Abstract
Myeloid cells play a key role in the outcome of anti-tumor immunity and response to anti-cancer therapy, since in the tumor microenvironment they may exert both stimulatory and inhibitory pressures on the proliferative, angiogenic, metastatic, and immunomodulating potential of tumor cells. Therefore, understanding the mechanisms of myeloid regulatory cell differentiation is critical for developing strategies for the therapeutic reversal of myeloid derived suppressor cell (MDSC) accumulation in the tumor-bearing hosts. Here, using an in vitro model system, several potential mechanisms of the direct effect of paclitaxel on MDSC were tested, which might be responsible for the anti-tumor potential of low-dose paclitaxel therapy in mice. It was hypothesized that a decreased level of MDSC in vivo after paclitaxel administration might be due to (i) the blockage of MDSC generation, (ii) an induction of MDSC apoptosis, or (iii) the stimulation of MDSC differentiation. The results revealed that paclitaxel in ultra-low concentrations neither increased MDSC apoptosis nor blocked MDSC generation, but stimulated MDSC differentiation towards dendritic cells. This effect of paclitaxel was TLR4-independent since it was not diminished in cell cultures originated from TLR4−/− mice. These results support a new concept that certain chemotherapeutic agents in ultra-low non-cytotoxic doses may suppress tumor progression by targeting several cell populations in the tumor microenvironment, including MDSC.
Keywords: Myeloid-derived suppressor cells, paclitaxel, dendritic cells, chemomodulation
Introduction
Myeloid regulatory and antigen-presenting cells play a principal role in the induction, maintenance, and outcome of the anti-tumor immune response and in the response to anti-cancer therapy, since in the tumor microenvironment they can collectively exert both stimulatory and inhibitory pressures on the proliferative, angiogenic, and immunomodulating potential of tumor cells, as well as tumor ability to spread and metastasize. Depending on their nature, activity, and location, dendritic cells (DC), macrophages, granulocytes, and immature myeloid cells can promote the differentiation, expansion, and polarization of CD4+ T-helper (TH)-1, TH2, TH17, and regulatory (Treg) T-cells or modulate the proliferation and activity of CD8+ cytotoxic T-lymphocytes (CTL).
Myeloid regulatory cells may also directly affect tumor cell pro-liferation, spreading, and interaction with other critical intra-tumoral stromal elements, such as fibroblasts and the endothelial cellular network. For instance, immunogenic (M1), alternatively activated pro-tumorigenic (M2) macrophages, Tie2-expressing monocytes and recently identified regulatory macrophages have been reported to control both tumor progression and the development of the anti-tumor immunity (Nagaraj et al., 2009; Jaiswal et al., 2010; Mantovani and Sica, 2010). Alternatively-activated tumor-associated macrophages have been implicated in carcinogenesis through macrophage/monocyte colony stimulating factor (M-CSF), tumor necrosis factor (TNF)-α, interleukin (IL)-10, and transforming growth factor (TGF)-β in the angiogenic switch through vascular endothelial growth factor (VEGF) as well as in the local invasion and metastasis through cathepsins B and S. They also contribute trophic functions to the emergence of nascent tumor clones, phagocytose apoptotic tumor cells, recruit other hematopoietic cells, influence the tissue response to hypoxia and, together with Treg, suppress TH1 cell and CTL anti-tumor responses (Gordon and Martinez, 2010). DC, a highly plastic group of mostly myeloid inherently efficient antigen-presenting regulators, incorporate resident DC, migratory DC, and inflammatory DC capable of eliciting lymphocyte responses including activation of CD4+ TH1 and TH2 cells (Watowich and Liu, 2010). In contrast to these conventional DC, plasmacytoid DC, immature conventional DC, and regulatory DC may exhibit potent immunosuppressive and/or tolerogenic properties in the tumor milieu and block proliferation of antigen-specific and non-specific CD4+ and CD8+ T-cells, support polarization and activation of Treg lymphocytes and stimulate intra-tumoral neovascularization (Shurin et al., 2006, 2011, 2012; Murdoch et al., 2008; Tisch, 2010). TGFβ within the tumor microenvironment may induce a population of tumor-associated neutrophils (TAN) with a pro-tumorigenic phenotype (N1 cells), while a TGFβ blockade resulted in the recruitment and activation of TAN with an anti-tumor phenotype (N2) (Fridlender et al., 2009). Thus, diversity is a hallmark of tumor-associated myeloid-derived cells endowed with suppressive activity in the blood, in lymphoid tissues, and in tumors (Mantovani, 2010).
MDSC, the most known group of myeloid regulatory cells, are the heterogeneous population of myeloid progenitor and immature myeloid cells that share a common property of suppressing immune responses. MDSC use a variety of mechanisms to suppress tumor immunity (Condamine and Gabrilovich, 2010). Monocytic and granulocytic MDSC both utilize a mechanism in which arginase catabolizes L-arginine, depleting it in the local environment. T-Cells deprived of L-arginine are deficient for CD3ζ chain and are arrested in the G0–G1 phase of the cell cycle (Rodriguez et al., 2007; Ostrand-Rosenberg, 2010). MDSC also prevent T-cell activation by sequestering cystine and limiting the availability of cysteine (Ostrand-Rosenberg, 2010; Srivastava et al., 2010). Granulocytic MDSC express high levels of reactive oxygen species which induce apoptosis of T-cells and also nitrate T-cell receptors (Gabrilovich and Nagaraj, 2009). Monocytic MDSC produce NO and block T-cell proliferation. MDSC also perturb T-cell activation by secreting IL-10, prostaglandins, and other immunosuppressive mediators and by inducing Foxp3+-regulatory T-cells (Treg) (Eruslanov et al., 2010; Fujimura et al., 2010).
Understanding the diversity of tumor-associated myeloid lineages and the mechanisms of myeloid regulatory cell differentiation and inter-differentiation is crucial for developing therapeutic strategies controlling their differentiation, polarization, and accumulation in the tumor microenvironment. For instance, MDSC differentiate into mature granulocytes, macrophages, or DC under normal conditions, but under chronic inflammatory conditions, including cancer, differentiation of MDSC into mature myeloid cells is blocked and the population is expanded (Almand et al., 2001; Mirza et al., 2006; Ochoa et al., 2007; Narita et al., 2009). Elimination of MDSC dramatically improved immune responses in tumor-bearing mice and in cancer patients; in some models, it resulted in a direct anti-tumor effect (Youn and Gabrilovich, 2010). Therefore, several approaches have been offered to inhibit MDSC maturation (e.g., STAT3 inhibitors, RTK inhibitor Sunitinib), deplete MDSC (e.g., gemcitabine, 5-FU, Alemtuzumab), block MDSC functions (e.g., nitroaspirines, PDE5 and COX-2 inhibitors), or force MDSC differentiation (e.g., Vitamins A and D3) (Suzuki et al., 2005; Gabrilovich and Nagaraj, 2009; Ko et al., 2009, 2010; Pulaski et al., 2009; Ugel et al., 2009; Condamine and Gabrilovich, 2010). Interestingly, certain chemotherapeutic agents from different classes have been reported to deplete MDSC and inhibit MDSC formation (Suzuki et al., 2005; Le et al., 2009; Kodumudi et al., 2010) by inducing cell death in MDSC or their precursors. However, nothing is known about the effects of non-cytotoxic low-dose chemotherapeutic drugs on MDSC formation or differentiation (Naiditch et al., 2011).
A growing interest in the immunostimulatory properties of several chemotherapeutic agents used in ultra-low non-cytotoxic doses has been supported by recent reports showing that paclitaxel, doxorubicin, methotrexate, and other agents can up-regulate maturation and function of murine and human DC (Kaneno et al., 2009; Shurin et al., 2009), regulate signal transduction pathways in DC (Shurin et al., 2008), increase tumor recognition by DC and CTL (Kaneno et al., 2011), and suppress formation of regulatory DC both in vitro and in vivo (Shurin et al., in press). We have recently demonstrated that paclitaxel in an ultra-low non-cytotoxic dose in vivo significantly delays the development of spontaneous melanoma in mice, which is associated with decreased tumor infiltration by MDSC (Sevko et al., in press). However, the mechanism of this effect remains unclear.
Here, using an in vitro model system, we tested several potential mechanisms of a direct effect of paclitaxel on MDSC, which might be involved in paclitaxel-induced down-regulation of MDSC and its anti-tumor potential in mice. We hypothesized that decreased level of MDSC in vivo after paclitaxel administration might be due to either the (i) blockage of MDSC generation, (ii) induction of MDSC apoptosis, or (iii) stimulation of MDSC differentiation. Our results revealed that paclitaxel in ultra-low concentrations neither increased MDSC apoptosis nor blocked MDSC generation but stimulated MDSC differentiation towards DC.
Materials and methods
Animals
Male C57BL/6 mice (6–8-week-old, Taconic Farms, Germantown, NY) were housed in a pathogen-free AAALAC-accredited facility under controlled temperature (22°C), relative humidity (55.5%), and 12-h light/dark cycles with a commercial rodent diet and water available ad libitum. C57BL/10ScNJ mice with a deletion of the Tlr4 gene (Jackson Lab., Bar Harbor, Maine) were used for the generation of TLR4−/− cells. All animal procedures were conducted under an animal protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC).
MDSC cultures
Myeloid derived suppressor cells (MDSC) could be generated from the bone marrow precursors using granulocyte-macrophage/monocyte colony stimulating factor (GM-CSF), GM-CSF + IL-6 or different variations of growth factors and cytokines (e.g., c-kit ligand, IL-3, IL-6, thrombopoietin, VEGF, and FLT3L) (Rössner et al., 2005; Marigo et al., 2010; Zhou et al., 2010). The appearance of cells with similar properties and phenotype can be obtained from GM-CSF-driven, bone marrow-derived cultures by the addition of tumor-conditioned medium (Kusmartsev and Gabrilovich, 2006). In addition, bone marrow MDSC could be maintained in cultures supplemented with GM-CSF (Dolcetti et al., 2010).
Therefore, to collect, maintain, and generate MDSC cultures, bone marrow cells were isolated from murine femur and tibia, depleted of erythrocytes, CD4+, CD8+, and B220+ cells, and cultured overnight in RPMI 1640 medium supplemented with 10% fetal bovine serum, 50 μg gentamicin/ml, 1 mM sodium pyruvate, 2 mM L-glutamine, and 0.1 mM non-essential amino acids (all from Invitrogen, Grant Island, NY) at 37°C in 5% CO2. The non-adherent cells were collected and seeded in complete medium containing 1000 U GM-CSF/ml alone or in combination with 5 ng IL-6/ml (both agents from PeproTech, Rocky Hill, NJ) for 5–7 days. Paclitaxel (Mayne Pharma, Salisbury South, Australia) was added on Day 1 or Day 4, and the MDSC were then harvested and analyzed on Days 5, 6, and 7 of the culture regimen.
Flow cytometry
To assess MDSC phenotype, cultured cells were washed in FACS buffer (Hank’s balanced salt solution [HBSS] containing 0.1% [w/v] bovine serum albumin and 0.1% [w/v] NaN3) and stained for MDSC markers (CD11b, Gr-1, and Ly6C). Furthermore, the differentiation of MDSC into DC was analyzed by staining for DC-related markers (CD11b, CD11c, CD80, CD86, I-Ab, and CD40). All antibodies used for these staining protocols were purchased in fluorochrome-conjugated forms (as rat antimouse monoclonal antibodies) from BD Biosciences (San Diego, CA). Cells were analyzed on a FACScan flow cytometer using the Cell Quest 1.0 software (all from BD Biosciences). A minimum of 10,000 events/sample was acquired. The results were expressed as the percentage of positive cells.
Apoptosis measurement
To analyze the level of apoptosis induced by paclitaxel, MDSC were treated with 1, 50, or 200 nM paclitaxel for 24 h and then double-stained with FITC-conjugated Annexin V (BD Biosciences) and 10 μg propidium iodide/ml (Sigma, Saint Louis, MO), as described in Balkir et al. (2004). The levels of cell apoptosis were determined by FACScan with Cell Quest 1.0 software. Early and late apoptotic cells were defined as AnnexinV+PI− or AnnexinV+PI+, respectively. A minimum of 10,000 events/sample was acquired.
Mixed leukocyte reaction (MLR)
To determine functional activity of MDSC and DC, cells were separated using anti-CD11c-coated micromagnetic beads (Miltenyi Biotec., Bergisch Gladbach, Germany) and co-incubated for 48 h with syngeneic splenocytes (106 cell/ml) pre-activated with Con A (2.5 μg/ml, 5 h, Sigma) at 1:30 E:T ratio. [3H]-thymidine (1 μCi/200 μl, NEN, PerkinElmer Inc., Waltham, MA) was added for 16 h prior to cell harvesting on GF/C glass fiber filters (Whatman Intl. Ltd., Maidstone, UK) using a MACH III microwell harvester (Tomtec, Hamden, CT). Incorporation of 3H-thymidine was determined on a MicroBeta TRILUX liquid scintillation counter (Wallac, Gaithersburg, MD) and expressed as count per minute (cpm).
Statistical analysis
Statistical analysis of experimental data was performed with software package GraphPad Prism (GraphPad Software, La Jolla, CA). For all analyses, the level of significance was set at a probability of p < 0.05. ANOVA was used for comparison of multiple groups. For single comparisons of two groups, a Student’s t-test was used after evaluation of normality. All experiments were repeated at least 3–4 times. Data are presented as the means ± SEM.
Results
Generation of MDSC
Our recent studies revealed that the treatment of ret transgenic mice developing spontaneous melanoma with low dose paclitaxel resulted in a significant decrease in MDSC numbers in the tumor microenvironment and in the inhibition of tumor development (Sevko et al., in press). Here we investigated a direct effect of paclitaxel in low non-cytotoxic concentrations on MDSC in vitro. By taking advantage of already knowing what are low non-cytotoxic concentrations of paclitaxel, i.e., those that do not exert any cytotoxic or cytostatic effect on tumor cell lines, bone marrow hematopoietic cells, or DC (Shurin et al., 2008, 2009), we have verified whether paclitaxel is able to (i) block MDSC generation, (ii) induce apoptosis in MDSC, or (iii) accelerate differentiation of MDSC.
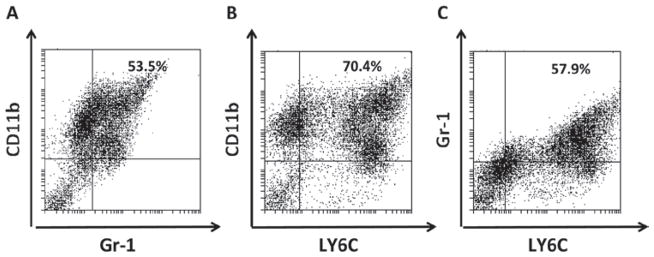
Enriched populations of MDSC and hematopoietic progenitor cells were isolated from bone marrow. To maintain MDSC in cultures and induce differentiation of myeloid precursors into MDSC, cells were cultured with a high-concentration of GM-CSF alone as described by Rössner et al. (2005), GM-CSF combined with IL-6 as described in Rössner et al. (2005) and Zhou et al. (2010), or ret melanoma cell line-conditioned medium. Flow cytometry analysis of cells expressing Gr-1 and CD11b and sub-type marker Ly6C was performed on Day 5 to confirm the phenotypic appearance of MDSC in cultures. No marked differences in the percentage of Gr-1+CD11b+ MDSC in cultures supplemented with GM-CSF alone vs [GM-CSF + IL-6] vs [GM-CSF + tumor-conditioned medium] were detected. Gr-1+CD11b+ MDSC represented typically 45–60% of cells (Figure 1a). Both monocytic LY6Chigh and granulocytic LY6Clow sub-populations of MDSC were observed in these culture conditions (Figures 1b and 1c). Therefore, experiments focusing on the effect of paclitaxel on MDSC were carried out with MDSC prepared with the bone marrow-derived cells cultured with GM-CSF alone.
Figure 1.
Phenotype of ex vivo generated MDSC. MDSC were cultured for 5 days and stained for (a) CD11b, Gr-1, and (b and c) a sub-type marker Ly6C, as described in the Materials and methods. Data from one representative staining are shown. Similar results were obtained in five independent experiments.
Effect of paclitaxel on the generation of MDSC
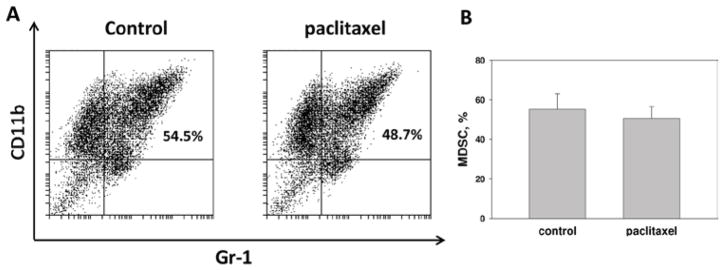
We first tested whether paclitaxel in low non-cytotoxic concentrations might suppress the generation of MDSC. The bone marrow-derived MDSC cultures (prepared as described above) were treated with medium (control) or paclitaxel (0.2 and 1 nM) on Day 1 and characterized by flow cytometry on Day 5 (Figure 2). The results of several independent experiments revealed that the proportion of MDSC in cultures was not significantly altered by the addition of paclitaxel (Figure 2a). However, one can notice a tendency to a slight decrease in the percentage of MDSC in 1 nM paclitaxel-treated cultures, i.e., 55.2 (± 7.7) vs 50.9 (± 5.6) (p > 0.05) (Figure 2b). Thus, paclitaxel in low concentrations does not directly affect the generation of MDSC in the bone marrow-derived cell cultures.
Figure 2.
Paclitaxel does not block the generation of MDSC ex vivo. MDSC cultures were treated with 1 nM paclitaxel on Day 1 and compared with untreated cells on Day 5. (a) Data from one representative CD11b/Gr-1 staining are shown. (b) The results of three independent experiments are shown as the means (± SEM).
Paclitaxel fails to induce apoptosis in MDSC
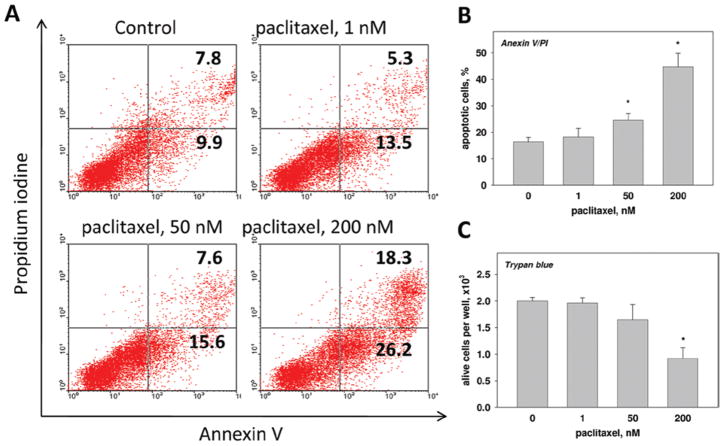
Then we tested whether paclitaxel in low concentrations might induce cell death in MDSC cultures. MDSC were treated with 0, 1, 50, or 200 nM paclitaxel for 4 days followed by the Annexin/PI staining (Figure 3). Addition of 1 nM paclitaxel did not significantly alter the percentage of early apoptotic (Annexin V+/PI−) cells (13.1 [± 2.1]% vs 10.5 [± 2.2]% in the control; p > 0.1), although 50 nM paclitaxel slightly increased the percentage of dying cells (16.7 [± 2.6]%; p > 0.05) and displayed a significant pro-apoptotic effect in 200 nM (28.3 [± 3.4]; p < 0.05). Late apoptotic (Annexin V+/PI+) cells represented 5–8% of the populations when cultures were treated with 0, 1, or 50 nM paclitaxel; treatment with 200 nM paclitaxel increased that group to ~ 20% (19.8 [± 3.1]% vs 7.4 [± 1.3]% in the control (p < 0.05; Figure 3a). Figure 3b summarizes the results of three experiments showing that paclitaxel in low (1 nM) concentration did not alter the amount of total (early and late) apoptotic cells compared to untreated cells (18.2 [± 3.3]% vs 16.4 [± 1.6]%; p > 0.1), whereas at higher concentrations (50 and 200 nM) it significantly induced apoptosis in MDSC (24.6 [± 2.4]% and 47.7 [± 5.1]%, respectively; p < 0.05). Trypan blue staining confirmed these findings, revealing that paclitaxel at 200 nM killed > 50% of the cells in the MDSC cultures (Figure 3c). Therefore, these results suggest that low concentrations of paclitaxel do not directly induce apoptosis in MDSC in vitro.
Figure 3.
Paclitaxel fails to induce apoptosis in MDSC. MDSC were treated with different concentrations of paclitaxel or medium alone (control) for 24 h and Annexin V/PI staining was used to measure the percentage of apoptotic cells (a, b), whereas trypan blue staining was used to measure live cell numbers (c), as described in the Materials and methods. Data from one representative Annexin V/PI staining are shown (a). The results of three independent experiments are shown (b, c) as the means ± SEM. * p < 0.05 vs control. (See colour version of this figure online at www.informahealthcare.com/imt)
Paclitaxel stimulates differentiation of MDSC
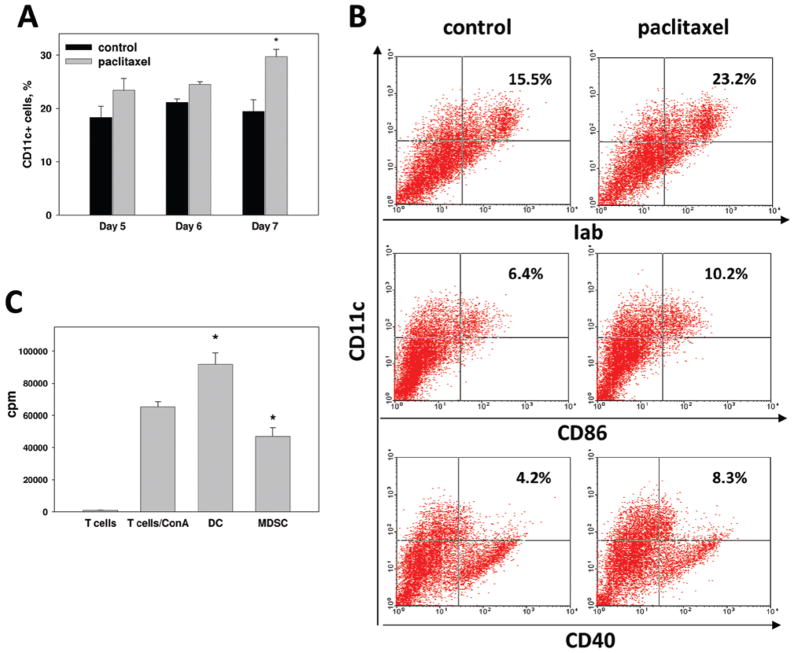
Our next hypothesis, which could explain the down-regulation of MDSC accumulation in tumor-bearing mice, was that paclitaxel may stimulate final differentiation of MDSC into DC. To this end, we treated cells with 0.2 and 1 nM paclitaxel on Day 4 and performed flow cytometry staining for DC-associated marker CD11c on Days 5, 6, and 7 (Figure 4a). On Days 5 and 6, i.e., 24 and 48 h after addition of 1 nM paclitaxel to MDSC cultures, the number of CD11c+ cells was slightly increased to 125% of the corresponding control values. However, 72 h after the treatment, the levels of CD11c+ DC increased significantly from 19.4 (± 2.2)% to 29.7 (± 1.4)% (p < 0.01). Similar, although less dramatic results were obtained with the addition of 0.2 nM paclitaxel to MDSC cultures (data not shown). To confirm the ability of 1 nM paclitaxel to stimulate the final differentiation of MDSC towards DC in vitro, we characterized control and treated cultures using additional DC-related phenotypic markers, including CD86, MHC Class II, and CD40 (Figure 4b). The statistical analysis of the results from five experiments revealed a significantly increased numbers of semi-mature MHC-II+ and co-stimulatory molecule-positive DC in MDSC cultures 72 h after addition of paclitaxel (p < 0.05). Figure 4b shows the representative results, suggesting that the treatment increased the percentage of CD11c+MHC-II+CD86lowCD40low DC in MDSC cultures. Similar results were obtained with 0.2 nM paclitaxel, although the percentage of CD11c+ DC and expression of MHC Class II and co-stimulatory molecules were moderately lower. Finally, to determine the functional activity of paclitaxel-induced DC, CD11c+ cells (DC) were sorted from MDSC cultures and tested in syngeneic MLR assay with pre-activated T-cells. Figure 4c shows that isolated DC, as expected, stimulated T-cell proliferation, while DC-depleted MDSC displayed T-cell inhibitory activity. Thus, paclitaxel in ultra-low concentrations significantly up-regulated the differentiation of immunosuppressive MDSC into functionally active immunostimulatory DC in vitro.
Figure 4.
Paclitaxel stimulates differentiation of MDSC into DC. (a) MDSC cultures were treated with 1 nM paclitaxel and the amount of CD11c+ DC was assessed after 24–72 h by FACScan, as described in Materials and methods. Results from four independent experiments are shown as the mean (± SEM). (b) Additional phenotyping of DC revealed that they express MHC Class II molecules and low levels of CD86 and CD40. (c) CD11c+ DC were isolated from paclitaxel-treated MDSC cultures using magnetic beads (DC) and co-cultured with ConA-activated syngeneic splenocytes (T-cells/ConA). DC-depleted MDSC served as a control (MDSC). T-cell proliferation was assessed by [3H]-thymidine incorporation and express as count per minute (cpm). * p < 0.05 vs corresponding control (a) or ConA-stimulated T-cell proliferation (c). (See colour version of this figure online at www.informahealthcare.com/imt)
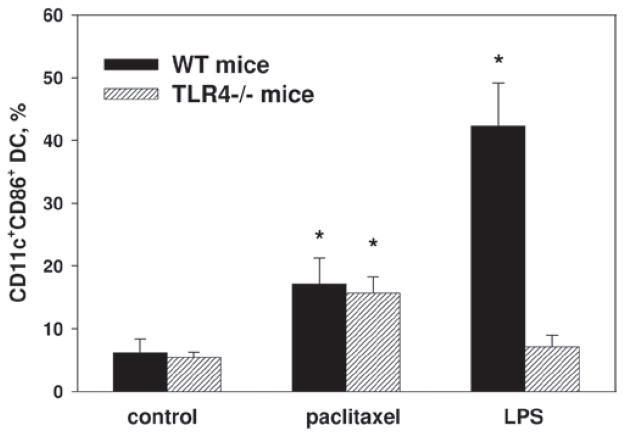
Paclitaxel modulates MDSC differentiation in a TLR4-independent manner
Since paclitaxel in ultra-low concentrations augments final differentiation of MDSC into DC, assessed by increased percentage of CD11c+ cells expressing MHC Class II and co-stimulatory molecules, we addressed a question on the involvement of TLR4 signaling in the described modulation. It has been recently demonstrated that paclitaxel in sub-therapeutic doses and μM concentrations can mimic the action of TLR agonists such as LPS and induce maturation of mouse macrophages and DC (Manthey et al., 1994; Kalechman et al., 1996; Chan and Yang, 2000; Byrd-Leifer et al., 2001).
To this end, we repeated the experiments with MDSC prepared from TLR4−/− mice. Although the effect of LPS on MDSC differentiation into DC was markedly abrogated in TLR4−/− cultures in comparison to wild-type (WT) cultures (up to 6-times; p < 0.01), significant stimulation of DC accumulation induced by 1 nM paclitaxel was similar in TLR4−/− and WT samples (17.1 [± 4.2]% and 15.7 [± 2.6]% of CD11c+CD86+ cells in WT and TLR4−/− treated cultures vs 6.2 [± 2.1]% and 5.4 [± 0.9]% in corresponding non-treated cultures) (Figure 5). Thus, the effect of paclitaxel at 1 nM concentration on MDSC differentiation into DC was not mediated by TLR4 signaling.
Figure 5.
Paclitaxel induces differentiation of MDSC into DC in TLR4-independent manner. MDSC cultures were prepared from the bone marrow cells obtained from wild-type (WT) and TLR4−/− mice and cultured with GM-CSF alone or GM-CSF + IL-6. At Day 5, cells were treated with 1 nM paclitaxel for 72 h and DC were assessed by FACScan as CD11c+ cells co-expressing CD86, CD80, CD40, and MHC Class II and expressed as the means (± SEM) (n = 3). * p < 0.05 vs corresponding control.
In summary, our experiments revealed that paclitaxel in low concentrations did not induce apoptosis in MDSC or blocked their generation from hematopoietic precursors. However, paclitaxel was able to up-regulate MDSC differentiation into DC.
Discussion
Immunosuppressive regulatory cells (such as MDSC that are generated and accumulated during tumor development and progression) inhibit anti-tumor immunity and represent a crucial obstacle to the clinical performance of immunotherapy and chemo-immunotherapy of cancer (Youn and Gabrilovich, 2010). However, a growing body of evidence demonstrates that the clinical efficacy of several types of chemotherapeutic approaches not only relies on the direct cytotoxic or cytostatic effects on the malignant cells, but also on the ability of chemotherapeutic agents to promote anti-tumor immune responses.
New data also indicates that several conventional chemo- and radiotherapy protocols can selectively eliminate immune regulatory cells and revert the immunosuppressive state associated with tumor progression, suggesting that chemotherapeutic agents may directly or indirectly affect formation and function of immunosuppressive cells, including MDSC (Naiditch et al., 2011). In fact, recent in vivo studies showed an effect of chemotherapeutic treatment on the population of MDSC and thus an enhancement of the immune response against tumors. For instance, 5-Fluorouracil (5-FU) has been identified as an anti-cancer agent that selectively induced MDSC apoptotic cell death in vitro and in vivo. The elimination of MDSC by 5-FU increased interferon (IFN)-γ secretion by tumor-specific CTL infiltrating the tumor and promoted T-cell-dependent anti-tumor responses in vivo, suggesting an opportunity to reverse the tumor-mediated immunosuppression (Vincent et al., 2010; Apetoh et al., 2011). Gemcitabine, given at a dose similar to the equivalent dose used in patients, was able to dramatically and specifically reduce the number of MDSC found in the spleens of animals bearing large tumors with no significant reductions in CD4+ T-cells, CD8+ T-cells, natural killer (NK) cells, macrophages, or B-cells. The loss of MDSC was accompanied by an increase in the anti-tumor activity of CD8+ T-cells and activated NK cells (Suzuki et al., 2005). The administration of oxaliplatin (5 mg/kg, intraperitoneally) and its effect on liver metastases was also associated with a reduction in MDSC and a shift in the tumor microenvironment towards a more pro-immunogenic phenotype (Gonzalez-Aparicio et al., 2011). Of note, elimination of MDSC by chemotherapeutic agents used in conventional or moderately low doses relies on their cytotoxic effects and may be associated with acute or cumulative toxicities limiting the clinical development or utilization of such approaches.
Several anti-neoplastic chemotherapeutic agents have been recently reported to display potent immunostimulatory properties when used in ultra-low doses. These unexpected effects did not relay on cytotoxic or cytostatic effects of tested agents on the immune, hematopoietic precursor, or tumor cells, but induced maturation and activation of DC or increased antigen processing and recognition of tumor cells by tumor-specific T-cells (Shurin et al., 2008, 2009; Kaneno et al., 2009, 2011). This phenomenon, which is not associated with apoptosis of tumor or immune cells, was termed ‘chemomodulation’ or ‘chemoimmunomodulation’ to distinguish its nature from conventional cytotoxicity-based chemotherapy. We have recently revealed a decreased infiltration of the tumor microenvironment by MDSC after treatment with paclitaxel in ultra-low non-cytotoxic doses in vivo (Sevko et al., in press). These results suggest that there are several potential targets for chemomodulation in the tumor microenvironment adding MDSC to DC and tumor cells. However, the mechanisms of MDSC down-regulation by ultra-low-dose paclitaxel in vivo have not been determined. Several potential pathways may explain this new phenomenon, including the inhibition of MDSC generation, induction of MDSC apoptosis or stimulation of the final differentiation of MDSC.
Here, we showed that paclitaxel might directly affect MDSC in vitro. The generation of MDSC from the bone marrow precursors was not altered by 1 nM paclitaxel treatment (see Figure 2). Furthermore, treatment with paclitaxel did not increase the rate of apoptosis in MDSC cultures (see Figure 3), ruling out the second potential mechanism of MDSC down-regulation by paclitaxel. However, our results revealed that the treatment with 1 nM paclitaxel stimulated final differentiation of MDSC towards DC (see Figures 4 and 5). These results provide one possible pathway that may be responsible for down-regulation of MDSC levels in animals treated with ultra-low-dose paclitaxel.
Our results also raise an important question about the involvement of TLR4 signaling in the immunomodulating effects of paclitaxel. Pfannenstiel et al. (2010) recently reported that paclitaxel treatment (at 100 nM) of DC precursors prior to maturation (induced by lipopolysac-charide [LPS]) enhanced the expression of CD80, CD40, and MHC Class II, and that this was blocked by anti-TLR4 antibodies in vitro. As we consistently used significantly lower concentrations of paclitaxel in the current experiments, we have tested whether the effect of paclitaxel on DC might still be mediated by TLR4 when the drug is used at these lower (0.5–1 nM) doses. Using the same TLR4 blocking antibodies described by Pfannenstiel et al. and LPS as a control, we showed that the effect of ultra-low concentrations of paclitaxel on DC maturation is TLR4-independent (Zhang et al., in press).
To confirm these results, we repeated the experiment with DC from TLR4−/− B6/10ScNJ mice. Although the effect of LPS on DC generation was markedly abrogated in TLR4−/− DC in comparison to WT DC, a significant up-regulation of DC maturation markers induced by 0.5–1 nM paclitaxel was similar in the TLR4−/− and WT DC (Zhang et al., in press). Thus, our results suggest that the effects of paclitaxel at low nM concentrations on murine DC are not mediated by TLR4 signaling. John et al. (2010) also reported that the paclitaxel-induced increase in HLA Class II expression on human DC was not blocked by anti-TLR4 antibodies. Furthermore, here, using the same experimental approach, we report that stimulation of MDSC differentiation into DC by paclitaxel is not mediated by TLR4 (see Figure 5), a finding consistent with others who reported that docetaxel (a paclitaxel analog that does not interact with TLR4) polarized MDSC toward cells expressing MHC Class II, CD11c, and CD86 (Kodumudi et al., 2010).
In summary, these studies have shown that paclitaxel (Taxol) at low nM concentrations is able to push differentiation of MDSC into DC without altering MDSC longevity in vitro. These results not only help to explain how treatment with ultra-low doses of paclitaxel may decrease MDSC numbers in mice (Sevko et al.), but provide new evidence of multi-targeted potential of using ultra-low doses of chemotherapeutic agents for modulating the tumor micro-environment as a neoadjuvant approach. Understanding the mechanisms involved in modulation of immune regulatory and effector cells in the tumor microenvironment by ultra-low doses of chemotherapeutic agents, i.e., chemomodulation, in vivo, will provide non-toxic alternatives for different types of anti-cancer therapy.
Acknowledgments
This project was supported by NIH RO1 CA154369 grant (to M.R.S.), DKFZ-MOST Cooperation in Cancer Research (grant CA128, to V.U.), Dr Mildred Scheel Foundation for Cancer Research (grant 108992, to V.U.), and the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Immunotherapy of Cancer (to V.U.).
Footnotes
An Article Based Upon Original Research Presented at the 2nd International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM), Budapest, Hungary, May 2011
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Vegran F, Ladoire S, Ghiringhelli F. Restoration of anti-tumor immunity through selective inhibition of myeloid derived suppressor cells by anti-cancer therapies. Curr Mol Med. 2011;11:365–372. doi: 10.2174/156652411795976574. [DOI] [PubMed] [Google Scholar]
- Balkir L, Tourkova IL, Makarenkova VP, Shurin GV, Robbins PD, Yin XM, Chatta G, Shurin MR. Comparative analysis of dendritic cells transduced with different anti-apoptotic molecules: Sensitivity to tumor-induced apoptosis. J Gene Med. 2004;6:537–544. doi: 10.1002/jgm.545. [DOI] [PubMed] [Google Scholar]
- Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Chan OT, Yang LX. The immunological effects of taxanes. Cancer Immunol Immunother. 2000;49:181–185. doi: 10.1007/s002620000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2010;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE catabolism in myeloid cells. J Leukocyte Biol. 2010;88:839–848. doi: 10.1189/jlb.1209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGFβ: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T, Mahnke K, Enk AH. Myeloid-derived suppressor cells and their role in tolerance induction in cancer. J Dermatol Sci. 2010;59:1–6. doi: 10.1016/j.jdermsci.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Aparicio M, Alzuguren P, Mauleon I, Medina-Echeverz J, Hervas-Stubbs S, Mancheno U, Berraondo P, Crettaz J, Gonzalez-Aseguinolaza G, Prieto J, Hernandez-Alcoceba R. Oxaliplatin in combination with liver-specific expression of interleukin-12 reduces the immunosuppressive microenvironment of tumors and eradicates metastatic colorectal cancer in mice. Gut. 2011;60:341–349. doi: 10.1136/gut.2010.211722. [DOI] [PubMed] [Google Scholar]
- Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–219. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J, Ismail M, Riley C, Askham J, Morgan R, Melcher A, Pandha H. Differential effects of paclitaxel on dendritic cell function. BMC Immunol. 2010;11:14. doi: 10.1186/1471-2172-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechman Y, Shani A, Dovrat S, Whisnant JK, Mettinger K, Albeck M, Sredni B. The anti-tumoral effect of the immunomodulator AS101 and paclitaxel (Taxol) in a murine model of lung adenocarcinoma. J Immunol. 1996;156:1101–1109. [PubMed] [Google Scholar]
- Kaneno R, Shurin GV, Kaneno FM, Naiditch H, Lou J, Shurin MR. Chemotherapeutic agents in low non-cytotoxic concentrations increase immunogenicity of human colon cancer cells. Cell Oncol. 2011;34:97–106. doi: 10.1007/s13402-010-0005-5. [DOI] [PubMed] [Google Scholar]
- Kaneno R, Shurin GV, Tourkova IL, Shurin MR. Chemomodulation of human dendritic cell function by anti-neoplastic agents in low non-cytotoxic concentrations. J Transl Med. 2009;7:58. doi: 10.1186/1479-5876-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JS, Bukowski RM, Fincke JH. Myeloid-derived suppressor cells: A novel therapeutic target. Curr Oncol Rep. 2009;11:87–93. doi: 10.1007/s11912-009-0014-6. [DOI] [PubMed] [Google Scholar]
- Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–3536. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: Suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–4594. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–331. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T-cells from tumor-bearing mice. Int Immunopharmacol. 2009;9:900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Manthey CL, Perera PY, Salkowski CA, Vogel SN. Taxol provides a second signal for murine macrophage tumoricidal activity. J Immunol. 1994;152:825–831. [PubMed] [Google Scholar]
- Mantovani A. The growing diversity and spectrum of action of myeloid-derived suppressor cells. Eur J Immunol. 2010;40:3317–3320. doi: 10.1002/eji.201041170. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: Balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPβ transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-transretinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumor angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Collazo M, Corzo CA, Youn JI, Ortiz M, Quiceno D, Gabrilovich DI. Regulatory myeloid suppressor cells in health and disease. Cancer Res. 2009;69:7503–7506. doi: 10.1158/0008-5472.CAN-09-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiditch H, Shurin MR, Shurin GV. Targeting myeloid regulatory cells in cancer by chemotherapeutic agents. Immunol Res. 2011;50:276–285. doi: 10.1007/s12026-011-8213-2. [DOI] [PubMed] [Google Scholar]
- Narita Y, Wakita D, Ohkur T, Chamoto K, Nishimura T. Potential differentiation of tumor bearing mouse CD11b+Gr-1+ immature myeloid cells into both suppressor macrophages and immunostimulatory dendritic cells. Biomed Res. 2009;30:7–15. doi: 10.2220/biomedres.30.7. [DOI] [PubMed] [Google Scholar]
- Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S. Myeloid-derived suppressor cells: More mechanisms for inhibiting anti-tumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannenstiel LW, Lam SSK, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol. 2010;263:79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulaski HL, Spahlinger G, Silva IA, McLean K, Kueck AS, Reynolds RK, Coukos G, Conejo-Garcia JR, Buckanovich RJ. Identifying alemtuzumab as an anti-myeloid cell anti-angiogenic therapy for the treatment of ovarian cancer. J Transl Med. 2009;7:49. doi: 10.1186/1479-5876-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, Ochoa AC. L-Arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössner S, Voigtlander C, Wiethe C, Hanig J, Seifarth C, Lutz MB. Myeloid dendritic cell precursors generated from bone marrow suppress T-cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol. 2005;35:3533–3544. doi: 10.1002/eji.200526172. [DOI] [PubMed] [Google Scholar]
- Sevko A, Kremer V, Falk C, Umansky L, Shurin MR, Shurin GV, Umansky V. Application of paclitaxel in low noncytotoxic doses supports vaccination with melanoma antigens in mice. J Immunotoxicol. doi: 10.3109/1547691X.2012.655343. in press. [DOI] [PubMed] [Google Scholar]
- Shurin GV, Ouellette CE, Shurin MR. Regulatory dendritic cells in the tumor immunoenvironment. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-011-1138–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in non-cytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin GV, Tourkova IL, Shurin MR. Low-dose chemotherapeutic agents regulate small Rho GTPase activity in dendritic cells. J Immunother. 2008;31:491–499. doi: 10.1097/CJI.0b013e318176fae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin MR, Naiditch H, Zhong H, Shurin GV. Regulatory dendritic cells: New targets for cancer immunotherapy. Cancer Biol Ther. 2011;11:988–992. doi: 10.4161/cbt.11.11.15543. [DOI] [PubMed] [Google Scholar]
- Shurin MR, Shurin GV, Lokshin A, Yurkovetsky ZR, Gutkin DW, Chatta G, Zhong H, Han B, Ferris RL. Intratumoral cytokines/chemokines/growth factors and tumor infiltrating dendritic cells: Friends or enemies? Cancer Metastasis Rev. 2006;25:333–356. doi: 10.1007/s10555-006-9010-6. [DOI] [PubMed] [Google Scholar]
- Shurin MR, Naiditch H, Gutkin DW, Umansky V, Shurin GV. ChemoImmunoModulation: Immune Regulation by the Antineoplastic Chemotherapeutic Agents. Current Medicinal Chemistry. doi: 10.2174/092986712800099785. in press. [DOI] [PubMed] [Google Scholar]
- Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances anti-tumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- Tisch R. Immunogenic versus tolerogenic dendritic cells: A matter of maturation. Int Rev Immunol. 2010;29:111–118. doi: 10.3109/08830181003602515. [DOI] [PubMed] [Google Scholar]
- Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, Zilio S, Bronte V. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9:470–481. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rebe C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T-cell-dependent anti-tumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- Watowich SS, Liu YJ. Mechanisms regulating dendritic cell specification and development. Immunol Rev. 2010;238:76–92. doi: 10.1111/j.1600-065X.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: The blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, French DL, Ma G, Eisenstein S, Chen Y, Divino CM, Keller G, Chen S, Pan P. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010;28:620–632. doi: 10.1002/stem.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Han B, Shurin MR, Shurin GV. Paclitaxel controls polarization of regulatory dendritic cells in vitro and in vivo in the lung metastasis model in mice. Submitted. [Google Scholar]