Abstract
Retinal amacrine cells are thought to lack chromatic or color–selective light responses and play only a minor role in color processing. We now show that a type of mammalian (Ictidomys tridecemlineatus) amacrine cell selectively carries a blue–On signal, which is received from a blue or short–wavelength sensitive (S–) cone On bipolar cell. This glycinergic inhibitory “S–cone amacrine cell” is ideally placed for driving “blue–Off” responses in downstream ganglion cells.
Blue–green dichromacy is the fundamental color mechanism in mammals, and provides advantages relative to monochromacy with respect to color discrimination and circadian entrainment1–3. Two blue–green opponent responses have been recorded in retinal ganglion cells: blue–On/green–Off4–6 and blue–Off/green–On3, 7–9, signaling blue light On and Off, respectively. A distinctive S–cone On bipolar cell (SCB) 10–12, exclusively receives S–cone input and relays blue–On signals to the small bistratified ganglion cell6, which also receives yellow–Off signals from Off cone bipolar cells (that contact both M– and L–cones). In contrast, the retinal pathway that mediates the blue–Off response has not yet been identified. Typically, retinal Off signals originate from Off cone bipolar cells. However, an S–cone selective Off bipolar cell has not been identified. Alternatively, an inhibitory amacrine cell can invert blue–On signals, obviating the need for an S–cone Off bipolar cell. However, amacrine cells are not usually considered to play a direct role in color vision insofar as color selective amacrine cell responses have not been recorded 13.
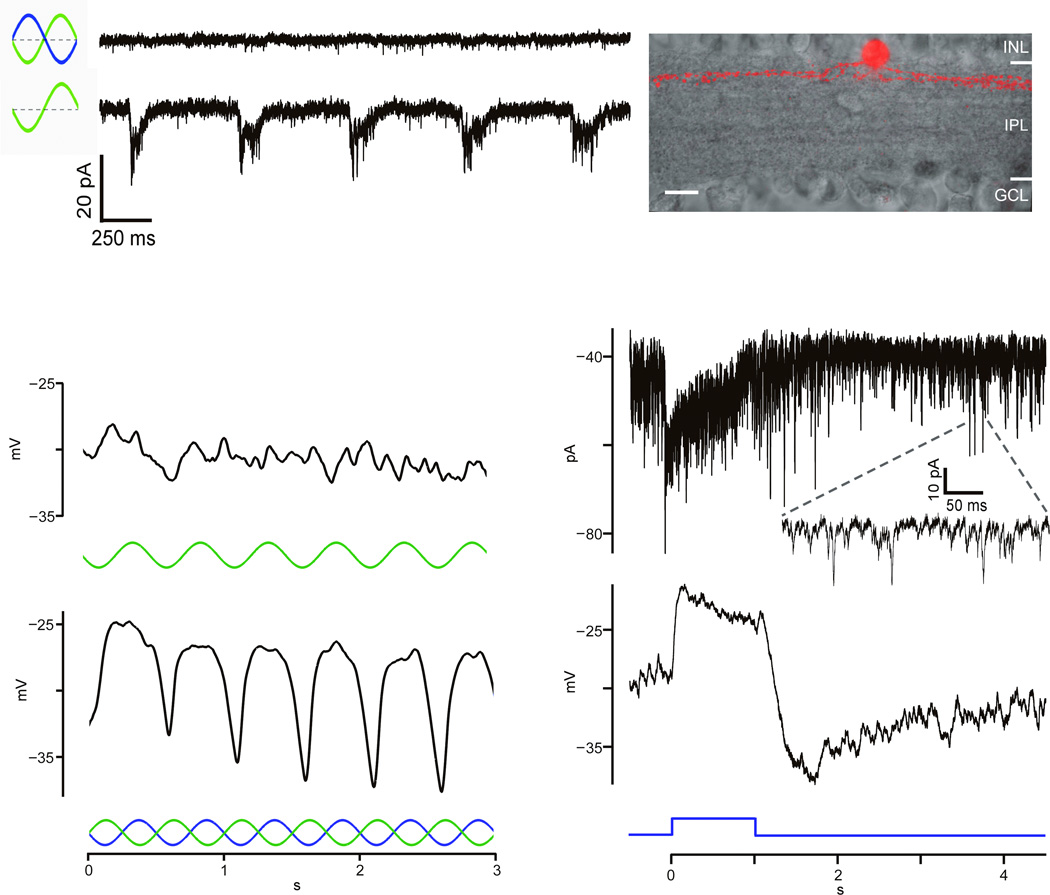
We recorded from amacrine cells in slices from the cone–dominant ground squirrel to identify those receiving S–cone selective inputs (Suppl. Methods). To search for S–cone mediated amacrine cell signals, we used a “silent substitution” technique that selectively stimulated S–cones (Suppl. Fig. 1). Most amacrine cells either lacked significant S–cone input or received non–selective cone inputs (Fig. 1a; Suppl. Fig. 2). A small number, however, demonstrated selective responses to the S–cone isolating stimulus (SCIS; Fig. 1b and Suppl. Fig. 2), and were thus referred to as “S–cone amacrine cells” (SCAs; n=12). SCA voltage responses followed blue light modulation, whereas green light alone elicited little response, indicating prominent chromatic selectivity (Fig. 1b). SCAs exhibited abundant spontaneous synaptic currents presumably triggered by frequent excitatory inputs (EPSCs) from bipolar cells (Fig. 1c). The SCA response to a 1s blue light pulse was a relatively sustained depolarization with a hyperpolarizing overshoot at pulse offset (Fig. 1c), which may temporarily pause the SCA’s synaptic output. Assuming that the SCA output is inhibitory, suppression of inhibition can provide an excitatory drive to downstream neurons.
Figure 1. SCA light responses.
a) An amacrine cell is unresponsive to SCIS (upper) but responsive to green LED (lower); one–tier ramification (right). b) Sample SCA unresponsive to green LED stimulus (upper) but responsive to SCIS (lower). c) Sample SCA current (upper) and voltage (lower) responses to a 1s blue light pulse. Inset:spontaneous EPSCs.
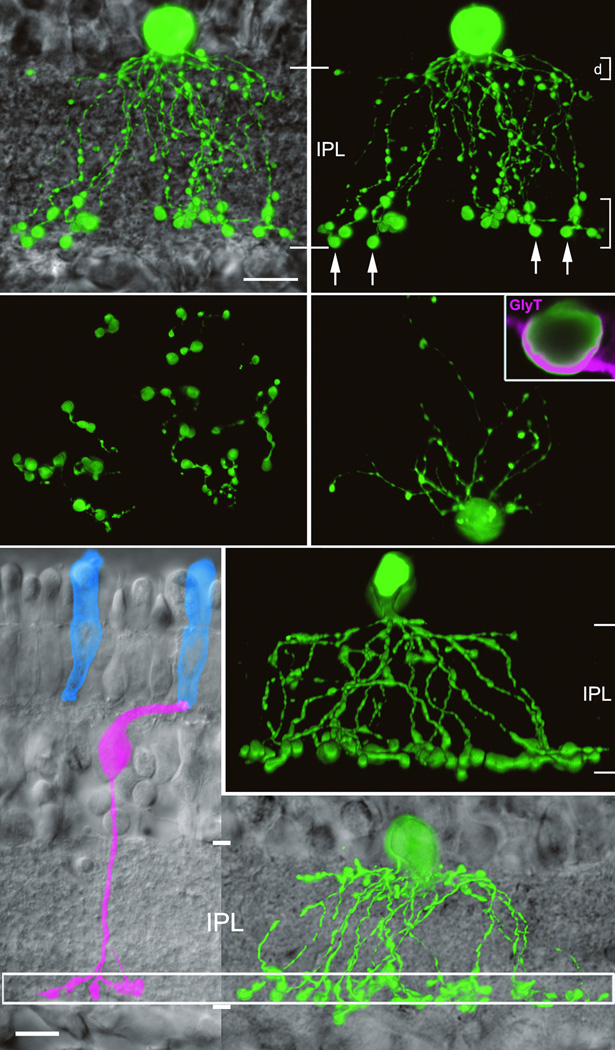
Tracer filled SCAs (Fig. 2) had a narrow dendritic field (60.5 ± 5.2µm, n=10) and a two–tier ramification near the inner and outer IPL boundaries (Fig. 2a). Dendrites descended through the IPL, bestowing a bell–shaped profile in vertical sections (Fig. 2a,b,f,g). The upper dendritic tier was slender and sparse compared to the lower tier, which contained large varicosities (Fig. 2a,b,c,f,g). The varicosities stratified at the same level as SCB axon terminals (Fig. 2e,g). SCAs label with an antibody to the glycine transporter (Inset Fig. 2d, n=4), a characteristic of glycinergic amacrine cells which suggests that SCAs can inhibit downstream neurons.
Figure 2. SCA morphology.
a) Tracer–filled SCA (green) against DIC background. b) Dual dendritic arbors shown as flatmounts in c) and d). Arrows indicate sample varicosities likely to be synaptic sites. Inset d) SCAs immunolabeled by anti– glycine transporter (magenta). f,g)Additional examples of SCAs. e) An SCB (magenta) contacts a single S–cone (blue). Aligning IPLs of e and f, SCA varicosities co–stratify with SCB axon terminals (box).
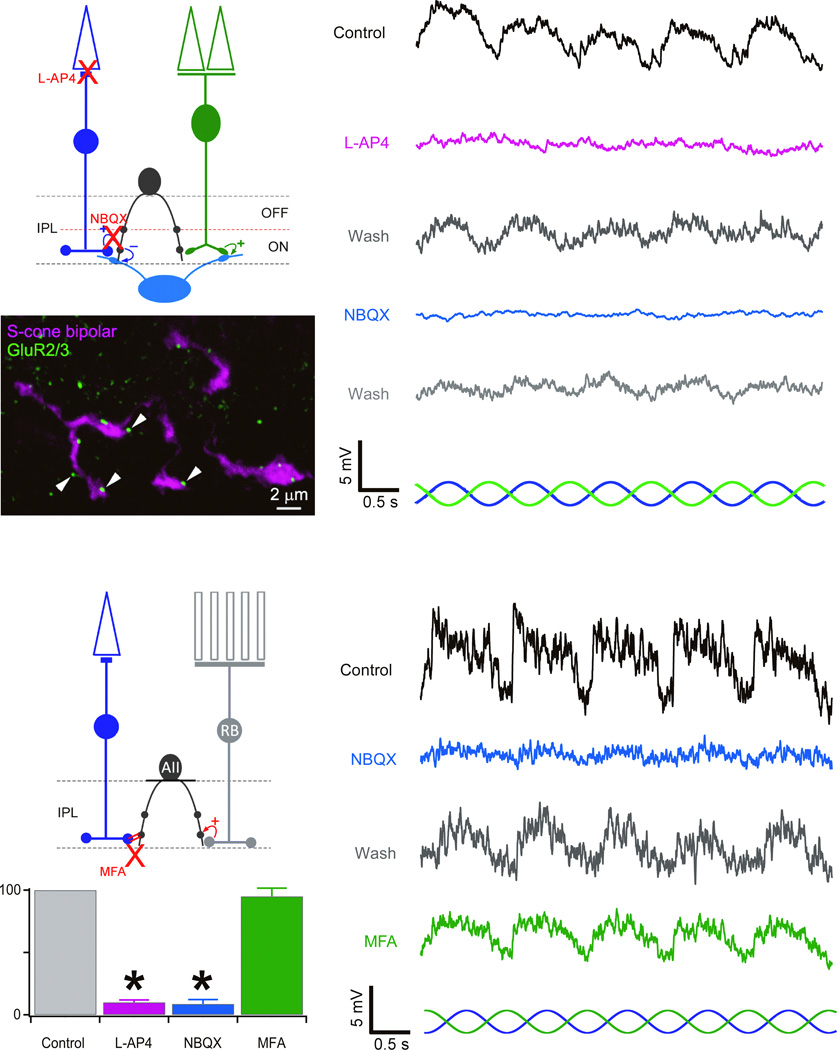
Consistent with its proposed position in the S–cone pathway (Fig. 3a), SCA responses to the SCIS were abolished by 4 µM L–AP4, which blocks synaptic transmission between S–cones and SCBs (10.0% ± 1.9% of control, n=3; Fig. 3a). SCA responses to the SCIS were reversibly abolished by 5 µM of the AMPA/kainate receptor antagonist NBQX which should block signaling at the bipolar to amacrine cell synapse (8.7% ± 3.5% of control, n=5; Fig. 3a). In addition, SCB axon terminals are physically opposed to puncta that immunolabel for AMPA–type glutamate receptor subunit GluR2/3 (Fig. 3a).
Figure 3. SCA pharmacology.
a) Proposed SCA pathway; below: SCB axon terminals (magenta) opposite puncta immunolabeled for GluR2/3 (green). Right: abolition of SCA responses to the SCIS ) by L–AP4 and NBQX. b) AII pathway. Graphics summarize abolition of SCA responses to SCIS by L–AP4 and NBQX, but not MFA . Mean ± S.D.; *p<0.01.
The presumed SCA role in the S–cone pathway mirrors that of AII amacrine cells in the rod pathway (Fig. 3b), which form gap junctions with SCBs and may carry S–cone signals14. Although the block of the SCIS input to SCAs by NBQX, suggested otherwise, we wanted to further rule out an identity between SCAs and AIIs. Indeed, SCA responses to the SCIS were unaffected by 100 µM of the gap junction blocker MFA (95.0% ± 6.6% of the control, n=3; Fig. 3b), which, in the same retinal slice, blocked gap junctional conductances between cones (data not shown). We therefore conclude that SCAs are not AIIs, but a novel chromatic–selective amacrine cell.
Could SCAs supply a blue–Off signal to blue–Off/green–On ganglion cells? Anatomical descriptions of blue–Off ganglion cells suggest that they are monostratified cells with dendrites ramifying near the inner border of the IPL7, 15, matching the stratification of the lower tier of SCA dendrites. Therefore, it is possible that SCA varicosities make synapses with dendrites of the blue–Off ganglion cell (Suppl. Fig. 3, a→b). Alternatively, SCAs may inhibit green–On bipolar cell terminals, which are presynaptic to the ganglion cell (Suppl. Fig. 3, a→c→d).
Another potential target of SCAs is the intrinsically photosensitive retinal ganglion cell (ipRGC)2, 3 that, in the primate retina, had blue–Off/yellow–On cone responses in addition to intrinsic photosensitivity3. Dendrites of ipRGCs ramify at the inner and outer borders of the IPL, co–stratifying with SCA dendrites. If SCAs provide blue–Off inputs to ipRGCs, they could function in the ancient blue–yellow color system that synchronizes the biological clock with the environment by signaling dawn–dusk spectral shifts1–3.
In sum, we present evidence for a color coding amacrine cell, which is well–positioned to provide “blue–Off” signals in the color pathway of the mammalian retina.
Supplementary Material
ACKNOWLEDGEMENTS
We thank S. DeVries, B. Szmajda, and T. Rosenberg for critically reading the manuscript. Special thanks to D. Merriman for editing. This work is supported by the Intramural Research Program of the National Eye Institute.
Footnotes
AUTHOR CONTRIBUTIONS
S.C. and W.L. conceived the project and designed the experiments. S.C. performed the experiments. S.C. and W.L. analyzed the data. W.L. wrote the manuscript.
REFERENCES
- 1.Mollon JD. J Exp Biol. 1989;146:21. doi: 10.1242/jeb.146.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Neitz J, et al. Vision Res. 2011;51:633. doi: 10.1016/j.visres.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dacey DM, et al. Nature. 2005;433:749. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 4.Dacey DM, et al. Nature. 1994;367:731. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- 5.Chichilnisky EJ, et al. Nat Neurosci. 1999;2:889. doi: 10.1038/13189. [DOI] [PubMed] [Google Scholar]
- 6.Calkins DJ, et al. J Neurosci. 1998;18:3373. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin L, et al. J Neurosci. 2009;29:2706. doi: 10.1523/JNEUROSCI.5471-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmi JM, et al. J Vis. 2002;2:608. doi: 10.1167/2.9.3. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs GH, et al. Vision Res. 1980;20:9. doi: 10.1016/0042-6989(80)90136-4. [DOI] [PubMed] [Google Scholar]
- 10.Kouyama N, et al. J Neurosci. 1992;12:1233. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, et al. Nat Neurosci. 2006;9:669. doi: 10.1038/nn1686. [DOI] [PubMed] [Google Scholar]
- 12.Mariani AP. Nature. 1984;308:184. doi: 10.1038/308184a0. [DOI] [PubMed] [Google Scholar]
- 13.Calkins DJ, et al. Nature. 1996;381:613. doi: 10.1038/381613a0. [DOI] [PubMed] [Google Scholar]
- 14.Field GD, et al. Nat Neurosci. 2009;12:1159. doi: 10.1038/nn.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dacey DM. In: The Cognitive Neurosciences. Gazzaniga MS, editor. Cambridge: MIT Press; 2004. pp. 281–301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.