or its feruloylated derivatives at O-2 or O-3 positions of the xylose residues. Endoxylanase hydrolyses the arabinoxylans randomly producing XOs of varying degree of polymerization (D.P.2-10), and they come under the broad definition of NDOs. The NDOs resist digestion by small intestinal digestive enzymes [3].
Carbohydrates reaching the colon undergo different degree of fermentation and results in the production of short chain fatty acids (SCFA) such as acetate, propionate and butyrate, which provide metabolic energy for the host and help in the acidification of the bowel [4].
Apart from their prebiotic effect, NDOs are believed to alleviate disease symptoms such as diabetes, arteriosclerosis and colon cancer. Prebiotic effect of fructo-oligosacharides (FOS) and galacto-oligosaccharides (GOS) were reported [5–7]. However the use of XOs as prebiotic has not yet been effectively exploited. In the present study an endoxylanase (E.C.3.2.1.8) purified from 96 h ragi malt [8] has been used to obtain XO(s) from soluble polysaccharides (SP) isolated from ragi bran and elucidate its structural characterization. The potential of XOs as effective prebiotic components is also proved in the present study.
Materials and Methods
Materials
Ragi (Eleusine coracana, Indaf-15) seeds were procured from V.C. farm, University of agricultural science, Bangalore, Karnataka, India. HPLC (μ-Bondapak aminopropyl) and GLC (OV-225 and PEG-20 M) columns were obtained from Shimadzu Co., Kyoto, Japan. Termamyl (E.C.3.2.1.1; from Bacillus licheniformis) and glucoamylase (E.C.3.2.1.3; from Aspergillus niger) were purchased from Sigma, MO, USA. Microbial cultures were procured from National Dairy Research Institute, Karnal.
Preparation of Ragi Bran
Ragi seeds were cleaned before use. Bran was prepared from finger millet according to the protocol described by Shobana et al. [9]. Ragi seeds were moistened by spraying with 7% (w/v) water equilibrated for 10 min and pulverized in a carborundum disc mill. The resultant meal was sifted through an 85-mesh stainless steel sieve (180 mm openings) and the tailings were again pulverized and sifted through the same sieve. The process of pulverizing the tailings and sieving were repeated two more times. The millet endosperm flour fraction that passed through the first second and third stages of sieving was pooled and termed as refined finger millet flour. The unsievable tailings (which are 85-mesh size) at the end of the third pulverizing were collected and termed as ragi bran.
Isolation of Soluble (SP) and Insoluble Polysaccharides (IP) from Ragi Bran
Ragi bran (100 g) was suspended in acetate buffer (400 ml, 0.05 M, pH 5.0), stirred for 2 h and digested with termamyl (500 μl) at 95°C for 2 h followed by glucoamylase (2 ml) at 55°C for 48 h. The resultant hydrolysate obtained after centrifugation (10,000×g, 10 min) was concentrated to 50 ml and precipitated with 3 vol. of ethanol. Precipitate was separated out, dialyzed and lyophilized to obtain SP. The residue obtained after centrifugation was dried by solvent exchange, i.e., washing with ethanol (80%) followed by 95% and methanol (96%) and diethyl ether (99%). The dried residue obtained after diethyl ether washing is designated as insoluble polysaccharides (IP).
Chemical Composition Analysis
Polysaccharides (10 mg) isolated from ragi bran were derivatised into alditol acetates [10] and analyzed using Shimadzu GLC system (GC-15A) with flame ionization detector and OV-225 column (8 ft × 1/8′′). GLC was carried out using a Shimadzu 14-B gas liquid chromatograph equipped with flame ionization detector at 200°C column temperature and 250°C injector and detector port temperatures. Nitrogen (40 ml/min) was used as carrier gas Protein concentration was determined by Bradford method [11] with bovine serum albumin (BSA) as standard.
Characterization and Purification of Xylobiose
SP (20 mg) isolated from ragi bran was subjected to enzymatic hydrolysis using purified ragi xylanase (240 μg) [8] at 50°C for 150 min. The reaction was stopped by adding three volumes of ethanol (70%) to precipitate the undegraded polysaccharides which were subsequently separated from the hydrolytic products by centrifugation (10,000×g, 10 min). The hydrolysate containing the oligosaccharides was concentrated to 1 ml by flash evaporation (Buchi Rotavapor111) at 30°C. Ethanol was completely removed by flash evaporation and the oligosaccharide was redissolved in DDH2O (1 ml) and separated on Biogel P-2 column (0.9 × 105 cm) using water as the eluent at a flow rate of 6 ml h−1 [12]. The oligosaccharide collected by Biogel P-2 purification was concentrated by lyophilization, passed through a Millipore filter (0.2 μm), resolved and identified by Shimadzu HPLC system equipped with refractive index detector and μ-Bondapak-NH2 column (4.1 mm × 30 cm) using acetonitrile: water (75:25) at a flow rate of 0.7 ml/min.
Mass spectrum of the oligosaccharide purified through Biogel P-2 column followed by HPLC was measured by Alliance, Waters 2695 mass spectrometer using positive mode electrospray ionization with the following operational conditions i.e. capillary voltage 3.5 kV, core voltage 100 V, source temperature 80°C, disolvation temperature 150°C, core gas (Argon) 35 lth−1 and disolvation gas (Nitrogen) 500 lt h−1.
The 1H NMR spectrum of purified oligosaccharide (1.5 mg in D2O) was recorded using Bruker500 spectrometer operating at 500 MHz at 27°C with tetra methyl silane (TMS) as the internal standard. 16 pulses were collected with pulse retention time of 5 s and pulse angle 30°.
In Vitro Fermentation Experiments
Bifidobacterium adolescentis NDRI 236, Bifidobacterium bifidum 229 ATCC 29521, Bifidobacterium bifidum NCDO 2715, Lactobacillus brevis 01 NDRI strain RTS, Lactobacillus plantarum 020 NDRI strain 184, Pediococcus pentosaceus 035 NCDO 813, Pediococcus pentosaceus ATCC 8081 were the microorganisms used for the present study.
Individual cultures were grown in MRS broth and subjected to centrifugation (3,000×g, 15 min 15°C) after 24 h of incubation at 37°C. The resultant cells were suspended in 0.85% saline. Serial dilutions (10−6) were prepared to get the requisite cell population.
In vitro experiments were carried out using crude and purified XO(s) liberated from ragi bran SP by. The crude oligosaccharides refer to the mixture of oligosaccharides obtained after ragi bran WEP hydrolysis by the action of ragi xylanase and the purified refer to the oligosaccharide collected through Biogel-P-2 column purification. The XOs liberated from ragi bran by xylanase treatment were filtered through membrane filter (0.22 μm, Millipore) and added at 0.25% level to deMan Rogosa and Sharpe (MRS) broth (without dextrose, 2 ml) and inoculated with 100 μl of culture suspension giving 200 CFU (Colony Forming Unit) and incubated at 37°C for 48 h. With respect to Bifidobacterium cultures Cysteine–HCl (20 μl) was added to the culture broth and incubation was carried out in an anaerobic chamber. Growth characteristics were monitored by measuring pH and absorbance (600 nm) of culture broth after 48 h of incubation. Microbial cultures after 48 h of incubation were centrifuged (3000 × g, 20 min, 15°C) and oven dried to determine the dry cell mass. The resultant supernatants were analyzed for SCFA.
Enzyme Assays
Aliquots were taken out from culture broth (24 h) and assayed for following enzymes. For xylanase assay, larchwood xylan (0.5% in acetate buffer, pH 5.0, 0.1 M; Sigma, MO, USA) was incubated with 20 μl of sample at 50°C for 1 h. The reaction was stopped by the adding dinitrosalicylic acid (DNS, 1.0 ml) and the reducing sugar liberated was quantified by Dinitro salicylic acid method, [13]. One unit of activity is defined as the amount of enzyme required to release 1 μmol of xylose/min under the experimental conditions.
The substrate, p-nitrophenyl β-d-xylopyranoside (0.5 ml, 2 mmol in sodium phosphate buffer) (Sigma, MO, USA) was incubated with sample (20 μl) for 1 h at 37°C for the estimation of β-d-xlopyransidase activity [14]. The reaction was stopped by adding saturated solution of sodium tetraborate (0.5 ml) and absorbance was read at 400 nm. One unit of activity is defined as the amount of enzyme required to liberate 1 μmol of p-nitrophenol/min under assay conditions. p-nitrophenol (2-10 μg/0.5 ml) was used as standard. p-nitrophenyl α-l-arabinofuranoside, α-d-galactopyranoside and β-d-galactopyranoside (Sigma, MO, USA) were taken as the substrates [14] for α-l-arabinofuranosidase, α-d-galactopyranosidase and β-d-galactopyranosidase assay and the reactions were performed as mentioned above. For the estimation of acetyl esterase activity, p-nitrophenyl acetate (Sigma, MO, USA) was taken as the substrate and assay was performed as mentioned above except the reaction time was reduced to 30 min. All the assays were performed in triplicates.
SCFA Analysis
The culture supernatant obtained by centrifuging (3000 × g, 20 min, 15°C) the culture broth after 48 h incubation was acidified with 50% sulphuric acid and extracted with diethyl ether [15] and analyzed for SCFA by GLC on PEG-20 M with column, injector and detector temperatures of 120, 220 and 230°C respectively [16]. Acetate, propionate and butyrate were used as the standards.
Results and Discussion
Isolation and Neutral Sugar Composition of Ragi Bran SP
SP yielded from destarched ragi bran was around 14.4%. SP yield was found to be less compared to wheat bran [17]. Studies have been also carried out on maize bran [18] and water soluble non-starch polysaccharides from native and germinated ragi [19].
The neutral sugar composition of SP and IP isolated from ragi bran were shown in Table 1. Arabinose and xylose were found to be the major monosaccharides in the ragi bran SP with trace amounts of galactose, glucose, rhamnose and mannose. Ara/xyl ratio in SP was found to be 1:1.8, while in the case of IP it is 1:1.2. Ara/xyl ratio reported for arabinoxylans from barley and malt were close to 0.65 [20] whereas, in wheat bran, the ratio was in accordance with the present study [17].
Table 1.
Neutral sugar composition of non-starch polysaccharides obtained from ragi bran
| Sample | Rha | Ara | Xyl | Man | Gal | Glu |
|---|---|---|---|---|---|---|
| Ragi | ||||||
| SP | 5.4 | 41.8 | 22.9 | 4.71 | 14.9 | 10.4 |
| IP | 0.92 | 10.4 | 9.1 | 4.3 | 2.9 | 72.4 |
Rha Rhamnose, Ara arabinose, Xyl xylose, Man mannose, Gal galactose, Glu glucose, SP soluble polysaccharides, IP insoluble polysaccharides
Liberation of XO from Ragi Bran SP by Xylanase Hydrolysis and their Purification
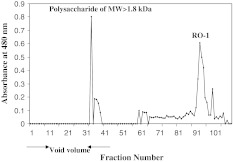
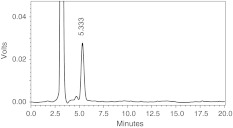
Oligosaccharides (17.9%) liberated from ragi bran by the action of purified ragi xylanase were separated on Biogel P-2. The partially degraded polysaccharides (molecular weight >1.8 kDa) were separated out in the void volume and single oligosaccharide designated as RO-1 was collected (Fig. 1) and its purity was confirmed by HPLC (Fig. 2). RO-1 was found to be exclusively consisting of xylose as analysed by GLC. Wheat bran subjected to endoxylanase treatment also showed the similar hydrolysis pattern hydrolysis wherein xylotriose and xylobiose were the major products [17].
Fig. 1.
Elution profile of xylo-oligosaccharides liberated from ragi bran WEP on Biogel P-2, RO-1, represents the major oligosaccharide liberated from ragi bran WEP. Water was used as the eluent at a flow rate of 6 ml h−1
Fig. 2.
HPLC profile of RO-1liberated from ragi bran WEP. Acetonitrile:water (75:25) is used as the eluent at a flow rate of 0.7 ml/min
ESI–MS of the HPLC Purified Oligosaccharide, RO-1
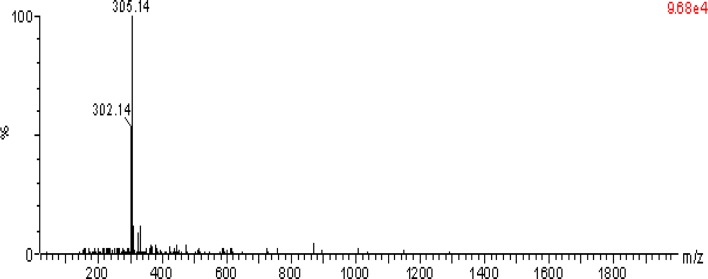
RO-1 showed preponderantly the presence of ion at m/z 305.14, which was identified as a disaccharide (150 × 2 = 300 − 18 (elimination of water molecule) = 282 + 23 (sodium adduct) = 305) (Fig. 3). The cleavage pattern of ragi bran water extractable polysaccharides (WEP) done by the method [21] confirmed the endo mode of action of xylanase.
Fig. 3.
ESI–MS of HPLC purified RO-1; measured by Alliance, Waters 2695 mass spectrometer using positive mode electrospray ionization
1HNMR Spectrum of the HPLC Purified Oligosaccharide, RO-1
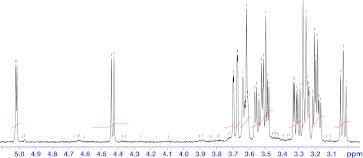
The structure of the purified oligosaccharide (RO-1) was elucidated by 1H NMR and the signals were falling in the range of 3.0–5.0 ppm (Fig. 4). The 1H NMR spectrum showed prominent chemical shifts for anomeric protons at 3.248, 3.542 and 3.7 ppm corresponding to H-2, H-3 and H-4 of β-d-xylopyranose residue respectively [22, 23]. The chemical shifts corresponding to arabinose residues and glucuronic acids were not detected in the spectrum. Hence the structure of the purified oligosaccharide is as follows, which is 1,4-linked β-d-xylobiose.
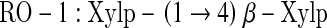 |
Fig. 4.
1H NMR spectrum of RO-1. HPLC Purified oligosaccharide (1.5 mg) was dissolved in D2O and Tetramethyl silane (TMS) was used as the internal standard
Growth Characteristics in Terms of OD and pH of the Culture Broth (48 h) and Cell Mass as Shown by Different Microorganisms Grown on Crude and Purified Xylobiose
In vitro experiments carried out using crude XOs and purified xylobiose proved their prebiotic nature with respect to Bifidobacteria and Lactobacilli sp. in terms of the growth characteristics pattern. SCFAs produced as a result of the fermentation of NDOs result in the reduction of the pH of culture broth. Such decrease in pH can be used as an indication of the prebiotic effect of the oligosaccharides incorporated in the culture broth [24, 25]. A decrease in the pH of the culture broth and increase in OD were observed for all the strains grown on crude XOs and RO-1 (Table 2) after 48 h incubation. Maximum OD was observed with respect to Pediococcus pentosaceus ATCC 8081. A concomitant increase in the dry cell mass was also observed compared to the control after 48 h incubation proving that XOs were utilized by the beneficial microbes and has enhanced their growth. Purified xylobiose is more effective than crude XOs wherein the OD of the culture broth and bacterial cell mass were found to be slightly high. The growth of the microorganism on particular oligosaccharide may be strain specific [26]. Pediococus pentosaceus NCDO 813 showed increased growth compared to Pediococus pentosaceus ATCC 8081. But with respect to B. bifidum the two different strains tested did not show much difference in their growth.
Table 2.
Growth characteristics of microorganisms grown on crude and purified oligosaccharide(s) liberated from ragi bran WEP after 48 h of incubation
| Microorganism | OD | pH | Cell mass (mg) |
|---|---|---|---|
| B. adolescentis NDRI 236 | 0.070 ± 0.006a | 7.2 ± 0.4 | 1.3 ± 0.1 |
| 0.483 ± 0.02b | 5.8 ± 0.15 | 6.0 ± 0.3 | |
| (0.563) ± 0.02c | (5.6) ± 0.1 | (7.0) ± 0.23 | |
| B. bifidum ATCC 29521 | 0.068 ± 0.005a | 6.9 ± 0.3 | 1.0 ± 0.07 |
| 0.737 ± 0.04b | 5.7 ± 0.1 | 7.0 ± 0.2 | |
| B. bifidum NCDO 2715 | 0.06 ± 0.003a | 7.5 ± 0.3 | 1.1 ± 0.08 |
| 0.705 ± 0.02b | 5.8 ± 0.26 | 6.0 ± 0.32 | |
| L. plantarum NDRI strain 184 | 0.039 ± 0.003a | 6.9 ± 0.4 | 1.0 ± 0.05 |
| 0.496 ± 0.01b | 5.9 ± 0.18 | 5.3 ± 0.2 | |
| (0.634) ± 0.03c | (5.8) ± 0.16 | (7.0) ± 0.3 | |
| L. brevis 01 | 0.04 ± 0.002a | 7.1 ± 0.2 | 1.4 ± 0.07 |
| 0.517 ± 0.025b | 6.0 ± 0.16 | 5.0 ± 0.37 | |
| P. pentosaceus NCDO 813 | 0.031 ± 0.003a | 7.5 ± 0.2 | 1.0 ± 0.04 |
| 0.388 ± 0.01b | 6.9 ± 0.14 | 6.0 ± 0.4 | |
| P. pentosaceus ATCC 8081 | 0.035 ± 0.002a | 6.9 ± 0.15 | 1.2 ± 0.05 |
| 0.752 ± 0.05b | 5.6 ± 0.2 | 7.0 ± 0.18 |
aControl(media without sugar supplement)
b Crude oligosaccharides
c Values in parenthesis are for purified xylobiose; The values are represented with standard error, n = 3. The OD of 48 h culture broth was measured spectrophotometrically at 600 nm
Enzyme Activities in the 24 h Microbial Culture Broth
The 24 h cultures showed xylanase, xylosidase, arabinofuranosidases, α and β-galactosidase and acetyl esterase activities. High activity of xylanase (1020–1690 μU ml−1) was detected in the culture broth of all the tested microorganisms grown on crude XOs (Table 3). Acetyl esterase activity was found to be negligible (0.24–0.36 μU ml−1). Similar result was observed in the case of B. adolescentis NDRI 236 and L. plantarum grown on RO-1. The hydrolytic enzymes produced by the microorganisms help in the digestion of NDOs, which escape digestion in the upper gastrointestinal tract. SCFA are produced as a result of the fermentation of NDOs.
Table 3.
Enzyme activities (μU/ml) in 24 h old culture broth of microorganisms grown on crude XOs and xylobiose
| Microorganism | Xylanase | Xylosidase | Arabinofuranosidase | α-galactosidase | β-galactosidase | Acetyl esterase |
|---|---|---|---|---|---|---|
| B. adolescentis NDRI 236 | 1020 ± 10 | 3.9 ± 0.02 | 11.4 ± 0.2 | 3.6 ± 0.03 | 5.1 ± 0.12 | 0.24 ± 0.02 |
| (1090) ± 12a | (3.8) ± 0.5 | (12.6) ± 0.16 | (3.1) ± 0.02 | (3.6) ± 0.04 | (0.38) ± 0.03 | |
| B. bifidum ATCC 29521 | 1160 ± 14 | 3.3 ± 0.05 | 5.4 ± 0.26 | 2.7 ± 0.03 | 4.5 ± 0.04 | 0.36 ± 0.02 |
| B. bifidum NCDO 2715 | 1690 ± 15 | 2.4 ± 0.02 | 6.6 ± 0.2 | 2.7 ± 0.02 | 4.2 ± 0.02 | 0.24 ± 0.02 |
| L. plantarum NDRI strain 184 | 1260 ± 13 | 2.7 ± 0.04 | 6.9 ± 0.3 | 2.6 ± 0.02 | 3.4 ± 0.16 | 0.35 ± 0.04 |
| (1690) ± 12a | (2.7) ± 0.04 | (12.6) ± 0.12 | (3.1) ± 0.02 | (3.6) ± 0.2 | (0.38) ± 0.05 | |
| L. brevis 01 | 1200 ± 10 | 2.7 ± 0.02 | 8.1 ± 0.32 | 3.0 ± 0.03 | 3.3 ± 0.2 | 0.24 ± 0.03 |
| P. pentosaceus NCDO 813 | 1200 ± 14 | 3.1 ± 0.04 | 7.2 ± 0.2 | 2.7 ± 0.04 | 3.9 ± 0.04 | 0.35 ± 0.02 |
| P. pentosaceus ATCC 8081 | 1200 ± 13 | 2.7 ± 0.02 | 7.5 ± 0.12 | 2.9 ± 0.03 | 4.5 ± 0.05 | 0.36 ± 0.02 |
The values are represented with standard error, n = 3
aValues in parenthesis are for purified xylobiose and the one without parenthesis are for crude oligosaccharides
SCFA in the 48 h Microbial Culture Broth Analyzed by GLC
Acetic, propionic and butyric acids are the major SCFA produced during fermentation of carbohydrates in the large bowel [27]. Acetate was the chief SCFA released by the microorganisms due to fermentation of both crude XOs and RO-1 (Table 4). Butyrate was analyzed in the culture broth of B. bifidum ATCC 29521 (0.86%) and L. plantarum whereas, propionate was detected only in the culture broth of L. plantarum NDRI strain 184 grown on crude XOs. B. adolescentis NDRI 236 and L. plantarum were taken to study the prebiotic effect of the purified oligosaccharide due to its less quantity. Acetate (100%) was detected in the culture broth of B. adolescentis NDRI 236 grown on RO-1, whereas acetate (81.4%) and butyrate (3.0%) were the end products of fermentation by L. plantarum. Lactate concentration was not tested in the present study. Our results are in accordance with the in vivo study by Pan et al. wherein they found that oligosaccharides (FOS and GOS) change the concentrations of SCFAs and the microbial population of mouse bowel and acetate, propionate, and butyrate in the cecum were increased with administration of oligosaccharides [28]. Hence the present study suggests that oligosaccharide fermentation patterns obtained in vitro could be used to predict behavior in vivo.
Table 4.
Proportions of acetic, propionic and butyric acid as a percentage of total SCFA formed after 48 h incubation in the culture broth of microbes incorporated with crude and purified oligosaccharide(s)
| Microorganism | Acetate | Propionate | Butyrate |
|---|---|---|---|
| B. adolescentis NDRI 236 | 100 ± 0.00 | – | – |
| (100) ± 0.00a | – | – | |
| B. bifidum ATCC 29521 | 99.1 ± 4.3 | – | 0.86 ± 0.02 |
| B. bifidum NCDO 2715 | 100 ± 0.00 | – | – |
| L. plantarum NDRI strain 184 | 57.7 ± 2.7 | 24.4 ± 2.2 | 18.0 ± 1.02 |
| (96.43 ± 3.35)a | – | (3.57) ± 0.23 | |
| L. brevis 01 | 100 ± 0.00 | – | – |
| P. pentosaceus NCDO 813 | 100 ± 0.00 | – | – |
| P. pentosaceus ATCC 8081 | 100 ± 0.00 | – | – |
The values are represented as mean values mol% ± SD, n = 3
aValues in parenthesis are for purified xylobiose
Acknowledgements
Authors thank Dr. V. Prakash, FRSc, Director CFTRI for his keen interest in the work and encouragement. M.C. thanks the Council of Scientific and Industrial Research (CSIR, New Delhi) for the grant of junior and senior fellowships. The authors are grateful to Dr. M.C Varadaraj, Head, HRD, CFTRI for providing lab facilities to in vitro prebiotic studies.
References
- 1.Gibson GR, Roberfroid B. Dietary modulation of the human colonic microflora: Introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 2.Charalampopoulos D, Wang R, Pandiella SS, Webb C. Application of cereals and cereal components in functional foods: a review. Int J Food Microbiol. 2002;79:131–141. doi: 10.1016/S0168-1605(02)00187-3. [DOI] [PubMed] [Google Scholar]
- 3.Laere KMJ, Hartemink R, Bosveld M, Schols HA, Voragen AGJ. Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria. J Agric Food Chem. 2000;48:1644–1652. doi: 10.1021/jf990519i. [DOI] [PubMed] [Google Scholar]
- 4.Swennen K, Courtin CM, Delcour JA. Non-digestible oligosaccharides with prebiotic properties. Crit Rev Food Sci Nutr. 2006;46:459–471. doi: 10.1080/10408390500215746. [DOI] [PubMed] [Google Scholar]
- 5.Bouhnik Y, Flourie BD, Agay-Abensour L, Pochart P, Gramet G, Durand M, Rambaud JC. Administration of transgalacto-oligosaccharides increases faecal Bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J Nutr. 1997;127:444–448. doi: 10.1093/jn/127.3.444. [DOI] [PubMed] [Google Scholar]
- 6.Delzenne NM, Kok N. Effects of fructans-type prebiotics on lipid metabolism. Am J Clin Nutr. 2001;73:456S–458S. doi: 10.1093/ajcn/73.2.456s. [DOI] [PubMed] [Google Scholar]
- 7.Rastall RA. Functional oligosaccharides: application and manufacture. Annu Rev Food Sci Technol. 2010;1:305–339. doi: 10.1146/annurev.food.080708.100746. [DOI] [PubMed] [Google Scholar]
- 8.Manisseri C, Muralikrishna G. Characterization of purified xylanase from finger millet (Eleusine coracana-Indaf 15) malt. Eur Food Res Technol. 2008;227:587–597. doi: 10.1007/s00217-007-0760-3. [DOI] [Google Scholar]
- 9.Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of a-glucosidase and a-amylase. Food Chem. 2009;115:1268–1273. doi: 10.1016/j.foodchem.2009.01.042. [DOI] [Google Scholar]
- 10.Sawardekar JS, Slonekar JM, Jeanes A. Quantitative determination of monosaccharides as their alditol acetates by gas-liquid chromatography. Anal Chem. 1965;37:1602–1604. doi: 10.1021/ac60231a048. [DOI] [Google Scholar]
- 11.Bradford MM. A rapid sensitive method for quantitation of microgram quantities of proteins utilizing the principles of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Guillon F, Tranquet O, Quillien L, Utille J-P, Ortiz JJO, Saulnier L. Generation of polyclonal and monoclonal antibodies against arabinoxylans and their use for immunocytochemical location of arabinoxylans in cell walls of endosperm of wheat. J Cereal Sci. 2004;40:167–182. doi: 10.1016/j.jcs.2004.06.004. [DOI] [Google Scholar]
- 13.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 14.Beldman G, Osuga D, Whitaker JR. Some characteristics of β-d-xylopyranosidases, α-l-arabinofuranosidases and an arabinoxylan α-l-arabinofuranohydrolase from wheat bran and germinated wheat. J Cereal sci. 1996;23:169–180. doi: 10.1006/jcrs.1996.0017. [DOI] [Google Scholar]
- 15.Karppinen S, Liukkonen K, Aura AM, Forssell P, Poutanen K. In vitro fermentation of polysaccharides of rye, wheat and oat brans and inulin by human faecal bacteria. J Sci Food Agric. 2000;80:1469–1476. doi: 10.1002/1097-0010(200008)80:10<1469::AID-JSFA675>3.0.CO;2-A. [DOI] [Google Scholar]
- 16.Silvi S, Rumney CJ, Cresci A, Rowland IR. Resistance starch modifies gut microflora and microbial metabolism in human flora associated rats inoculated with faeces from Italian and UK donors. J Appl Microbiol. 1999;6:521–530. doi: 10.1046/j.1365-2672.1999.00696.x. [DOI] [PubMed] [Google Scholar]
- 17.Benamrouche S, Cronier D, Debeire P, Chabbert B. A chemical and histological study on the effect of (1 → 4)-β-endo-xylanase treatment on wheat bran. J Cereal sci. 2002;36:253–260. doi: 10.1006/jcrs.2001.0427. [DOI] [Google Scholar]
- 18.Saulnier L, Vigouroux J, Thibault J-F. Isolation and partial characterization of feruloylated oligosaccharides from maize bran. Carbohydr Res. 1995;272:241–253. doi: 10.1016/0008-6215(95)00053-V. [DOI] [PubMed] [Google Scholar]
- 19.Rao RSP, Muralikrishna G. Water soluble feruloyl arabinoxylans from rice and ragi: changes upon malting and their consequence on antioxidant activity. Phytochem. 2006;67:91–99. doi: 10.1016/j.phytochem.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 20.Dervilly G, Leclercq C, Zimmermann D, Roue C, Thibault J–F, Saulnier L. Isolation and characterization of high molar mass water-soluble arabinoxylans from barley and barley malt. Carbohydr plym. 2002;47:143–149. doi: 10.1016/S0144-8617(01)00172-2. [DOI] [Google Scholar]
- 21.Fernandez LEM, Obel N, Scheller V, Roepstorff P. Differentiation of isomeric oligosaccharide structures by ESI tandem MS and GC-MS. Carbohydr Res. 2004;339:655–664. doi: 10.1016/j.carres.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann RA, Leeflang BR, Barse MMJ, Kamerling JP, Vliegenthart JFG. Characterization by 1H-N.M.R. spectroscopy of oligosaccharides, derived from arabinoxylans of white endosperm of wheat, that contains the elements →4)[α-l-Araf-(1 → 3)]-β-d-Xylp-(1 → or → 4)[α-l-Araf- (1 → 2)][α-l-Araf-(1 → 3)]-β-d-Xylp-(1→. Carbohydr Res. 1991;221:63–81. doi: 10.1016/0008-6215(91)80049-S. [DOI] [PubMed] [Google Scholar]
- 23.Gruppen H, Hoffman RA, Kormelink FJM, Voragen AGJ, Kamerling JP, Vliegenthart JFG. Characterisation by 1H-N.M.R. spectroscopy of enzymatically derived oligosaccharides from alkali-extractable wheat-flour arabinoxylan. Carbohydr Res. 1992;233:45–64. doi: 10.1016/S0008-6215(00)90919-4. [DOI] [PubMed] [Google Scholar]
- 24.Berggren AM, ME Bjorck I, Nyman EMGL. Short-chain fatty acid content and ph in caecum of rats given various sources of carbohydrates. J Sci Food Agric. 1993;63:397–406. doi: 10.1002/jsfa.2740630405. [DOI] [Google Scholar]
- 25.Morisse JP, Maurice R, Boilletot E, Cotte JP. Assessment of the activity of a fructooligosaccharide on different caecal parameters in rabbits experimentally infected with E. coli 0.103. Ann Zootech. 1993;42P:81–87. doi: 10.1051/animres:19930109. [DOI] [Google Scholar]
- 26.Holtl SM, Miller-Fosmorel CM, Cote GL. Growth of various intestinal bacteria on alternansucrase derived oligosaccharides. Lett Appl Microbiol. 2005;40:385–390. doi: 10.1111/j.1472-765X.2005.01681.x. [DOI] [PubMed] [Google Scholar]
- 27.Ruppin H, Bar-Meir Soergel KH, Wood CM, Schmitt MG. Absorption of short chain fatty acids by the colon. Gastroenterol. 1980;78:1500–1507. [PubMed] [Google Scholar]
- 28.Pan X-D, Chen F-Q, Wu T-X, Tang H-G, Zhao Z-Yu. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J Zhejiang Univ Sci B. 2009;10(4):258–263. doi: 10.1631/jzus.B0820261. [DOI] [PMC free article] [PubMed] [Google Scholar]