Abstract
The objective of this study was to investigate the efficiency of multifunctional PEG-based hemoglobin conjugates crosslinked with antioxidant enzymes for their ability to protect an oxygen carrier (hemoglobin) and insulin secreting islets from the combination of hypoxic and free radical stress under simulated transplantation conditions. In this study, RINm5F cells and isolated pancreatic islets were challenged with oxidants (H2O2 or xanthine and xanthine oxidase) and incubated with conjugates (Hb-Hb or Hb-SOD-CAT) in normoxia (21% oxygen) or hypoxia (6% or 1% oxygen). Hemoglobin protection, intracellular free radical activity and cell viability in RINm5F cells measured by methemoglobin, DCF-DA and MTT assay respectively showed that cells were better protected by conjugates containing antioxidant enzymes. Insulin secretion from islets and qualitative confocal evaluation of viability showed beta cells were protected by conjugates containing antioxidant enzymes when exposed to induced stress. Our study suggested that antioxidant enzymes play a significant role in hemoglobin protection and thus extended cell protection.
Keywords: Tissue Engineering, Islets, Hypoxia, Oxygen Carrier, Antioxidants, Poly(ethylene glycol) Cross-linking
Introduction
Islet transplantation can circumvent the need for daily injections of exogenous insulin in patients with type 1 insulin-dependent diabetes mellitus(Meloche 2007). Even though this approach has proven to be successful, its widespread use is limited by a shortage of islet donors and long-term islet viability(Beck et al. 2007). Use of non-human islets can some extent solve islet scarcity problems in transplantation (Giraldo et al. 2010). Unfortunately the long-term viability of de-vascularized islets following transplantation continues to hamper the achievement of successful long-term normoglycemia(Bae 2004). Islet damage can be caused by factors such as ischemia(Linn et al. 2006), exposure to reactive oxygen species immediately following islet isolation or during ex-vivo cell culture(Lepore et al. 2004; Rao et al. 2005), hypoxia induced free radical damage by mitochondrial metabolism(Ko et al. 2008; Li and Jackson 2002) or by low partial oxygen pressures at the transplantation sites(Carlsson et al. 1998; Li et al. 2004) which all contribute to the loss of islet viability and impede successful islet transplantation. The extremely low levels of anti-oxidative enzymes such as superoxide and minimal or non-detectable levels of catalase (Lenzen et al. 1996) coupled with minimal antioxidant capability of islets necessitates the addition of an antioxidant defense mechanism(Mohseni Salehi Monfared et al. 2009) to protect the isolated islets from free radical damage.
Previous results (Chae et al. 2002; Chae et al. 2004) has shown that poly(ethylene glycol) (PEG) stabilized hemoglobin functioned as an artificial oxygen carrier to isolated cells. However, the long-term use of co-encapsulating PEG-crosslinked hemoglobin with islets is limited due to the continuous conversion of hemoglobin to methemoglobin by autooxidation(Simoni et al. 2009; Winterbourn 1990) and free radical damage(Vallelian et al. 2008). To overcome the problem of free radical damage to hemoglobin, we synthesized a dicarboxymethylated poly(ethylene glycol) (PEG) (supplemental figure 1) which was used to link antioxidant enzymes (i.e., superoxide dismutase (SOD) and catalase (CAT)) to hemoglobin (Hb), creating a Hb conjugate system (Hb-SOD-CAT). Previous in vitro studies showed hemoglobin was effectively protected from free radicals in the conjugate system(Nadithe and Bae 2010) and the low p50 design (p50 indicates O2 tension at which hemoglobin is half-saturated) helped release of higher amounts of oxygen when exposed to low partial oxygen pressures or hypoxic stress(Nadithe and Bae 2011). In this research, we further tested the robustness and effectiveness of SOD and CAT in protecting RINm5F cells or islets and hemoglobin in normoxia (21%) or hypoxia (6% or 1%) and free radical-rich incubation conditions that were relevant to an in vivo transplantation(Carlsson et al. 2000; Veriter et al. 2011).
Materials and Methods
Materials
Fresh bovine red blood cell suspension was purchased from Innovative Research (Novi, Michigan IC100-0410). Poly(ethylene glycol) (2 kDa), potassium tertiary butoxide, ethyl bromoacetate, N-hydroxysuccinimide (NHS), N,N’-dicyclohexylcarbodiimide (DCC), superoxide dismutase from bovine erythrocytes (SOD-S7571), bovine liver catalase (CAT-C40), hydrogen peroxide (H2O2), xanthine (X), xanthine oxidase (XO), acridine orange (AO), propidium iodide (PI), (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)(MTT), RPMI1640 powder, bovine serum albumin (BSA), and Dulbecco’s phosphate buffered saline (DPBS) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, USA). Penicillin-streptomycin, fetal bovine serum (FBS), trypsin/EDTA-0.25% solution, and 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) were purchased from Invitrogen, Inc. (Carlsbad, CA). Dialysis membranes were purchased from Spectrum Labs (Rancho Dominguez, CA). Amicon Ultra-15 Centrifugal Filters (50 and 100 kDa) were purchased from Millipore (Billerica, MA). 125I-Insulin radioimmunoassay (RIA) kit was purchased from MP Biomedicals (Irvine, CA).
Conjugation of Hb to SOD and CAT
As described previously(Chae et al. 2002), the hydroxyl end groups of PEG (2kDa) were chemically modified to PEG dicarboxylic acid and activated using NHS and DCC. The activated, PEG-NHS, was characterized by 1H NMR and thin-layer chromatography. Hemoglobin was isolated from bovine red blood cells using previously described methods(Chae et al. 2002; Nadithe and Bae 2010). For the conjugate formulation containing hemoglobin only (Hb-Hb), isolated hemoglobin was cross-linked using activated PEG at a fixed weight ratio of Hb:PEG (1:10). For the conjugate formulation containing antioxidant enzymes, the enzymes (SOD and CAT) were mixed with hemoglobin at a fixed ratio of 150000 to 300000 units using the same Hb:PEG (1:10) ratio described previously(Nadithe and Bae 2010). The reaction was carried out for 3 hours at 4 °C with constant stirring and the product was dialyzed against DPBS for 24 hours using dialysis membranes (MWCO 100 kDa). The desired conjugate concentration (0.1mM) was achieved by using Amicon™ ultracentrifugation filters. The product was filter-sterilized using 0.22 μm syringe filter and stored at 4 °C until further use. Methemoglobin Quantification: Methemoglobin in each sample was determined using previously described methods(Patton and Palmer 2005). Briefly, diluted samples were transferred into a UV-visible cuvette such that the absorbance reading (A1) at 630 nm was less than one OD unit. Then the sample was mixed with 50 μL of potassium cyanide solution (1 part 10% KCN and 1 part PBS pH 7.5) and reacted for 5-10 minutes. A second absorbance (A2) was taken at 630 nm and the methemoglobin was calculated using equation (1):
| (1) |
where the two absorbance values (A1 and A2) were subtracted from each other and divided by the extinction coefficient (3.7 cm · mM−1). To determine the total hemoglobin content in the samples, each sample was mixed with 50 μL of 20% K3Fe(CN)6 to convert any remaining oxy- and deoxy-hemoglobin species to methemoglobin. After adding another 50 μL of 10% KCN absorbance (A3) was measured at 540 nm. The hemoglobin content was calculated by dividing A3 value by the cyanomethemoglobin extinction coefficient of 11 cm−1 · mM−1 and multiplying by the dilution factor (Equation 2). The percent of methemoglobin in the sample was calculated by taking the ratio of total methemoglobin to hemoglobin and multiplying by 100.
| (2) |
Hypoxic and Oxidative Stress
Hypoxic stress was induced using a tri-gas incubator (Thermo Fisher Scientific Inc., Pittsburgh, PA, USA) with continuous flushing of ultra pure N2 to obtain the target hypoxic levels (6% or 1% oxygen). At equilibrium, the incubator contained 6% or 1% O2, 5% CO2 and 89% or 94% N2. Normoxia (21% O2) were maintained using a mixture of 95% air and 5% CO2. Oxidative stress was induced either from superoxide anions generated by 1 mM X and 10 mU/mL XO system(Rodrigues and Gomes 2010), or hydroxyl free radicals from 1 mM H2O2(von Sonntag 2008). For all oxidant-challenging experiments, RINm5F cells or islets were cultured in the presence of oxidants for 24 hours in the incubator at 37 °C (n = 3).
Experimental Groups
Experimental groups used optimized hemoglobin conjugates ratio of 1:10 Hb:PEG selected following our previous studies showing low methemoglobin (Nadithe and Bae 2010) and efficient oxygen carrier in hypoxic conditions(Nadithe and Bae 2011). The following groups were selected for evaluating the protective activity of Hb-conjugates: (1) RINm5F cells or islets with medium only, (2) RINm5F cells or islets with medium containing either 1 mM H2O2 or 1 mM X and 10 mU/mL XO, (3) RINm5F cells or islets with medium containing 0.1 mM conjugated Hb-Hb plus 1 mM H2O2 or 1 mM X and 10 mU/mL XO and (4) RINm5F cells or islets with medium containing 0.1 mM hemoglobin conjugated with antioxidants (Hb-SOD-CAT) plus 1 mM H2O2 or 1 mM X and 10 mU/mL XO.
Cell Culture (pancreatic islets and RINm5F cell line)
Pancreatic islets were obtained either by isolation from male Sprague-Dawley rats (200-250 grams) using collagenase digestion and discontinuous Ficoll density gradient separation (Chae et al. 2002; Chae et al. 2004) or purchased directly from the Joslin Diabetes Center, Boston, MA. Isolated islets or RINm5F cells were cultured in RPMI1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin in a cell culture incubator maintained at 37° C containing 95% air / 5% CO2 at normal humidity. Islets were cultured for 24 – 48 hours before use in experiments. Normalized islet equivalents (islet size of 100 μm) were used to account for the natural variability in islet size. RINm5F cells were harvested using 0.25% trypsin/10 mM EDTA and seeded (1×105 cells per well) in 24-well tissue culture plates and cultured for 24 hours prior to experiments.
Measuring Intracellular ROS levels
For the detection of intracellular reactive oxygen species (ROS), RINm5F cells were challenged with oxidants in the presence of conjugates for 24 hours and then washed with DPBS. Cells were then incubated with 20 μM of the cell-permeant ROS indicator H2DCF-DA prepared in 1 mL PBS for 60 minutes at 37 °C. Oxidized fluorescent derivate dichlorofluorescein (DCF) by intracellular free radicals was detected using a plate reader at excitation/emission wavelength of 485/530 nm(Tulsawani et al. 2010).
MTT assay for Cell Viability
RINm5F cells were incubated for 24 hours with oxidants and formulations, then cell viability (metabolic activity) were determined by using MTT reduction assay(Hayon et al. 2003). MTT was added to the wells at a final concentration of 0.5 mg/ml and cells were incubated for 3 hours at 37 °C under normoxia. Following incubation the medium was removed and the formed formazan crystals were solubilized by adding 1mL of DMSO (dimethyl sulfoxide) and incubating the plates for 10 minutes at 37 °C. Absorbance was measured at 570 nm using a microplate reader (SpectraMax M2, Molecular devices; Sunnyvale, CA). The data is shown as cell viability percentage (mean ± S.D.) accumulated from three independent experiments.
Insulin Secretion Assay
Fifty islets were incubated with oxidants and hemoglobin conjugates for 24 hours in either hypoxia or normoxia. After 24 hours, culture medium was removed and the islets were carefully rinsed with DPBS and placed in Kreb’s Ringers HEPES (KRH) solution containing Ca+2, 15.6 g/dL glucose (450G) and 0.5%w/v BSA (KRH = 4.74 mM KCl, 1.19 mM KH2PO4, 1.19 mM MgCl2.6H2O, 2.54 mM CaCl2, 25 mM NaHCO3, 10 mM HEPES)(Kang and Bae 2006). After 60 minutes incubation the medium was carefully collected and analyzed for insulin content using RIA techniques as per the manufacturer’s instructions. Insulin secretion was normalized relative to control islets (21% oxygen) that had been incubated in medium only (no oxidant) and the percentage of insulin secretion is expressed as mean ± S.D. (n = 3).
Confocal Microscopy
Isolated pancreatic islets were placed in LabTek chambers (VWR, Bridgeport, NJ), incubated with Hb conjugates and challenged with oxidants for 24 hours and then stained to visually analyze viability using confocal microscopy. A combination of Acridine Orange (AO) (to stain live cells) and Propidium Iodide (PI) (to stain apoptotic cells) (100 μl AO/PI solution; 0.67 μg/ml AO and 75 μg/ml PI in PBS) dyes were used for staining. Permeabilized islets were incubated with the dyes in the dark at room temperature for 10 minutes prior to imaging(Kim et al. 2005). Islets were excited at 500 nm and 536 nm and emission was detected at 530 nm and 620 nm for AO and PI respectively to obtain confocal images using a Fluorview confocal microscope (FV300, Olympus IX 81 microscope). Image analysis was conducted using Image J software (http://rsbweb.nih.gov/ij/).
Statistical Analysis
The statistical significance between experimental groups (control, oxidant, oxidant and Hb-Hb, oxidant and Hb-SOD-CAT) and different partial oxygen pressures (1%, 6%, or 21%) were analyzed by analysis of variance to compare group means followed by Holm-Sidak test. A p value < 0.05 was considered to be statistically significant.
Results
Methemoglobin content in hemoglobin conjugates
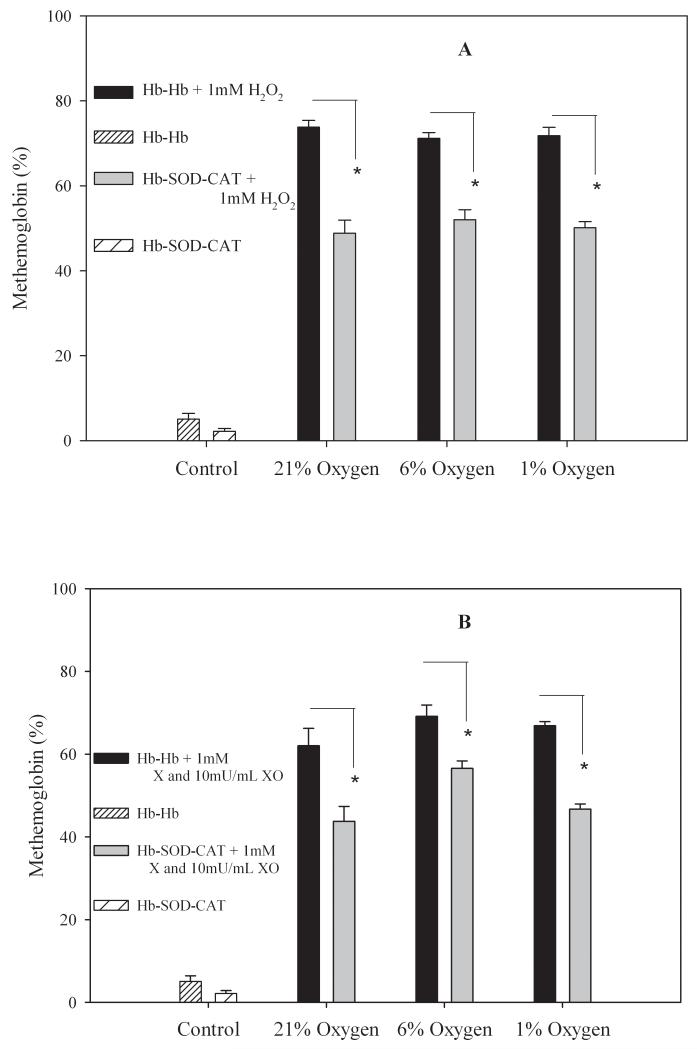
Conjugates exposed to oxidants showed substantial qualitative damage (supplemental information). When conjugates were exposed to 1 mM H2O2 for 24 hours at 37 °C, the percentage of methemoglobin found in the Hb-Hb conjugates was 74 ± 2% at 21% oxygen, 71 ± 1% at 6% oxygen, and 72 ± 2% at 1% oxygen levels, while for Hb-SOD-CAT conjugates the content was considerably lower at 48 ± 3% for 21% oxygen, 52 ± 2% at 6% oxygen, and 50 ± 1% at 1% oxygen levels (Figure 1A). For conjugates exposed to superoxide anion the percentage methemoglobin formed in Hb-Hb was 62 ± 4%, 69 ± 3% and 67 ± 1% at 21%, 6%, and 1% oxygen respectively while for Hb-SOD-CAT conjugates the methemoglobin content was 44 ± 4%, 56 ± 2% and 47 ± 1% at 21%, 6% and 1% oxygen respectively (Figure 1B). These results indicates that CAT and SOD remained active following conjugation and more importantly their presence decreased damage caused by the presence of hydrogen peroxide or superoxide ions in the medium. Statistical analysis showed that there were significant differences in the amount of methemoglobin formation in Hb-Hb compared to Hb-SOD-CAT conjugates at each of three oxygen pressures tested (p<0.01). There was no statistical significance when compared at different oxygen partial pressures (p>0.5) between the control conjugate samples and either peroxide or superoxide anion challenged conjugates. Overall results show methemoglobin formation was 20% lower when antioxidant enzymes were present versus the conjugates without antioxidant enzymes regardless of the oxygen level, confirming that there was a significant benefit from including antioxidants.
Figure 1.
Intracellular free radical activity
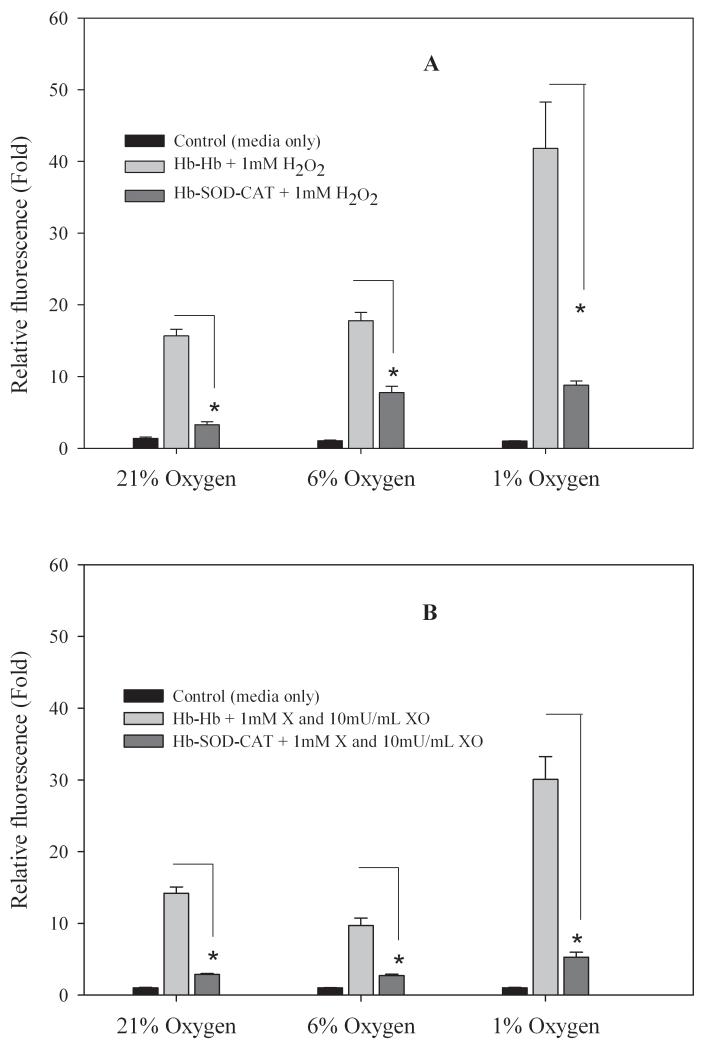
Intracellular free radical activity was measured to evaluate the capability of cells to adapt to external stressors. When RINm5F cells were incubated with 0.1 mM Hb-Hb and challenged with 1 mM H2O2 the relative fluorescence was 16 ± 1, 18 ± 1 and 42 ± 6 fold at 21%, 6%, and 1% oxygen respectively but when cells were incubated with 0.1 mM Hb-SOD-CAT the relative fluorescence fold levels were only 3 ± 0.5, 8 ± 1 and 9 ± 1 at 21%, 6%, and 1% oxygen respectively relative to untreated control cells incubated in medium only (Figure 2A). A similar trend was found when cells were challenged with superoxide anion; for cells incubated with 0.1 mM Hb-Hb relative fluorescence values were 14 ± 1, 10 ± 1 and 30 ± 3 at 21%, 6%, and 1% oxygen respectively but when cells were incubated with 0.1 mM Hb-SOD-CAT the relative fluorescence levels were only 3 ± 0.5, 3 ± 0.5 and 5 ± 1 at oxygen pressures of 21%, 6% or 1% respectively relative to untreated control cells(Figure 2B). Cells treated with oxidants only for 24 hours were washed away during the assay procedure, indicating that most likely there was widespread cell death which led to complete cell detachment. For cells exposed to hydrogen peroxide there were significant differences in fluorescence levels detected between cells incubated with media only (no conjugates) and Hb-Hb (p<0.01), and between cells incubated with media only (no conjugates) and Hb-SOD-CAT (p<0.01). For cells challenged with superoxide anion, there were significant differences in fluorescence between cells incubated with media only (no conjugates) and Hb-Hb (p<0.01), and between cells incubated with media only (no conjugates) and Hb-SOD-CAT at only 1% oxygen (p<0.01).
Figure 2.
Viability of RINm5F cells
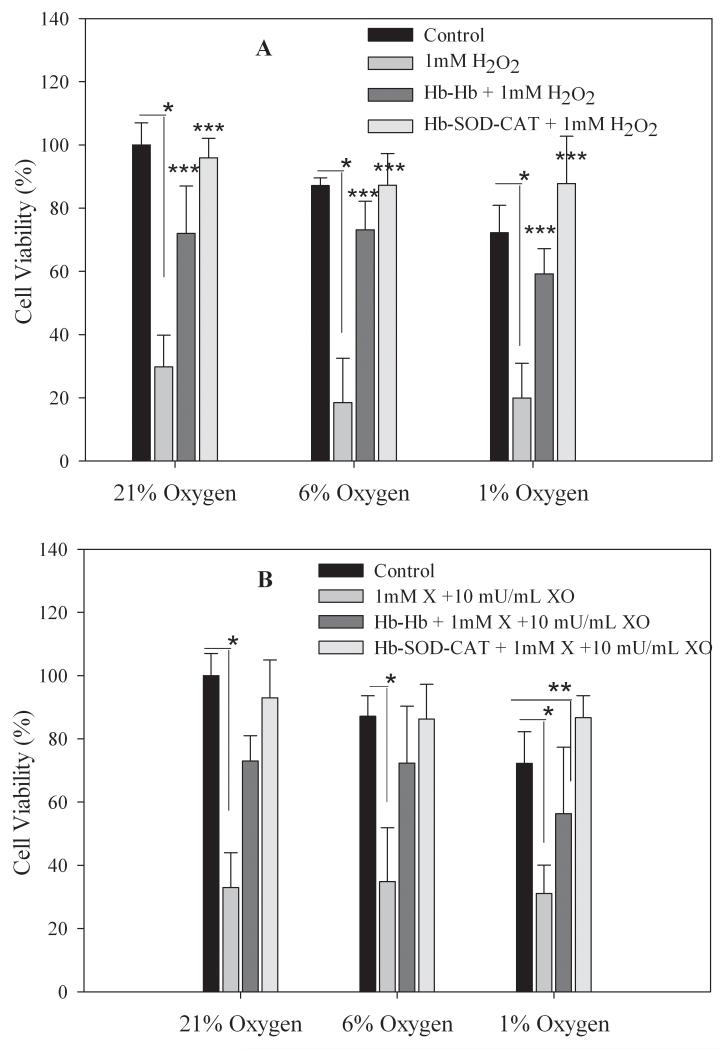
The viability of RINm5F cells decreased significantly from 100% (untreated cells incubated in 21% oxygen) to 30 ± 10% (21% oxygen), 18 ± 14% (6% oxygen) and 20 ± 11% (1% oxygen) when cells were challenged with 1 mM H2O2 and incubated for 24 hours at 37 °C. Comparatively, cells incubated with 0.1 mM Hb-Hb and treated with 1 mM H2O2 had significantly higher cell viabilities of 72 ± 15% (21% oxygen), 73 ± 9% (6% oxygen) and 59 ± 8% (1% oxygen) (Figure 3A). Cell viabilities improved even further for cells incubated with 0.1 mM Hb-SOD-CAT and 1 mM H2O2 with cell viabilities of 96 ± 6% (21% oxygen), 87 ± 10% (6% oxygen) and 88 ± 15% (1% oxygen) which were comparable to untreated control cells incubated in medium at 21 % oxygen and higher than cells incubated with Hb-Hb conjugates. Statistical analysis showed that there was no significant difference (p > 0.1) in cell viability between control cells incubated at 21 % oxygen and cells incubated with antioxidant enzymes (Hb-SOD-CAT) and challenged with 1 mM H2O2 at any of the oxygen partial pressures tested (21%, 6% or 1%), indicating that the presence of SOD and CAT in the conjugate bestowed significant protection and greatly improved cell viability. There were statistically significant differences in viability between untreated control cells in 21 % oxygen and cells treated with 1 mM H2O2 (p<0.001); and between untreated control cells and cells incubated with 0.1 mM Hb-Hb and treated with 1 mM H2O2 at every partial pressure of oxygen tested (21%, 6% or 1%) (p<0.05), confirming the inability of Hb-Hb to protect cells from the combination of free radical and hypoxic stress.
Figure 3.
When RINm5F cells were incubated with superoxide anion generated by 1 mM X and 10 mU/mL XO cell viability decreased considerably to 33 ± 11%, 35 ± 17% and 31 ± 9% for 21%, 6% and 1% oxygen respectively (Figure 3B). Cells incubated with 0.1 mM Hb-Hb and exposed to superoxide anion had substantially higher cell viabilities of 73 ± 8%, 72 ± 18% and 56 ± 21% at 21%, 6% and 1% oxygen respectively. When cells were incubated with 0.1 mM Hb-SOD-CAT and challenged with superoxide anion, RINm5F cells maintained viabilities of 93 ± 12%, 86 ± 11% and 87 ± 7% at 21%, 6% and 1% oxygen. Statistical analysis showed that there was no significant difference in viability between untreated control cells incubated at 21% oxygen and cells incubated with Hb-SOD-CAT and challenged by superoxide anion at any of the partial oxygen pressures tested (21%, 6% or 1%) (p > 0.1), suggesting that the antioxidant enzymes were instrumental in removing superoxide anion. Cell viability was significantly lower in cells treated with superoxide anion compared to untreated control cells (21% oxygen) (p < 0.001) at every partial pressure tested (21%, 6% or 1%). Cell viabilities were also quite low for cells incubated with Hb-Hb and treated with superoxide anion at 1% oxygen (p < 0.05), indicating the inability of Hb-Hb alone to protect cells when exposed to the combination of free radical and severe hypoxic stress (1% oxygen).
Insulin release by pancreatic islets
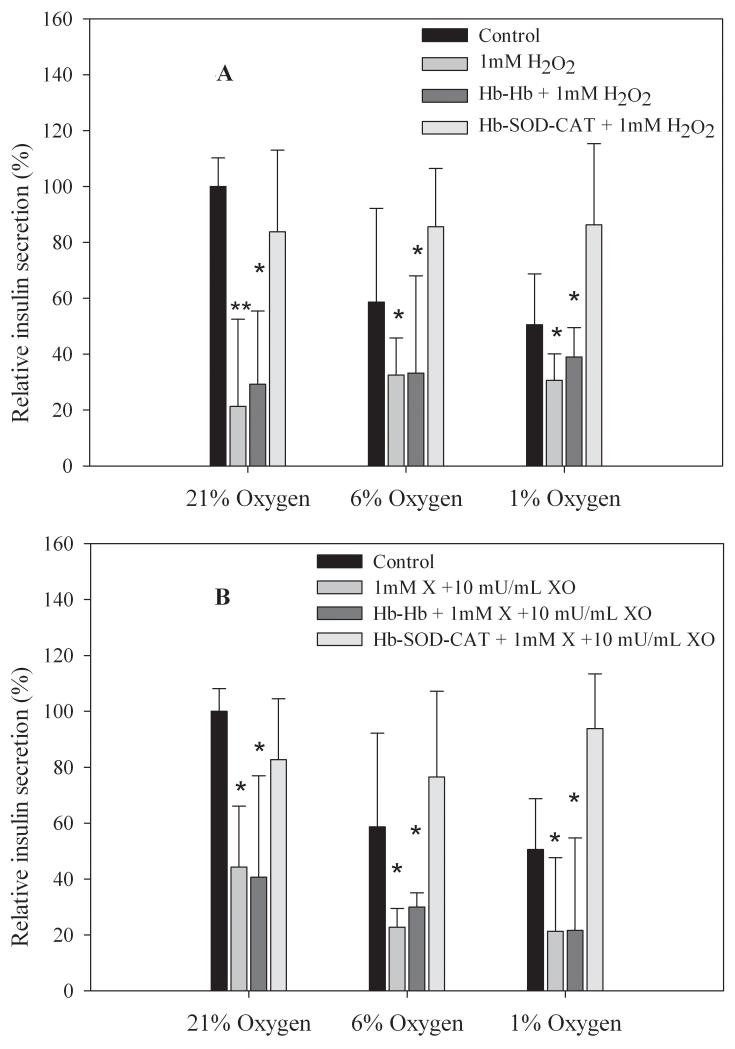
For comparison, insulin secretion was normalized to control untreated islets at 21% oxygen. Islets maintained at 21% oxygen and challenged with 1 mM H2O2, islets only (no conjugates) had greatly reduced insulin release of 21 ± 31%, islets incubated with Hb-Hb had slightly better insulin release of 29 ± 26%, while islets incubated with Hb-SOD-CAT released significantly higher levels of insulin (84 ± 29%) compared to untreated healthy control islets (Figure 4A). A similar trend was seen in comparing islets exposed to 1 mM H2O2 at 6% partial oxygen pressure, insulin release was only 32 ± 13% for islets alone (no conjugates), 33 ± 35% for islets incubated with Hb-Hb, but for islets incubated with Hb-SOD-CAT insulin secretion was well-maintained at 86 ± 21%. Islets exposed to 1 mM H2O2 at 1% partial oxygen pressure also showed insulin secretion levels similar to the other conditions; insulin secretion was 31 ± 10% for islets only (no conjugates), 39 ± 10% for islets incubated with Hb-Hb and 86 ± 29% for islets incubated with Hb-SOD-CAT. Compared to control islets (no conjugates, 21% oxygen), insulin secretion was significantly lower for islets incubated with 1 mM H2O2 (p < 0.005) and for islets incubated with Hb-Hb plus 1 mM H2O2 ( p < 0.01), suggesting a substantial loss of insulin secreting capability from islets regardless of the oxygen partial pressure tested (21%, 6% or 1%). The differences in insulin secretion for islets incubated with Hb-SOD-CAT and control was insignificant (p>0.4), demonstrating the direct correlation between the of antioxidant enzymes and the preservation of insulin secretion functionality.
Figure 4.
For islets challenged with superoxide anion (1 mM X and 10 mU/mL XO), islets showed greatly reduced insulin secretion at 44 ± 22% (21% oxygen), 23 ± 7% (6% oxygen), and 21 ± 26% (1% oxygen) when islets were exposed to superoxide anion only. Islets incubated with Hb-Hb and exposed to superoxide anion also had reduced insulin secretion of 41 ± 36% (21% oxygen), 30 ± 5% (6% oxygen), and 22 ± 33% (1% oxygen). However, islets incubated with Hb-SOD-CAT and exposed to superoxide anion retained insulin secretion ability with secretion levels of 83 ± 22% (21% oxygen), 77 ± 31% (6% oxygen), and 94 ± 20% (1% oxygen) (Figure 4B). Insulin secretion levels were not statistically different between control islets (no conjugates, 21% oxygen) and islets incubated with Hb-SOD-CAT and challenged with superoxide anion (p>0.2), demonstrating that islets were protected from free radical and hypoxic damage by the presence of the antioxidant enzymes at every partial oxygen pressure tested (21%, 6% and 1%). Insulin secretion levels dropped significantly in islets challenged with superoxide anion (p<0.01), and for islets incubated with Hb-Hb and challenged with superoxide anion (p<0.01) at every partial oxygen pressure tested (21%, 6% or 1%).
Confocal Microscopic Analysis of Pancreatic Islets
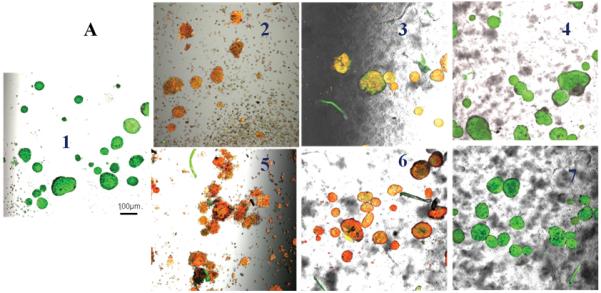
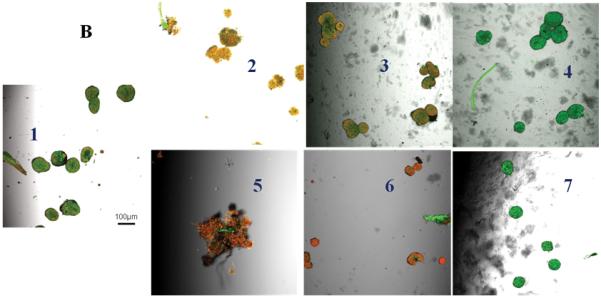
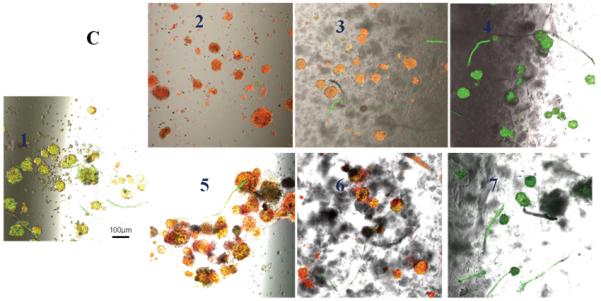
Untreated control pancreatic islets incubated at 21% oxygen had very high uptake of AO and no uptake of PI indicating 100% cell viability (Figure 5A). Islets incubated at 6% or 1% oxygen showed higher uptake of PI with little uptake of AO indicating the susceptibility of islets to hypoxia (Figure 5B & C). Islets only (no conjugates) and islets incubated with 0.1 mM Hb-Hb that were challenged with 1 mM H2O2 had high uptake of PI and minimal uptake of AO at every partial oxygen pressure tested (21%, 6% or 1% oxygen) indicating islet damage. Islets only (no conjugates) that were challenged with superoxide anion showed increased uptake of PI and minimal uptake of AO regardless of oxygen content, indicating islets were damaged by the superoxide anion. Islets incubated with 0.1 mM Hb-Hb and exposed to superoxide anion also showed uptake of PI regardless of oxygen content, and islets look qualitatively similar to superoxide challenged islets only (no conjugates). However, for islets that had been incubated with 0.1 mM Hb-SOD-CAT and challenged with 1 mM H2O2 or exposed to superoxide anion, PI uptake was minimal and AO uptake was high regardless of oxygen levels, indicating that the islets were well-protected from the combined stresses of peroxide or superoxide anion damage and hypoxia over 24 hours in 21%, 6% or 1% oxygen.
Figure 5.
Discussion
In this conjugate system the oxygen carrier hemoglobin is the most important component so it needs to be protected from free radical damage in order to extend its ability to release oxygen and protect the isolated islets from hypoxic stress. Studies from another ascorbate-glutathione system also strongly suggested the need for antioxidant system to inhibit the pro-oxidant effect of acellular hemoglobins(Simoni et al. 2009). Methemoglobin levels were higher in Hb-Hb conjugates without antioxidant enzymes SOD and CAT, indicating that hemoglobin was damaged considerably by H2O2 and superoxide anion. Although there was some methemoglobin formation in Hb-SOD-CAT conjugates following incubation with oxidants, our results show that conversion levels were significantly lower compared to Hb-Hb only. If desired, the amount of methemoglobin formation could be decreased further by increasing the amount of antioxidant enzymes present in the cross-linked conjugates. It was previously reported that hemoglobin systems formed higher levels of methemoglobin levels as the partial oxygen levels decreased(Takeoka et al. 1997). In contrast our results indicated that the damage caused by the level of oxygen (21%, 6% and 1%) was insignificant compared to amount of damage caused by H2O2 or superoxide anion levels over 24 hours at 37 °C. However, our results could be due to a combination of hypoxia and the oxidants so the individual contributions are unknown. Any detrimental effects caused specifically by partial oxygen pressures could become more evident if the incubation time is increased (greater than 24 hours) or by testing the conjugate in vivo. Overall our observations clearly demonstrate that the inclusion of antioxidant enzymes in the conjugates afforded significant protection of hemoglobin from both hypoxia and free radical stresses.
It has been reported that the typical H2O2 concentration in human plasma ranges between 4-5 μM and becomes increased under inflammatory conditions (Bragt and Bonta 1980; Nagababu and Rifkind 2000). One group showed that there was a substantial increase in H2O2 levels when diabetic pregnant mice were placed under hypoxic stress (Li et al. 2005), mimicking the hypoxic conditions that can occur during transplantation. In our experiments, hemoglobin conjugates were challenged with levels of H2O2 that were 200 times higher than the normal plasma range to test the robustness of the system; and the Hb-SOD-CAT conjugates demonstrated that the enzymes were able to preserve hemoglobin functionality in an extremely harsh radical-rich environment. We hypothesize that the oxidant conditions at the transplantation site in vivo may not be as harsh as the conditions used for our in vitro experiments, therefore our designed Hb-SOD-CAT conjugate will provide ample protection from environmental stress.
Intracellular ROS production was reduced when cells were incubated with Hb-SOD-CAT when compared to Hb-Hb, indicating that the Hb-SOD-CAT was efficient in protecting RINm5F cells from the harmful effect of the oxidants. Cell viability of RINm5F cells challenged with oxidants remained higher in cells treated with Hb-SOD-CAT compared to Hb-Hb, providing further evidence that crosslinked Hb-SOD-CAT offered superior protection from the combination of oxygen-induced stress (21%, 6% or 1% oxygen) and peroxide or superoxide anion free radical stress compared to the Hb-Hb conjugate. Observed higher cell viability with Hb-SOD-CAT is due to the protection of RINm5F cells from combined free radical and hypoxic stress. RINm5F cell viability was somewhat preserved even with Hb-Hb conjugates incubated with oxidants, probably because oxidants attacked hemoglobin first before attacking RINm5F cells thus sparing cells from initial hypoxic and free radical stress; as hemoglobin degraded then RINm5F cells were directly exposed to hypoxia and reactive species and increased cell death at latter stages. Based on these results, we expect for incubations extended beyond 24 hours, cells incubated with Hb-Hb conjugates without antioxidant enzymes would have 100% cell death. These results complement the intracellular free radical activity results from RINm5F cells incubated with conjugates and challenged with oxidants.
Functions such as insulin release are greatly affected in islets experiencing hypoxic and free radical-rich environments (de Groot et al. 2003; Dionne et al. 1993; Li et al. 2004). It has been shown that Ca+2 impairment mediated by the inhibition of glucose metabolism(Rebelato et al. 2010) or other alternative Ca+2 signal modulating mechanism in islets leads to impaired insulin secretion(Imoto et al. 2008). Transient normal mitochondrial ROS production is required for glucose induced insulin secretion in islets (Leloup et al. 2009) and for our experiments we did not evaluate the exact mechanism of insulin secretion in the presence of ROS. The high variability in our insulin data can partly be attributed to the differential insulin release from isolated islets(Kang and Bae 2009). Our results show that Hb-SOD-CAT protected or enhanced normalized insulin release from islets at all the partial oxygen pressures tested following challenges with free radicals in hypoxic conditions. Thus our results show the beneficial effect of conjugated antioxidant enzymes in Hb-SOD-CAT in retaining or enhancing the metabolic function of islets as monitored by insulin release.
It was shown mechanistically that hemoglobin was degraded by superoxide anion and hydrogen peroxide without cross-linked antioxidant enzymes (Nadithe and Bae 2010). Further, the oxygen supplying capability of hemoglobin to RINm5F cells was shown to be superior in low partial oxygen environment when antioxidant enzymes were cross-linked with Hb(Nadithe and Bae 2011). To some extent, islets may be protected in free radical environment with only SOD and CAT only but not when hypoxic condition is prevailing. And also Hb-Hb alone will not function for extended time period as free radical assault will damage hemoglobin. Islets incubated with Hb-SOD-CAT conjugates and exposed to H2O2 and superoxide anion showed minimal uptake of PI which could be due to direct protection of hemoglobin and islets from free radical damage by antioxidant enzymes followed by higher oxygen supply to islets by virtue of hemoglobins low p50 design (Nadithe and Bae 2011). These results strongly suggest that Hb-SOD-CAT may be an appropriate strategy to help prevent hypoxia-induced graft failure in conditions such as encapsulated islet transplantation(de Groot et al. 2003; de Groot et al. 2004; Ricci et al. 2005) and ischemia re-perfusion injury(Chang 2010). These Hb-SOD-CAT conjugates will be co-encapsulated with islets using poly-L-lysine and alginate polymeric materials as microcapsules and may be functional in future islet transplantation studies. We expect the hemoglobin in conjugates and islets be protected from free radical stress and thus hemoglobin continuously supplies oxygen to islets in hypoxic environment. This strategy has strong potential to prevent in vivo islet death at transplantation site and help insulin secretion from islets in treating type 1 diabetics.
Conclusion
In this work we demonstrated the effectiveness of a hemoglobin-antioxidant conjugate system (Hb-SOD-CAT) to improve the overall function of pancreatic islets when exposed to conditions similar to in vivo transplantation. Cells incubated with Hb-Hb only that was placed in hypoxic and free radical-rich environments without protection from antioxidant enzymes showed increased cell death from the combined stressors. The oxygen-supplying function of hemoglobin in hypoxic environments is more effective when antioxidant enzymes are present to protect or inhibit hemoglobin from damage. Our results clearly demonstrate that the beneficial effect of adding SOD and CAT confers protection of hemoglobin and islets against hypoxic and oxidative stresses and inhibited methemoglobin formation to extend the oxygen supplying capability of hemoglobin.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Han Chan Kang (University of Utah, Department of Pharmaceutics and Pharmaceutical Chemistry, Salt Lake City, UT) for his assistance in editorial review. Authors are also thankful to Dr. Christopher Rodesch and Keith R. Carney in the Fluorescence Microscopy Core Facility at the University of Utah. This study was supported by NIH grant DK56884.
References
- Bae YH. Caged pancreatic islet for IDDM. Yonsei Med J. 2004;45(Suppl):56–60. doi: 10.3349/ymj.2004.45.Suppl.56. [DOI] [PubMed] [Google Scholar]
- Beck J, Angus R, Madsen B, Britt D, Vernon B, Nguyen KT. Islet encapsulation: strategies to enhance islet cell functions. Tissue Eng. 2007;13(3):589–99. doi: 10.1089/ten.2006.0183. [DOI] [PubMed] [Google Scholar]
- Bragt PC, Bonta IL. Oxidant stress during inflammation: anti-inflammatory effects of antioxidants. Agents Actions. 1980;10(6):536–9. doi: 10.1007/BF02024159. [DOI] [PubMed] [Google Scholar]
- Carlsson PO, Liss P, Andersson A, Jansson L. Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes. 1998;47(7):1027–32. doi: 10.2337/diabetes.47.7.1027. [DOI] [PubMed] [Google Scholar]
- Carlsson PO, Palm F, Andersson A, Liss P. Chronically decreased oxygen tension in rat pancreatic islets transplanted under the kidney capsule. Transplantation. 2000;69(5):761–6. doi: 10.1097/00007890-200003150-00015. [DOI] [PubMed] [Google Scholar]
- Chae SY, Kim SW, Bae YH. Effect of cross-linked hemoglobin on functionality and viability of microencapsulated pancreatic islets. Tissue Eng. 2002;8(3):379–94. doi: 10.1089/107632702760184655. [DOI] [PubMed] [Google Scholar]
- Chae SY, Kim YY, Kim SW, Bae YH. Prolonged glucose normalization of streptozotocin-induced diabetic mice by transplantation of rat islets coencapsulated with crosslinked hemoglobin. Transplantation. 2004;78(3):392–7. doi: 10.1097/01.tp.0000128617.14309.26. [DOI] [PubMed] [Google Scholar]
- Chang TM. Blood replacement with nanobiotechnologically engineered hemoglobin and hemoglobin nanocapsules. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(4):418–30. doi: 10.1002/wnan.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M, Schuurs TA, Keizer PP, Fekken S, Leuvenink HG, van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12(8):867–75. [PubMed] [Google Scholar]
- de Groot M, Schuurs TA, van Schilfgaarde R. Causes of limited survival of microencapsulated pancreatic islet grafts. J Surg Res. 2004;121(1):141–50. doi: 10.1016/j.jss.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- Giraldo JA, Weaver JD, Stabler CL. Tissue engineering approaches to enhancing clinical islet transplantation through tissue engineering strategies. J Diabetes Sci Technol. 2010;4(5):1238–47. doi: 10.1177/193229681000400525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayon T, Dvilansky A, Shpilberg O, Nathan I. Appraisal of the MTT-based assay as a useful tool for predicting drug chemosensitivity in leukemia. Leuk Lymphoma. 2003;44(11):1957–62. doi: 10.1080/1042819031000116607. [DOI] [PubMed] [Google Scholar]
- Imoto H, Sasaki N, Iwase M, Nakamura U, Oku M, Sonoki K, Uchizono Y, Iida M. Impaired insulin secretion by diphenyleneiodium associated with perturbation of cytosolic Ca2+ dynamics in pancreatic beta-cells. Endocrinology. 2008;149(11):5391–400. doi: 10.1210/en.2008-0186. [DOI] [PubMed] [Google Scholar]
- Kang HC, Bae YH. Polymeric gene transfection on insulin-secreting cells: sulfonylurea receptor-mediation and transfection medium effect. Pharm Res. 2006;23(8):1797–808. doi: 10.1007/s11095-006-9027-0. [DOI] [PubMed] [Google Scholar]
- Kang HC, Bae YH. Transfection of insulin-secreting cell line and rat islets by functional polymeric gene vector. Biomaterials. 2009;30(14):2837–45. doi: 10.1016/j.biomaterials.2009.01.035. [DOI] [PubMed] [Google Scholar]
- Kim YY, Chae SY, Kim S, Byun Y, Bae YH. Improved phenotype of rat islets in a macrocapsule by co-encapsulation with cross-linked Hb. J Biomater Sci Polym Ed. 2005;16(12):1521–35. doi: 10.1163/156856205774576682. [DOI] [PubMed] [Google Scholar]
- Ko SH, Ryu GR, Kim S, Ahn YB, Yoon KH, Kaneto H, Ha H, Kim YS, Song KH. Inducible nitric oxide synthase-nitric oxide plays an important role in acute and severe hypoxic injury to pancreatic beta cells. Transplantation. 2008;85(3):323–30. doi: 10.1097/TP.0b013e31816168f9. [DOI] [PubMed] [Google Scholar]
- Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, Colombani AL, Ktorza A, Casteilla L, Penicaud L. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58(3):673–81. doi: 10.2337/db07-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20(3):463–6. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- Lepore DA, Shinkel TA, Fisicaro N, Mysore TB, Johnson LE, d’Apice AJ, Cowan PJ. Enhanced expression of glutathione peroxidase protects islet beta cells from hypoxia-reoxygenation. Xenotransplantation. 2004;11(1):53–9. doi: 10.1111/j.1399-3089.2004.00082.x. [DOI] [PubMed] [Google Scholar]
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282(2):C227–41. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- Li R, Chase M, Jung SK, Smith PJ, Loeken MR. Hypoxic stress in diabetic pregnancy contributes to impaired embryo gene expression and defective development by inducing oxidative stress. Am J Physiol Endocrinol Metab. 2005;289(4):E591–9. doi: 10.1152/ajpendo.00441.2004. [DOI] [PubMed] [Google Scholar]
- Li X, Chen H, Epstein PN. Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. J Biol Chem. 2004;279(1):765–71. doi: 10.1074/jbc.M307907200. [DOI] [PubMed] [Google Scholar]
- Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, Brandhorst D. Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol. 2006;144(2):179–87. doi: 10.1111/j.1365-2249.2006.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche RM. Transplantation for the treatment of type 1 diabetes. World J Gastroenterol. 2007;13(47):6347–55. doi: 10.3748/wjg.v13.i47.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni Salehi Monfared SS, Larijani B, Abdollahi M. Islet transplantation and antioxidant management: a comprehensive review. World J Gastroenterol. 2009;15(10):1153–61. doi: 10.3748/wjg.15.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadithe V, Bae YH. Synthesis and characterization of hemoglobin conjugates with antioxidant enzymes via poly(ethylene glycol) cross-linker (Hb-SOD-CAT) for protection from free radical stress. Int J Biol Macromol. 2010;47(5):603–13. doi: 10.1016/j.ijbiomac.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadithe V, Bae YH. Hemoglobin Conjugates with Antioxidant Enzymes (Hemoglobin-Superoxide Dismutase-Catalase) via Poly(Ethylene Glycol) Crosslinker for Protection of Pancreatic Beta RINm5F Cells in Hypoxia. Tissue Eng Part A. 2011;17(19-20):2453–62. doi: 10.1089/ten.tea.2010.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagababu E, Rifkind JM. Reaction of hydrogen peroxide with ferrylhemoglobin: superoxide production and heme degradation. Biochemistry. 2000;39(40):12503–11. doi: 10.1021/bi992170y. [DOI] [PubMed] [Google Scholar]
- Patton JN, Palmer AF. Photopolymerization of bovine hemoglobin entrapped nanoscale hydrogel particles within liposomal reactors for use as an artificial blood substitute. Biomacromolecules. 2005;6(1):414–24. doi: 10.1021/bm049432i. [DOI] [PubMed] [Google Scholar]
- Rao P, Maeda H, Yutong X, Yamamoto M, Hirose N, Sasaguri S. Protective effect of a radical scavenger, MCI-186 on islet cell damages induced by oxidative stress. Transplant Proc. 2005;37(8):3457–8. doi: 10.1016/j.transproceed.2005.09.072. [DOI] [PubMed] [Google Scholar]
- Rebelato E, Abdulkader F, Curi R, Carpinelli AR. Low doses of hydrogen peroxide impair glucose-stimulated insulin secretion via inhibition of glucose metabolism and intracellular calcium oscillations. Metabolism. 2010;59(3):409–13. doi: 10.1016/j.metabol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Ricci M, Blasi P, Giovagnoli S, Rossi C, Macchiarulo G, Luca G, Basta G, Calafiore R. Ketoprofen controlled release from composite microcapsules for cell encapsulation: effect on post-transplant acute inflammation. J Control Release. 2005;107(3):395–407. doi: 10.1016/j.jconrel.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Rodrigues JV, Gomes CM. Enhanced superoxide and hydrogen peroxide detection in biological assays. Free Radic Biol Med. 2010;49(1):61–6. doi: 10.1016/j.freeradbiomed.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Simoni J, Villanueva-Meyer J, Simoni G, Moeller JF, Wesson DE. Control of oxidative reactions of hemoglobin in the design of blood substitutes: role of the ascorbate-glutathione antioxidant system. Artif Organs. 2009;33(2):115–26. doi: 10.1111/j.1525-1594.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- Takeoka S, Sakai H, Kose T, Mano Y, Seino Y, Nishide H, Tsuchida E. Methemoglobin formation in hemoglobin vesicles and reduction by encapsulated thiols. Bioconjug Chem. 1997;8(4):539–44. doi: 10.1021/bc970091y. [DOI] [PubMed] [Google Scholar]
- Tulsawani R, Kelly LS, Fatma N, Chhunchha B, Kubo E, Kumar A, Singh DP. Neuroprotective effect of peroxiredoxin 6 against hypoxia-induced retinal ganglion cell damage. BMC Neurosci. 2010;11:125. doi: 10.1186/1471-2202-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallelian F, Pimenova T, Pereira CP, Abraham B, Mikolajczyk MG, Schoedon G, Zenobi R, Alayash AI, Buehler PW, Schaer DJ. The reaction of hydrogen peroxide with hemoglobin induces extensive alpha-globin crosslinking and impairs the interaction of hemoglobin with endogenous scavenger pathways. Free Radic Biol Med. 2008;45(8):1150–8. doi: 10.1016/j.freeradbiomed.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Veriter S, Aouassar N, Adnet PY, Paridaens MS, Stuckman C, Jordan B, Karroum O, Gallez B, Gianello P, Dufrane D. The impact of hyperglycemia and the presence of encapsulated islets on oxygenation within a bioartificial pancreas in the presence of mesenchymal stem cells in a diabetic Wistar rat model. Biomaterials. 2011 doi: 10.1016/j.biomaterials.2011.02.061. [DOI] [PubMed] [Google Scholar]
- von Sonntag C. Advanced oxidation processes: mechanistic aspects. Water Sci Technol. 2008;58(5):1015–21. doi: 10.2166/wst.2008.467. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC. Oxidative reactions of hemoglobin. Methods Enzymol. 1990;186:265–72. doi: 10.1016/0076-6879(90)86118-f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.