Abstract
Therapeutic options for infections caused by gram-negative organisms expressing plasmid-mediated AmpC β-lactamases are limited because these organisms are usually resistant to all the β-lactam antibiotics, except for cefepime, cefpirome and the carbapenems. These organisms are a major concern in nosocomial infections and should therefore be monitored in surveillance studies. Hence, this study was aimed out to determine the prevalence of plasmid-mediated AmpC β-lactamases in E. coli and K. pneumoniae from a tertiary care in Bangalore. A total of 63 E. coli and 27 K. pneumoniae were collected from a tertiary care hospital in Bangalore from February 2008 to July 2008. The isolates with decreased susceptibility to cefoxitin were subjected to confirmation test with three dimensional extract tests. Minimum inhibitory concentrations (MICs) were determined by agar dilution method. Conjugation experiments, plasmid profiling and susceptibility testing were carried out to investigate the underlying mechanism of resistance. In our study, 52 (57.7%) isolates showed resistance to cefoxitin, the occurrence of AmpC was found to be 7.7% of the total isolates. Plasmid analysis of the selected isolates showed the presence of a single plasmid of 26 kb in E. coli and 2 Kb in K. pneumoniae. Plasmid-mediated AmpC β-lactamases were found in 11.1% of K. pneumoniae and in 6.3% of E. coli. Curing and conjugation experiments showed that resistance to cephamycins and cephalosporins was plasmid-mediated. Our study has demonstrated the occurrence of plasmid-mediated AmpC in E. coli and K. pneumoniae which illustrates the importance of molecular surveillance in tracking AmpC-producing strains at general hospitals and emphasizes the need for epidemiological monitoring.
Keywords: AmpC, E. coli, K. pneumoniae, Cefoxitin, Antibiotic resistance, Plasmid curing
Introduction
Bacteria over expressing AmpC β-lactamases are of major clinical concern because they confer resistance to a wide variety of β-lactams, β-lactam/β-lactamase inhibitor combinations, and monobactams, but are susceptible to fourth generation cephalosporins and carbapenems [1–3]. Constitutive over expression of AmpC β-lactamases in gram-negative organisms occurs either by deregulation of the ampC chromosomal gene or by acquisition of a transferable ampC gene on a plasmid or other transferable element. The transferable ampC gene products are commonly called plasmid-mediated AmpC β-lactamases [3–5]. Emergence of plasmid-mediated AmpC β-lactamase producing strains is well recognized [6–8]. Phenotypically bacteria resemble chromosomal class C β-lactamase producers in that they confer resistance to cephalosporins and cephamycins such as, cefoxitin, and are not inhibited by β-lactam/β-lactamase inhibitors [9].
AmpC β-lactamase genes are native to the chromosomes of many gram-negative bacilli but are missing from Klebsiella spp. and are rarely hyper-expressed in E. coli. Mobilization from the genome of species typically carrying inducible/de-repressed blaampC such as Citrobacter freundii and Morganellamorganii by plasmids into K. pneumoniae and E. coli in which they confer novel resistance pattern [7, 8, 10, 11].
Despite of increased interest in the recognition of plasmid-mediated AmpC β-lactamases in E. coli and Klebsiella spp., the worldwide distribution and prevalence of AmpC mediated resistance is fragmentary. This is due in part to the limited number of surveillance studies seeking clinical strains producing AmpC β-lactamases and the difficulty that laboratories have in accurately detecting these resistance mechanism(s) particularly as there are no published CLSI criteria [12]. In addition it has been stated that the detection of AmpC β-lactamases production is challenging since the hyperproduction of chromosomal AmpC in association with OMP F porin loss in E. coli or porin deficiency in K. pneumoniae can produce similar resistance phenotypes. Therefore, detection of plasmid-mediated AmpC enzyme producing isolates is considered critical for epidemiological studies and hospital infection control, because the genes can be spread to other organisms. Hence, the study was conducted to determine the prevalence of plasmid-mediated AmpC β-lactamases in clinical isolates of Escherichia coli and Klebsiella Pneumoniae and their antibiotic susceptibility pattern.
Materials and Methods
Clinical Strains
In a duration of six months (February 2008–July 2008), 90 consecutive non repetitive clinical isolates of E. coli (n = 63) and K. pneumoniae (n = 27) were obtained from Sri Bhagawan Mahaveer Jain hospital, Bangalore. The sources of isolates were from urine, throat swab, pus. Significant proportions of the strains were from the hospitalized patients (41). All isolates were identified, using the standard conventional microbiological techniques [13].
Antibiotic Susceptibility Testing
All the bacterial isolates were tested for cefoxitin disk (30 μg) resistance. The isolates with cefoxitin zone diameter less than 18 mm were further subjected to detailed susceptibility testing by standard disk diffusion method to note the concomitant resistance to other antibiotics. Disk diffusion tests were performed and the results were interpreted according to the guidelines of the CLSI [14]. The antibiotics used include nine β-lactams—ampicillin (10 μg), aztreonam (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg) cefuroxime (30 μg), cefotaxime (30 μg), cefepime (30 μg), imipenem, and amoxicillin/clavulanic acid (10 μg) and three non-β-lactam antibiotics, gentamicin (10 μg), amikacin (30 μg), and ciprofloxacin (5 μg). E. coli ATCC 25922 was used as a quality control strain.
MICs Determination
MICs of antimicrobial agents was carried out by the agar dilution method with Mueller–Hinton agar according to the 2005 CLSI recommendations [15], including ten β-lactams—ampicillin (10 μg), aztreonam (30 μg), ceftazidime (30 μg), ceftriaxone (30 μg) cefuroxime (30 μg), cefotaxime (30 μg), cefepime (30 μg), cefoxitin (30 μg), imipenem, and amoxicillin/clavulanic acid (10 μg) and three non-β-lactam antibiotics—gentamicin (10 μg), amikacin (30 μg), and ciprofloxacin (5 μg). E. coli ATCC 25922 was used as a quality control strain.
Phenotypic AmpC Detection
Since there are no published CLSI criteria for phenotypic screening or confirmatory test for AmpC β-lactmases, a modified three dimensional test was used for the detection of AmpC enzymes in cefoxitin resistant isolates [16].
Plasmid Analysis and Curing Experiments
The selected AmpC producing strains of E. coli and K. pneumoniae were screened for plasmids by the alkaline lysis method [17]. Plasmid curing experiments was also preformed as described by Shahid et al. [18]. Ethidium bromide was used as the curing agent. The minimum inhibitory concentration of ethidium bromide was determined for the bacterial isolates in trypticase soya broth and the highest concentration permitting the growth was used. The plasmid cured colonies were then tested for antibiotic susceptibility. Concomitant loss of antibiotic resistance was compared with the loss of plasmid content.
Transconjugation Experiments
To determine whether the resistance was transferable, transconjugation experiments were performed on the AmpC producing isolates. Seven AmpC positive isolates were used as donors and E. coli K12 (F−SR lac−) resistant to streptomycin, was used as the recipient strain. Equal volumes of the donor and the recipient strain cultures in Luria–Bertani (LB) broth were mixed. The mixtures were then incubated at 37°C for 16 h. The transconjugants were selected on MacConkey agar plates supplemented with streptomycin (500 μg/ml) and ceftazidime (2 μg/ml) and were analyzed for plasmids as described above. Recipient E. coli K12 (F−SR lac−) was screened for antibiotic susceptibility by agar dilution method with reference to the guidelines of CLSI, the presence of plasmids before the conjugation, followed by similar screening of the transconjugants after mating experiments.
Results
Clinical Specimens and Bacterial Species Distribution
Out of the 63 isolates of E. coli, 52 isolates were from urine, 4 from throat swab and 7 from pus. Of the 27 K. pneumoniae isolates, 22 isolates were from urine, 2 from throat swab and 3 from pus. Among 63 E. coli and 27 K. pneumoniae isolates, 52 isolates (57.77%) showed cefoxitin zone diameter ≤18 mm and were considered as screen positive for AmpC production. 7 of the 52 cefoxitin resistant clinical isolates yielded positive AmpC by the three dimensional extract test. The occurrence rate of AmpC producers in E. coli and K. pneumoniae was 7.77% (7/90), including 4 in E. coli and 3 in K. pneumoniae. Out of the four isolates of E. coli, three (75%) were from urine samples and one (25%) was from pus sample. Among the K. pneumoniae isolates, one (33.3%) was from urine sample and two (66.6%) were from pus samples.
Characterististics of Antibiotic Resistance in AmpC Producing Strains
The results of antibiotic resistance rates and patterns of cefoxitin resistant isolates (n = 52) are shown in Tables 1 and 2. All the isolates tested in the present study for antibiotic susceptibility were multidrug resistant, the majority of isolates (61.53%) being resistant to nine or more of the 12 antibiotics tested, followed by resistance to eight drugs (17.3%), seven and six drugs (5.76%) and three drugs (11.53%). Concomitant high resistance to ampicillin, amoxicillin/clavulanic acid, ciprofloxacin, aztreonam, gentamicin, was present in 98, 86.53, 88.46, 82.69, and 48% isolates.
Table 1.
Resistance rates of other antibiotics tested in cefoxitin resistant isolates (n = 52)
| Antibiotics tested | % Resistance |
|---|---|
| Aztreonam | 82.69 |
| Amoxicillin/clavulanic acid | 86.53 |
| Ampicillin | 98.0 |
| Ceftazidime | 82.69 |
| Cefotaxime | 90.38 |
| Ceftriaxone | 84.61 |
| Cefuroxime | 92.30 |
| Cefepime | 67.30 |
| Imipenem | 0 |
| Gentamicin | 48.0 |
| Amikacin | 7.69 |
| Ciprofloxacin | 88.46 |
Table 2.
Resistance patterns of cefoxitin-resistant isolates (n = 52)
| Resistant to | Resistance patterns (n) | Total |
|---|---|---|
| Ten drugs | Ca Ci Ce Cpm Cu A Ac Ao Cf G (15) Ca Ci Ce Cu A Ac Ao Cf G Ak (2) |
17 |
| Nine drugs | Ca Ci Ce Cpm Cu A Ac Cf G (03) Ca Ci Ce Cpm Cu A Ac Ao Cf (10) Ca Ci Ce Cpm Cu A Cf G Ak (02) |
15 |
| Eight drugs | Ca Ci Ce Cpm Cu A Ac Cf (03) Ca Ci Ce Cpm Cu A Ao Cf (02) Ca Ci Ce Cu A Ac Cf G (01) Ca Ci Ce Cu A Ac Ao Cf (02) Ci Ce Cu A Ac Ao Cf G (01) |
09 |
| Seven drugs | Ca Ci Ce Cu A Ac Ao (02) Ca Ci Ce A Ao Cf G (01) |
03 |
| Six drugs | Ce Cu A Ac Ao Cf (03) | 03 |
| Three drugs | Cu Cf Ao (01) Cu A Ao (01) A Ac Ao (03) |
05 |
Antibiotics key: Ca ceftazidime, Ci ceftriaxone, Ce cephotaxime, Cu cefuroxime, Cpm cefepime, A ampicillin, Ao aztreonam, Ac amoxicillin/clavulanic acid, G gentamicin, Ak amikacin, Cf ciprofloxacin
Out of the seven AmpC producers resistance to cefipime was noticed in three (42.85%) isolates. All of the seven AmpC producing strains were susceptible to imipenem. Table 3 shows the MIC of the seven AmpC producing isolates. These seven isolates showed very similar susceptibility profiles, characterized by elevated MICs for aztreonam, ceftazidime, cefotaxime, cefoxitin, cefuroxime while MICs of imipenem for all of them were 0.5 μg/ml. The MICs of cefepime varied among the strain isolated.
Table 3.
Antibiotic resistance and MIC distributions of the seven plasmid mediated AmpC producing E. coli and K. pneumoniae
| Antimicrobial agents | Resistance rate (%) | MIC distribution (μg/ml) |
|---|---|---|
| Aztreonam | 85.7 | >128 |
| Amoxicillin/clavulanic acid | 100 | 64 |
| Ampicillin | 85.7 | >128 |
| Ceftazidime | 85.7 | 128 |
| Cefotaxime | 85.7 | 256 |
| Cefoxitin | 100 | 256 |
| Ceftriaxone | 71.42 | >64 |
| Cefuroxime | 85.7 | >128 |
| Cefepime | 42.9 | 64(1), 32 (1), 8(1) |
| Imipenem | 0 | 0.5 |
| Gentamicin | 28.6 | 128 |
| Amikacin | 14.2 | 4 |
| Ciprofloxacin | 71.4 | 64 |
Plasmid Analysis and Plasmid Curing
Plasmid analysis of the isolates showed the presence of a single plasmid of ~26 Kb in E. coli and 2 Kb in K. pneumoniae. Curing experiments were attempted in these isolates to determine the change in the plasmid content associated with the antibiotic resistance pattern. Curing experiments using the intercalating dye ethidium bromide was successful only in 85.7% (6/7) isolates. The MICs of the ethidium bromide ranged from 400 to 600 μg/ml for these isolates. The loss of antibiotic resistance was concominant with the loss of plasmid content. It was noted that all the isolates that lost plasmids became susceptible to cefoxitin and gentamicin.
Transfer of AmpC β-Lactamases and Antimicrobial Resistance in Transconjugation
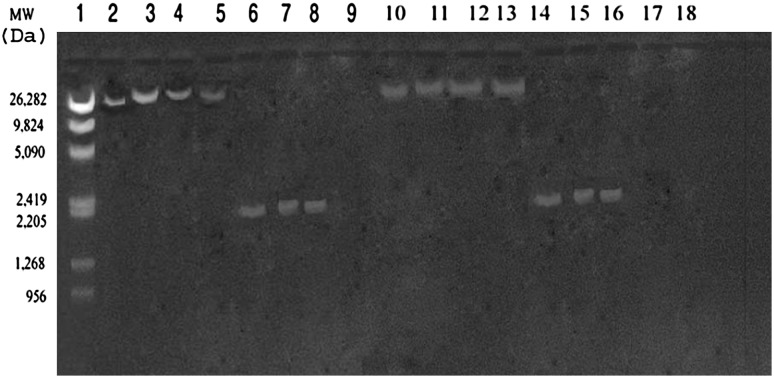
All the AmpC producing isolates subjected to transconjugation experiments were able to transfer resistance to cefoxitin and cephaolosporins. The MIC of cefoxitin against the transconjugants is ≥256 μg/ml, representing a 32-fold increase relative to that of the recipient E. coli K12 (F−SR lac−) strain (8 μg/ml). All transconjugants showed elevated MICs for ampicillin, aztreonam, ceftazidime, cefotaxime, cefoxitin, ceftriaxone and gentamicin Additionally, MICs of the transconjugants to β-lactam in combination with β-lactamase inhibitors showed no significant synergy, which is one of the characteristics of the AmpC enzyme. The recipient strain E.coli K12 (F−SR lac−) did not show the presence of any plasmid before the transconjugation experimentation. The plasmid profile of the donors and transconjugants are shown in Fig. 1.
Fig. 1.
Agarose gel (0.8%) showing 26 and 2 Kb plasmids in donors (Lane 2–8) and their respective transconjugants (Lane 10–16). Molecular weight marker λ/Mlu Hind digest along with their size (in Daltons) is shown in lane 1. The recipient strain (E. coli K12F−SR lac−) screened for plasmids before transconjugation experiment was found devoid of the same (lane 9)
Discussion
Plasmid-mediated AmpC β-lactamases have been found worldwide but are less common than extended-spectrum β-lactamases (ESBLs), and in E. coli, they appear to be less often a cause of cefoxitin resistance than an increased production of chromosomal AmpC β-lactamase [19 and references cited therein]. Failure to detect AmpC β-lactamase producing strains has contributed to their uncontrolled spread and therapeutic failures [20]. Hence, their appearance in hospital settings should be identified quickly so that appropriate antibiotic usage and containment measures can be implemented.
In India, AmpC producing strains of Enterobacteriaceae have emerged as a challenge in hospitalized as well as community based patients [21]. In 2003, 20.7% AmpC producers were found among gram-negative isolates in Guru Tegh Bahadur Hospital, Delhi [22]. In the same year, Subha et al. found AmpC β-lactamase production in 24.1% of Klebsiella spp. and 37% of E. coli in Chennai [23]. Around the same time, Shahid et al. reported 20 P. aeruginosa isolates as producing AmpC beta lactamases in Aligarh [18]. In 2005, data from Chennai revealed AmpC production in 20.8% Klebsiella spp. and 16.6% E. coli [24]. In the present study 7.77% of the isolates were found to be to be AmpC producers which were less than that reported from Delhi, Chennai and Aligarh. This difference may be due to the difference in selection criteria of isolates, the variation in the ability to produce AmpC β-lactamases among different gram negative bacteria and different clinical specimens. The data generated in our study revealed a relatively higher percentage of gram negative isolates producing these enzymes when compared to earlier report of 3.3% for E. coli and 2.2% for K. pneumoniae isolates in Karnataka [25], this recent increase in AmpC producing isolates may be indicative of the ominous trend of more and more isolates acquiring resistance mechanisms rendering the antimicrobial armamarium ineffective.
Of the 52 cefoxitin-resistant isolates, 45 (86.53%) were negative for AmpC β-lactamase production by three dimensional method. Cefoxitin resistance in these isolates could be due to the lack of permeation porins [22]. In these strains, cefoxitin resistance can be explained by the loss of porins. Hernandez et al. demonstrated that interruption of a porin gene by insertion sequences is a common type of mutation that causes the loss of porin expression and increased cefoxitin resistance [26]. Interestingly, no AmpC harboring isolates revealed susceptibility to cefoxitin in our study or else, as we have used only cefoxitin resistance-based screening test.
Previous studies suggested that cefepime might be effective for the treatment of infections caused by AmpC producing organisms. However, in this study, 42.85% (3 of 7) AmpC β-lactamase producers were resistant to cefepime. Song et al. reported that the association of plasmid-mediated AmpC β-lactamases with ESBLs may cause the failure of treatment and there is also a report indicating the high inoculum effect of cefepime in plasmid-mediated AmpC β-lactamase producing isolates [27–29]. Similar data has been published in the MYSTIC Program in Europe and the US (1997–2004) [30]. The available data suggest that carbapenems are more effective than cefepime in treating serious infections that involve large numbers of AmpC producing organisms.
In our study, imipenam was the only drug found unaffected by the action of these enzymes. These findings are in concordance with the studies conducted by other authors, who also reported a rate of 100% sensitivity to imipenam [31–35]. These carbapenam agents may be beneficial in treatment of AmpC infection; however, indiscriminate use of these agents may promote increased resistance to carbapenam.
The transconjugation study showed that resistance genes producing AmpC in seven isolates were conjugated successfully, suggesting that it is located on a transferable element such as a conjugative plasmid. The transfer of plasmids encoding β-lactamase genes into E. coli K12 (F−SR lac−) was accompanied by resistance to cephalosporins as well as decreased susceptibility to gentamicin. Thus it indicated that the resistance to β-lactams and aminoglycosides coexisted.
Plasmid analysis of the representative isolates showed the consistent presence of a single plasmid. Successfully cured isolates became susceptible to cephamycins and cephalosporins thus providing evidence of carriage of blaampC genes on the plasmid. Dissemination of these AmpC β-lactamase encoding plasmids is thought to facilitate the spread of resistance against a wide range of antibiotics among K. pneumoniae and E. coli.
Cephalosporin resistance among K. pneumoniae and E. coli has increased worldwide [36] as shown in the present antimicrobial susceptibility data, but the rates of resistance to cephalosporins, including that to cefepime, are high. However, all AmpC producing isolates remain susceptible to imipenam. Multi-resistant organisms should be treated with antibiotic regimens other than cephalosporins. Continuous or frequent use of cephalosporins probably leads to higher resistance rates of AmpC producing isolates of Enterobacteriaceae.
Therefore, it would be wise to perform surveillance of clinical isolates to monitor resistance levels in the different wards [37]. In addition, present findings may have important implications in the control of AmpC β-lactamase-producing K. pneumoniae and E. coli strains, which are likely to be overlooked in hospitals. In order to prevent the spread of resistant hospital flora, we suggest restriction of the prescription of broad-spectrum antimicrobial agents is necessary [38].
This is a preliminary study designed with an objective to detect the possible occurrence of AmpC β-lactamases in a tertiary care hospital and to institute antibiotic policy to minimize the emergence of antimicrobial resistance. This is perhaps the first report of Plasmid-mediated AmpC β-lactamase production among gram-negative clinical isolates from this city.
References
- 1.Girlich D, Naas T, Bellais S, Poirel L, Karim A, Nordmann P. Heterogeneity of AmpC cephalosporinases of Hafnia alvei clinical isolates expressing inducible or constitutive ceftazidime resistance phenotypes. Antimicrob Agents Chemother. 2000;44:3220–3223. doi: 10.1128/AAC.44.11.3220-3223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez–Perez FJ, Hanson ND. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002;40:2153–2162. doi: 10.1128/JCM.40.6.2153-2162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson KS, Smith Moland E. Version 2000: the new beta lactamases of Gram-negative bacteria at the dawn of the new millennium. Microbes Infect. 2000;2:1225–1235. doi: 10.1016/S1286-4579(00)01276-4. [DOI] [PubMed] [Google Scholar]
- 4.Bush K. New beta-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin Infect Dis. 2001;32:1085–1089. doi: 10.1086/319610. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind A, Chong Y, Lee K. Plasmid-encoded AmpC beta-lactamases: how far have we gone 10 years after the discovery? Bonsai Med J. 1998;39:520–525. doi: 10.3349/ymj.1998.39.6.520. [DOI] [PubMed] [Google Scholar]
- 6.Gazouli M, Tzouvelekis LS, Prinarakis E, Miriagou V, Tzelepi E. Transferable cefoxitin resistance in Enterobacteria from Greek hospitals and characterization of a plasmid-mediated group 1 beta-lactamase (LAT-2) Antimicrob Agents Chemother. 1996;40:1736–1740. doi: 10.1128/aac.40.7.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez M, Tran JH, Chow N, Jacoby GA. Epidemiology of conjugative plasmid-mediated AmpC beta-lactamases in the United States. Antimicrob Agents Chemother. 2004;48(2):533–537. doi: 10.1128/AAC.48.2.533-537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC-type beta-lactamases. Antimicrob Agents Chemother. 2002;46(1):1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/AAC.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coundron PE, Hanson ND, Climo MW. Occurrence of extended-spectrum and AmpC β-lactamases in blood stream isolates of Klebsiella pneumoniae: isolates harbor plasmid-mediated FOX-5 and ACT-1 AmpC β-lactamsases. J Clin Microbiol. 2003;41:772–777. doi: 10.1128/JCM.41.2.772-777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moland ES, Black JA, Ourada J, Reisbig MD, Hanson ND, Thomson KS. Occurrence of newer β-lactamases in Klebsiella pneumoniae isolated from 24 US hospitals. Antimicrob Agents Chemother. 2002;46:3837–3842. doi: 10.1128/AAC.46.12.3837-3842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson KS. Controversies about extended-spectrum and AmpC beta-lactamases. Emerg Infect Dis. 2001;7:333–336. doi: 10.3201/eid0702.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collee JG, Miles RS, Watt B. Tests for identification of bacteria. In: Collee JG, Marmion BP, Fraser AG, Simmons A, editors. Mackie and McCartney practical medical microbiology. Singapore: Longman Singapore Publishers; 1996. pp. 131–150. [Google Scholar]
- 14.Clinical and Laboratory Standards (2006) Performance standards for antimicrobial susceptibility testing: sixteenth informational supplement. Clinical and laboratory Standards Institute, Wayne, M110–S16
- 15.Clinical and Laboratory Standards (2005) Performance standards for antimicrobial susceptibility testing. Fifteenth informational supplement. Clinical and Laboratory Standards Institute, Wayne, M100–S15
- 16.Shahid M, Malik A, Agrawal M, Singhal S. Phenotypic detection of the extended-spectrum and AmpC β-lactamases by a new spot inoculation method and modified three-dimensional extract test: comparison with conventional three-dimensional extract test. J Antimicrob Chemother. 2004;54:684–687. doi: 10.1093/jac/dkh389. [DOI] [PubMed] [Google Scholar]
- 17.Davis LG, Dibner MD, Battey JF. Large scale alkaline lysis method of plasmid purification. In: Davis LG, Dibner MD, Battery JF, editors. Basic methods in molecular biology, Sect. 8-3. New York: Elsevier; 1986. p. 99. [Google Scholar]
- 18.Shahid M, Malik A, Sheeba Multidrug-resistant Pseudomonas aeruginosa strains harboring R plasmids and AmpC β-lactamases isolated from hospitalized burn patients in a tertiary care hospital of North India. FEMS Microbiol Lett. 2003;228:181–186. doi: 10.1016/S0378-1097(03)00756-0. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby GA. AmpC β-lactamases. Clin Microbiol Rev. 2009;22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singhal S, Mathur T, Khan S, Upadhyay DJ, Chugh S, Gaind R, Rattan A. Evaluation of methods for AmpC β-lactamase in gram-negative clinical isolates from tertiary care hospitals. Indian J Med Microbiol. 2005;23:120–124. doi: 10.4103/0255-0857.16053. [DOI] [PubMed] [Google Scholar]
- 21.Gupta V. An update on newer β-lactamases. Indian J Med Res. 2007;126:417–427. [PubMed] [Google Scholar]
- 22.Manchanda V, Singh NP. Occurrence and detection of AmpC β lactamases among gram negative clinical isolates using a modified three dimensional test at Guru Tegh Bahadur Hospital, Delhi, India. J Antimicrob Chemother. 2003;51:415–418. doi: 10.1093/jac/dkg098. [DOI] [PubMed] [Google Scholar]
- 23.Subha A, Devi VR, Ananthan AmpC β lactamase producing multidrug resistant strains of Klebsiella spp and E. coli isolated from children under five in Chennai. Indian J Med Res. 2003;117:13–18. [PubMed] [Google Scholar]
- 24.Ananthan S, Subha A. Cefoxitin resistance mediated by loss of a porin in clinical strains of Klebsiella pneumoniae and E. coli. Indian J Med Microbiol. 2005;23(1):20–23. doi: 10.4103/0255-0857.13867. [DOI] [PubMed] [Google Scholar]
- 25.Ratna AK, Menon I, Kapur I, Kulkarni R. Occurrence and detection of AmpC β lactamases at a referral hospital in Karnataka. Indian J Med Res. 2003;118:29–32. [PubMed] [Google Scholar]
- 26.Hernandez A, Benedl VJ, Martinez LM, Pascual A, Aguilar A, Tomas M, Alberti S. Development of resistance during antimicrobial therapy caused by insertion sequence interruption of porin genes. Antimicrob Agents Chemother. 1999;43:937–939. doi: 10.1128/aac.43.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang CI, Pai H, Kim SH, Kim HB, Kim EC, Oh MD, Choe KW. Cefepime and the inoculum effect in tests with Klebsiella pneumoniae producing plasmid-mediated AmpC-type beta-lactamase. J Antimicrob Chemother. 2004;54:1130–1133. doi: 10.1093/jac/dkh462. [DOI] [PubMed] [Google Scholar]
- 28.Queenan AM, Foleno B, Gownley C, Wira E, Bush K. Effects of inoculum and beta-lactamase activity in AmpC and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae clinical isolates tested by using NCCLS ESBL methodology. J Clin Microbiol. 2004;42:269–275. doi: 10.1128/JCM.42.1.269-275.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song W, Moland ES, Hanson ND, Lewis JS, Jorgensen JH, Thomson KS. Failure of cefepime therapy in treatment of Klebsiella pneumoniae bacteremia. J Clin Microbiol. 2005;43:4891–4894. doi: 10.1128/JCM.43.9.4891-4894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goossens H, Grabein B. Prevalence and antimicrobial susceptibility data for extended spectrum beta lactamase and AmpC producing Enterobacteriaceae from the MYSTIC program in Europe and the US (1997–2004) Diagn Microbiol Infect Dis. 2005;53(4):257–264. doi: 10.1016/j.diagmicrobio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Thomson KS, Sanders CC. Detection of extended spectrum β-lactamases in members of family Enterobacteriaceae: comparison of the double disk and three dimensional tests. Antimicrob Agents Chemother. 1992;36:1877–1882. doi: 10.1128/AAC.36.9.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pena C, Pujol M, Ardanuy C. Epidemiology and successful control of a large outbreak due to Klebsiella pneumoniae producing extended spectrum β-lactamases. Antimicrob Agents Chemother. 1998;42:53–58. doi: 10.1128/aac.42.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winokur PL, Canton R, Casellas JM, Legakis N. Variations in the prevalence of strains expressing an extended spectrum β-lactamase phenotype and characterisation of isolates from Europe, the Americans and the Western Pacific region. Clin Infect Dis. 2001;32:594–603. doi: 10.1086/320182. [DOI] [PubMed] [Google Scholar]
- 34.Abigail S, Mathai E, Jesudason MV, John TJ. Ceftazidime resistance among Klebsiella pneumoniae in South India. Indian J Med Res. 1995;102:53–55. [PubMed] [Google Scholar]
- 35.Subha A, Ananthan S. Extended spectrum β-lactamase mediated resistance to third generation cephalosporins among Klebsiella pneumoniae in Chennai. Indian J Med Microbiol. 2002;20:92–95. [PubMed] [Google Scholar]
- 36.Tan TY, Ng SY, Teo L, Koh Y, Teok CH. Detection of plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis. J Clin Pathol. 2008;61:642–644. doi: 10.1136/jcp.2007.053470. [DOI] [PubMed] [Google Scholar]
- 37.Peterson LR. Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistance. Clin Microbiol Infect. 2005;11(Suppl 5):4–16. doi: 10.1111/j.1469-0691.2005.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paterson DL. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis. 2004;38(Suppl 4):S341–S345. doi: 10.1086/382690. [DOI] [PubMed] [Google Scholar]