Abstract
Hepatic ischemia and reperfusion injury (IRI) remains an important challenge in clinical orthotopic liver transplantation (OLT). Tissue inhibitor of metalloproteinase-1 (TIMP-1) is the major endogenous regulator of matrix metalloproteinase-9 (MMP-9). In this study, we investigated the functional significance of TIMP-1 expression in a well-established mouse model of partial liver IRI. Compared to wild-type mice, TIMP-1−/− mice showed further impaired liver function and histological preservation after IRI. Notably, TIMP-1 deficiency led to lethal liver IRI, as over 60% of the TIMP-1−/− mice died post-reperfusion, whereas all TIMP-1+/+ mice recovered and survived surgery. Lack of TIMP-1 expression was accompanied by markedly high levels of MMP-9 activity, which facilitates leukocyte transmigration across vascular barriers in hepatic IRI. Indeed, TIMP-1−/− livers were characterized by massive leukocyte infiltration and by upregulation of proinflammatory mediators, including TNF-α, IFN-γ, and iNOS, post-IRI. The inability of TIMP-1−/− mice to express TIMP-1 increased the levels of active caspase-3 and depressed the expression of Bcl-2 and the phosphorylation of Akt, emphasizing an important role for TIMP-1 expression on hepatocyte survival. Using independent parameters of regeneration, 5-bromodeoxyuridine (BrdU) incorporation, proliferating cell nuclear antigen (PCNA) expression, and histone H3 phosphorylation, we provide evidence that hepatocyte progression into S phase and mitosis was impaired in TIMP-1 deficient livers after IRI. Inhibition of the cell cycle progression by TIMP-1 deficiency was linked to depressed levels of cyclins-D1 and -E and to disrupted c-Met signaling pathway, as evidenced by reduced phosphorylated c-Met expression and elevated c-Met ectodomain shedding post-liver IRI. In conclusion, these results support a critical protective function for TIMP-1 expression on promoting survival and proliferation of liver cells and on regulating leukocyte recruitment and activation in liver IRI.
Hepatic ischemia/reperfusion injury (IRI) occurs during trauma, shock, orthotopic liver transplantation (OLT), and other surgical procedures where blood supply to liver is temporarily interrupted 1. Hepatic IR-related damage is the result of various factors that include leukocyte migration, release of cytokines and free radicals 1, 2.
Leukocytes migration across endothelial and extracellular matrix (ECM) barriers is dependent on cellular adhesion-release and focal matrix degradation mechanisms 3. While adhesion molecules are important for the successful leukocyte transmigration by providing leukocyte attachment to the endothelium, there is a growing body of evidence suggesting that matrix metalloproteinases (MMP) are critical for facilitating leukocyte movement across vascular barriers 3. In this regard, our previous studies showed an important role for leukocyte-expressed MMP-9, or gelatinase B, as a key mediator of leukocyte transmigration leading to liver injury 4.
Tissue inhibitors of metalloproteinases (TIMPs) are a family of naturally occurring inhibitors of MMPs. Alterations in the MMP-TIMP balance have been linked to pathological conditions that require disruption of the basement membrane, such as tumor invasion, angiogenesis, and wound healing 5. There are at least four identified members (TIMP 1-4) in the TIMP family, varying in tissue specific expression and in their ability to inhibit various MMPs 6. Among the different TIMPs, TIMP-1 is of particular interest; TIMP-1 is a 28.5 kDa soluble glycoprotein known to inhibit MMP-9 with high affinity, without interacting with MMP-2, or gelatinase A (the other member of the gelatinase family), as it lacks the required C-terminal MMP-2 interacting residues 7, 8.
In addition to its ability to inhibit MMP activity, TIMP-1 possesses other biological activities, such as cell growth regulation, that are just beginning to be recognized and characterized 9. The specific effects of the TIMPs are likely depending on the cell context and on the pathological condition. While TIMP-1 has been detected in the plasma of patients after liver transplantation 10, and in rat liver grafts after IRI 11, its role in liver IRI, or in OLT, remains to be established. Therefore, in the present study, we used mice lacking TIMP-1 to examine the significance of TIMP-1 expression in hepatic IRI.
MATERIALS AND METHODS
Mice and Model of Hepatic IRI
Male TIMP-1−/− Knockout (KO) mice in the C57BL/6 background (B6.129S4-Timp1tm1Pd/J) and respective TIMP-1+/+ wild-type C57BL-6 controls were obtained from the Jackson Laboratory. Hepatic IRI was performed as previously described 4. Briefly, arterial and portal venous blood supplies were interrupted to the cephalad lobes of the liver for 90 minutes using an atraumatic clip and mice were sacrificed after reperfusion. The animal studies were performed according to approved guidelines by the American Association of Laboratory Animal Care.
Assessment of Liver Damage
Serum alanine transaminase (ALT) and serum aspartate transaminase (AST) levels were measured with an auto analyzer by ANTECH Diagnostics (Los Angeles, CA), as described 4. Liver specimens were fixed with a 10% buffered formalin solution, embedded in paraffin and processed for H&E staining; to determine the percentage of necrotic area, ten random sections per slide were evaluated in duplicate using NIH IMAGE (Image-J).
Immunohistochemistry
Immunostaining was performed in cryostat sections, as described 4, 11. Mac-1 (M1/70), and Ly-6G (1A8), from BD Biosciences, TIMP-1 (Ab86482; Abcam), MMP-9 (AF909; R&D Systems), and cleaved-caspase-3 (ASP175; Cell Signaling) antibodies were used at optimal dilutions. Sections were blindly evaluated by counting ten HPFs/section in triplicates. Dual/triple staining was detected by immunofluorescence with Alexa Fluor 594-red anti-goat IgG (H+L) (Molecular Probes), and Texas Red anti-rat IgG (H+L) antibodies (Vector Laboratories). Alexa Fluor 488 phalloidin (Molecular Probes) and Vectashield mounting media with DAPI (Vector Laboratories) were used for F-actin and nuclear staining, respectively. Slides were analyzed using a Leica Confocal Microscope (UCLA Brain Research Institute).
Parameters of regeneration
Mice were injected i.p. with 50mg/kg of BrdU (Sigma) 2h prior to liver harvest, as described 12. BrdU incorporation, PCNA, and phosphorylated histone H3 were detected by immunohistochemistry in paraffin sections using anti-BrdU (Bu20a; Neomarkers), anti-PCNA (PC-10; Neomarkers), and anti-pH3 (Ser10; Cell Signaling) antibodies. Proliferation indexes were determined in triplicate and quantified under light microscopy by counting ten, randomly chosen, HPFs/section. Data were expressed as the percentage of BrdU, PCNA, or pH3 stained hepatocytes per total number of hepatocytes.
Myeloperoxidase (MPO) Assay
Myeloperoxidase activity was evaluated in frozen tissue homogenized in an iced solution of 0.5% hexadecyltrimethyl-ammonium and 50 mmol/L of potassium phosphate buffer solution 4. After centrifugation, the supernatants were mixed in a solution of hydrogen peroxide-sodium acetate and tetramethyl benzidine (Sigma). The quantity of enzyme degrading 1 μmol/L of peroxide/minute at 25°C per gram of tissue was defined as 1U of MPO activity.
Western blot and Zymography Analysis
Western blots and Zymography were performed as described 4, 11. Proteins (40 μg/sample) in sodium dodecyl sulfate (SDS)-loading buffer were electrophoresed through 12% S D S-polyacrylamide gel electrophoresis (PAGE) and transferred to PVDF membranes. Membranes were incubated with specific antibodies against cleaved caspase-3 (ASP175), phospho-AKT (D9E; C31E5E), AKT (C67E7), phospho-c-Met (D26 and 130H2), c-Met (25H2) (Cell Signaling), Bcl-2 (Abcam) and cyclin D1 (BD Biosciences). After development, membranes were stripped and reblotted with anti-actin antibody (Santa Cruz).
Gelatinolytic activity was detected in liver extracts (100μg) by 10% SDS-PAGE contained 1mg/ml of gelatin (Invitrogen), under non-reducing conditions. After incubation in development buffer (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, and 0.02% NaN3, pH 7.5), gels were stained with Coomassie brilliant blue R-250 (Bio-Rad), and destained with methanol/acetic acid/water (20:10:70). Prestained molecular weight markers (Bio-Rad) and MMP-9 (BIOMOL International) served as standards. Relative quantities of protein were determined using a densitometer (NIH Image J software)
RNA Extraction and Reverse Transcriptase PCR
RNA was extracted from livers with Trizol (Life Technologies) as described 4. Reverse transcription was performed using 5 μg of total RNA in a first-strand cDNA synthesis reaction with SuperScript III RNaseH Reverse Transcriptase (Life Technologies), as recommended by the manufacturer. The cDNA product was amplified by PCR using primers specific for each target cDNA.
Data Analysis
Data in the text and figures are expressed as mean ± standard deviation. Statistical comparisons between groups of normally distributed data were performed with the Student t-test using statistical package SPSS (SPSS Inc., Chicago, IL). Kaplan-Meier analysis was used to determine statistical significance of the differences in mouse survival. P values of less than 0.05 were considered statistically significant.
RESULTS
Time Course of TIMP-1 Expression in Wild-Type Livers Post-IRI
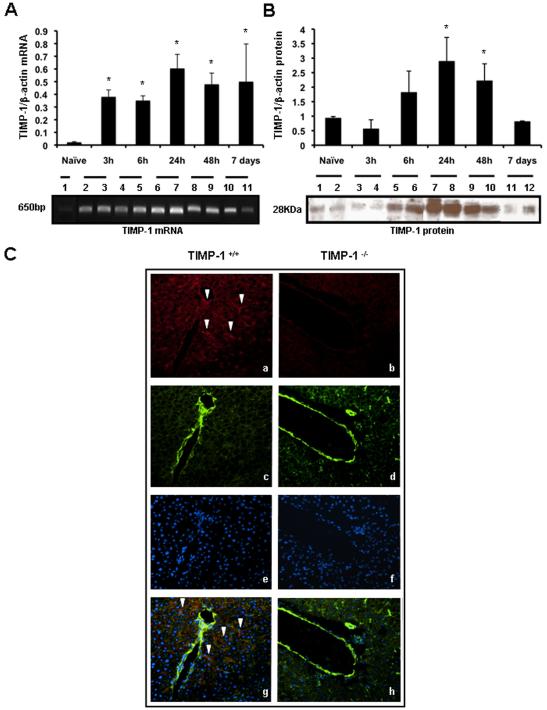
TIMP-1 mRNA was almost undetectable in naïve livers and it was significantly upregulated in TIMP-1+/+ livers from 3h to 7d post-reperfusion, (Fig. 1A). TIMP-1 protein expression was mildly detected in TIMP-1+/+ naïve livers and it was markedly increase in livers after 6h of reperfusion, particularly at 24h and 48h post-IRI (Fig. 1B). Immunofluorescence analysis showed TIMP-1 staining in the surviving parenchyma predominantly around the portal triads of wild-type livers (Fig. 1C); TIMP-1+ staining was mostly detected in cells along hepatic sinusoids, likely HSC, and in scattered hepatocytes. In vitro studies have linked TIMP-1 production to hepatic stellate cells (HSC) and to hepatocytes 13. Conversely, TIMP-1 staining was absent in TIMP-1−/− livers after IRI (Fig. 1C).
Figure 1.
Time course of TIMP-1 expression in TIMP-1+/+ livers post-IRI. TIMP-1 mRNA expression (panel A) was almost absent in naïve wild-type livers (lane1) and it was significantly upregulated at 3h (lanes 2 and 3), 6h (lanes 4 and 5), 24h (lanes 6 and 7), 48h (lanes 8 and 9) and 7d (lanes 10 and 11) after liver IRI. TIMP-1 protein (panel B) was mildly expressed in naïve wild-type livers (lanes 1 and 2) and in livers at 3h (lanes 3 and 4) and 7h (lanes 10 and 11) post-IRI; however, it was markedly increased in livers at 6h (lanes 5 and 6), 24h (lanes 7 and 8) and 48h (lanes 9 and 10) after IRI. Panel C shows representative immunofluorescence staining in TIMP-1+/+ (a, c, e, and g) and TIMP-1−/− (b, d, f, and h) livers at 6h post-IRI; TIMP-1 in red (a, b; Alexa Fluor 594), F-actin in green (c, d; Alexa Fluor 488 phalloidin), nuclear stain in blue (e,f; Dapi), and staining overlay (g,h); TIMP-1 positive staining was mostly detected in the surviving parenchyma surrounding the vasculature of wild-type livers post-IRI, whereas TIMP-1 staining was undetectable in the TIMP-1−/− livers, (arrows denote TIMP-1 staining; n=4/group; *p<0.05 relative to naïve livers).
TIMP-1 Deficient Mice Had Reduced Survival Rate after Hepatic IRI
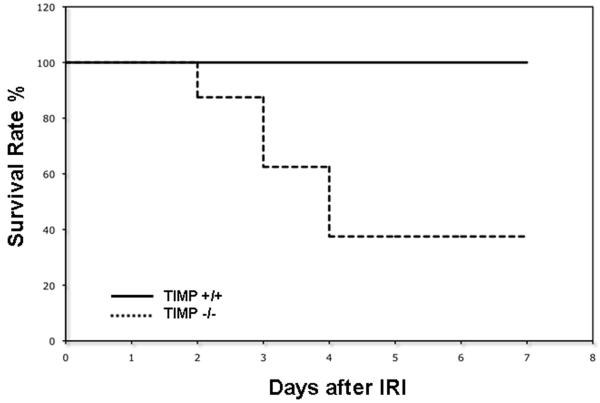
To test the significance of TIMP-1 expression in liver IRI, our experiments included TIMP-1 deficient and respective wild-type (TIMP-1+/+) control mice. The model of partial liver IRI is nonlethal 14; regardless of the significant liver damage detected during the first few days of hepatic IRI, virtually every animal survives after reperfusion. Notably, TIMP-1 deficiency resulted in an unanticipated reduced survival rate post-IRI (37% vs. 100%; p<0.05). Only 3 out of the 8 TIMP-1−/− mice survived after reperfusion, while all 8 TIMP-1+/+ (WT) animals recovered from injury and survived up to 7 days post-IRI (Fig 2). TIMP-1−/− mice failed to recover from the injury and succumbed between the second and fourth day post-IRI. Therefore, these results indicate an important role for TIMP-1 expression in hepatic IRI.
Figure 2.
Mouse survival after liver IRI. TIMP-1−/− deficient mice (dotted line) showed a significantly reduced survival rate at 7 days post-IRI, as compared with TIMP-1 +/+ mice (solid line); TIMP-1−/− animal survival was 37% versus 100% in the respective controls (n=8/group; p<0.05).
Liver Damage Was Increased in TIMP-1 Deficient Mice After IRI
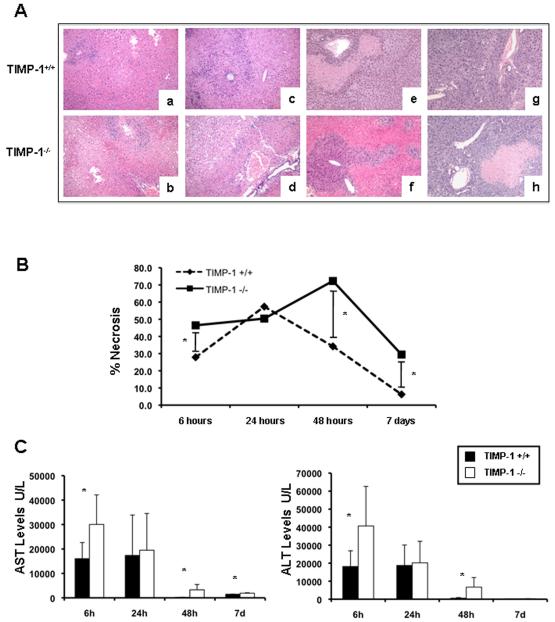
There were no detectable differences in liver histology and transaminase levels between naive TIMP-1−/− and naive WT mice. Wild-type livers were characterized by significant sinusoidal congestion and extensive necrosis after reperfusion; however, TIMP-1 deficiency was associated with further lobular architecture disruption at 6h, 48h, and 7d post-IRI (Fig. 3A). Indeed, TIMP-1−/− mice demonstrated 2-3-fold higher levels of hepatocellular necrosis (p<0.05) when compared with TIMP-1+/+ mice at 48h post-IRI (Fig. 3B). TIMP-1−/− mice that survived surgery showed improved liver histology at 7d post-IRI; however, levels of liver necrosis were still higher in these mice when compared to respective WT controls (Fig. 3A and B). The serum transaminase levels (U/L) were significantly increased in TIMP-1 mice at 6h (sAST: 30,040±12,104 vs. 16,033±6,598, p<0.05; sALT: 40,660±21,970 vs. 18,148±8,727, p<0.05), 48h (sAST: 3,290±2,170 vs. 197.75±82.44, p<0.05; sALT: 6,720±5,298 vs. 571.25±348.9, p<0.05), and 7d (sAST: 1,909±155 vs. 1,472±62, p<0.05; sALT: 254±88 vs. 119±42, p<0.05) post-IRI, (Fig. 3C). All together, these data emphasize the concept that TIMP-1 has a protective function in hepatic IRI.
Figure 3.
Liver histological preservation and serum transaminases in TIMP-1−/− and TIMP-1+/+ mice. Representative H&E staining (panel A) of TIMP-1+/+ (a, c, e, and g) and TIMP-1−/− (b, d, f, and h) livers at 6h (a, and b), 24h (c, and d), 48h (e, and f), and 7d (g, and h) post-I/R injury; TIMP-1 deficiency was associated with further disruption of lobular architecture as compared with TIMP-1+/+ livers, particularly at 6h, 48h post-IRI, and 7d. The percentage of hepatocellular necrosis (panel B) was increased 2-3 fold in the TIMP-1−/− livers at 48h after IRI. sAST and sALT levels (panel C) were measured in blood samples retrieved after IRI; transaminase levels were significantly increased in TIMP-1−/− mice at 6h, 48h, and 7d post IRI, as compared with respective TIMP-1+/+ controls (n=4-6 mice/group *p<0.05).
MMP-9 Expression and Activity Was Upregulated in TIMP-1 Deficient Livers After IRI
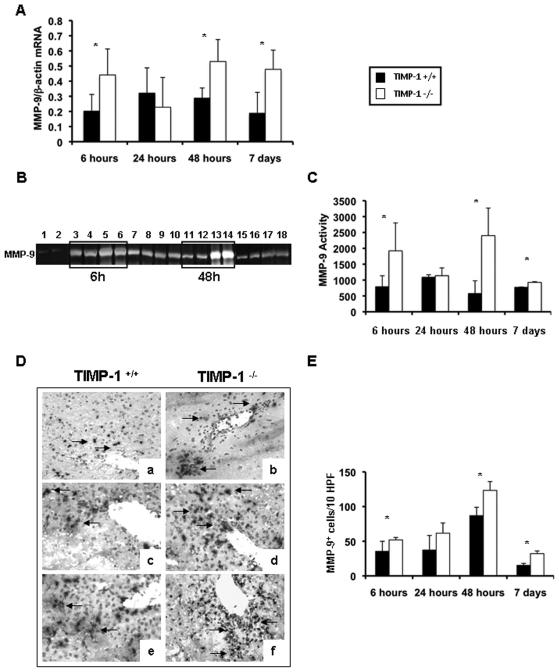
TIMP-1−/− mice showed significantly up-regulated MMP-9/β-actin mRNA expression at 6h (0.44±0.17 vs. 0.20±0.11; p<0.05), 48h (0.53±0.15 vs. 0.29±0.07; p<0.05), and 7d (0.48±0.13 vs. 0.19±0.14; p<0.05) after IRI, (Fig. 4A). Moreover, zymography analysis showed that MMP-9 activity was almost undetected in naïve livers and highly expressed in TIMP-1−/− and WT livers post-IRI; however, MMP-9 activity was markedly upregulated in the livers of TIMP-1−/− mice after 6h (p<0.05) and 48h (p<0.05) of reperfusion, as compared to controls (Fig. 4B). Indeed, the MMP-9 activity increase observed in the TIMP-1−/− mice was over 4-folds of that obtained in the control animals at 48h post-IRI (Fig. 4C). Finally, MMP-9+ leukocytes were present in significantly higher numbers in TIMP-1−/− livers at 6h (52±3 vs. 35±14; p<0.05), 48h (123±13 vs. 87±12; p<0.05), and 7d (32±4 vs. 15±3; p<0.05) post-IRI, (Fig. 4D and E). Thus, TIMP-1 deficiency is correlated to increased levels of MMP-9 expression/activity in hepatic IRI.
Figure 4.
MMP-9 expression and activity in TIMP-1−/− and TIMP-1+/+ mice. MMP-9 mRNA expression (panel A), as detected by RT-PCR analysis, was significantly upregulated in TIMP-1−/− mice at 6h, 48h, and 7d after IR injury, as compared to the respective wild-type controls. MMP-9 activity (panels B and C), analyzed by zymography in TIMP-1+/+ (lanes 1, 3, 4, 7, 8, 11, 12, 15, and 16) and TIMP-1 −/− (lanes 2, 5, 6, 9, 10, 13, 14, 17, and 18) livers; MMP-9 activity was almost absent in naïve livers of TIMP-1+/+ (lane 1) and TIMP-1−/− (lane 2) mice and highly detectable in TIMP-1+/+ and TIMP-1−/− livers at 6h (lanes 3-6), 24h (lanes 7-10), 48h (lanes 11-14), and 7d (lanes 15-18) post-IRI; however, compared to controls, MMP-9 activity was markedly upregulated in TIMP-1−/− livers at 6h, 48h, and 7d after IRI. MMP-9+ cells (panel D and E) in wild-type controls (a, c, e) and TIMP-1−/− livers (b, d and f) at 6h (a, and b), 24h (c, and d), and 48h (e, and f) post-IRI; MMP-9+ cells were detected in significantly higher numbers in TIMP-1−/− livers, particularly at 6h, 48h, and 7d post-reperfusion, (n=4-5/group; *p<0.05).
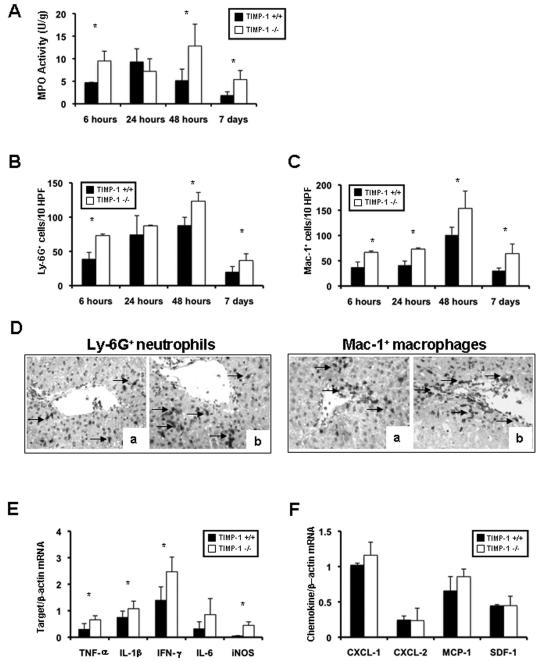
Deficiency in TIMP-1 Enhanced Myeloperoxidase Activity and Leukocyte Accumulation/Activation in Hepatic IRI
MPO activity (U/g) was increased in TIMP-1−/− livers (9.5±2.1 vs. 4.7±0.07; p<0.05) at 6h post-reperfusion, as compared with TIMP-1+/+ controls (Fig. 5A). MPO activity was comparable in both TIMP-1−/− and control livers at 24h post-IRI. However, MPO activity in TIMP-1−/− livers increased again over controls at 48h (12.8±4.9 vs. 5.1±2.6; p<0.05) and 7d (5.4±2.0 vs. 1.8±0.8; p<0.05) post-IRI (Fig. 5A). MPO activity correlated with Ly-6G+ cell numbers; Ly-6G neutrophils were increased in the absence of TIMP-1 at 6h (73±2 vs. 39±10; p<0.05), 48h (123±13 vs. 88±12; p<0.05), and 7d (37±9 vs. 20±8; p<0.05) post-IRI (Fig. 5B, and D). Moreover, TIMP-1 deficiency also caused a substantial increase of infiltrating Mac-1 macrophages at 6h (67±3 vs. 37±10; p<0.05), 24h (73±2 vs. 41±8; p<0.05), 48h (154±34 vs. 101±15; p<0.05), and 7d (64±19 vs. 30±5; p<0.05) post-IRI (Fig. 5C and D). The extent of leukocyte infiltration correlated with proinflammatory cytokine expression. TNF-α (0.66±0.15 vs. 0.37±0.28; p<0.05), IL-1β (1.08±0.29 vs. 0.75±0.24 p<0.05), and IFN-γ (1.08±0.29 vs. 0.75±0.24; p<0.05) were significantly upregulated in TIMP-1−/− livers at 6h post-IRI (Fig 5E). TIMP-1−/− livers at 48h (IL-1β: 0.21±0.04 vs. 0.10±0.02; p<0.05) and 7d (IL-1β: 0.20±0.04 vs. 0.14±0.03 and TNF-α: 0.32±0.07 vs. 0.21±0.04; p<0.05) post-IRI were also characterized by significantly increased proinflammatory cytokine expression. Further, iNOS expression, which associates to liver injury 15, showed a ≈2.5-fold increase (p<0.05) in 6h TIMP-1−/− livers. In contrast, IL-10, well-known for its protective role in hepatic IRI 16, was downregulated in TIMP-1 −/− livers at 48h (0.26±0.13 vs. 0.65±0.14; p<0.05) and 7d (0.43±0.21 vs. 0.82±0.14; p<0.05) post-IRI.
Figure 5.
Intrahepatic MPO enzyme activity and leukocyte infiltration/activation in TIMP-1−/− and TIMP-1+/+ mice. MPO enzymatic activity (panel A), an index of neutrophil infiltration, was markedly upregulated in TIMP-1−/− livers when compared to wild-type controls at 6h, 48h, and 7d after IRI. Ly-6G+ neutrophil infiltration (panel B) was significantly increased in livers of TIMP-1−/− mice at 6h, 48h and 7d post-IRI. Mac-1+ macrophages (panel C) were detected in higher numbers in livers of TIMP-1−/− mice at 6h, 24h, 48h and 7d post-IRI. Panel D illustrates immunostaining of Ly-6G neutrophils (left) and Mac-1 macrophages (right) in TIMP-1+/+ livers (a) and TIMP-1−/− (b) at 6h after IRI. Pro-inflammatory mediators (panel E) in TIMP-1+/+ and TIMP-1−/− livers; TNF-α, IL-1β, IFN-γ, and iNOS mRNA levels were significantly upregulated in TIMP-1−/− deficient livers at 6h post-IRI, as compared to respective controls. Chemokine gene evaluation (panel F) showed comparable expressions of CXCL-1, CXCL-2, MCP-1 and SDF-1 in TIMP-1+/+ and TIMP-1−/− livers after reperfusion (n=4-5/group; *p<0.05).
TIMP-1 Deficiency Did Not Alter the Expression of Major Chemokines in Hepatic IRI
To determine whether TIMP-1 deficiency affects chemokine expression, we assessed major cell activating chemokines linked to liver IRI (Fig. 5F). CXCL-1 (1.16±0.19 vs. 1.02±0.03) and CXCL-2 (0.24±0.18 vs. 0.24±0.06), were comparably expressed in both TIMP-1−/− and wild-type livers at 6h post-IRI. Moreover, TIMP-1−/− and WT livers also expressed similar levels of MCP-1 (0.86±0.11 vs. 0.66±0.20) and SDF-1 (0.45±0.13 vs. 0.45±0.02) 6h post-reperfusion. The expression levels of these chemokines were also comparable in TIMP-1−/− and WT livers at 24h, 48h, and 7d post-IRI (data not shown).
Deficiency in TIMP-1 Impaired Liver Regeneration after IRI
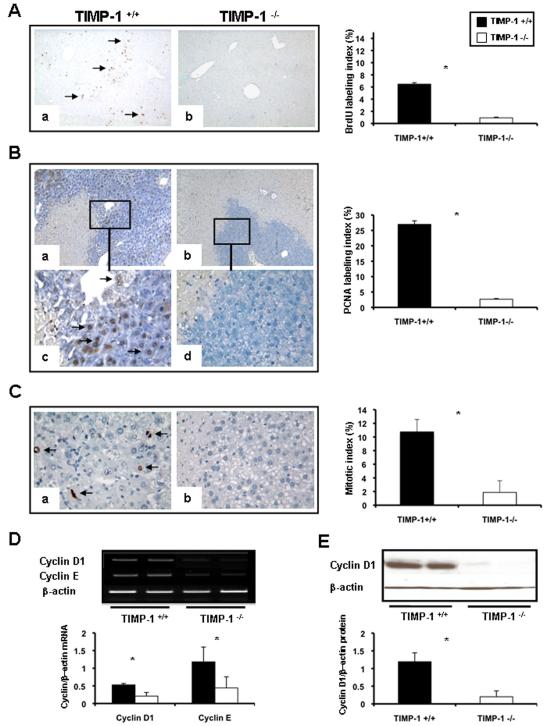
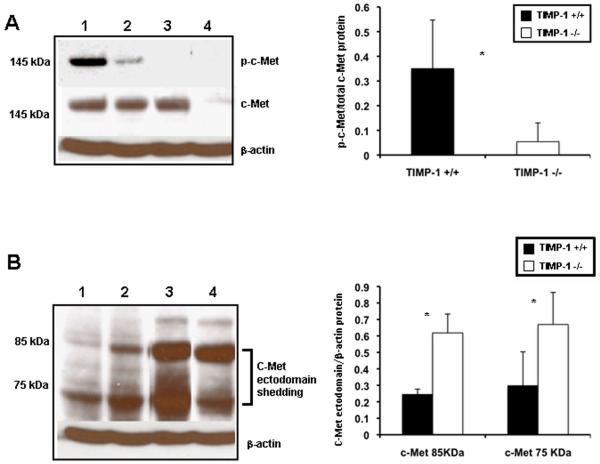
To determine whether TIMP-1 deficiency interferes with cell proliferation, the percentage of cells in S phase, the BrdU and PCNA labeling indexes, and the percentage of phosphorylated histone H3 (P-H3)-positive cells, the mitotic index (MI), were evaluated after liver IRI. BrdU (0.53±0.11 vs.1.70±0.13; p<0.05), PCNA (0.51±0.46 vs. 5.02±2.98; p<0.05) and MI (0.50±0.46 vs. 2.96±1.67) indexes were modestly detected at 24h post-IRI, with decreased proliferation indexes in the TIMP-1 −/− livers when compared to controls. While BrdU (0.92±0.11 vs. 6.46±0.24; p<0.05), PCNA (2.65±0.33 vs. 26.96±2.74; p<0.05), and MI (1.87±1.71 vs. 10.74±1.82; p<0.05) indexes were still almost negligible in TIMP-1−/− livers at 48h post-IRI, they were significantly increased in TIMP-1+/+ controls (Fig 6A-C). Several TIMP-1 −/− animals died between the second and fourth day post-IRI; nonetheless, TIMP-1−/− mice that survived surgery exhibited some evidence of delayed liver regeneration, as the mitotic index (7.16±2.47 vs. 3.39±1.17) was enhanced in these animals at 7d post-IRI. Moreover, cyclin D1, a regulator of the G1-to-S phase transition 17, and cyclin E, also necessary for entry into S phase 18, were downregulated at mRNA level in TIMP-1−/− livers (cyclin D1: 0.21±0.04 vs. 0.53±0.11; p<0.05; cyclin E: 0.44±0.32 vs. 1.18±0.42; p<0.05) at 48h post-reperfusion (Fig 6D). Cyclin D1 was almost absent in TIMP-1−/− livers at protein level (0.20±0.26 vs. 1.19±0.25; p<0.05), contrasting with an approximate 6-fold increased expression detected in the wild-type livers at 48h post-IRI (Fig. 6E). c-Met-HGF interactions result in c-Met phosphorylation, which is the central stimulus for the G1–S progression of hepatocytes 19. The inability of TIMP-1−/− mice to express TIMP-1 led to markedly decreased HGF/c-Met signaling, as evidenced by the markedly reduced levels of phosphorylated c-Met (0.05±0.07 vs. 0.35±0.20; p<0.05) in their livers at 48h post-IRI, (Fig. 7A). Further, c-Met ectodomain shedding, a process by which proteins are proteolytically released from the cell surface, negatively regulates c-Met signaling 20. In our settings, absence of TIMP-1 resulted in significantly enhanced c-Met ectodomain shedding in liver IRI, (Fig. 7B). Therefore, these results evidence that loss of TIMP-1 interferes with liver regeneration after IRI.
Figure 6.
Expression of hepatic regenerative markers in TIMP-1−/− and TIMP-1+/+ mice. Hepatocyte BrdU incorporation (panel A), PCNA labeling (panel B), and phosphorylated histone H3-positive cells (panel C) in TIMP-1+/+ (a, and c) and TIMP-1−/− (b, and d) livers at 48h post-IRI; PCNA staining (c, and d) is shown in higher magnification to better illustrate positive (c) and virtually negative (d) PCNA hepatocyte-labeling in the surviving parenchyma of TIMP-1+/+ and TIMP-1−/− livers, respectively. TIMP-1−/− livers showed markedly diminished BrdU, PCNA, and mitotic labeling indexes, as compared to controls. The densitometric ratios of cyclin D1/β-actin and cyclin E/β-actin mRNA (panel D) were significantly depressed in TIMP-1−/− livers at 48h post-IRI. Cyclin D1 at protein level (panel E) was also profoundly depressed in TIMP-1−/− livers at 48h post-IRI, (n=4-5/group; *p<0.05).
Figure 7.
cMet phosphorylation and c-Met ectodomain shedding in TIMP-1−/− and TIMP-1+/+ livers. c-Met, the high affinity tyrosine kinase receptor for hepatocyte growth factor, was readily phosphorylated in TIMP-1+/+ wild-type livers (lanes 1, and 2), contrasting with the almost lack of c-Met phosphorylation detected in TIMP-1−/− livers (lanes 3, and 4) post-IRI; the densitometric phospho-c-Met/c-Met ratio was decreased several-fold in the TIMP-1−/− livers at 48h post-IRI, as compared to respective WT controls. Moreover, the c-Met ectodomain fragments 85 kDa and 75 kDa (panel B) were particularly elevated in TIMP-1−/− livers (lanes 3, and 4) at 48h post-IRI when compared with respective matched wild-type controls (lanes 1, and 2), (n=4-5/group; *p<0.05).
Lack of TIMP-1 Exacerbates Caspase-3 Activation in Liver IRI
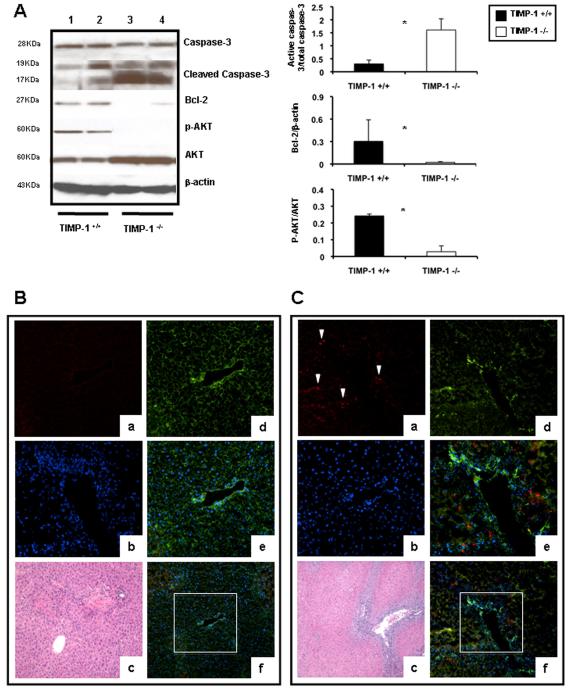
Caspase-3 is expressed in tissues as an inactive 32-kDa precursor, which is cleaved to generate a 17-kDa mature active form during apoptosis 21. The active caspase-3 was absent in naïve livers and increased in TIMP-1−/− and wild-type livers at 6h post-reperfusion; however, 17KDa caspase-3 expression was significantly higher (0.55±0.22 vs. 0.12±0.08; p<0.05) in the livers of TIMP-1−/− mice, as compared to controls. Notably, the active 17KDa caspase-3 was particularly increased in livers of mice deficient in TIMP-1 (1.79±0.24 vs. 0.27±0.16; p<0.05) at 48h, preceding TIMP-1−/− mouse death post-IRI, (Fig. 8A). Immunofluorescence analysis of cleaved-17KDa caspase-3 indicated that while the active form of capase-3 was minimally expressed in scattered cells in wild-type livers at 48h post-IRI, it was readily detected in the TIMP-1 deficient livers in the still surviving tissue areas (Fig. 8B and C). Moreover, Bcl-2, a known inhibitor of cell death, was almost absent in the TIMP-1−/− livers at 48h post-IRI (0.13±0.08 vs. 0.69±0.19; p<0.05) (Fig. 8A). Finally, phosphorylation of Akt, a 57-kD protein-serine/threonine kinase with pro-survival associated functions 22, was depressed in TIMP-1−/− livers (0.10±0.07 vs. 0.44±0.30; p<0.05) at 48h post-IRI (Fig. 8A). At 7d post-IRI, Bcl-2 was still reduced (≈0.6-fold; p<0.05) in TIMP-1−/− livers, compared to controls. Hence, these results support a major protective role for TIMP-1 expression in hepatic IRI.
Figure 8.
Apoptotic and pro-survival markers in livers of TIMP-1−/− and TIMP-1+/+ mice. Caspase-3, Bcl2, and Akt expressions (panel A) in wild-type (lanes 1 and 2) and TIMP-1−/− (lanes 3 and 4) livers at 48h post-IRI; the densitometric ratio of active caspase-3/total caspase-3 was significantly increased in TIMP-1−/− livers at 48h post-IRI, whereas the densitometric ratios of Bcl-2/β-actin and pAkt/Akt were markedly reduced in these livers. Representative triple immunofluorescence in TIMP-1+/+ (panel B) and TIMP-1−/− (C) livers of cleaved caspase-3 in red (a, Alexa Fluor 594), F-actin in green (d, Alexa Fluor 488 phalloidin), nuclear stain in blue (b, Dapi), and staining overlay (e, and f); H&E staining (c); active caspase-3 was predominantly detected in the still surviving tissue adjacent to the large vessels and surrounded by extensive areas of necrosis in TIMP-1−/− livers at 48h post-IRI, (n=4-5/group; *p<0.05).
DISCUSSION
The understanding of the functions of TIMPs in liver IRI has the potential to contribute to the development of novel therapeutic approaches to prevent hepatic IRI, and consequently, to improve the outcome of liver transplantation. In this study, we investigated the functional significance of TIMP-1 expression in a well-established 90 min mouse model of partial liver warm IRI 4.
Interactions between ECM components and cell adhesion receptors regulate leukocyte functions; therefore, it is not unanticipated that enzymatic degradation of ECM can alter leukocyte behaviors 23. Indeed, cells employ proteolytic enzymes, particularly MMPs, to control the ECM turnover, to release growth factors, and to migrate across ECM 24. There is a growing body of evidence supporting key functions for MMP expression in the pathogenesis of liver diseases 3, 25, 26. In this regard, we have previously shown that MMP-9 regulates leukocyte recruitment and contributes to hepatic IRI 4. While TIMP-1 can inhibit a broad range of MMPs, it is particularly potent for MMP-9 27. However, compared to MMP-9, the role of its natural inhibitor, TIMP-1, is virtually unknown in liver IRI. TIMP-1 expression is very low in naïve livers and it is induced after liver IRI; though, it is still insufficient to prevent an elevated MMP activity in liver IRI 11. In the present study, we show that TIMP-1 deficiency resulted in further exaggerated upregulation of MMP-9 activity and, more strikingly, it led to a poor survival rate after reperfusion. This is particularly interesting having in consideration that the model of partial liver IRI is nonlethal 14. Indeed, all TIMP-1+/+ mice survived hepatic IRI despite the significant liver damage detected in the livers after reperfusion; in contrast, only 3 out of 8 TIMP-1−/− mice survived more than 4 days after liver IRI. In general, TIMP-1−/− mice showed additional impairment of liver function and more severe lesions, which likely led to their death between the second and fourth day post-reperfusion.
While infiltrating leukocytes are recognized as mediators of hepatic IRI 3, 28, the mechanisms involved in their recruitment to sites of inflammatory stimulation in liver are still far from being understood. TIMP-1−/− livers showed massive leukocyte accumulation post-IRI. This latter feature, together with the findings that MMP-9 enzymatic activity was significantly increased in the TIMP-1−/− livers, strongly support an important regulatory role for TIMP-1 on leukocyte recruitment in hepatic IRI. Local concentrations of TIMP-1 are important for regulating MMP-9 activity in vivo 29, and TIMP-1 has also been implicated in leukocyte infiltration into the damaged brain 30. In addition to amplified leukocyte migration, TIMP-1 deficient mice showed significantly increased levels of proinflammatory mediators after liver injury. IFN-γ and iNOS, which have been linked to tissue injury, including hepatic injury 15, 31, were markedly upregulated in the TIMP-1−/− post-IRI. Moreover, TNF-α, whose expression is often associated with neutrophil infiltration and liver damage 32, was also significantly increased in the TIMP-1 livers after reperfusion.
Impaired liver regeneration/repair is one of the most frequent features in acute liver failure. Adult hepatocytes, which make up to 80% of hepatic cells, are long lived and normally do not undergo cell division; however, they maintain the ability to proliferate in response to injury 33. Using three independent parameters of regeneration (BrdU, PCNA, and mitotic indexes), we provide evidence that hepatocyte progression into S phase and mitosis was disrupted in TIMP-1 deficient mice during the first 48h post-IRI. Cyclins D1 and E, which are necessary for entry into S phase 17, 18, were profoundly depressed in the TIMP-1 deficient livers post-IRI. It is known that inhibition of cyclin D1 leads to growth arrest and to impaired hepatic regeneration 34. It is perhaps important to stress that the role of TIMP-1 in liver regeneration may depend on the type of injury as TIMP-1 can negatively affect regeneration after substantial hepatic resection 35.
Our results agree with previous findings indicating that TIMP-1 has a growth-promoting activity in a broad variety of cells 9, 36, 37, including in hepatocytes 38, and that TIMP-1 can stimulate the HGF/cMet pathway by inhibiting MMP-mediated c-Met shedding 39. Activation of the HGF/cMet signaling pathway requires phosphorylation of c-Met, which is needed for efficient liver regeneration 40. In our settings, the inability of TIMP-1−/− mice to express TIMP-1 led to virtually undetectable phosphorylated c-Met levels after liver reperfusion. Further, TIMP-1 deficiency resulted in increased proteolytic cMet ectodomain shedding, which may account in part for the reduced levels of phosphorylated c-Met post-liver IRI; soluble c-Met shed ectodomains act as decoy receptors by interfering with HGF binding to c-Met 20. Therefore, our work strongly supports the view that TIMP-1−/− livers have an impaired capability to regenerate after IRI.
In addition to impaired liver regeneration, cell death by necrosis, apoptosis, or necroapoptosis, is a prominent feature of liver IRI 14, 41. The expression of TIMP-1 was detected in the surviving parenchyma of wild-type mice after the ischemic insult, suggesting a potential role for TIMP-1 in conferring resistance to cell death. Indeed, TIMP-1−/− deficient livers exhibited increased liver necrosis, particularly at 48h post-IRI. Moreover, caspase-3 activation, the executor of apoptosis 42, was significantly increased in TIMP-1−/− livers as compared with control littermates after IRI, and it was accompanied by a decrease in Bcl-2 expression. While morphologic alterations of apoptosis are mostly mediated by caspases 42, Bcl-2 is an integral membrane anti-apoptotic protein expressed even in healthy cells 43. In this regard, it has been reported that TIMP-1 can inhibit apoptosis in a wide variety of cell types, including stellate cells 44, B cells 45, epithelial cells, 46 and mesangial cells 47, through MMP-dependent and -independent mechanisms. Moreover, it has also been shown that exogenous TIMP-1 confers resistance against apoptosis in isolated endothelial cells via activation of the PI-3/Akt signaling pathway 48. Akt is a 57-kD protein-serine/threonine kinase with pro-survival functions 22. In our settings, lack of TIMP-1 expression resulted in almost completely depleted Akt phosphorylation, without changing total Akt protein levels, suggesting that TIMP-1 activates the Akt signaling pathway in hepatic IRI. TIMP-1 inhibition of cell death can also be mediated via its regulatory role on MMP enzymatic activity. The extracellular matrix proteolysis mediated by MMPs can lead to detachment of liver cells resulting in apoptosis, by a phenomenon called “anoikis” 49. Indeed, we have previously shown that MMP-9, in addition to facilitate leukocytes infiltration in livers after IRI, induces hepatocyte apoptosis after IRI 15.
In summary, these studies demonstrate an important protective role for TIMP-1 expression in liver IRI. Overall, we show that TIMP-1 has relevant functions on promoting cell survival and proliferation of liver cells and on regulating leukocyte recruitment and activation in liver IRI. The inability of TIMP-1−/− mice to express TIMP-1 resulted in enhanced liver damage and in lethal hepatic IRI. Moreover, our data provide the rationale for studies, currently under development in our laboratory, aimed at efficiently overexpressing TIMP-1 in vivo as a potential therapeutic approach to improve hepatic IRI.
Supplementary Material
Acknowledgments
This work was supported by the following grants from the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) R01AI057832, UCLA Academic Senate, and the Pfleger Foundation (to A.J.C.). S.D. was supported in part by a doctoral fellowship from the Fundação para a Ciência e Tecnologia (FCT), Portugal.
REFERENCES
- 1.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–36. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 2.Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–63. doi: 10.1053/jlts.2003.50105. [DOI] [PubMed] [Google Scholar]
- 3.Coito AJ. Leukocyte transmigration across endothelial and extracellular matrix protein barriers in liver ischemia/reperfusion injury. Curr Opin Organ Transplant. 2011;16:34–40. doi: 10.1097/MOT.0b013e328342542e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2008;47:186–98. doi: 10.1002/hep.21922. [DOI] [PubMed] [Google Scholar]
- 5.Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 6.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 7.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Morgunova E, Tuuttila A, Bergmann U, Tryggvason K. Structural insight into the complex formation of latent matrix metalloproteinase 2 with tissue inhibitor of metalloproteinase 2. Proc Natl Acad Sci U S A. 2002;99:7414–9. doi: 10.1073/pnas.102185399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuyvenhoven JP, Molenaar IQ, Verspaget HW, Veldman MG, Palareti G, Legnani C, Moolenburgh SE, Terpstra OT, Lamers CB, van Hoek B, Porte RJ. Plasma MMP-2 and MMP-9 and their inhibitors TIMP-1 and TIMP-2 during human orthotopic liver transplantation. The effect of aprotinin and the relation to ischemia/reperfusion injury. Thromb Haemost. 2004;91:506–13. doi: 10.1160/TH03-05-0272. [DOI] [PubMed] [Google Scholar]
- 11.Moore C, Shen XD, Gao F, Busuttil RW, Coito AJ. Fibronectin-alpha4beta1 integrin interactions regulate metalloproteinase-9 expression in steatotic liver ischemia and reperfusion injury. Am J Pathol. 2007;170:567–77. doi: 10.2353/ajpath.2007.060456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–7. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 13.Torres L, Garcia-Trevijano ER, Rodriguez JA, Carretero MV, Bustos M, Fernandez E, Eguinoa E, Mato JM, Avila MA. Induction of TIMP-1 expression in rat hepatic stellate cells and hepatocytes: a new role for homocysteine in liver fibrosis. Biochim Biophys Acta. 1999;1455:12–22. doi: 10.1016/s0925-4439(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 14.Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223–31. doi: 10.1002/hep.510300513. [DOI] [PubMed] [Google Scholar]
- 15.Hamada T, Duarte S, Tsuchihashi S, Busuttil RW, Coito AJ. Inducible nitric oxide synthase deficiency impairs matrix metalloproteinase-9 activity and disrupts leukocyte migration in hepatic ischemia/reperfusion injury. Am J Pathol. 2009;174:2265–77. doi: 10.2353/ajpath.2009.080872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshidome H, Kato A, Edwards MJ, Lentsch AB. Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: implications of a central role for nuclear factor kappaB. Hepatology. 1999;30:203–8. doi: 10.1002/hep.510300120. [DOI] [PubMed] [Google Scholar]
- 17.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–7. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 18.Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–24. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 20.Petrelli A, Circosta P, Granziero L, Mazzone M, Pisacane A, Fenoglio S, Comoglio PM, Giordano S. Ab-induced ectodomain shedding mediates hepatocyte growth factor receptor down-regulation and hampers biological activity. Proc Natl Acad Sci U S A. 2006;103:5090–5. doi: 10.1073/pnas.0508156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Z, Hendrickson EA, Bremner TA, Wyche JH. A sequential two-step mechanism for the production of the mature p17:p12 form of caspase-3 in vitro. J Biol Chem. 1997;272:13432–6. doi: 10.1074/jbc.272.20.13432. [DOI] [PubMed] [Google Scholar]
- 22.Mullonkal CJ, Toledo-Pereyra LH. Akt in ischemia and reperfusion. J Invest Surg. 2007;20:195–203. doi: 10.1080/08941930701366471. [DOI] [PubMed] [Google Scholar]
- 23.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woessner JF., Jr. That impish TIMP: the tissue inhibitor of metalloproteinases-3. J Clin Invest. 2001;108:799–800. doi: 10.1172/JCI13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dechene A, Sowa JP, Gieseler RK, Jochum C, Bechmann LP, El Fouly A, Schlattjan M, Saner F, Baba HA, Paul A, Dries V, Odenthal M, Gerken G, Friedman SL, Canbay A. Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology. 2010;52:1008–16. doi: 10.1002/hep.23754. [DOI] [PubMed] [Google Scholar]
- 26.Bissell DM. Inflammation and hepatic fibrosis. Semin Liver Dis. 2010;30:211–4. doi: 10.1055/s-0030-1255350. [DOI] [PubMed] [Google Scholar]
- 27.Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J Neurosci Res. 2003;74:801–6. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–9. [PubMed] [Google Scholar]
- 29.Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crocker SJ, Whitmire JK, Frausto RF, Chertboonmuang P, Soloway PD, Whitton JL, Campbell IL. Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. Am J Pathol. 2006;169:2104–16. doi: 10.2353/ajpath.2006.060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–80. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 32.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–62. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–47. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007;104:17081–6. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammed FF, Pennington CJ, Kassiri Z, Rubin JS, Soloway PD, Ruther U, Edwards DR, Khokha R. Metalloproteinase inhibitor TIMP-1 affects hepatocyte cell cycle via HGF activation in murine liver regeneration. Hepatology. 2005;41:857–67. doi: 10.1002/hep.20618. [DOI] [PubMed] [Google Scholar]
- 36.Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- 37.Fata JE, Leco KJ, Moorehead RA, Martin DC, Khokha R. Timp-1 is important for epithelial proliferation and branching morphogenesis during mouse mammary development. Dev Biol. 1999;211:238–54. doi: 10.1006/dbio.1999.9313. [DOI] [PubMed] [Google Scholar]
- 38.Kopitz C, Gerg M, Bandapalli OR, Ister D, Pennington CJ, Hauser S, Flechsig C, Krell HW, Antolovic D, Brew K, Nagase H, Stangl M, von Weyhern CW, Brucher BL, Brand K, Coussens LM, Edwards DR, Kruger A. Tissue inhibitor of metalloproteinases-1 promotes liver metastasis by induction of hepatocyte growth factor signaling. Cancer Res. 2007;67:8615–23. doi: 10.1158/0008-5472.CAN-07-0232. [DOI] [PubMed] [Google Scholar]
- 39.Schelter F, Kobuch J, Moss ML, Becherer JD, Comoglio PM, Boccaccio C, Kruger A. A disintegrin and metalloproteinase-10 (ADAM-10) mediates DN30 antibody-induced shedding of the met surface receptor. J Biol Chem. 2010;285:26335–40. doi: 10.1074/jbc.M110.106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101:4477–82. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 42.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 44.Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069–76. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 45.Guedez L, Stetler-Stevenson WG, Wolff L, Wang J, Fukushima P, Mansoor A, Stetler-Stevenson M. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest. 1998;102:2002–10. doi: 10.1172/JCI2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu XW, Taube ME, Jung KK, Dong Z, Lee YJ, Roshy S, Sloane BF, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells from extrinsic cell death: a potential oncogenic activity of tissue inhibitor of metalloproteinase-1. Cancer Res. 2005;65:898–906. [PubMed] [Google Scholar]
- 47.Lin H, Chen X, Wang J, Yu Z. Inhibition of apoptosis in rat mesangial cells by tissue inhibitor of metalloproteinase-1. Kidney Int. 2002;62:60–9. doi: 10.1046/j.1523-1755.2002.00403.x. [DOI] [PubMed] [Google Scholar]
- 48.Boulday G, Fitau J, Coupel S, Soulillou JP, Charreau B. Exogenous tissue inhibitor of metalloproteinase-1 promotes endothelial cell survival through activation of the phosphatidylinositol 3-kinase/Akt pathway. Ann N Y Acad Sci. 2004;1030:28–36. doi: 10.1196/annals.1329.004. [DOI] [PubMed] [Google Scholar]
- 49.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.