Abstract
Both clinical and experimental evidence has revealed that calorie restriction (CR) is capable of improving heart function. However, most the reports are focused on the effect of CR on the pathological states such as obesity while the effect of CR on heart function in otherwise healthy subjects are not well understood. This study examined the long-term CR effect on cardiac contractile function and possible underlying mechanisms involved. C57BL/6 mice were subjected to a 40% CR or ad libitum feeding for 20 weeks. Echocardiographic and cardiomyocyte contractile properties were evaluated. Intracellular signaling pathways were examined using western blot analysis. Our results showed that CR overtly lessened glucose intolerance, body and heart weights (although not heart size), lowered fat tissue density, decreased left ventricular (LV) wall thickness (septum and posterior wall) in both systole and diastole, and reduced LV mass (not normalized LV mass) without affecting fractional shortening. Cardiomyocyte cell length and cross-sectional area were reduced while peak shortening amplitude was increased following CR. CR failed to affect maximal velocity of shortening/relengthening, duration of shortening and relengthening. Immunoblotting data depicted decreased and increased phosphorylation of Akt/GSK-3β and AMPK/ACC, respectively, following CR. CR also dampened the phosphorylation of mTOR, ERK1/2 and c-Jun while it increased the phosphorylation of JNK. Last but not least, CR significantly promoted cardiac autophagy as evidenced by increased expression of LC3B-II (and LC3B-IIto-LC3B-I ratio) and Beclin-1. In summary, our data suggested that long-term CR may preserve cardiac contractile function with improved cardiomyocyte function, lessen cardiac remodeling and promote autophagy.
Keywords: Calorie restriction, Cardiac function, Remodeling, Insulin signaling, Autophagy
INTRODUCTION
Both clinical and experimental studies have shown that calorie restriction is capable of extending lifespan, lowering the onset of chronic diseases as well as the overall disease morbidity and mortality1, 2. Caloric restriction has been shown to exert some profound cardiovascular effects including lowered blood pressure3, decreased systemic inflammation4, and improved cardiac diastolic parameters5. However, the precise impact of caloric restriction on cardiac contractile function and geometry has not been elucidated. Up-to-date, the majority of studies dealing with caloric restriction in the heart are somewhat focused on pathological conditions including aging, obesity and diabetes mellitus6–8. The impact of caloric restriction on heart function of normal or healthy subjects remains somewhat elusive. It was reported that a short-term low-calorie diet (471 Kcal/d for 3 d) triggers accumulation of myocardial triglycerides and suppresses left ventricular diastolic function in otherwise healthy subjects9, 10. However, long-term caloric restriction (at 80% of control diet for 9 months) was shown to improve diastolic function in healthy subjects11.
To better evaluate the effect of long-term caloric restriction on heart function under physiological condition, the present study was designed to place adult C57BL/6 mice on a calorically restricted diet (60% of the control diet) for 20 weeks prior to assessment of myocardial and cardiomyocyte function. Cardiac geometry, glucose tolerance and fat tissue density were examined after long-term caloric restriction. To explore possible mechanisms of action involved in caloric restriction-induced changes in cardiac function and geometry, if any, essential signaling pathways responsible for cardiac function including Akt, AMP-dependent protein kinase (AMPK), glycogen synthase kinase-3β (GSK-3β), mammalian target of rapamycin (mTOR), c-Jun, c-Jun NH2-terminal kinase (JNK), extracellular signal-regulated protein kinase (ERK) and autophagy were scrutinized in mouse hearts following long-term caloric restriction. Autophagy is essential to cell survival, and the interruption of which triggers ventricular dysfunction and heart failure12, 13. In particular, autophagy may be positively regulated by AMPK, while being negatively regulated by mTOR, a downstream signaling molecule of the cell survival factor Akt14–16. In addition, AMPK and Akt signaling are often inversely correlated such that Akt negatively regulates AMPK phosphorylation14–16. To this end, changes in the signaling cascade of Akt, AMPK and the AMPK substrate acetyl-CoA carboxylase (ACC) were evaluated in the heart following chronic caloric restriction.
METHODS
Experimental animals
The experimental procedure described in this study was approved by our Institutional Animal Use and Care Committee (University of Wyoming, Laramie, WY) and was in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85-23, revised 1996). In brief, 4-month-old adult male C57BL/6 mice were housed in individual cages and were fed ad libitum (AL) for 2 weeks. The average caloric intake was calculated from the daily food intake over these 2 weeks. Mice were then randomly divided into two groups. Control mice were fed AL for the next 20 weeks whereas caloric restriction mice were fed 90% of the average value of calorie for 1 week (10% restriction for acclimation) followed by 60% of calorie for 20 weeks (40% restriction). The diet was enriched in vitamins and minerals to ensure constant daily intake of vitamins and minerals during the caloric restriction.
Body fat composition measurement
Body composition was measured using Dual Energy X-ray Absorptiometry (DEXA), which is a clinical measure of lean tissue mass, adipose tissue mass, and bone mineral mass and density. A low level pencil-beam x-ray moved transversely from the head to the tail across the sedated mouse. Difference in absorbance of the X-ray was detected according to tissue density. Percent fat was calculated using fat and body mass17.
Intraperitoneal glucose tolerance test (IPGTT)
Following 20 weeks of caloric restriction or AL diet feeding, mice were fasted for 12 hrs before an intraperitoneal injection of glucose (2 g/kg body weight). Blood glucose levels were determined by clipping the mouse tail immediately before glucose challenge, as well as at 0, 30, 60 and 120 min thereafter. Blood glucose levels were determined using an ACCU-CHEK Advantage Glucose Analyzer (Roche Diagnostics Corporation, IN)18.
Echocardiographic assessment
Cardiac geometry and function were evaluated in anesthetized (Avertin 2.5%, 10 µl/g bw, i.p.) mice using a 2-D guided M-mode Sonos 5500 echocardiography (Phillips Medical Systems, Andover, MD) equipped with a 15-6 MHz linear transducer. Hearts were imaged in 2-D mode in the parasternal long-axis view with a depth of 2 cm. A M-mode cursor was then positioned perpendicular to interventricular septum and posterior wall of the left ventricular (LV) at the level of the papillary muscles in the 2-D mode. The sweep speed was 100 mm/sec at the M-mode. Diastolic wall thickness, LV end diastolic dimension (EDD) and LV end systolic dimension (ESD) were measured from leading edge to leading edge in accordance with the Guidelines of the American Society of Echocardiography. The percentage of LV fractional shortening was calculated as [(EDD-ESD)/EDD] × 100. Heart rates were averaged over 10 cardiac cycles18.
Cardiomyocyte Isolation
After ketamine/xylazine sedation, mouse hearts were removed and perfused with Krebs-Henseleit bicarbonate buffer containing (in mM) 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES, and 11.1 glucose, with 5% CO2- 95% O2. Hearts were subsequently digested with a Krebs-Henseleit bicarbonate buffer containing 223 U/ml collagenase D (Boehringer Mannheim, Indianapolis, IN) for 20 min. After perfusion, left ventricles were removed and minced before being filtered. Extracellular Ca2+ was slowly added back to 1.25 mM. Myocytes with obvious sarcolemmal blebs or spontaneous contractions were not used. Myocytes were used within 6 hours of isolation18.
Cell shortening/relengthening
Mechanical properties of myocytes were assessed using an IonOptix™ soft-edge MyoCam system (IonOptix, Milton, MA) as described previously18. Myocytes were placed in a chamber mounted on the stage of an Olympus IX-70 microscope and superfused (~2 ml/min at 25°C) with a buffer containing (in mM): 131 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES. Myocytes were field stimulated at 0.5 Hz unless otherwise stated. Cell shortening and relengthening were assessed using the following indices: peak shortening19, time-to-PS (TPS), time-to-90% relengthening (TR90), and maximal velocities of shortening/relengthening (± dL/dt).
Western blot analysis
Myocardial protein from left ventricles was prepared as described18. Samples with equal amount of proteins were separated on 7–12% SDS-polyacrylamide gels in a minigel apparatus (Mini-PROTEAN II, Bio-Rad) and transferred to nitrocellulose membranes. The membranes were blocked with 5% milk in TBS-T, and were incubated overnight at 4°C with anti-Akt, anti-phosphorylated Akt (pAkt, Thr308), anti-GSK-3β, anti-phosphorylated GSK-3β (pGSK-3β, Ser9), anti-mTOR, anti-phosphorylated mTOR (pmTOR, Ser2448), anti-ERK1/2, anti-phosphorylated ERK1/2 (pERK1/2, Thr202 and Tyr204), anti-AMPK, anti-phosphorylated AMPK (pAMPK, Thr172), anti-ACC, anti-phosphorylated ACC (pACC, Ser79), anti-LC3B, anti-Beclin-1, anti-ATG5, anti-ATG7 and anti-GAPDH (loading control) antibodies. After washing blots to remove excessive primary antibody binding, blots were incubated for 1 hr with horseradish peroxidase (HRP)–conjugated secondary antibody (1:5,000). Antibody binding was detected using enhanced chemiluminescence (Amersham Pharmacia, Piscataway, NJ), and film was scanned and the intensity of immunoblot bands was detected with a Bio-Rad Calibrated Densitometer (Model: GS-800).
Data Analysis
Data were presented as Mean ± SEM. Statistical significance (p < 0.05) for each variable was estimated by t-test.
RESULTS
General biometric and echocardiographic characteristics
General biometric profiles of control and caloric restriction mice are shown in Table 1. Caloric restricted mice displayed smaller body and heart weights as well as fat tissue density without change in heart size (heart weight normalized to body weight). Caloric restriction did not affect fasting blood glucose levels. Echocardiographic assessment revealed that caloric restriction significantly altered cardiac geometry including decreased left ventricular wall and septal thickness during systole and diastole (PWS, PWD, IVSS, and VSD) as well as left ventricular end diastolic diameter (EDD) without affecting left ventricular end systolic diameter (ESD) and fractional shortening. Consistent with the change in gross heart weight, calculated LV mass was significantly decreased associated with unchanged LV mass in caloric restricted mice compared with control mice.
Table 1.
General biometric and echocardiographic characteristics in mice
| Parameters | Control | CR |
|---|---|---|
| Body Weight (g) | 36.4 ± 2.9 | 22.0 ± 1.2 * |
| Heart Weight (millgram) | 151 ± 8 | 109 ± 5 * |
| Heart /Body Weight (mg/g) | 4.76 ± 0.20 | 4.78 ± 0.17 |
| Fat tissue Density (%) | 30.1 ± 4.1 | 12.4 ± 3.6 * |
| Fasting Blood Glucose (mg/dl) | 107.7 ± 3.6 | 118.0 ± 8.7 |
| LVEDD (mm) | 2.39 ± 0.08 | 2.02 ± 0.12 * |
| LVESD (mm) | 1.16 ± 0.06 | 1.11 ± 0.09 |
| IVSD (mm) | 1.28 ± 0.03 | 1.09 ± 0.04 * |
| IVSS (mm) | 1.83 ± 0.05 | 1.51 ± 0.08 * |
| PWD (mm) | 1.24 ± 0.06 | 1.02 ± 0.02 * |
| PWS (mm) | 1.59 ± 0.07 | 1.16 ± 0.04 * |
| Fractional Shortening (%) | 50.6 ± 1.5 | 45.6 ± 2.7 |
| LV Mass (milligram) | 105.8 ± 5.4 | 65.6 ± 5.0 * |
| LV Mass/ Body Weight (mg/g) | 3.13 ± 0.23 | 3.00 ± 0.20 |
CR: Caloric restriction; LVEDD: left ventricular end diastolic diameter; LVESD: left ventricular end systolic diameter; IVSD = septum thickness in diastole; IVSS = septum thickness in systole; PWD = left ventricular posterior wall in diastole; PWS = left ventricualr posterior wall in systole; LV = left ventricular. Mean ± SEM, n = 6–9 mice per group,
p < 0.05 vs. control group.
Intraperitoneal glucose tolerance test
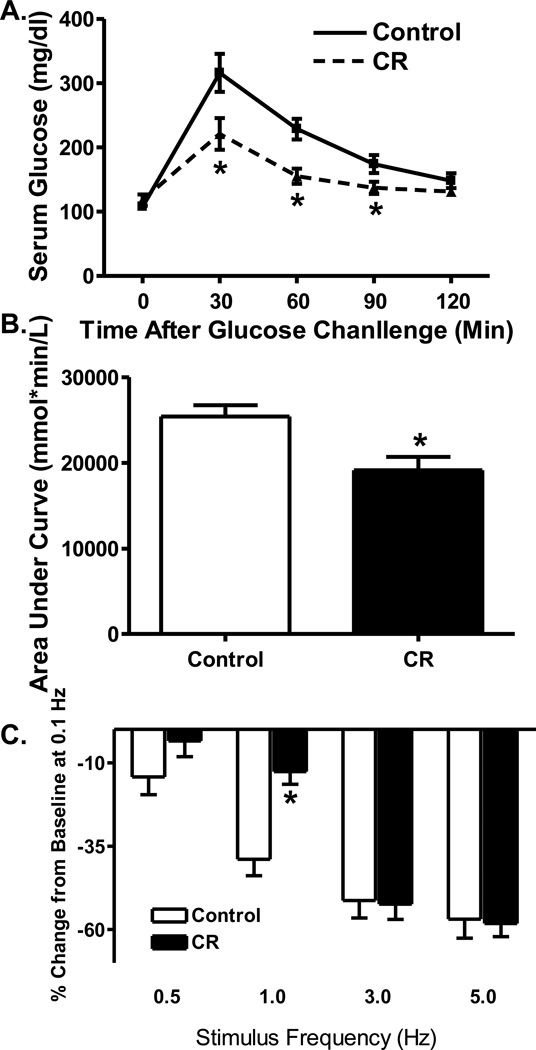
To assess the status of glucose tolerance, IPGTT was performed in caloric restricted and control mice. As shown in Fig. 1A, blood glucose levels were significantly reduced at 30, 60 and 90 minutes following glucose challenge in caloric restriction mice compared with control mice. This is further supported by the area under the glucose curve with an overt decrease in the caloric restriction group compared with controls (Fig. 1B). These data suggested an improved insulin sensitivity following caloric restriction.
Fig. 1.
Effect of caloric restriction on intraperitoneal glucose tolerance test (IPGTT) and cardiomyocyte peak shortening-stimulus frequency relationship. A: Time course of serum glucose level after glucose challenge (2 g/kg, i.p., b.w.); B: Area under the curve (AUC); and C: Changes in peak shortening amplitude (normalized to that of 0.1 Hz from the same cell) at various stimulus frequencies (0.1 – 5.0 Hz). Mean ± SEM, n = 6–7 mice (panels A–B) and 23 cells (panel C) per group, * p < 0.05 vs. control group.
Cardiomyocyte size and contractile properties
Cardiomyocyte size and contractile properties following caloric restriction are shown in Table 2. Our data revealed that caloric restriction significantly reduced resting cell length and cross-sectional area with little effect on cell width. Caloric restriction significantly increased peak shortening amplitude without elicit any effect on maximal velocity of shortening/relengthening (± dL/dt), duration of shortening (TPS) and relengthening (TR90).
Table 2.
Cardiomyocyte geometry and contractile properties
| Parameters | Control | CR |
|---|---|---|
| Cell Cross-Sectional Area (µm2) | 3021 ± 114 | 2281 ± 82* |
| Resting Cell Length (µm) | 138.4 ± 3.0 | 118.0 ± 2.8* |
| Resting Cell Width (µm) | 23.5±0.6 | 22.6±0.9 |
| Peak Shortening (%) | 5.4 ± 0.3 | 6.5 ± 0.3* |
| − dL/dt (µm/sec) | 155.2 ± 11.1 | 159.40 ± 9.0 |
| +dL/dt (µm/sec) | 162.2 ± 9.5 | 174.0 ± 8.6 |
| Time to Peak 90.0% (msec) | 87 ± 4 | 88 ± 4 |
| Time to Baseline 90.0% (msec) | 139 ± 9 | 147 ± 10 |
CR: Caloric restriction; ± dL/dt: maximal velocity of shortening/relengthening; Mean ± SEM, n = 97 cells from 3 mice per group,
p < 0.05 vs. control group.
Stimulation frequency-cardiomyocyte shortening response
Rodent hearts normally contract at very high frequencies, whereas our mechanical recording was performed at 0.5 Hz. To evaluate the impact of caloric restriction on cardiac contractile function under higher frequencies, we increased stimulus frequency up to 5.0 Hz (300 beats per min) and recorded the steady state peak shortening. Cardiomyocytes were initially stimulated to contract at 0.5 Hz for 5 min to ensure a steady state before commencing the frequency response. Fig. 1C displays a negative staircase of PS with the increased stimulus frequency in both groups. Interestingly, the amplitude of decline in PS was significantly less in CR group at 1.0 Hz (although not at all other stimulus frequencies). These data favor a possible improved intracellular Ca2+ cycling or stress tolerance capacity by CR at relatively low stimulus frequency.
Effect of caloric restriction on Akt and AMPK signaling cascades
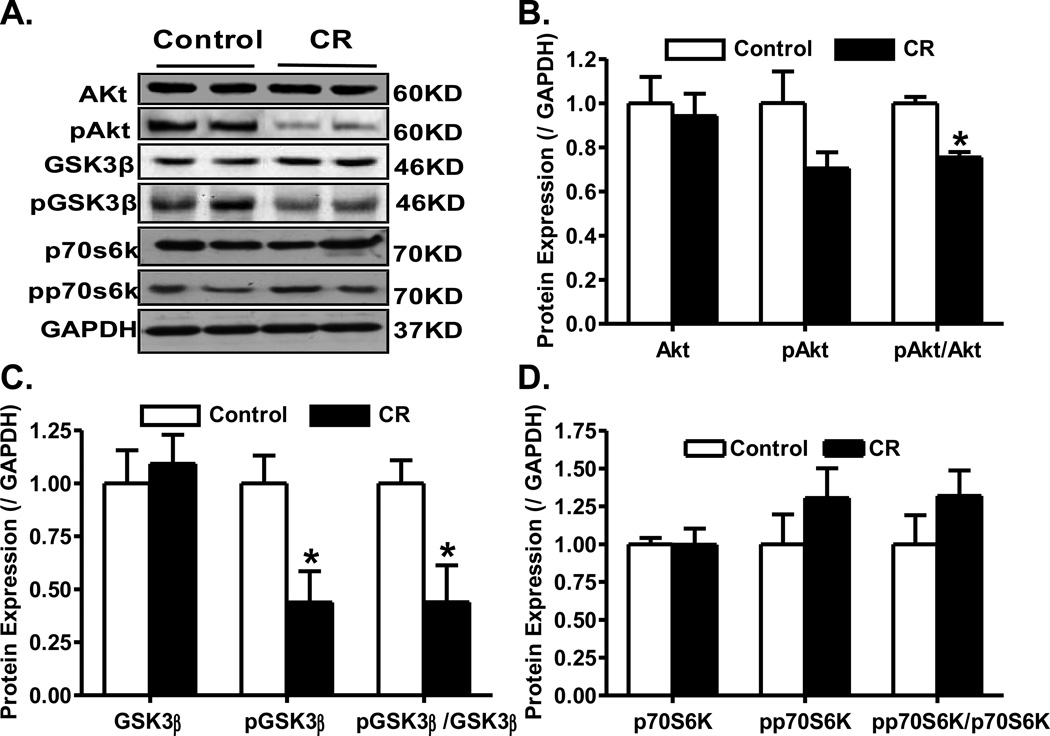
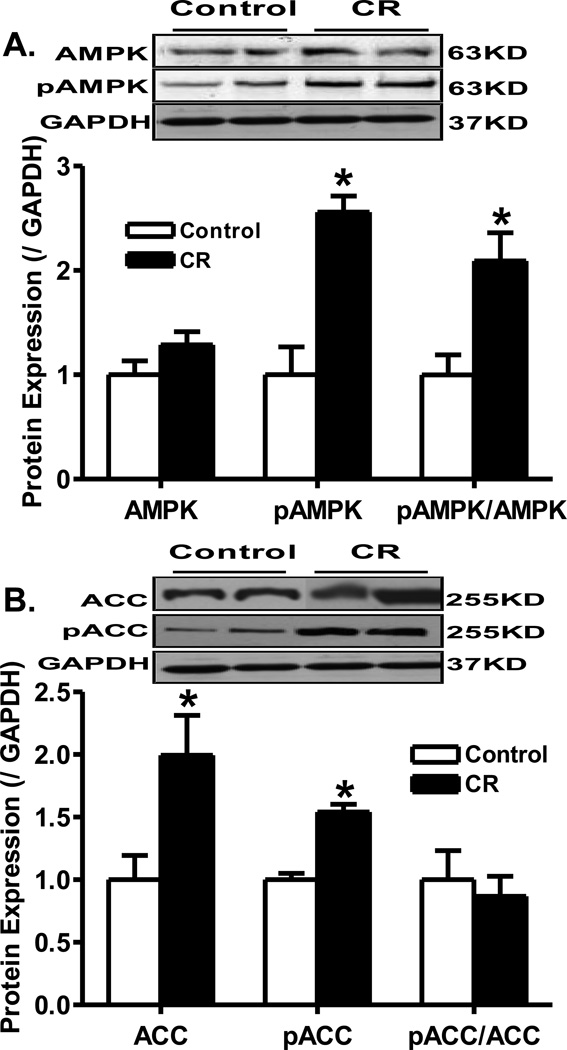
To elucidate the possible signaling mechanisms involved in caloric restriction-induced effect on myocardial function, Western blot analysis was performed on signaling molecules governing myocardial function including Akt and AMPK. Immunoblotting data revealed that caloric restriction significantly down-regulated phosphorylation of Akt (pAkt-to-Akt ratio), and the Akt downstream signaling molecule GSK-3β (pGSK-3β and pGSK-3β-to- GSK-3β ratio) without affecting phosphorylation of another Akt downstream signal p70s6k. Caloric restriction did not affect the expression of pan Akt, GSK-3β and p70s6k (Fig. 2). To the contrary, caloric restriction significantly enhanced the phosphorylation of AMPK and ACC (both absolute levels and normalized values). Caloric restriction did not affect the expression of AMPK although it significantly upregulated the level of pan ACC (Fig. 3).
Fig. 2.
Protein expression of pan and phosphorylated Akt, GSK3β and p70s6k in myocardium from control and caloric restriction mice. A: Representative gel blots of Akt, pAkt, GSK3β, pGSK3β, p70s6k, pp70s6k and GAPDH (loading control) using specific antibodies; B: Levels of pan and phosphorylated Akt as well as pAkt-to-Akt ratio; C: Levels of pan and phosphorylated GSK3β as well as pGSK3β-to-GSK3β ratio; and D: Levels of pan and phosphorylated p70S6K as well as pp70S6K-to-p70S6K ratio. Mean ± SEM, n = 4–5 per group, * p < 0.05 vs. control group.
Fig. 3.
Protein expression of pan and phosphorylated AMPK and ACC in myocardium from control and caloric restriction mice. A: Levels of pan and phosphorylated AMPK as well as pAMPK-to-AMPK ratio; B: Levels of pan and phosphorylated ACC as well as pACC-to-ACC ratio. Insets: Representative gel bots of AMPK, pAMPK, ACC, pACC and GAPDH (loading control) using specific antibodies. Mean ± SEM, n = 4–5 per group, * p < 0.05 vs. control group.
Effect of caloric restriction on mTOR, ERK1/2, c-Jun and JNK signaling
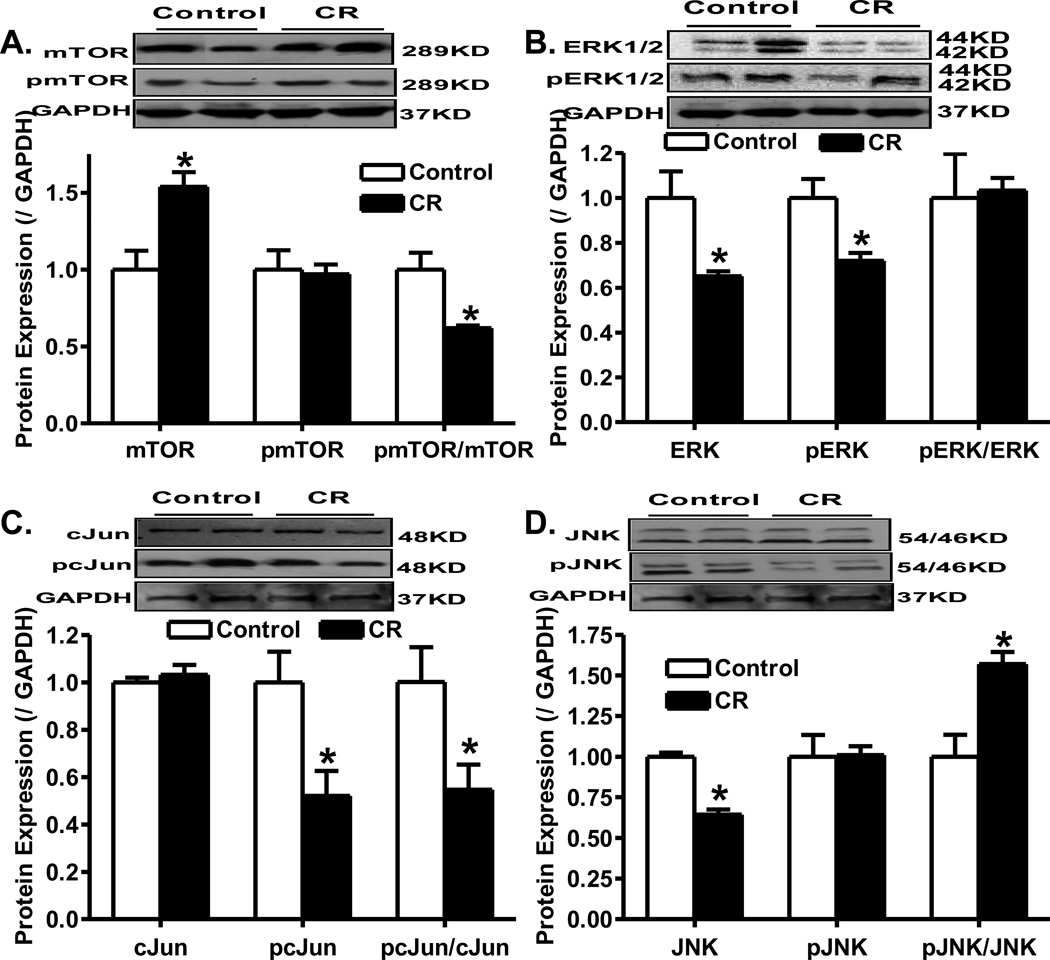
To elucidate the potential signaling mechanisms involved in cardiac growth, the mTOR, ERK1/2, c-Jun and JNK signaling cascades were examined in the hearts following caloric restriction. Results shown in Fig. 4 showed that caloric restriction significantly reduced the phosphorylation of mTOR (pmTOR-to-mTOR ratio), ERK (pERK) and c-Jun (pc-Jun and pc-Jun-to-c-Jun ratio) while it significantly enhanced JNK phosphorylation (pJNK-to-JNK ratio). Moreover, caloric restriction significantly upregulated pan mTOR as well as downregulated the expression of ERK and JNK without affecting the pan expression of c-Jun (Fig. 4).
Fig. 4.
Western blot analysis of pan and phosphorylated forms of mTOR, ERK1/2, cJun and JNK in myocardium from control and caloric restriction mice. A: Levels of pan and phosphorylated mTOR as well as pmTOR-to-mTOR ratio; B: Levels of pan and phosphorylated ERK1/2 as well as pERK1/2-to-ERK1/2 ratio; C: Levels of pan and phosphorylated cJun as well as pcJun-to-cJun ratio; and D: Levels of pan and phosphorylated JNK as well as pJNK-to-JNK ratio; Insets: Representative gel blots of pan and phosphorylated mTOR, ERK1/2, cJun and JNK using specific antibodies. GAPDH was used as the loading control. Mean ± SEM, n = 4–5 per group, * p < 0.05 vs. control group.
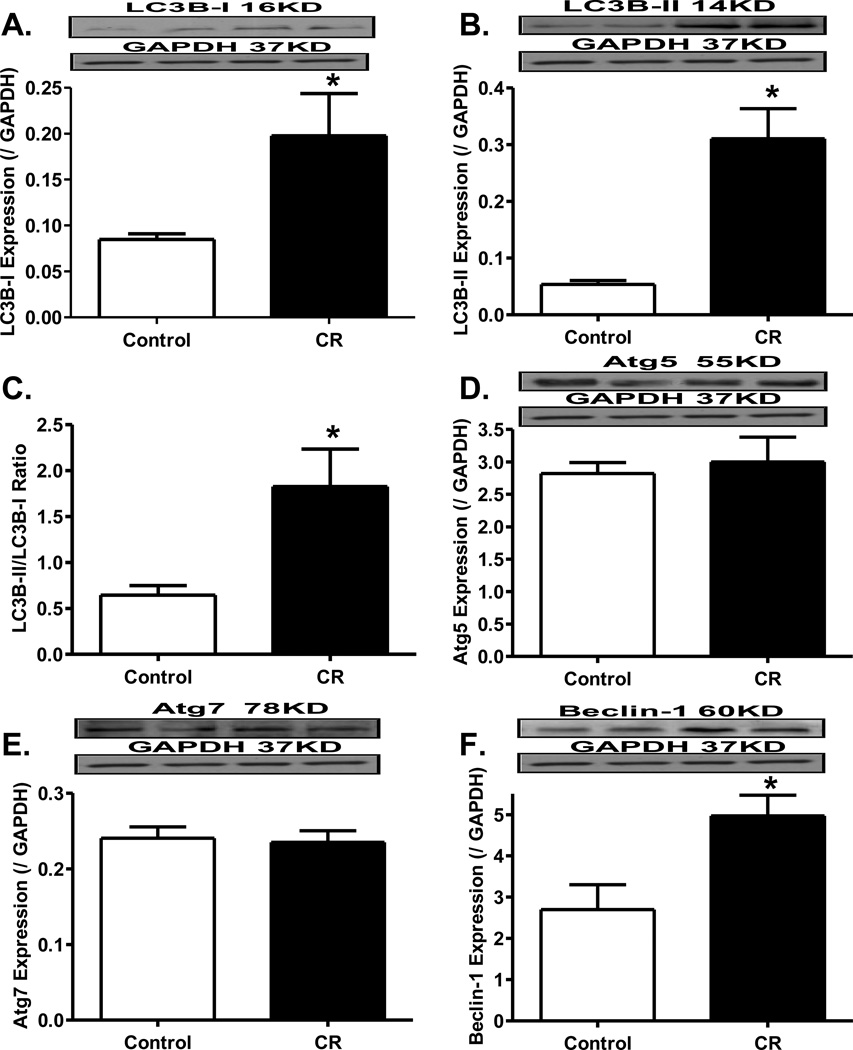
Effect of caloric restriction on myocardial autophagy
Data shown in Fig. 5 revealed that caloric restriction significantly facilitated myocardial autophagy as evidenced by the increased levels of LC3B-I, LC3B-II, LC3B-II-to-LC3B-I ratio, and Beclin-1 with little changes in Atg5 and Atg7.
Fig. 5.
Influence of caloric restriction on autophagy. Myocardial autophagy was evaluated by the conversion of LC3-I to LC3-II (panel A), expression of Beclin (panel B), Atg5 (panel C), Atg7 (panel D) proteins. Insets: Representative immunoblots of LC3-II, Beclin, Atg5 and Atg7 using specific antibodies. Mean ± SEM. n = 4–5 mice per group, * p < 0.05 vs. control group.
DISCUSSION
The salient findings from this study indicated that long-term caloric restriction improved glucose tolerance, reduced fat tissue density, decreased left ventricular (LV) wall thickness (septum and posterior wall) in systole and diastole, reduced heart weight and LV mass (not normalized heart size or LV mass), preserved echocardiographic contractile function, and improved cardiomyocyte contractile function. These geometric and mechanical responses were associated with decreased phosphorylation of Akt, GSK-3β, mTOR, c-Jun and increased phosphorylation of AMPK, ACC and JNK following long-term caloric restriction. In addition, cardiac autophagy was induced following long-term caloric restriction. These findings depicted that long-term caloric restriction may preserve cardiac contractile function and lessen cardiac remodeling, contributing to the reduced risk of heart hypertrophy and cardiac contractile dysfunction.
Caloric restriction has been defined as a reduction in calorie intake below the usual ad libitum intake without malnutrition4. In our study, daily caloric intake was restricted to 60% of the average ad libitum food intake. In response to the apparent energy deficiency, mice displayed a drastic decrease in body weight, fat mass and heart weight. Consistent with the notion that food restriction improves insulin sensitivity20, 21, our IPGTT results indicated that blood glucose levels were much lower at 30, 60 and 90 minutes after glucose challenge in caloric restricted mice compared with control mice. This is supported by a smaller area under the glucose curve in caloric restricted group. This result favors significantly improved insulin sensitivity in caloric restricted group. Although the precise nature of improved insulin sensitivity following caloric restriction is still elusive, reduced body weight and adiposity are expected to play a pivotal role.
Calorie restriction has been demonstrated to improve cardiac function in humans and experimental animal models22, 23. However, a majority of reports are focused on the effect of caloric restriction in pathological conditions including aging and obesity6–8. Little is known with regards to the effect of caloric restriction on heart geometry and function as well as the underlying mechanism involved in physiological condition6, 9, 10. Findings from our study revealed that long-term caloric restriction preserved myocardial contractile function and improved cardiomyocyte function, induced cardiomyocyte autophagy while lessening the remodeling process. LV fractional shortening was unaltered following caloric restriction. LV mass was significantly reduced following caloric restriction, in line with the lowered heart weight. It is possible that the dramatic change in body weight may be responsible for the reduced LV dimensions (LVEDD, septal and posterior wall thickness) following caloric restriction.
Our study described for the first time the cardiomyocyte contractile performance following caloric restriction. Cardiomyocyte contractile properties were analyzed including peak shortening amplitude, maximal velocity of shortening/relengthening (± dL/dt), time-to-PS (TPS), and time-to-90% relengthening (TR90). Our results revealed that caloric restriction significantly increased PS without affecting all other indices. Given that the resting cell length is significantly decreased, it is possible that the increased peak shortening amplitude was a result of reduced resting cell length. Echocardiographic and cardiomyocyte contractile properties favor a much preserved cardiac contractile function following long-term caloric restriction. The reduced resting cardiomyocyte length and cross-sectional area were likely to be responsible for reduced heart weight and LV mass. This adaptive cardiac remodeling process should significantly contribute to the preserved heart function under nutrition restriction. Along the same line, Shinmura and colleagues recently reported that caloric restriction (60% restriction which is similar to our present study) ameliorates the physiological decline in cardiac diastolic function due to aging. These authors reported identical echocardiographic left ventricular systolic function and a better diastolic function following caloric restriction. Moreover, intracellular Ca2+ clearance was facilitated while aging-induced loss of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) was attenuated by caloric restriction24, suggesting a role of intracellular Ca2+ homeostasis in long-term caloric restriction-induced beneficial response in the heart.
Insulin plays an important role in regulating cardiac function through a fine control of glucose and fatty acid metabolism25, 26. Under physiological conditions, glucose serves as the main carbohydrate metabolized by myocardium. Our present data revealed lessened phosphorylation of Akt and its downstream signaling molecule GSK-3β following caloric restriction. Akt is essential in mediating the effects of insulin on glucose transport, glycogen synthesis and gluconeogenesis27, 28. Akt, through phosphorylation and thereby inactivation of GSK-3β, participates in cell survival and growth, glycogen synthase activation and glycogen synthesis29, 30. Akt has been shown to be an important mediator of growth to control cellular hypertrophy in cardiac, skeletal and smooth muscles31. Akt signaling is usually up-regulated when the heart rapidly grows and may be down-regulated by caloric restriction32, suggesting a role of Akt in nutrient-dependent regulation of cardiac growth. Consistent with this notion, we found decreased Akt signaling in the heart following long-term caloric restriction, supporting a role of Akt in caloric restriction-induced regulation of cardiac geometry. Despite the unchanged pan GSK-3β level, levels of pGSK-3β and the pGSK-3β-to-GSK-3β ratio were decreased following caloric restriction. These results suggested a role of GSK-3β downstream of Akt in the regulation of heart geometry and function following caloric restriction. Our data failed to identify any change in the pan and phosphorylated p70s6k protein, not favoring a role of p70s6k in caloric restriction-induced cardiac geometric and functional responses.
mTOR is a large and evolutionarily-conserved member of the phosphatidylinositol kinase-related kinase family downstream of Akt with multiple biological functions such as control of cellular growth and proliferation via protein translational regulation33. Inhibition of mTOR retards protein synthesis and cell growth. Our finding showed that decreased pmTOR-to-total mTOR ratio and an upregulated pan mTOR expression following caloric restriction, supporting a possible role of mTOR in the regulation of cardiac growth and function downstream of Akt in our current experimental setting. Our result also revealed decreased total and phosphorylated ERK1/2 levels (although without changes in the pERK1/2-to-ERK1/2 ratio) following caloric restriction. ERK1/2 belong to a mitogen-activated protein kinase (MAPK) family34. Active ERK1/2 induce reprogramming of gene expression through phosphorylation of various intracellular target proteins and transcription factors to initiate cell growth and proliferation35. Activation of ERK1/2 is usually associated with pro-hypertrophic responses such as cardiomyocyte growth36. The lessened ERK1/2 levels depict a possible role of ERK in the cardiac growth repression following caloric restriction.
AMPK is an essential regulator of energy balance often activated by a wide variety of metabolic stresses37. AMPK activation is rather important to the heart function through the activation of energy-generating pathways and inhibition of energy-consuming pathways. Activation of AMPK increases fatty acid oxidation through phosphorylation and inhibition of acetyl-CoA carboxylase (ACC) which catalyzes the conversion of acetylCoA to malonyl-CoA38. In the present study, our results displayed increased AMPK activation following caloric restriction despite unchanged pan AMPK expression. Caloric restriction increased the levels of pan and phosphorylated ACC although the pACC-to-total ACC ratio remains unchanged following caloric restriction. These data suggested a possible role of activation of AMPK and ACC in preserving the cardiac contractile function following long-term caloric restriction. It has been reported that activation of Akt is associated with inhibition of AMPK16. In addition, AMPK acts as a nutrient-dependent regulator of mTOR39. During nutrient deprivation conditions, AMPK can be activated by upstream kinases and function to repress activation of mTOR, ultimately reducing the overall cellular energy expenditure40, 41. Given the reciprocal responses in Akt and AMPK following caloric restriction found in our study, a possible crosstalk may exist between Akt and AMPK signaling cascades to modulate the cardiac metabolism and growth following caloric restriction intervention.
In this study, levels of autophagy-related proteins were found to be elevated (LC3B and Beclin-1), or unchanged (Atg5 and Atg7) in hearts following the 20-week caloric restriction. Autophagy is a tightly regulated intracellular process for the degradation of cellular constituents42, 43. The role of autophagy in cardiomyocyte survival and function has been consolidated in autophagy-deficient animals and cell models12, 44. Our findings support the notion that constitutive cardiomyocyte autophagy is required for protein quality control, normal cellular structure and function. Wohlgemuth and colleagues reported that lifelong 40% caloric restriction drastically increased the expression of autophagic markers in the heart45. However, most of the reports on autophagy in the heart have been focused on mitochondrial morphology and turnover with age and other pathological conditions, such as cardiomyopathy or ischemia-reperfusion46, 47. Further study is warranted to better elucidate the role of autophagy induction in the preservation of cardiac function through removal of damaged cellular components in nutrient deficient states.
In conclusion, data from our current study suggest that long-term caloric restriction preserves cardiac contractile function and lessens cardiac remodeling under physiological state possibly through regulation of insulin signaling in particular the Akt, GSK-3β, AMPK and mTOR signaling cascades. Regulation of autophagy and stress signaling pathways such as ERK may play a pivotal role in the cardiac geometric and functional responses although further study is needed to consolidate their participation in such regulatory processes. These data should shed some light towards a better understanding of regulation of cardiac geometry and function under both physiological state and pathological conditions with a drastic change in nutrients.
ACKNOWLEDGMENTS
This work was supported in part by NIH/NCRR P20 RR016474.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 2.Nutrition classics. The American Journal of Cancer, Volume XXXVIII, March, 1940: The initiation and growth of tumors. Introduction. I. Effects of underfeeding. By Albert Tannenbaum. Nutr Rev. 1987;45(1):20–22. doi: 10.1111/j.1753-4887.1987.tb06069.x. [DOI] [PubMed] [Google Scholar]
- 3.Young JB, Mullen D, Landsberg L. Caloric restriction lowers blood pressure in the spontaneously hypertensive rat. Metabolism. 1978;27(12):1711–1714. doi: 10.1016/0026-0495(78)90256-1. [DOI] [PubMed] [Google Scholar]
- 4.Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93(1–3):87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 5.Taffet GE, Pham TT, Hartley CJ. The age-associated alterations in late diastolic function in mice are improved by caloric restriction. J Gerontol A Biol Sci Med Sci. 1997;52(6):B285–B290. doi: 10.1093/gerona/52a.6.b285. [DOI] [PubMed] [Google Scholar]
- 6.Niemann B, Chen Y, Issa H, Silber RE, Rohrbach S. Caloric restriction delays cardiac ageing in rats: role of mitochondria. Cardiovasc Res. 88(2):267–276. doi: 10.1093/cvr/cvq273. [DOI] [PubMed] [Google Scholar]
- 7.Hammer S, van der Meer RW, Lamb HJ, de Boer HH, Bax JJ, de Roos A, Romijn JA, Smit JW. Short-term flexibility of myocardial triglycerides and diastolic function in patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2008;295(3):E714–E718. doi: 10.1152/ajpendo.90413.2008. [DOI] [PubMed] [Google Scholar]
- 8.Viljanen AP, Karmi A, Borra R, Parkka JP, Lepomaki V, Parkkola R, Lautamaki R, Jarvisalo M, Taittonen M, Ronnemaa T, Iozzo P, Knuuti J, Nuutila P, Raitakari OT. Effect of caloric restriction on myocardial fatty acid uptake, left ventricular mass, and cardiac work in obese adults. Am J Cardiol. 2009;103(12):1721–1726. doi: 10.1016/j.amjcard.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Hammer S, van der Meer RW, Lamb HJ, Schar M, de Roos A, Smit JW, Romijn JA. Progressive caloric restriction induces dose-dependent changes in myocardial triglyceride content and diastolic function in healthy men. J Clin Endocrinol Metab. 2008;93(2):497–503. doi: 10.1210/jc.2007-2015. [DOI] [PubMed] [Google Scholar]
- 10.van der Meer RW, Hammer S, Smit JW, Frolich M, Bax JJ, Diamant M, Rijzewijk LJ, de Roos A, Romijn JA, Lamb HJ. Short-term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes. 2007;56(12):2849–2853. doi: 10.2337/db07-0768. [DOI] [PubMed] [Google Scholar]
- 11.Riordan MM, Weiss EP, Meyer TE, Ehsani AA, Racette SB, Villareal DT, Fontana L, Holloszy JO, Kovacs SJ. The effects of caloric restriction- and exercise-induced weight loss on left ventricular diastolic function. Am J Physiol Heart Circ Physiol. 2008;294(3):H1174–H1182. doi: 10.1152/ajpheart.01236.2007. [DOI] [PubMed] [Google Scholar]
- 12.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13(5):619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 13.Yitzhaki S, Huang C, Liu W, Lee Y, Gustafsson AB, Mentzer RM, Jr, Gottlieb RA. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol. 2009;104(2):157–167. doi: 10.1007/s00395-009-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Rehman SK, Zhang W, Wen A, Yao L, Zhang J. Autophagy is a therapeutic target in anticancer drug resistance. Biochim Biophys Acta. 2010;1806(2):220–229. doi: 10.1016/j.bbcan.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280(37):32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 16.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278(41):39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 17.Ren J, Dong F, Cai GJ, Zhao P, Nunn JM, Wold LE, Pei J. Interaction between age and obesity on cardiomyocyte contractile function: role of leptin and stress signaling. PLoS One. 2010;5(4) doi: 10.1371/journal.pone.0010085. e10085. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell. 2010;9(4):592–606. doi: 10.1111/j.1474-9726.2010.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119(14):1941–1949. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Dean DJ, Brozinick JT, Jr, Cushman SW, Cartee GD. Calorie restriction increases cell surface GLUT-4 in insulin-stimulated skeletal muscle. Am J Physiol. 1998;275(6 Pt 1):E957–E964. doi: 10.1152/ajpendo.1998.275.6.E957. [DOI] [PubMed] [Google Scholar]
- 21.Zhu M, de Cabo R, Anson RM, Ingram DK, Lane MA. Caloric restriction modulates insulin receptor signaling in liver and skeletal muscle of rat. Nutrition. 2005;21(3):378–388. doi: 10.1016/j.nut.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86(1):7–13. doi: 10.1093/ajcn/86.1.7. [DOI] [PubMed] [Google Scholar]
- 23.Han X, Ren J. Caloric restriction and heart function: is there a sensible link? Acta Pharmacol Sin. 2010;31(9):1111–1117. doi: 10.1038/aps.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol. 2011;50(1):117–127. doi: 10.1016/j.yjmcc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Ren J, Sowers JR, Walsh MF, Brown RA. Reduced contractile response to insulin and IGF-I in ventricular myocytes from genetically obese Zucker rats. Am J Physiol Heart Circ Physiol. 2000;279(4):H1708–H1714. doi: 10.1152/ajpheart.2000.279.4.H1708. [DOI] [PubMed] [Google Scholar]
- 26.Awan MM, Saggerson ED. Malonyl-CoA metabolism in cardiac myocytes and its relevance to the control of fatty acid oxidation. Biochem J. 1993;295(Pt 1):61–66. doi: 10.1042/bj2950061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13(10):444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 28.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava AK, Pandey SK. Potential mechanism(s) involved in the regulation of glycogen synthesis by insulin. Mol Cell Biochem. 1998;182(1–2):135–141. [PubMed] [Google Scholar]
- 30.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 31.Muslin AJ, DeBosch B. Role of Akt in cardiac growth and metabolism. Novartis Found Symp. 2006;274:118–126. discussion 126–131, 152-115, 272-116. [PubMed] [Google Scholar]
- 32.Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, Rosenzweig A, Kahn CR, Abel ED, Walsh K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277(40):37670–37677. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 33.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 34.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26(22):3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 35.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 36.Muslin AJ. MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci (Lond) 2008;115(7):203–218. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100(3):328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 38.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5'-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270(29):17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 39.Lindsley JE, Rutter J. Nutrient sensing and metabolic decisions. Comp Biochem Physiol B Biochem Mol Biol. 2004;139(4):543–559. doi: 10.1016/j.cbpc.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281(28):18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- 41.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36(12):2445–2462. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9(10):1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Vatta M. Danon disease as a cause of autophagic vacuolar myopathy. Congenit Heart Dis. 2007;2(6):404–409. doi: 10.1111/j.1747-0803.2007.00132.x. [DOI] [PubMed] [Google Scholar]
- 45.Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA., Jr Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10(3):281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 46.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102(39):13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J. 2001;65(11):965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]