Abstract
Objective
Many forms of arthritis are accompanied by significant chronic joint pain. Here we studied whether there is significant sprouting of sensory and sympathetic nerve fibers in the painful arthritic knee joint and whether nerve growth factor (NGF) drives this pathological reorganization.
Methods
A painful arthritic knee joint was produced by injection of complete Freund’s adjuvant (CFA) into the knee joint of young adult mice. CFA-injected mice were then treated systemically with vehicle or anti-NGF antibody. Pain behaviors were assessed and at 28 days following the initial CFA injection, the knee joints were processed for immunohistochemistry using antibodies raised against calcitonin gene-related peptide (CGRP; sensory nerve fibers), neurofilament 200 kDa (NF200; sensory nerve fibers), growth associated protein-43 (GAP43; sprouted nerve fibers), tyrosine hydroxylase (TH; sympathetic nerve fibers), CD31 (endothelial cells) or CD68 (monocytes/macrophages).
Results
In CFA-injected mice, but not vehicle-injected mice, there was a significant increase in the density of CD68+ macrophages, CD31+ blood vessels, CGRP+, NF200+, GAP43+, and TH+ nerve fibers in the synovium as well as joint pain-related behaviors. Administration of anti-NGF reduced these pain-related behaviors and the ectopic sprouting of nerve fibers, but had no significant effect on the increase in density of CD31+ blood vessels or CD68+ macrophages.
Conclusions
Ectopic sprouting of sensory and sympathetic nerve fibers occurs in the painful arthritic joint and may be involved in the generation and maintenance of arthritic pain.
Introduction
Although arthritis is the most common musculoskeletal disorder in the world, afflicting 50 million people in the USA alone (1), our understanding and ability to treat arthritic joint pain and disease remains strikingly poor. A number of factors have frustrated efforts to understand what drives arthritic joint pain including conflicting observations in epidemiologic studies, protracted disease duration, and the poor correlation between joint damage and arthritic pain (2). Currently, there is a remarkable lack of well-tolerated and effective analgesic therapies (3–5). Compounding these difficulties, human tissue used for experimental analyses is typically obtained from patients with advanced disease at joint replacement surgery, thereby limiting insight to the mechanisms that contribute to the development and maintenance of human chronic arthritic joint pain.
Arthritic joint disease presents a variety of symptoms that impact the use and function of the joint including stiffness, inflammation, swelling and pain (6). From the perspective of most patients, the symptom that has the greatest impact on their functional status is joint pain (1). Unfortunately, there exists a particular dearth in understanding the specific mechanisms that drive arthritic joint pain, a unifying theory as to what drives arthritic joint pain, and what key processes need to be targeted to effectively attenuate this pain.
In the present study we use a mouse model of a painful arthritic joint that is generated by injecting complete Freund’s adjuvant (CFA) into the articular space of the left knee joint of young adult mice. This procedure generates highly robust and reproducible pain-related behaviors including increased flinching as well as reduced weight bearing and use of the arthritic joint. Using this model we tested the hypothesis that following joint injury/inflammation, neurotrophic factors are released that induce a robust and inappropriate sprouting of sensory and sympathetic nerve fibers in the arthritic joint. These newly sprouted nerve fibers are not only present in a higher density per unit area than is found in the normal joint but they will also be present in inappropriate areas (i.e. synovium, meniscus, in the space normally occupied by articular cartilage) so that normally non-noxious loading of the joint will now be perceived as painful. This hypothesis would suggest that the extent of joint destruction alone will not predict the frequency and severity of pain, as a significant component of chronic arthritic joint pain would be maintained by an active and pathological neurochemical and/or morphological remodeling of sensory and sympathetic nerve fibers in the painful joint.
Methods
Animals
Experiments were performed on a total of 40 adult male C57BL/6J mice (Jackson Laboratories), initially at 8 weeks of age, weighing 20–25 g. The mice were housed in accordance with the National Institutes of Health guidelines under specific pathogen free conditions in autoclaved cages maintained at 22°C with a 12-hour alternating light and dark cycle and were given autoclaved food and water ad libitum. The Institutional Animal Care and Use Committee at the Minneapolis VA Medical Center approved all procedures.
Complete Freund’s adjuvant (CFA) injection
A modified version of a previously validated model of arthritic inflammation of the knee joint (7) was produced by performing a single intra-articular injection of CFA (5μg in 10μl) every 7 days, over a period of 28 days (4 injections total). Briefly, mice were anesthetized using 2–3% isoflurane mixed with air. An injection of saline solution (sham animals) or CFA was given using a 30 gauge, ½ inch needle that was fitted with cannulation tubing such that only 2.5 mm of the needle was allowed to puncture the joint. Ten microliters of CFA or saline were injected through the patellar ligament into the articular space, using the femoral condyles as a guide.
Experimental groups
Mice were divided into 4 groups: naive (n=10), sham (intra-articular injection of saline; n=10), CFA-injected mice + vehicle (saline solution, i.p., administered at day 5, 10, 15 and 25 post-initial injection of CFA; n=10) and CFA-injected + anti-NGF (i.p., administered at day 5, 10, 15 and 25 post-initial injection of CFA; n=10).
Treatment with anti-nerve growth factor antibody
The anti-nerve growth factor (NGF) sequestering antibody (mAb 911), kindly provided by Dr. David Shelton (Rinat/Pfizer), is effective in blocking the binding of NGF to both TrkA (tropomyosin receptor kinase A) and p75 NGF receptors and inhibiting TrkA autophosphorylation (8). The anti-NGF antibody possesses a plasma half-life of 5–6 d in the mouse andit does not appreciably cross the blood brain barrier (9). The dose used (10 mg/kg, i.p.) was selected due to its reported efficacy in attenuating skeletal pain in rodents (9). Anti-NGF therapy was administered within 4 hrs after initial intra-articular CFA injection and additionally on day 5, 10, 15, and 25 post-initial CFA injection.
Behavioral measures of arthritic joint pain
Behavioral measures of arthritic joint pain, including spontaneous pain (flinching) and stimulus-evoked pain (limb use and rotorod analysis), were performed on days 0, 3, 10, 17 and 24 post-initial intra-articular CFA injection, and the ability of the animal to place weight on the arthritic limb vs. non-arthritic limb (dynamic weight bearing) was performed on day 0, 4, 9, 16, and 23 post-initial intra-articular CFA injection. At least 6 animals were used in each behavioral experiment group.
The number of spontaneous flinches, representative of spontaneous nocifensive behavior was recorded over a 2-minute observation period. Flinches were defined as the number of times the animal raised its hind paw.
Normal limb use during spontaneous ambulation over a 2 minute period in an open field was used as an indicator of stimulus-evoked pain and was scored on a scale of 5 to 0: (5) normal use, (4) partial limp, but not pronounced, (3) pronounced limp, (2) limp and guarding behavior, (1) partial non-use of limb in locomotor activity, and (0) complete lack of limb use.
Forced ambulatory limb use was determined during a 2 minute period using rotorod analysis (Columbus Instruments). The animals were placed on the rod with X2 speed, 8.0 acceleration, and 2.5 sensitivity control settings and the score was recorded using the 5-0 limb use scale described above.
Dynamic weight bearing analysis of the arthritic limb was performed using a Dynamic Weight Bearing device (Bioseb) as similar pedobarographic analysis is used clinically to determine weight bearing of patients with arthritic knee joints (10). Using a synchronized video recording of the 5 minute test and the scaled map of the detected zones, each presumed paw detection was validated by an observer and identified as a left or right and fore or hind paw.
Immunohistochemistry
Mice were deeply anesthetized by carbon dioxide asphyxiation, delivered using a compressed gas cylinder, at day 28 post-initial CFA injection and perfused intracardially with 20ml of 0.1M phosphate buffered saline (PBS, pH=7.4 at 4°C) followed by 30ml of 4% formaldehyde/12.5% picric acid solution in 0.1M PBS (pH=6.9 at 4°C). Ipsilateral and contralateral knee joints were harvested following perfusion and post-fixed for at least 12 hours in the perfusion fixative. The process of post-fixing, decalcification and sectioning of the bones/joints was performed as previously described (11).
To qualitatively and quantitatively assess the changes in the density and morphology of nerve fibers that innervate the knee joint, macrophage infiltration and aberrant neovascularization of the synovium, 20μm-thick frozen sections of the bone/joint were processed according to our previously published procedures (11). Frozen bone/knee joint sections were incubated with an antibody against calcitonin-gene related peptide (CGRP, polyclonal rabbit anti-rat CGRP, 1:10,000; Sigma Chemical Co.; catalog #C8198) to label unmyelinated and thinly myelinated primary afferent sensory nerve fibers, and an antibody against neurofilament 200 kDa (NF200, chicken anti- NF200, 1:5000; Neuromics; catalog #CH22104) to label myelinated primary afferent sensory nerve fibers. Sympathetic nerve fibers were labeled with an antibody against tyrosine hydroxylase (TH, polyclonal rabbit anti-rat TH, 1:1,000; Chemicon; catalog #AB152). Sprouted nerve fibers were labeled with an antibody against growth associated protein-43 (GAP-43, rabbit anti-GAP43, 1:1000; Millipore; catalog #AB5220). Blood vessels were labeled with an antibody against platelet endothelial cell adhesion molecule (rat anti-mouse CD31, 1:500; BD PharMingen; catalog #550274). Monocytes/macrophages were indentified with an antibody against a myeloid glycoprotein (rat anti-mouse CD68; 1:2000; AbD Serotec; catalog #MCA1957). Additionally, 4 to 5 sequential frozen bone sections from animals in each experimental group were cut at 10μm-thick and stained with hematoxylin-eosin (H&E) to visualize gross pathological changes induced by CFA.
Quantification of nerve fiber density, sprouting, macrophage infiltration and neovascularization
Approximately 30 separate, 20 μm-thick frozen sections were obtained from each knee joint. Three confocal images (Olympus America Inc, software v. 5.0) from different sections separated by at least 100 μm were obtained for each marker. Sections were initially scanned at low power (x100) to identify areas with the highest capillary or nerve fiber density in the synovium (hot spots) and one image per section was acquired within the medial synovial hot spot. While nerve fibers and blood vessels were observed throughout the inflamed synovium, neovascularization and nerve sprouting was consistently present in the synovium adjacent to the meniscus and therefore most of the hot spots were found in this area.
The average volume of CFA-inflamed synovium analyzed was 315 μm (length), 315 μm (width), 20 μm (depth). The Z-stacked images were analyzed with Image-Pro Plus v. 6.0 (Media Cybernetics) and nerve fibers and blood vessels were manually traced to determine the length of nerve fibers or blood vessels within each tissue section. Nerve sprouting or neovascularization was reported as density of nerve fibers or blood vessels per volume of synovium (mm/mm3)(11). CD68+ macrophages were quantified in each layer of the inflamed synovium from z-series images (400x magnification) of each field of view using Imaris Pro Software v. 6.0 (Bitplane AG). Only CD68+ cells that displayed visible nuclei as determined by counterstaining with 4′,6-diamidino-2-phenylindole (DAPI) were counted. Data from at least 3 slices per knee joint were averaged and expressed as total number of CD68+ macrophages per tissue volume (mm3).
Statistics
The SPSS statistics package (v. 12, SPSS Inc) was used to perform statistical tests. One-way ANOVA was used to compare behavioral results and immunohistochemical measures between the experimental groups. For multiple comparisons, the Fisher’s PLSD (Protected Least Significant Difference) post hoc test was used with a significance level set at P<0.05. In all cases, the investigator responsible for behavioral testing, plotting, measuring, and counting was blind to the experimental group identifier of each animal.
Results
A model of an arthritic joint induced by unilateral CFA injection produces infiltration of macrophages, aberrant neovascularization, sensory and sympathetic nerve fiber sprouting, and joint-related pain behaviors
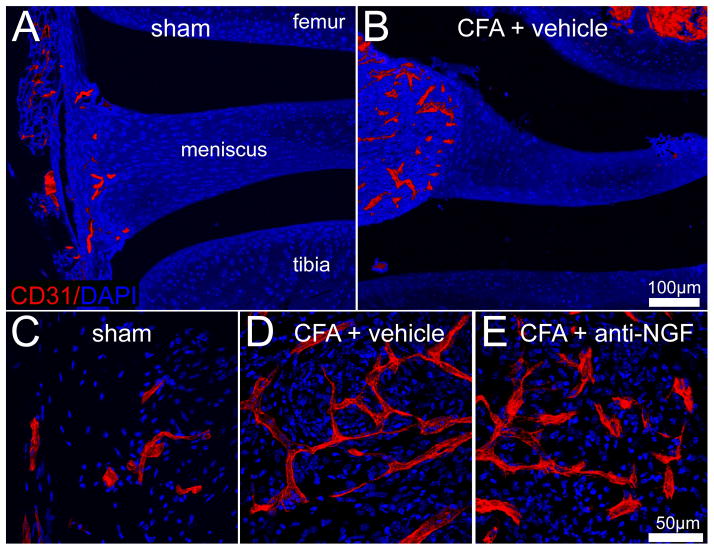
CFA-induced changes in structures of the knee joint, including bone, synovium, and meniscus (Figure 1) were examined histologically and immunohistochemically in CFA-injected mice at 28 days post-initial intra-articular CFA injection and compared to naïve and sham mice. As results for naïve and sham animals were essentially identical, results from naïve animals are not shown. In sham mice, low levels of CD68+ macrophages and CD31+ blood vessels were observed in the synovial-meniscal interface (Figure 2, 3, and 5). In addition, CGRP+, GAP43+, NF200+ sensory and TH+ sympathetic nerve fibers were also evident at low levels in the bone proximal to the joint and the synovial-meniscal interface in these sham mice (Figure 4 and 5).
Figure 1.
A schematic diagram illustrating structural changes (A, B) that are observed in H&E stained sections from a mouse model of the arthritic knee joint (C, D, E). Twenty-eight days following initial complete Freund’s adjuvant (CFA) injection into the intra-articular space of the mouse knee joint there is significant inflammation of the synovium and displacement of the meniscus (D) as compared to the sham (saline injection only) joint (C). Administration of anti-NGF therapy (10mg/kg, i.p., given 4hrs and day 5, 10, 15, 20, 25 post-initial CFA injection) does not significantly reduce these CFA-induced structural changes (E). The outlined box in (A) illustrates the region from which the subsequent brightfield and confocal images and data for figures 1–5 were obtained. Schematic diagram adapted from Wieland, HA et al., Nat Rev Drug Discov. 2005 Apr;4(4):331–44.
Figure 2.
Confocal images showing that following CFA injection into the intra-articular space of the knee joint there is an influx of macrophages into the synovium that is not reduced with anti-NGF therapy. Representative confocal images of CD68+ macrophages (red/orange) and DAPI labeled nuclei (blue) in mouse knee joint sections (20 μm thick) from sham (A, B), CFA-injected mice treated with vehicle (C, D) and CFA-injected animals treated with anti-NGF therapy (E, F). Twenty-eight days following the initial CFA injection, a significant infiltration of CD68+ macrophages into the synovium is observed as compared to sham animals. Note, as viewed in the low power confocal images (A, C) the CD68+ macrophages are localized in the synovium and are not observed in the meniscus. Administration of anti-NGF therapy does not significantly reduce the number of CD68+ macrophages within the synovium of the CFA-induced arthritic joint (E, F).
Figure 3.
Confocal images showing that following CFA injection into the knee joint there is an increaed vascularization of the synovial tissue that is not reduced with anti-NGF therapy. Representative confocal images of a vascular endothelial marker, CD31+ (red) and DAPI labeled nuclei (blue) in knee sections (20μm-thick) from sham (A, C), CFA-injected mice treated with vehicle (B, D), and CFA-injected animals treated with anti-NGF therapy (E). In sham mice, a low level vascularization by CD31+ vessels is observed in the synovial space of the knee joint. Twenty-eight days following the initial CFA injection, a significant number of CD31+ vessels have developed and have an enlarged and disorganized morphology as compared to sham animals. Note, as viewed in the low power confocal images (A, B) the CD31+ vessels are localized in the synovium and are not observed in the meniscus of the joint. Administration of anti-NGF therapy does not significantly reduce the number of CD31+ blood vessels in the synovium of CFA-injected mice (E) as compared to CFA-injected mice treated with vehicle (D).
Figure 5.
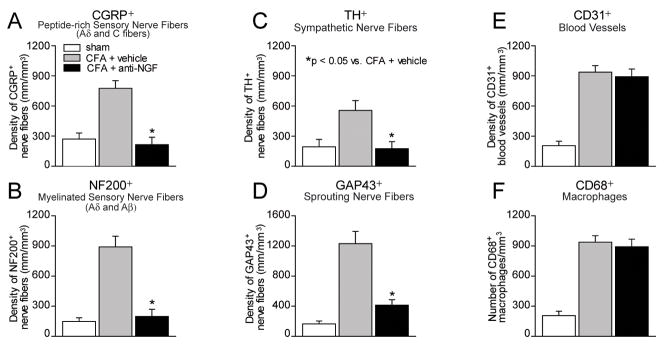
Histograms showing that administration of NGF sequestering therapy reduces CFA-induced nerve sprouting of sensory and sympathetic nerve fibers but not the aberrant neovascularization and macrophage infiltration in the arthritic joint. Quantitative analysis reveals that at day 28 post-initial injection of CFA there are significant increases in density (mm/mm3) of sensory (CGRP+; A, NF200+; B), sympathetic (TH+; C) nerve fibers, nerve fibers undergoing sprouting (GAP43+; D), increases in vascularization (CD31+; E), and significant influx of macrophages (CD68+; F), in the synovium of the arthritic joint. Administration of anti-NGF therapy significantly reduces the number/density of CGRP+ (A) and NF200+ (B) sensory, TH+ sympathetic (C), and GAP43+ (D) nerve fibers within the synovium of the CFA-induced arthritic joint as compared to CFA-injected mice treated with vehicle. In contrast, preventive sequestration of NGF does not reduce the number or alter the morphology of the CFA-induced aberrant CD31+ neovascularization (E) or the number of CD68+ macrophages (F) in the synovium of the arthritic joint. Each bar represents the mean of at least 6 mice ± SEM. *P<0.05.
Figure 4.
Confocal images showing that CFA-induced inflammation of the joint induces sprouting of CGRP+, NF200+ and GAP43+ nerve fibers in the synovium and this sprouting is significantly attenuated by anti-NGF therapy. Representative confocal images of NF200 and CGRP labeled sensory nerve fibers and GAP43+ nerve fibers (yellow/orange), and DAPI labeled nuclei (blue) in 20μm thick knee joint sections from sham (A, D, G respectively), CFA injected mice treated with vehicle (B, E, H), and CFA-injected animals treated with anti-NGF therapy (C, F, I). In sham mice, a low level, regular pattern of innervation by NF200+, CGRP+, and GAP43+ fibers is observed in the synovial space of the knee joint. Twenty-eight days following the initial CFA injection, a significant number of NF200+, CGRP+, and GAP43+ nerve fibers have sprouted and have a disorganized appearance as compared to sham animals. Note, NF200+, CGRP+, and GAP43+ sprouted nerve fibers are localized in the synovium and are not observed in the meniscus of the joint. Administration of anti-NGF therapy significantly reduces the number/density of NF200+ (C) CGRP+ (F) and GAP43+ (I) nerve fibers within the synovium of CFA-injected animals as compared to CFA-injected animals treated with vehicle (B, E, H).
In contrast, 28 days following the initial CFA injection a substantial influx of CD68+ macrophages (Figure 2 and 5) and significant neovascularization (Figure 3 and 5) were observed within the synovium of the arthritic joint. Interestingly, these newly formed blood vessels exhibited a non-standard bifurcating pattern and uneven thickness of the vessel wall as compared to blood vessels in the synovium of sham mice (Figure 3). In addition, a robust sprouting of CGRP+, GAP43+, NF200+ sensory and TH+ sympathetic nerve fibers occurred within the synovium of the inflamed joint (Figure 4 and 5). These newly sprouted nerve fibers in the synovium of the arthritic joint were found in higher density, appearing highly disorganized as compared to the primarily linear morphology of these nerve fibers in the synovium of sham mice.
In addition to the cellular changes that occurred in the CFA-induced arthritic joint, significant pain-related behaviors were observed in CFA-injected mice treated with vehicle as compared to sham mice (Figure 6). Results from all pain-related behavioral analyses reveal a rapid escalation of these pain behaviors by day 3 post-initial CFA-injection and maintain significance at each behavioral test time point through the experimental period (day 24 post-initial CFA injection) as compared to sham mice.
Figure 6.
Unilateral injection of CFA into the mouse knee joint induces pain behaviors that are attenuated with systemic administration of anti-NGF therapy. The number of spontaneous flinches (A) significantly increased following the initial CFA injection and remained at a significant level at each experimental time point. In addition, open field limb use (B), ambulatory guarding (rotarod; C), and dynamic weight bearing of the ipsilateral hind limb (D, E) declined significantly following the initial CFA injection and continued to decline during the experimental period. Early/sustained treatment with anti-NGF reduced spontaneous flinching pain behaviors approximately 80% by day 3 post-initial CFA injection and this rate of pain reduction was maintained through the 24 day experimental period (A). Hind limb use and rotarod analysis results from animals with CFA-induced arthritic joint show that these pain-related behaviors were attenuated approximately 50% by treatment with anti-NGF (B, C). Dynamic weight bearing of the hind limb (both % weight and % time spent on ipsilateral hind limb) was attenuated approximately 75% in animals that received early/sustained treatment with anti-NGF by day 23 post-initial CFA injection (D, E) while the contralateral limb remained unaffected (F).
Systemic administration of anti-NGF attenuates CFA-induced nerve fiber sprouting and arthritic joint pain
Quantification of nerve fiber density following early/sustained treatment with anti-NGF revealed that this therapy prevented the sprouting of CGRP+, NF200+, GAP43+ sensory and TH+ sympathetic nerve fibers in the synovium of the inflamed joint as assayed at day 28 post-initial CFA injection (Figure 4C, F, I and 5A–D). Additional results suggest that early/sustained anti-NGF therapy did not affect the organization or density of CGRP+, NF200+, GAP43+ sensory, and TH+ sympathetic nerve fibers in the synovium of the contralateral knee joint as compared to ipsilateral knee joint of sham mice (data not shown).
Sustained treatment with anti-NGF significantly reduced spontaneous flinching pain behavior by day 3 post-initial CFA injection and this pain reduction was observed at each behavioral test time point through the experimental period of 24 days (Figure 6A). Hind limb use and rotorod analysis results in CFA-injected mice show that these pain-related behaviors were attenuated approximately 50% by treatment with anti-NGF as compared to CFA-injected mice treated with vehicle (Figure 6B, C). The pain-related level of dynamic weight bearing of the hind limb (both % weight on ipsilateral hind limb and % time spent on ipsilateral hind limb) was reduced by approximately 75% in animals that received early/sustained treatment with anti-NGF by day 23 post-initial CFA injection (Figure 6D, E) while the contralateral limb remained unaffected (Figure 6F).
Systemic administration of anti-NGF does not significantly change macrophage infiltration or the aberrant neovascularization in the CFA-induced arthritic joint
Quantification of the total length of CD31+ blood vessels per tissue volume within the synovium of the inflamed joint at day 28 post-initial CFA injection suggests that early/sustained anti-NGF treatment does not significantly reduce the number of vessels as compared to CFA-injected mice treated with vehicle (Figure 5E). Similarly, quantification of the total number of CD68+ macrophages per tissue volume within the synovium of the inflamed joint at day 28 post-initial CFA injection suggests that early/sustained anti-NGF treatment does not significantly reduce the number of macrophages as compared to CFA-injected mice treated with vehicle (Figure 5F).
Discussion
Disease progression and joint pain
Although chronic joint pain can be caused by a very diverse group of injuries, disorders, and aging, what most joint disorders have in common is that they are frequently accompanied by significant pain and impairment of physical function (12, 13). Currently, management of skeletal pain is often not completely effective due to a high incidence of dose-limiting side effects with the major therapies used to treat this pain i.e. non-steroidal anti-inflammatory drugs (NSAIDs) and opioids (4, 5, 14).
Currently, our understanding of what drives joint skeletal pain is that as joint and adjacent bone is injured due to trauma and/or aging, nerves that innervate the bone are first activated and sensitized by factors released by stromal/inflammatory/immune cells. As the joint continues to deteriorate, these “sensitized” nerve fibers then become activated when noxious or non-noxious mechanical stimuli is applied to the joint. When the cartilage deteriorates to the point where it is no longer intact, bone on bone interactions can occur, which may induce direct mechanical stimulation of these sensitized nerve fibers (6, 15). These changes in the discharge pattern of peripheral nerve fibers have also been shown to lead to neurochemical and cellular changes in the spinal cord and higher centers of the brain (i.e. central sensitization) that contribute to the generation and maintenance of chronic joint pain (16, 17).
While the above mechanisms certainly contribute to arthritic joint pain, the present data demonstrates that the nerve fibers that innervate the joint are not simply static structures but can undergo a remarkable reorganization in terms of altered morphology, an increase in the density of nerve fibers per unit area, and sprouting into areas of the joint which are either poorly or non-innervated. The populations of nerve fibers that are undergoing sprouting include CGRP+ and NF200+ sensory nerve fibers which correspond to unmyelinated/thinly myelinated and myelinated nerve fibers, respectively. In the present study, a robust sprouting of TH+ post-ganglionic sympathetic nerve fibers was observed in the inflamed synovium of the painful arthritic joint and chronic administration of anti-NGF therapy blocked this sprouting. Several reports have suggested that sympathectomy attenuates disease progression and/or pain in arthritis (18–20) although sympathectomy induced enhancement of disease progression and/or pain in arthritis has also been reported (21–23). Whether these differences are due to the different species, models of arthritis, or methods of producing a sympathectomy that were employed in these studies remains unclear. In light of these findings, future studies that more fully elucidate the role that sympathetic nerve fibers play in driving disease progression and pain in arthritis are clearly warranted.
It has previously been shown that the majority of both the sensory and sympathetic nerve fibers that innervate the skeleton express Tropomyosin receptor kinase A (TrkA) (24). Activation of TrkA, which is the cognate receptor for NGF, has been shown to induce sprouting in both developing and adult sensory and sympathetic nerve fibers (25, 26). Here, the administration of an anti-NGF antibody, which sequesters NGF and prevents its binding to TrkA, largely blocked the sprouting of sensory and sympathetic nerve fibers in the arthritic joint and significantly attenuated arthritic joint pain. In addition, we did not observe any changes in nerve fiber sprouting or pain behaviors in the contralateral joint, although this phenomenon has been described previously in other pain states (27).
Additionally, the administration of anti-NGF did not affect disease progression as evaluated by neovascularization and macrophage infiltration in the inflamed knee joint. These studies agree with previous studies in rodents that reported CFA-induced knee joint inflammation and CFA-induced hindpaw edema are not significantly modified by anti-NGF treatment (9, 28).
NGF sensitizes and induces nerve fiber sprouting in the arthritic joint
Based on the data presented here it is hypothesized that, in the adult arthritic joint, NGF released from inflammatory/immune/stromal cells induces a marked sprouting of TrkA+, but not TrkA−, sensory and sympathetic nerve fibers. These newly sprouted sensory and sympathetic nerve fibers have a distinctive morphology and are present at a higher density of nerve fibers/unit area than is found in the normal joint. As these newly sprouted fibers are present in areas of the joint that are subject to significant stress and load bearing, and NGF has been shown to induce a marked sensitization and alteration in the phenotype of sensory and sympathetic nerve fibers, these changes may contribute to arthritic joint pain.
Pathological nerve sprouting similar to that which is observed in the present mouse model of the painful arthritic joint is found in other non-skeletal and skeletal pain states both in rodents and humans. Thus, aberrant nerve sprouting has been shown to be present in humans in non-skeletal chronic pain states including interstitial cystitis, vulvodynia, and irritable bowel diseases (29). Previous studies have also reported that in humans with chronic discogenic pain there is growth of CGRP+ nerve fibers into normally aneural and avascular areas of the human intervertebral disc (30). Additionally, studies have shown that significant sprouting of CGRP+ nerve fibers occurs following bone fracture in rats as well as in the arthritic joints of humans and animals (31–35). Furthermore, other reports suggest that significant sprouting of TrkA+ nerve fibers can occur following tumor infiltration of the skeleton (11). Whether similar nerve sprouting can occur in bone following total knee replacement is not known. However, if it does occur, this may partially explain why some patients experience unsuccessful relief of pain following total knee replacement (36).
While sprouting of TrkA+ nerve fibers clearly can occur in the painful arthritic joint, what remains to be defined is what specific endogenous stromal, inflammatory and immune cells that are the major source of NGF, whether the availability of NGF in the injured and/or aged joint is a major determinant of the sprouting of TrkA+ nerve fibers, and whether there is a correlation between peripheral nerve fiber sprouting and the generation and/or maintenance of chronic arthritic joint pain.
Previous data suggests that release of NGF can contribute to the sensitization of TrkA+ sensory nerve fibers as NGF-TrkA activation of intracellular signaling cascades in the adult specifically modulate the sensitivity of primary afferent nociceptors to mechanical, thermal and chemical stimuli in vitro and in vivo (37, 38). NGF also binds TrkA receptors expressed on the peptidergic fiber terminal itself, resulting in sensitization or increased expression of a number of receptors and channels at the membrane surface, including transient receptor potential vanilloid 1 (TRPV1), acid-sensing ion channel-3 (ASIC-3), bradykinin receptors, voltage-gated sodium and calcium channels, and putative mechanotransducers, that may contribute to hypersensitivity after inflammation (25).
Following the period of immediate hypersensitivity with NGF release after tissue injury, early transcriptional changes also occur in the sensory signaling pathway. As NGF also signals via retrograde transport of the internalized NGF-TrkA complex, there is a delay (from hours to days) before some of NGF’s contribution to hypersensitivity is seen. After retrograde transport to the dorsal root ganglia, the signal from the NGF-TrkA complex can produce changes in sensory phenotype through the switching on (and off) of gene promoters, which leads to increased synthesis of neuropeptides (e.g., substance P [SP], CGRP, and brain-derived neurotrophic factor [BDNF]), and of receptors and ion channels that are expressed by nociceptors (39–42). Together, these data emphasize that while NGF-induced sprouting of TrkA+ nerve fibers may be involved in driving skeletal pain, NGF-induced sensitization and alteration of the phenotype of TrkA+ nerve fibers may play a significant role in the generation and maintenance of chronic arthritic joint pain.
Blockade of NGF/TrkA and the relief of skeletal pain
Several preclinical studies have suggested that blocking NGF/TrkA can be efficacious in attenuating skeletal pain. Thus, therapies blocking the NGF/TrkA pathway reversed the established hyperalgesia in a rodent model of autoimmune arthritis (9) and osteoarthritis (43) suggesting that NGF is involved in prolonged hyperalgesia. Additionally, a role for NGF in maintenance of hypersensitivity in chronic injury has also been demonstrated using several models of bone cancer (44, 45) and a model of bone fracture (46).
One rather unique aspect of the sensory innervation of bone and joint, which may in part explain why anti-NGF therapy is effective in relieving both malignant and non-malignant skeletal pain, is that the majority of unmyelinated (CGRP+) and myelinated (NF200+) sensory nerve fibers that innervate bone and joint appear to express TrkA (24). Accordingly, few unmyelinated non-peptidergic (IB4+/RET+) nerve fibers are present in bone (47, 48), therefore therapies that target NGF or TrkA may be particularly efficacious in relieving bone pain as the majority of nociceptors express TrkA and respond to NGF.
Recent human trials with an anti-NGF therapy using humanized anti-NGF monoclonal antibodies have demonstrated that these therapies are efficacious in relieving pain due to osteoarthritis (49, 50). In these studies, and in preclinical studies, anti-NGF therapy is anti-hyperalgesic (i.e. normalizing a decreased nociceptive threshold) as opposed to analgesic (i.e. increasing normal and sensitized nociceptive threshold). However, recent human clinical trials in elderly humans with osteoarthritis (OA) have been halted due to the need for earlier than expected joint replacement in a small subset of patients (49). What remains unclear is whether this earlier than expected joint replacement in patients being treated with anti-NGF is simply due to greater use of the diseased joint or to unforeseen adverse events on the bone itself such as a decrease in either the formation or maintenance of the vascular supply of the bone.
Conclusions and limitations
These results suggest that anti-NGF has a profound effect on blocking sprouting of TrkA+ nerve fibers and pain in the arthritic joint but had no detectable effect on the formation or maintenance of CD31+ blood vessels or the influx of CD68+ macrophages in the arthritic or normal joint and bone. The present results demonstrate that NGF activation plays a significant role in driving arthritic joint and skeletal pain. Issues including determining the efficacy of NGF/TrkA blockade on removing ectopic sprouting once it occurs, defining the sources of NGF in the arthritic joint, determining whether there is a significant sympathetic component to this pain, and examining if TrkA+ nerve sprouting changes with the progression of arthritis, remain important but unanswered questions.
Acknowledgments
Work supported by: The Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service Grants (O4380-I and A6707-R), National Institutes of Health Grants (NS23970, CA154550, CA157449) and by the Calhoun Fund for Bone Pain
References
- 1.Arthritis: The Nation’s Most Common Cause of Disability. 2011 http://www.cdc.gov/arthritis/
- 2.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365(9463):965–73. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 3.Bove SE, Flatters SJ, Inglis JJ, Mantyh PW. New advances in musculoskeletal pain. Brain Res Rev. 2009;60(1):187–201. doi: 10.1016/j.brainresrev.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanas A, Tornero J, Zamorano JL. Assessment of gastrointestinal and cardiovascular risk in patients with osteoarthritis who require NSAIDs: the LOGICA study. Ann Rheum Dis. 2010;69(8):1453–8. doi: 10.1136/ard.2009.123166. [DOI] [PubMed] [Google Scholar]
- 5.Feinberg SD. Prescribing analgesics. How to improve function and avoid toxicity when treating chronic pain. Geriatrics. 2000;55(11):44, 49–50, 53. passim. [PubMed] [Google Scholar]
- 6.Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of osteoarthritis and the genesis of pain. Med Clin North Am. 2009;93(1):83–100. xi. doi: 10.1016/j.mcna.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Gauldie SD, McQueen DS, Clarke CJ, Chessell IP. A robust model of adjuvant-induced chronic unilateral arthritis in two mouse strains. J Neurosci Methods. 2004;139(2):281–91. doi: 10.1016/j.jneumeth.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Hongo JS, Laramee GR, Urfer R, Shelton DL, Restivo T, Sadick M, et al. Antibody binding regions on human nerve growth factor identified by homolog- and alanine-scanning mutagenesis. Hybridoma. 2000;19(3):215–27. doi: 10.1089/02724570050109611. [DOI] [PubMed] [Google Scholar]
- 9.Shelton DL, Zeller J, Ho WH, Pons J, Rosenthal A. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain. 2005;116(1–2):8–16. doi: 10.1016/j.pain.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 10.Kul-Panza E, Berker N. Pedobarographic findings in patients with knee osteoarthritis. Am J Phys Med Rehabil. 2006;85(3):228–33. doi: 10.1097/01.phm.0000200377.52610.cd. [DOI] [PubMed] [Google Scholar]
- 11.Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171(2):588–98. doi: 10.1016/j.neuroscience.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 13.Sawatzky R, Liu-Ambrose T, Miller WC, Marra CA. Physical activity as a mediator of the impact of chronic conditions on quality of life in older adults. Health Qual Life Outcomes. 2007;5:68. doi: 10.1186/1477-7525-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinblatt ME. Nonsteroidal anti-inflammatory drug toxicity: increased risk in the elderly. Scand J Rheumatol Suppl. 1991;91:9–17. doi: 10.3109/03009749109096946. [DOI] [PubMed] [Google Scholar]
- 15.Kidd BL. Osteoarthritis and joint pain. Pain. 2006;123(1–2):6–9. doi: 10.1016/j.pain.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009 doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey VL, Dickenson AH. Behavioural and electrophysiological characterisation of experimentally induced osteoarthritis and neuropathy in C57Bl/6 mice. Mol Pain. 2009;5:18. doi: 10.1186/1744-8069-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herfort R, Nickerson SH. Relief of arthritic pain and rehabilitation of chronic arthritic patient by extended sympathetic denervation. Arch Phys Med Rehabil. 1959;40(4):133–40. [PubMed] [Google Scholar]
- 19.Howell TH. Relief of pain in rheumatoid arthritis with tetraethylammonium bromide. Lancet. 1950;1(6597):204. doi: 10.1016/s0140-6736(50)92051-4. [DOI] [PubMed] [Google Scholar]
- 20.Levine JD, Moskowitz MA, Basbaum AI. The contribution of neurogenic inflammation in experimental arthritis. J Immunol. 1985;135(2 Suppl):843s–847s. [PubMed] [Google Scholar]
- 21.Sluka KA, Lawand NB, Westlund KN. Joint inflammation is reduced by dorsal rhizotomy and not by sympathectomy or spinal cord transection. Ann Rheum Dis. 1994;53(5):309–14. doi: 10.1136/ard.53.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorton D, Lubahn C, Klein N, Schaller J, Bellinger DL. Dual role for noradrenergic innervation of lymphoid tissue and arthritic joints in adjuvant-induced arthritis. Brain Behav Immun. 1999;13(4):315–34. doi: 10.1006/brbi.1999.0564. [DOI] [PubMed] [Google Scholar]
- 23.Lam FY, Ferrell WR. Neurogenic component of different models of acute inflammation in the rat knee joint. Ann Rheum Dis. 1991;50(11):747–51. doi: 10.1136/ard.50.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castaneda-Corral G, Jimenez-Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, et al. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience. 2011;178:196–207. doi: 10.1016/j.neuroscience.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 26.Gallo G, Lefcort FB, Letourneau PC. The trkA receptor mediates growth cone turning toward a localized source of nerve growth factor. J Neurosci. 1997;17(14):5445–54. doi: 10.1523/JNEUROSCI.17-14-05445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci. 1999;22(3):122–7. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- 28.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62(2):327–31. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 29.Anand P, Bley K. Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107(4):490–502. doi: 10.1093/bja/aer260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197(3):286–92. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 31.Buma P, Verschuren C, Versleyen D, Van der Kraan P, Oestreicher AB. Calcitonin gene-related peptide, substance P and GAP-43/B-50 immunoreactivity in the normal and arthrotic knee joint of the mouse. Histochemistry. 1992;98:327–339. doi: 10.1007/BF00270017. [DOI] [PubMed] [Google Scholar]
- 32.Wu Z, Nagata K, Iijima T. Involvement of sensory nerves and immune cells in osteophyte formation in the ankle joint of adjuvant arthritic rats. Histochem Cell Biol. 2002;118(3):213–20. doi: 10.1007/s00418-002-0443-x. [DOI] [PubMed] [Google Scholar]
- 33.Suri S, Gill SE, Massena de Camin S, Wilson D, McWilliams DF, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66(11):1423–8. doi: 10.1136/ard.2006.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashraf S, Wibberley H, Mapp PI, Hill R, Wilson D, Walsh DA. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann Rheum Dis. 2011;70(3):523–9. doi: 10.1136/ard.2010.137844. [DOI] [PubMed] [Google Scholar]
- 35.Ghilardi JR, Freeman KT, Jimenez-Andrade JM, Mantyh WG, Bloom AP, Kuskowski MA, et al. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain. 2010;6:87. doi: 10.1186/1744-8069-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wylde V, Dieppe P, Hewlett S, Learmonth ID. Total knee replacement: is it really an effective procedure for all? Knee. 2007;14(6):417–23. doi: 10.1016/j.knee.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274(3):159–62. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- 38.Bennett DL, Koltzenburg M, Priestley JV, Shelton DL, McMahon SB. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur J Neurosci. 1998;10(4):1282–91. doi: 10.1046/j.1460-9568.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 39.Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav 1. 8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport. 2001;12(14):3077–80. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- 40.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22(24):10662–70. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fjell J, Cummins TR, Davis BM, Albers KM, Fried K, Waxman SG, et al. Sodium channel expression in NGF-overexpressing transgenic mice. J Neurosci Res. 1999;57(1):39–47. doi: 10.1002/(SICI)1097-4547(19990701)57:1<39::AID-JNR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337(6205):362–4. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- 43.McNamee KE, Burleigh A, Gompels LL, Feldmann M, Allen SJ, Williams RO, et al. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149(2):386–92. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, et al. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005;65(20):9426–35. doi: 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 45.Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115(1–2):128–41. doi: 10.1016/j.pain.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Jimenez-Andrade JM, Martin CD, Koewler NJ, Freeman KT, Sullivan LJ, Halvorson KG, et al. Nerve growth factor sequestering therapy attenuates non-malignant skeletal pain following fracture. Pain. 2007;133(1–3):183–96. doi: 10.1016/j.pain.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, et al. Expression and co-expression of VR1, CGRP, and IB4-binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine. 2005;30(13):1496–500. doi: 10.1097/01.brs.0000167532.96540.31. [DOI] [PubMed] [Google Scholar]
- 48.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, et al. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone. 2010;46(2):306–13. doi: 10.1016/j.bone.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–31. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnitzer TJ, Lane NE, Birbara C, Smith MD, Simpson SL, Brown MT. Long-term open-label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthritis Cartilage. doi: 10.1016/j.joca.2011.01.009. [DOI] [PubMed] [Google Scholar]