Summary
Background
Recombinant tissue plasminogen activator (rt-PA, alteplase) improved functional outcome in patients treated soon after acute ischaemic stroke in randomised trials, but licensing is restrictive and use varies widely. The IST-3 trial adds substantial new data. We therefore assessed all the evidence from randomised trials for rt-PA in acute ischaemic stroke in an updated systematic review and meta-analysis.
Methods
We searched for randomised trials of intravenous rt-PA versus control given within 6 h of onset of acute ischaemic stroke up to March 30, 2012. We estimated summary odds ratios (ORs) and 95% CI in the primary analysis for prespecified outcomes within 7 days and at the final follow-up of all patients treated up to 6 h after stroke.
Findings
In up to 12 trials (7012 patients), rt-PA given within 6 h of stroke significantly increased the odds of being alive and independent (modified Rankin Scale, mRS 0–2) at final follow-up (1611/3483 [46·3%] vs 1434/3404 [42·1%], OR 1·17, 95% CI 1·06–1·29; p=0·001), absolute increase of 42 (19–66) per 1000 people treated, and favourable outcome (mRS 0–1) absolute increase of 55 (95% CI 33–77) per 1000. The benefit of rt-PA was greatest in patients treated within 3 h (mRS 0–2, 365/896 [40·7%] vs 280/883 [31·7%], 1·53, 1·26–1·86, p<0·0001), absolute benefit of 90 (46–135) per 1000 people treated, and mRS 0–1 (283/896 [31·6%] vs 202/883 [22·9%], 1·61, 1·30–1·90; p<0·0001), absolute benefit 87 (46–128) per 1000 treated. Numbers of deaths within 7 days were increased (250/2807 [8·9%] vs 174/2728 [6·4%], 1·44, 1·18–1·76; p=0·0003), but by final follow-up the excess was no longer significant (679/3548 [19·1%] vs 640/3464 [18·5%], 1·06, 0·94–1·20; p=0·33). Symptomatic intracranial haemorrhage (272/3548 [7·7%] vs 63/3463 [1·8%], 3·72, 2·98–4·64; p<0·0001) accounted for most of the early excess deaths. Patients older than 80 years achieved similar benefit to those aged 80 years or younger, particularly when treated early.
Interpretation
The evidence indicates that intravenous rt-PA increased the proportion of patients who were alive with favourable outcome and alive and independent at final follow-up. The data strengthen previous evidence to treat patients as early as possible after acute ischaemic stroke, although some patients might benefit up to 6 h after stroke.
Funding
UK Medical Research Council, Stroke Association, University of Edinburgh, National Health Service Health Technology Assessment Programme, Swedish Heart-Lung Fund, AFA Insurances Stockholm (Arbetsmarknadens Partners Forsakringsbolag), Karolinska Institute, Marianne and Marcus Wallenberg Foundation, Research Council of Norway, Oslo University Hospital.
Introduction
Recombinant tissue plasminogen activator (rt-PA, alteplase) was licensed in North America in 1996 for intravenous use within 3 h of stroke in selected patients after the publication of the results of the trials undertaken by the National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, MD, USA, in 624 patients.1 A restricted conditional licence for the use of rt-PA within 3 h of ischaemic stroke was granted in Europe in 2002 after publication of two further trials of intravenous rt-PA administered up to 6 h after ischaemic stroke2,3 and a systematic review of 3156 patients.4,5 The European approval allowed treatment, within 3 h, of patients younger than 80 years who also met other strict criteria.
Although the licence restricted the use of rt-PA to within 3 h, the results of the trials6 suggested that rt-PA, given as late as 6 h after ischaemic stroke, might reduce the number of patients with poor functional outcomes.4,5 In that context, the Third International Stroke Trial (IST-3) was initiated in 2000, with the aim of providing evidence from a randomised trial for a wide range of patients, including those older than 80 years, treated between 3–6 h, and with comorbid disorders such as previous stroke. Meanwhile, a meta-analysis of individual patient data from completed trials undertaken in 2004 suggested that intravenous rt-PA might be of benefit up to 4·5 h after stroke,6 with the findings confirmed in 2008 in the ECASS 3 trial of patients younger than 80 years who were treated between 3·0–4·5 h after stroke.7,8 Despite this increasing evidence from 11 trials of 3977 patients who were treated up to 6 h after stroke,9 substantial uncertainties10 have been caused by the lack of precision in the overall estimates of treatment effects for the important outcomes of death and functional outcome, longest time window for net benefit, effect of treatment in people older than 80 years (only 94 patients in this age group were included in the completed trials), and the effect of factors such as stroke severity, stroke subtype, use of antiplatelet drugs before stroke, and other comorbidities (eg, previous stroke, diabetes, or hypertension).
We updated the systematic review of rt-PA to assess the overall effect of intravenous rt-PA when given up to 6 h after stroke for important early and late outcomes, and to address some of the previous key uncertainties.
Methods
We updated the systematic review of trials of rt-PA in acute ischaemic stroke, first published in 1992,11 updated periodically in the Cochrane Database of Systematic Reviews since then.9 We searched using multiple overlapping search methods up to March, 2012 (appendix). We translated non-English language publications and obtained additional information from principal investigators.
We included all randomised trials of rt-PA versus control in patients with definite ischaemic stroke, after having excluded intracerebral haemorrhage and other brain disorders on imaging that might account for the symptoms. Two reviewers independently assessed study quality, applied the inclusion criteria, extracted the data, and resolved discrepancies by discussion or asking the trial investigators. We excluded trials that did not provide relevant clinical outcomes or in which the latest follow-up was less than 1 month after treatment. We aimed to use intention-to-treat data, sought additional unpublished data when necessary, and verified the extracted data with the principal investigators of the trials when possible, avoiding double counting of data when multiple publications arose from the same trial.
We extracted data using a standard form for trial characteristics including inclusion and exclusion criteria; method of randomisation; latest time to treatment; rt-PA dose and administration; other medical treatments; methods and timing of follow-up assessments; and the demographic details of patients. We extracted data for the number of patients allocated rt-PA or control with key early or late outcomes (detailed below) including the numbers within prespecified categories of modified Rankin Scale (mRS) score, Oxford Handicap Score (OHS, a further modification of the mRS), or equivalent by the end of follow-up. We treated mRS and OHS scale points as being equivalent and translated Barthel Index scores to mRS: Barthel scores greater than 60 are equivalent to mRS 0–2 and Barthel scores greater than 90 are equivalent to mRS 0–1.8 We sought to extract data for outcomes in key subgroups defined by delays to treatment (0–3 h, 3–6 h); age (up to and including 80 years or >80 years); presence or absence of visible infarction on imaging; baseline stroke severity; and different antithrombotic regimens. Tabulations of IST-3 data were provided by the trial statistician.
We assessed early outcomes (within 7 days) including death from any cause; fatal intracranial haemorrhage (death attributed to intracranial haemorrhage identified on scanning or at post mortem); death not attributable to intracranial haemorrhage; symptomatic intracranial haemorrhage (as defined in individual trials, but usually as worsening of neurological status contemporaneous with the appearance of new haemorrhage on brain imaging that was sufficient to cause neurological deterioration); and symptomatic infarct swelling (worsening of neurological status contemporaneous with major infarct swelling on imaging). We assessed outcomes at the end of follow-up including death between 7 days and the end of follow-up; total number of deaths from all causes; alive and favourable outcome (defined as mRS or OHS 0–1); alive and independent (defined as mRS or OHS 0–2); and dependency (defined as mRS or OHS 3–5).
Statistical analyses were all prespecified in the review protocol.9 The primary analysis was for all patients treated up to 6 h after stroke. We also assessed the effect of rt-PA given within 3 h and between 3–6 h of the stroke, and in patients aged up to 80 years versus those older than 80 years; for analyses subdivided by time to treatment we have used time to randomisation in IST-3 because this provides an unbiased estimate of time to treatment, which is applicable to patients allocated rt-PA and open control. We calculated odds ratios (ORs) and 95% CI for each outcome, unadjusted for baseline variables, using standard fixed-effects methods,12 and calculated statistical heterogeneity using the χ2 statistic, mentioned when significant heterogeneity was present. We provided data for the comparison of the overall estimate from the 11 previous trials with IST-3 and with the updated overall estimate of effect. We calculated the absolute effects per 1000 patients treated. The figures were prepared in R (version 2.11.1).
Role of the funding source
The sponsors and funding agencies were not involved in the design or execution of the review or interpretation of the data. No drug manufacturing company was involved in the study design, data collection, analysis, and interpretation, writing of the report, or decision to submit this report for publication. All authors saw and approved the final version of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
12 trials of intravenous rt-PA versus control, with 7012 patients (appendix p 4), were included in this systematic review and meta-analysis.1–3,7,13–21 Key methodological characteristics are identified in table 1 and further details are reported elsewhere.9,21 Most data (5276 patients [75%]) were from trials that were done in Europe. We judged the risk of bias to be low across all trials. Most trials included patients with different stroke severities and aetiological subtypes (eg, lacunar in NINDS and IST-3), although the results were not reported by subtype in most trials. Centralised randomisation, by telephone or through a secure webserver to ensure allocation concealment, was used in two trials.7,21 The total rt-PA dose was about 0·6 mg/kg13,14 to 1·1 mg/kg.2 An identical-looking placebo was used in all except two trials.19,21 Final follow-up was at 1 month in two trials,13,14 6 months in the IST-3 trial, and 3 months in the other trials. In four trials, follow-up had to be done by a masked clinician who was not involved in the initial care of the patient;1,7,16–18 mostly postal or masked telephone follow-up was used in IST-3; follow-up masking was not specified in the remaining trials. Functional outcomes were not available for two trials.14,15 All trials included patients who were taking aspirin before the stroke; subcutaneous heparin was allowed within 24 h of rt-PA in three trials;2,3,7 and antithrombotic drugs were not allowed until 24 h after rt-PA in the other trials. Data for all outcomes were not provided in all trials but all available data were used.
Table 1.
Randomised controlled trials of rt-PA in acute ischaemic stroke, design characteristics, and other key factors
|
Trial design |
Patients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Method of randomisation | Dose | Control | Antithrombotic drug use | Age inclusion range | Stroke type | Exclusion criteria* for CT-defined ischaemia | Time after stroke | Final follow-up | Follow-up method (independent) | |
| Mori et al,13 1992 | 31 | Seq Pack | 0·6 mg/kg (34 mg) or 0·9 mg/kg (51 mg) | Placebo | Avoid for 24 h | 18–80 years | Carotid territory; ICA or MCA occlusion on angiography | Visible infarction | <6 h | 4 weeks | At clinic (not stated) |
| JTSG,14 1993 | 98 | Seq Pack | 0·6 mg/kg (34 mg) | Placebo | Avoid for 24 h | 18–80 years | Carotid territory; ICA or MCA occlusion on angiography | Visible infarction | <6 h | 4 weeks | At clinic (not stated) |
| Haley et al,15 1993 | 27 | Seq Pack | 0·85 mg/kg | Placebo | Avoid intravenous heparin for several hours | 18–80 years | Any ischaemic stroke | None | <3 h | 3 months | At clinic (not stated) |
| ECASS,2 1995 | 620 | Seq Pack | 1·1 mg/kg (maximum 100 mg) | Placebo | Aspirin or intravenous heparin not allowed; subcutaneous heparin allowed for <24 h; thereafter, any antithrombotic allowed | 18–80 years | Carotid territory | Visible infarction greater than a third of MCA territory | 6 h | 3 months | At clinic (not stated) |
| NINDS,1 1995 | 624 | Seq Pack | 0·9 mg/kg (maximum 90 mg) | Placebo | Avoid for 24 h | 18–80 years† | Any except very mild and very severe | None | 3 h | 3 months | At clinic (masked independent clinician) |
| ECASS II,3 1998 | 800 | Seq Pack | 0·9 mg/kg (maximum 90 mg) | Placebo | Aspirin or intravenous heparin not allowed; subcutaneous heparin allowed for <24 h | 18–80 years | Carotid territory | Visible infarction greater than a third of MCA territory | 6 h | 3 months | At clinic (not stated) |
| ATLANTIS A,16 2000 | 142 | Seq Pack | 0·9 mg/kg (maximum 90 mg) | Placebo | Avoid for 24 h | 18–80 years | As for NINDS | None | 6 h | 3 months | At clinic (masked independent clinician) |
| ATLANTIS B,17,18 1999 | 613 | Seq Pack | 0·9 mg/kg (maximum 90 mg) | Placebo | Avoid for 24 h | 18–80 years | As for NINDS | Visible infarction greater than a third of MCA territory | Most within 5 h | 3 months | At clinic (masked independent clinician) |
| ECASS 3,7 2008 | 821 | Central telephone or internet based | 0·9 mg/kg (maximum 90 mg) | Placebo | Avoid for 24 h | 18–80 years | As for NINDS | Visible infarction greater than a third of MCA territory | 3·0–4·5 h | 3 months | At clinic (masked independent clinician) |
| Wang et al,19 2003 | 100 | Seq Pack | 0·9 mg/kg (maximum 90 mg) | Open control | Avoid for 24 h | 18–80 years | As for NINDS | Any visible infarction | 6 h | 3 months | At clinic (not stated) |
| EPITHET,20 2008 | 101 | Seq Pack | 0·9 mg/kg (maximum 90 mg) | Placebo | Avoid for 24 h | 18–80 years† | As for NINDS | Visible infarction greater than a third of MCA territory | 3–6 h | 3 months | At clinic (not stated) |
| IST-3,21 2012 | 3035 | Central telephone or internet based | 0·9 mg/kg (maximum 90 mg) | Placebo first 276 patients, open control thereafter | Avoid for 24 h; start aspirin at 24 h unless contraindicated | ≥18 years | All subtypes | Visible infarct only if it appears >6 h after stroke—ie, incompatible with stated time after stroke | 6 h | 6 months | Centralised telephone or postal questionnaire (yes) |
rt-PA=recombinant tissue plasminogen activator. Seq Pack=randomised by taking the next of a sequentially numbered pack of active drug or placebo. ICA=internal carotid artery. MCA=middle cerebral artery.
Haemorrhage and mimics were excluded in all studies.
NINDS included 69 patients and EPITHET included 25 patients older than 80 years.
The effects of rt-PA on the early outcomes are shown in figure 1 and the absolute event rates per 1000 patients treated with estimates of heterogeneity are provided in table 2. Data for deaths within 7 days, available from eight trials,2,3,7,13,15,19–21 showed that 8·9% of patients allocated rt-PA and 6·4% allocated control died within 7 days (figure 1), an absolute increase of 25 deaths per 1000 individuals treated (table 2).
Figure 1.
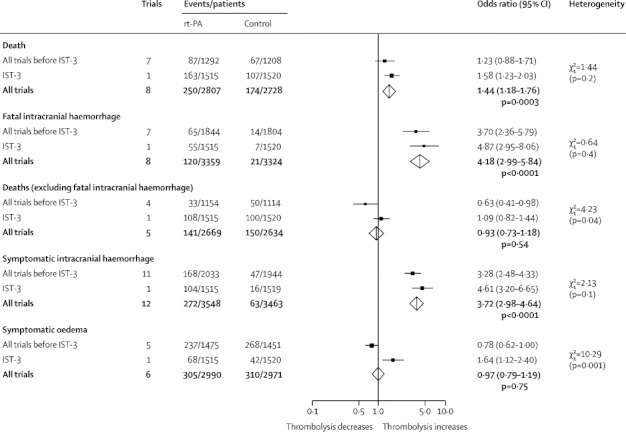
Effects of rt-PA on early outcomes (7 days)
Data are numbers, unless otherwise indicated. Degrees of freedom is 1. Treatment was administered up to 6 h after the stroke. rt-PA=recombinant tissue plasminogen activator. IST-3=Third International Stroke Trial.
Table 2.
Absolute effects of rt-PA per 1000 patients treated and between trial heterogeneity, all outcomes
| Effect per 1000 patients treated (95% CI) |
Heterogeneity |
||||
|---|---|---|---|---|---|
| χ2 | Df | p value | |||
| Events within 7 days, treatment up to 6 h after stroke | |||||
| Death (total) | 25 (11 to 39) | 7·34 | 7 | 0·39 | |
| Fatal intracranial haemorrhage | 29 (23 to 36) | 6·29 | 7 | 0·51 | |
| Death (not due to intracranial haemorrhage) | −4 (−16 to 8)* | 5·73 | 4 | 0·22 | |
| Symptomatic intracranial haemorrhage | 58 (49 to 68) | 15·24 | 11 | 0·17 | |
| Symptomatic oedema | −2 (−18 to 13)* | 17·47 | 5 | 0·004 | |
| Events by end of follow-up, treatment up to 6 h after stroke | |||||
| Deaths between 7 days and end of follow–up | −22 (−39 to −4) | 4·88 | 5 | 0·43 | |
| Deaths by end of follow–up | 7 (−11 to 25)* | 17·70 | 11 | 0·09 | |
| Alive and independent (mRS 0–2) | 42 (19 to 66) | 17·06 | 9 | 0·05 | |
| Alive and favourable outcome (mRS 0–1) | 55 (33 to 77) | 21·12 | 9 | 0·01 | |
| Dependent (mRS 3–5) | −50 (−73 to −27)* | 19·08 | 9 | 0·02 | |
| Outcome by time to treatment | |||||
| Alive, favourable outcome (mRS 0–1), <3 h | 87 (46 to 128) | 7·90 | 5 | 0·16 | |
| Alive and independent (mRS 0–2), <3 h | 90 (46 to 135) | 1·87 | 5 | 0·87 | |
| Alive and independent (mRS 0–2), 3–6 h | 18 (−10 to 45)* | 9·86 | 6 | 0·13 | |
| Dead by end of follow-up, <3 h | −15 (−55 to 25)* | 8·26 | 6 | 0·22 | |
| Dead by end of follow-up, 3–6 h | 18 (−3 to 39)* | 10·68 | 6 | 0·10 | |
| Symptomatic intracranial haemorrhage, <3 h | 68 (49 to 87) | 3·12 | 5 | 0·68 | |
| Symptomatic intracranial haemorrhage, 3–6 h | 58 (46 to 70) | 3·55 | 6 | 0·74 | |
| Outcome by age and time to treatment | |||||
| Treatment up to 6 h after stroke | |||||
| Alive and independent (mRS 0–2), ≤80 years | 43 (16 to 70) | 19·22 | 9 | 0·02 | |
| Alive and independent (mRS 0–2), >80 years | 38 (−3 to 79) | 2·14 | 2 | 0·34 | |
| Treatment up to 3 h | |||||
| Alive and independent (mRS 0–2), ≤80 years | 95 (35 to 155) | 3·64 | 5 | 0·60 | |
| Alive and independent (mRS 0–2), >80 years | 96 (35 to 157) | 0·67 | 1 | 0·41 | |
| Treatment between 3–6 h | |||||
| Alive and independent (mRS 0–2), ≤80 years | 23 (−8 to 54) | 8·91 | 6 | 0·18 | |
| Alive and independent (mRS 0–2), >80 years | −5 (−61 to 50) | 0 | 1 | 0·95 | |
rt-PA=recombinant tissue plasminogen activator. Df=degrees of freedom. mRS=modified Rankin Scale.
A minus indicates fewer events per 1000 patients treated with rt-PA.
Data for fatal intracranial haemorrhage within 7 days, available from eight trials,1–3,7,15–18,21 showed that 3·6% of patients allocated rt-PA and 0·6% of those allocated the control died from intracranial haemorrhage (figure 1), an absolute increase of 29 deaths per 1000 patients.
There was no evidence that rt-PA increased the number of deaths within 7 days from causes other than intracranial haemorrhage (figure 1), implying that the excess of early deaths with rt-PA is mostly from fatal intracranial haemorrhage, although it should be noted that data were only available from five trials.2,3,7,15,21 Although no between-trial heterogeneity was noted (table 2), there was marginal heterogeneity between previous trials as a group and IST-3 (p=0·04; figure 1).
Data for symptomatic intracranial haemorrhage within 7 days, available from all 12 trials, showed that 7·7% of patients allocated rt-PA and 1·8% allocated control developed this haemorrhage (figure 1; appendix p 5). The crude estimate of the absolute excess of symptomatic intracranial haemorrhage with rt-PA was 58 per 1000 patients treated (table 2).
Data for symptomatic infarct swelling within 7 days was available from six trials;1–3,7,17,18,21 10·2% allocated rt-PA and 10·4% allocated control developed infarct swelling (figure 1), with substantial between-trial heterogeneity (table 2) mostly due to differences between IST-3 and previous trials (p=0·001 figure 1).
The effects on outcomes at final follow-up are shown in figure 2 and the absolute event rates in table 2. Data for deaths between 7 days and the end of follow-up, available from eight trials,2,3,7,13,15,19–21 showed that 11·5% of patients allocated rt-PA and 13·6% allocated control died in this period (figure 2), a reduction of 22 deaths per 1000 individuals treated with rt-PA (table 2).
Figure 2.
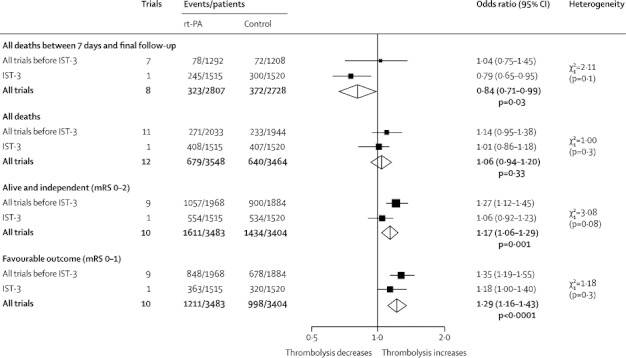
Effects of rt-PA on outcomes at final follow-up
Data are numbers, unless otherwise indicated. Treatment was administered up to 6 h after the stroke. rt-PA=recombinant tissue plasminogen activator. IST-3=Third International Stroke Trial. mRS=modified Rankin Scale.
Data for total number of deaths from all causes by the end of follow-up, available from all 12 trials, showed that 19·1% allocated rt-PA and 18·5% allocated control died (figure 2; appendix p 5), equivalent to a non-significant increase in deaths of seven per 1000 patients treated with rt-PA.
Data for patients who were alive and independent (mRS 0–2) were available from ten trials (appendix p 6).1–3,7,13,16–21 46·3% of patients allocated rt-PA and 42·1% allocated control were alive and independent (mRS 0–2) at the end of follow-up (figure 2), an absolute increase of 42 per 1000 people treated with rt-PA (table 2).
Data for patients who were alive with favourable outcome (mRS 0–1) were available in the same ten trials (appendix p 6). 34·8% of patients allocated rt-PA and 29·3% allocated control had mRS 0–1 at the end of follow-up (figure 2), equivalent to 55 more patients alive and almost symptom free per 1000 treated (table 2). Heterogeneity was noted between all trials (table 2) but not between previous trials combined and IST-3 (figure 2).
Data for patients treated within 3 h and between 3–6 h after stroke were available for three key outcomes (figure 3, table 2). Among patients treated within 3 h of stroke, using a cutoff of mRS 0–1 (six trials1–3,16,18,21), 283 (31·6%) of 896 allocated rt-PA and 202 (22·9%) of 883 allocated control achieved a favourable outcome (OR 1·61, 95% CI 1·30–1·99, p<0·0001), an absolute increase of 87 per 1000 individuals given rt-PA. Among patients treated within 3 h of stroke (six trials1–3,16,18,21), 40·7% allocated rt-PA and 31·7% allocated control achieved mRS 0–2 (figure 3), an absolute increase of 90 per 1000 patients treated (table 2). Of the patients treated between 3–6 h after stroke (seven trials2,3,7,16,17,20,21), 47·5% allocated rt-PA and 45·7% allocated control achieved mRS 0–2 (figure 2), an absolute increase of 18 per 1000 patients treated. The difference in ORs between the subgroups treated within 3 h and between 3–6 h was significant (χ2=9·49, 2 degrees of freedom (df), p=0·002). There were slightly fewer deaths by the end of follow-up in people treated within 3 h (seven trials;1–3,15,16,18,21 figure 3), equivalent to 15 fewer deaths per 1000 (table 2), but slightly more deaths in those treated between 3–6 h (seven trials;2,3,7,16,17,20,21 figure 3), equivalent to an excess of 18 per 1000 individuals who were treated with rt-PA. However, the difference in ORs between the subgroups treated within 3 h and between 3–6 h was not significant (χ2=3·20, df 2, p=0·07). No difference was noted in the summary ORs for symptomatic intracranial haemorrhage for those treated within 3 h (six trials;1–3,16,18,21 figure 3), equivalent to 68 more such haemorrhages per 1000 treated individuals (table 2), and for those treated between 3–6 h (seven trials;2,3,7,16,17,20,21 figure 3), equivalent to 58 more per 1000 treated patients (table 2; test for difference between subgroups χ2=0·57, df 2, p=0·45). The results were the same when the analysis was restricted to the trials of treatment in both time windows (data not shown). No heterogeneity was noted between trials, or between previous trials combined and IST-3, for any of the outcomes within 3 h and between 3–6 h.
Figure 3.
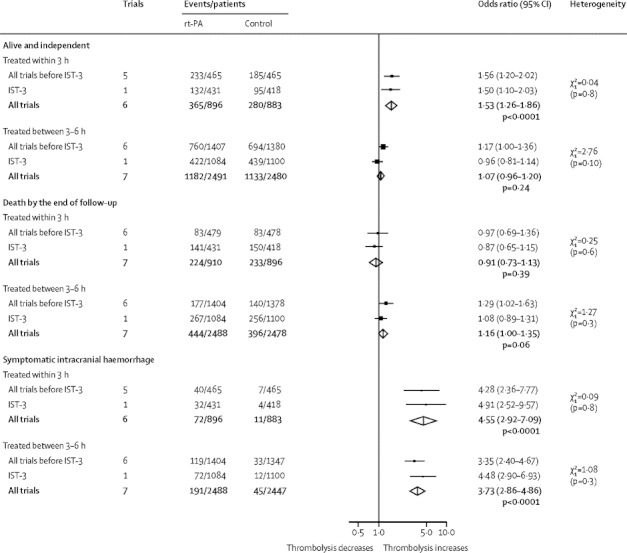
Effects of rt-PA on alive and independent (mRS 0–2) and death by the end of follow-up and on symptomatic intracranial haemorrhage within the first 7 days, by time to treatment
Data are numbers, unless otherwise indicated. rt-PA=recombinant tissue plasminogen activator. IST-3=Third International Stroke Trial. mRS=modified Rankin Scale.
Three trials included a total of 1711 patients older than 80 years (25 from EPITHET, 69 from NINDS, the rest from IST-3).1,20,21 Among patients older than 80 years who were treated within 6 h, 27·2% allocated rt-PA and 23·4% allocated control were mRS 0–2 (p=0·07; figure 4), an increase of 38 per 1000 individuals were alive and independent (table 2). Among patients aged 80 years and younger, 52·5% allocated rt-PA and 48·3% allocated control were alive and independent (mRS 0–2) at the end of follow-up (p=0·009), an increase of 43 per 1000 people (table 2). For patients older than 80 years treated within 3 h, 28·9% allocated rt-PA and 19·3% allocated control were alive and independent (figure 4; p=0·003), an increase of 96 per 1000 people (table 2). Among those 80 years and younger treated within 3 h, 49·6% allocated rt-PA and 40·1% allocated control were alive and independent (figure 4; p=0·001), an increase of 95 per 1000 people (table 2). Among people treated between 3–6 h after stroke, of those aged 80 years and younger 52·6% allocated rt-PA and 50·3% allocated control achieved mRS 0–2 at the end of follow-up compared with 25·9% allocated rt-PA and 26·4% allocated control who were older than 80 years (figure 4).
Figure 4.
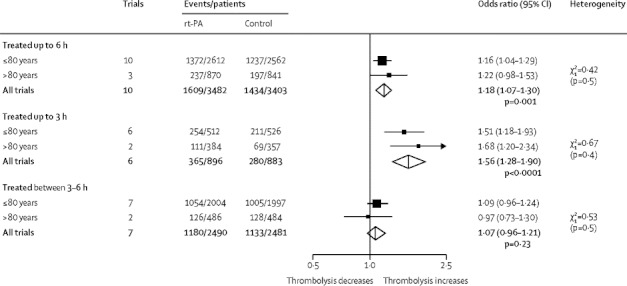
Effect of rt-PA on alive and independent at the end of follow-up, subgrouped by age and time to treatment
Data are numbers, unless otherwise indicated. rt-PA=recombinant tissue plasminogen activator. IST-3=Third International Stroke Trial.
Tabular data were too few to provide reliable evidence about the effects of antithrombotic drugs in addition to rt-PA, and were not available to assess the effect of presence or absence of visible infarction on imaging or by baseline stroke severity on main outcomes.
Discussion
The data reported in this systematic review and meta-analysis for 7012 patients showed that for every 1000 patients allocated intravenous rt-PA up to 6 h after stroke, 42 more patients were alive and independent (mRS 0–2); 55 more were alive with a favourable outcome (mRS 0–1) at the end of follow-up. This benefit occurred despite an increase in the number of early symptomatic intracranial haemorrhages and early deaths. These early hazards were offset by a reduction in the number of deaths between 7 days and the end of follow-up, and so, by the end of follow-up, no effect on deaths from all causes was apparent, and the number who were dependent (mRS 3–5) was reduced. Earlier treatment is better, leading to an increase in the number of patients who were alive with favourable outcomes (mRS 0–1) and alive and independent (mRS 0–2) with treatment within 3 h of stroke. Among the 1711 patients older than 80 years, the absolute benefits from rt-PA were at least as large as for the younger patients, especially with early treatment.
Do the effects of rt-PA in the 3035 patients in IST-3 (95% of whom did not meet contemporaneous European licence criteria) differ materially from the effects in the 3977 patients from 11 previous trials (who largely did meet these criteria)? The characteristics of patients in IST-3 that excluded them from the licence criteria were chiefly age and time: 53% were older than 80 years, 72% were treated more than 3 h after stroke onset. A large proportion had severe strokes and 146 (5%) had National Institutes of Health Stroke Scale greater than 25, hence the fairly high numbers of deaths in both the rt-PA and control groups. There was no evidence to suggest that any of the beneficial or adverse effects of rt-PA were qualitatively different in these outside-licence patients from individuals in earlier trials, except for non-intracranial-haemorrhage early deaths and symptomatic infarct swelling. The proportion of patients with symptomatic infarct swelling in IST-3 (3·6%) was less than that of previous trials (17·3%) but the results of IST-3 showed an increase in symptomatic infarct swelling with rt-PA, whereas those of previous trials had suggested a reduction (p=0·06). These differences could be attributable to several factors: the analysis of symptomatic oedema and its definition were prespecified in IST-3 but not in five of 11 previous trials that provided relevant data; patients in IST-3 were older, had more severe strokes (hence larger infarcts), and were treated later than in previous trials. These and other possibly aggregate characteristics could have changed the effect of rt-PA on symptomatic infarct swelling and in turn the effect on non-intracranial-haemorrhage early deaths; these points merit further analysis.
The updated review provides additional insights. First, the finding that fewer deaths occurred with rt-PA between 7 days and late follow-up in IST-3 confirms a pattern suspected in previous trials, and indicates that the excess hazard with rt-PA is largely attributable to symptomatic intracranial haemorrhage and occurs early, so that patients who survive the acute phase without intracranial haemorrhage benefit. One explanation for this might be that more disabled stroke survivors have an ongoing higher risk of death;22 rt-PA, by shifting the balance towards better functional outcome, reduces the risk of death in the long term. The longer follow-up in IST-3 (6 months vs 3 months in previous trials), might have allowed this net balance of early hazard versus later benefit to emerge more clearly. Second, the benefits of rt-PA occur in severe and milder strokes. Third, as suggested by the results of non-randomised studies,23,24 the relative and absolute benefits of rt-PA are at least as large in older as in younger people (figure 4, table 2). Fourth, the new data reinforce the evidence that benefit is greatest when rt-PA is given within 3 h. Therefore, efforts to reduce treatment delays should continue, particularly for older people.
The latest time window for benefit remains unclear. Although benefit from thrombolysis clearly declines with increasing delay to treatment,8 these data suggest that benefit probably extends beyond 4·5 h, possibly as late as 6 h in some patients, though the time probably varies with key individual or combined patients' characteristics, which were not possible to identify with just summary unadjusted data. Patients presenting later are different (eg, less severe stroke) than those presenting earlier. The lower benefit with later treatment is not due to more symptomatic intracranial haemorrhage because the odds of this haemorrhage with rt-PA are actually slightly lower in patients treated between 3–6 h than within 3 h (figure 3, table 2). Thus, non-significantly more deaths and the reduction in the OR for alive and independent at the end of follow-up for treatment between 3–6 h might indicate less benefit with later treatment—ie, less tissue to salvage, rather than simply more hazard.
An analysis of individual patient data from all 12 trials is essential to find out the effect of rt-PA in patients with different severities and subtypes of stroke (particularly lacunar stroke), by appearance of the ischaemic lesion or rest of the brain on imaging, in key subgroups of patients—eg, those in atrial fibrillation, on aspirin before stroke, with high blood pressure or diabetes—with narrow time windows and functional outcome strata, and by different doses of rt-PA, and to define the latest time for benefit. Identifying how to avoid symptomatic intracranial haemorrhage is critical, because it is the single largest hazard. Ongoing trials, Thrombolysis in Elderly Stroke Patients in Italy (TESPI),25 Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED), or EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND26) are addressing treatment in the elderly or at later times and ways to reduce the risk of haemorrhage—eg, whether a lower dose of rt-PA or blood pressure lowering can reduce symptomatic intracranial haemorrhage. Trials to investigate alternative doses, routes of thrombolytic drug administration, different thrombolytic drugs, and mechanical thrombectomy and advanced imaging could help identify patients for whom these alternative therapies might be more effective than is intravenous rt-PA. Information about the effect of thrombolysis on long-term survival free of dependence is sparse (with NINDS1 being the only trial so far in which outcomes at 12 months have been reported; for IST-3 the intention is to report functional outcome up to 18 months and overall survival thereafter21) but this study will be important for understanding the true health economic effect of thrombolytic treatment. If small gains in functional ability by 3–6 months translate into greater long-term survival free of disability,22 this is likely to reduce health-care costs and increase quality of life and cost-effectiveness.
Acknowledgments
Acknowledgments
UK Medical Research Council, Stroke Association, University of Edinburgh, National Health Service Health Technology Assessment Programme, Swedish Heart-Lung Fund, AFA Insurances Stockholm (Arbetsmarknadens Partners Forsakringsbolag), Karolinska Institute, Marianne and Marcus Wallenberg Foundation, Research Council of Norway, Oslo University Hospital funded this study. The authors of the Cochrane Review have not received financial support from any pharmaceutical company to undertake the review. We are extremely grateful to Lisa Blackwell, Clinical Trial Service Unit, Oxford, for preparing all the figures and Colin Baigent for very helpful comments during the preparation of this and the previous version of the Cochrane Review. We also thank Charles Warlow and Martin Dennis for helpful comments. We sincerely thank the principal investigators of previous trials for additional data during previous updates of the Cochrane Review and Steve Davis for data for patients older than 80 years in this update. We also thank the IST-3 Trial Steering Committee including the national coordinators and the data monitoring committee who commented on the draft report (details in reference 22).
Contributors
JMW wrote the protocol, undertook the searching, data extraction, and verification, sought additional information and verified information with trialists, analysed, drafted, and edited the report for the first published version and all the subsequent updates. VM and EB undertook searching, trial evaluation, data extraction, and verification in previous updates. GC provided data from IST-3. VM, EB, GdZ, PS, RIL, and GC did the data verification in this update and edited the report. All authors approved the report for submission.
Conflicts of interest
JMW, VM, EB, PS, RIL, and GC are all investigators in the IST-3 trial. JMW was a member of the expert neuroradiological adjudication committee for IST-3 and ECASS 3. GdZ was on the data and safety monitoring committees of the ECASS and ECASS II trials funded by Boehringer Ingelheim. JMW has received reimbursement for expenses for speaking at and attending meetings arranged by Boehringer Ingelheim, is not or has not been on any industry expert panels but did provide independent information to Boehringer Ingelheim to assist with the European license application for rt-PA. RIL has received payment in his role as conference Scientific Committee member and for occasional lectures from Boehringer Ingelheim; he has attended national stroke meetings that were organised and funded by Boehringer Ingelheim; and he is not a member of any industry advisory boards. EB and PS have received payment for lectures at meetings arranged by Boehringer Ingelheim, and reimbursement for costs for attending these meetings.
Supplementary Material
References
- 1.The National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group Tissue plasminogen activator for acute ischaemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Fieschi C. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Fieschi C. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Sandercock PAG, Berge E. Thrombolytic therapy with recombinant tissue plasminogen activator for acute ischemic stroke. Where do we go from here? A cumulative meta-analysis. Stroke. 2003;34:1437–1442. doi: 10.1161/01.STR.0000072513.72262.7E. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, del Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD000213. CD000213. [DOI] [PubMed] [Google Scholar]
- 6.Hacke W, Donnan G, Fieschi C. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 7.Hacke W, Kaste M, Bluhmki E. Thrombolysis with alteplase 3 to 4·5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 8.Lees KR, Bluhmki E, von Kummer R. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 9.Wardlaw JM, Murray VE, Berge E, del Zoppo GJ. Thrombolysis in acute ischaemic stroke. Cochrane Database Syst Rev. 2009;4 doi: 10.1002/14651858.CD000213.pub2. CD000213. [DOI] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Sandercock PA, Murray V. Should more patients with acute ischaemic stroke receive thrombolytic treatment? BMJ. 2009;339:b4584. doi: 10.1136/bmj.b4584. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw JM, Warlow CP. Thrombolysis in acute ischaemic stroke: does it work? Stroke. 1992;23:1826–1839. doi: 10.1161/01.str.23.12.1826. [DOI] [PubMed] [Google Scholar]
- 12.Antiplatelet Trialists' Collaboration Collaborative overview of randomised trials of antiplatelet therapy— I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ. 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 13.Mori E, Yoneda Y, Tabuchi M. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology. 1992;42:976–982. doi: 10.1212/wnl.42.5.976. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Hayakawa T, Kiuchi H, for the Japanese Thrombolysis Study Group Intravenous tissue plasminogen activator ameliorates the outcome of hyperacute embolic stroke. Cerebrovasc Dis. 1993;3:269–272. [Google Scholar]
- 15.Haley EC, Jr, Brott TG, Sheppard GL. Pilot randomized trial of tissue plasminogen activator in acute ischemic stroke. The TPA Bridging Study Group. Stroke. 1993;24:1000–1004. doi: 10.1161/01.str.24.7.1000. [DOI] [PubMed] [Google Scholar]
- 16.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0– to 6–hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Thrombolytic therapy in acute ischemic stroke study investigators. Stroke. 2000;31:811–816. doi: 10.1161/01.str.31.4.811. [DOI] [PubMed] [Google Scholar]
- 17.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 18.Albers GW, Clark WM, Madden KP, Hamilton SA. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke. 2002;33:493–495. doi: 10.1161/hs0202.102599. [DOI] [PubMed] [Google Scholar]
- 19.Wang SY, Wang XL, Zeng H. Early intravenous thrombolysis with recombinant tissue plasminogen activator for acute cerebral infarction. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2003;15:542–545. [PubMed] [Google Scholar]
- 20.Davis SM, Donnan G, Parsons MW. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 21.The IST-3 collaborative group The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012 doi: 10.1016/S0140-6736(12)60768-5. published online May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruins Slot K, Berge E, Dorman P. Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ. 2008;336:376–379. doi: 10.1136/bmj.39456.688333.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra NK, Ahmed N, Andersen G. Thrombolysis in very elderly people: controlled comparison of SITS International Stroke Thrombolysis Registry and Virtual International Stroke Trials Archive. BMJ. 2010;341:c6046. doi: 10.1136/bmj.c6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatnagar P, Sinha D, Parker RA, Guyler P, O'Brien A. Intravenous thrombolysis in acute ischaemic stroke: a systematic review and meta-analysis to aid decision making in patients over 80 years of age. J Neurol Neurosurg Psychiatry. 2011;82:712–717. doi: 10.1136/jnnp.2010.223149. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzano S, Toni D, for the TESPI trial Investigators TESPI (Thrombolysis in Elderly Stroke Patients in Italy): a randomized controlled trial of alteplase (rt-PA) versus standard treatment in acute ischaemic stroke in patients aged more than 80 years where thrombolysis is initiated within three hours after stroke onset. Int J Stroke. 2012;7:250–257. doi: 10.1111/j.1747-4949.2011.00747.x. [DOI] [PubMed] [Google Scholar]
- 26.Ma H, Parsons MW, Christensen S. A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) Int J Stroke. 2012;7:74–80. doi: 10.1111/j.1747-4949.2011.00730.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


