Abstract
Peripheral leukocyte recruitment in neuroinflammatory conditions can exacerbate brain tissue damage by releasing cytotoxic mediators and by increasing vascular permeability. Cyclooxygenase (COX)-derived prostaglan-dins promote the migration of several immune cells in vitro, however, the specific roles of COX-1 and -2 on leukocyte recruitment in vivo have not been investigated. To examine the specific effects of COX-1 or COX-2 deficiency on neuroinflammation-induced leukocyte infiltration, we used a model of intracerebroventricular lipopolysaccharide (LPS)-induced neuroinflammation in COX-1−/−, COX-2−/−, and their respective wild-type (WT) (+/+) mice. After LPS, leukocyte infiltration and inflammatory response were attenuated in COX-1−/− and increased in COX-2−/− mice, compared with their respective WT controls. This influx of leukocytes was accompanied by a marked disruption of blood–brain barrier and differential expression of chemokines. These results indicate that COX-1 and COX-2 deletion differentially modulate leukocyte recruitment during neuroinflammation, and suggest that inhibition of COX-1 activity is beneficial, whereas COX-2 inhibition is detrimental, during a primary neuroinflammatory response.
Keywords: LPS, cyclooxygenase, neuroinflammation, leukocyte
Introduction
Cyclooxygenase (COX) catalyze the rate-limiting step in the conversion of arachidonic acid to prostaglandins (PGs) and thromboxanes, lipid mediators involved in several physiological and pathological processes.1,2 Inflammation, associated with an increased expression of COX and elevated levels of PGs, has been implicated in a variety of acute and chronic neurologic and neurodegenerative disorders, including Alzheimer’s disease,3 Parkinson’s disease,4 and amyotrophic lateral sclerosis.5 The two distinct COX isoforms, COX-1 and COX-2, share 60% homology in their amino acids sequence and have comparable kinetics.1 However, the two isoforms differ in regulatory mechanisms, tissue distribution, preferential coupling to upstream and downstream enzymes,6 and in modulating lipopolysaccharide (LPS)-induced neuroinflammatory response.7,8
Peripheral blood leukocytes infiltrate the central nervous system (CNS) after various inflammatory stimuli and can exacerbate brain tissue injury by releasing various cytotoxic and inflammatory mediators and increasing vascular permeability.9,10 Interestingly, COX-derived PGE2 in the brain promotes migration in vitro of endothelial,11 monocyte-derived dendritic,12,13 mast cells,14 and macrophages,15 as well as breakdown of the blood–brain barrier (BBB).16 Supporting a role for prostaglandin in BBB breakdown and leukocyte infiltration, administration of non-steroidal anti-inflammatory drugs that inhibit COX activity provides partial amelioration of BBB disruption,17–19 and reduces leukocyte accumulation at the inflammatory site 20–22 and this effect is reversed by local application of PGE2.20
We have shown earlier that glial activation, cytokine production, oxidative stress, and neuronal damage in response to LPS are attenuated in COX-1−/− mice and increased in COX-2−/− mice compared to their respective wild-type (WT) controls.7,8 As we have shown earlier that proinflammatory cytokines and chemokines, which are involved in leukocyte influx into the inflamed brain, are reduced in COX-1−/− mice and increased in COX-2−/− mice,7,8 we hypothesized that COX-1 and COX-2 deficiency differentially modulate leukocyte recruitment and BBB permeability in response to LPS-induced neuroinflammation.
Thus, we examined the effect of COX-1 or COX-2 genetic deletion on leukocyte infiltration into the brain after intracerebroventricular (i.c.v.) LPS injection. I.c.v. injection of LPS has been used by our and other groups as a model of direct activation of brain innate immunity.7–8,23 Centrally injected LPS causes a robust change in brain expression of genes involved in inflammatory response, learning, and memory.24 Our data indicate that LPS-induced leukocyte infiltration was less severe in COX-1−/− mice, and significantly increased in COX-2−/− mice compared to their respective WT mice, and that these changes were accompanied by a differential expression of specific chemokines and BBB disruption.
Materials and methods
Mice and stereotaxic i.c.v. injection
Three-month-old male homozygous (COX-1−/− and COX-2−/− mice and their respective WT mice (COX-1 +/+ and COX-2 +/+) on a C57BL/6–129/Ola genetic background were used.25,26 Stereotaxic microinjection of LPS was performed as described earlier.8 Briefly, mice were anesthetized with ketamine (100 mg kg−1) and xylazine (10 mg kg−1, i.p.) and positioned in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA, USA). Vehicle (sterile saline, 5 μl) or LPS (Escherichia coli serotype 055:B5; 5 μg in 5 μl of sterile saline) was administered into the cerebral lateral ventricle using a 10 μl syringe with a fine needle (World Precision Instruments, Sarasota, FL, USA) and a syringe pump (Stoelting, Wood Dale, IL, USA) at a rate of 1 μl min−1. This dose of LPS and time point (24 h) have been shown earlier to induce a robust neuroinflammatory response and neuronal damage from our and other laboratories.7,8,23,24,27–29 The coordinates for the sterotaxic injections were −2.3 mm dorsal/ ventral, −1.0 mm lateral, and −0.5 mm anterior/posterior from the bregma.30 The needle was kept in this position for an additional 5 min after injection and then retrieved slowly out of the brain. All animal experiments were approved by the National Institutes of Health (NIH) Animal Care and Use Committee in accordance with NIH guidelines on the care and use of laboratory animals.
Histology
After transcardial perfusion with phosphate-buffered saline and subsequent 4% paraformaldehyde, we obtained 30 μm cryosections from the brains.8 Sections were incubated in primary antibodies, Iba-1 (Wako, Osaka, Japan), 7/4 (Serotec, Raleigh, NC, USA), and myeloperoxidase (MPO) (Cell Sciences, Canton, MA, USA) overnight at 4°C followed by appropriate biotinylated secondary antibody (Vector Laboratories, Burlingame, CA, USA). Primary antibody incubation omitted for assessment of BBB disruption using biotinylated anti-mouse IgG (Vector Laboratories).31 Sections were then incubated in Vectastain ABC solution (Vector Laboratories) and developed by diaminobenzidine incubation (Sigma). As cerebral neutrophil infiltration was obvious on digital imaging, the number of 7/4+ cells per section was quantified as described earlier.32,33 The number of neutrophils per section was quantified by counting the number of 7/4-stained cell bodies within 0.16 mm2 area of the CA3. For each measurement, two blinded independent investigators counted three sections (−1.58, −1.7, and −1.82 mm from the bregma) per brain and three to four brains per group.
Flow cytometry
Phosphate-buffered saline-perfused brains from individual mice were processed separately. Single-cell suspensions were obtained by homogenization through 70-μm cell strainers (BD Biosciences) in RPMI medium containing 10% FCS and blocked and stained for surface markers as described earlier.34,35 Cells were labeled with anti-CD45-phycoerythrin, anti-CD11b-FITC, and anti-Gr-1-allophycocynin antibody, or isotype-matched control antibodies (BD Biosciences) and then analyzed using a FACScan flow cytometry and Cell-Quest Pro software (BD Biosciences). For analysis of microglia, macrophages, and granulocytes, cells were gated on side scatter versus CD11b, followed by forward scatter versus CD11b, and forward scatter versus CD45. Microglia were separated from infiltrated leukocytes based on CD45 expression, with low levels of CD45 (CD45low) identifying CD11b+ microglia and high levels of CD45 (CD45high) identifying CD11b+ macrophages.35–37 Granulocytes were distinguished from macrophages based on expression of Gr-1.38 Quadrants were set on the basis of fluorescence levels using isotype-matched control antibodies and the percentage of CD11b+ CD45high and CD45highGr-1high cells were presented using earlier described methods.34,39
Enzyme-linked immunosorbent assay
The levels of CCL2, CXCL2 (R&D Systems, Minneapolis, MN, USA), and MPO (Cell Sciences) in plasma and brain were measured with commercially available enzyme-linked immunosorbent assay (ELISA) kits, as described earlier.7 All steps of this assay were performed following the manufacturer’s recommendations.
Quantitative real-time PCR
Brain total RNA was extracted using RNeasy Lipid Tissue Midi kit (Qiagen, Valencia, CA, USA) as directed by the manufacturer. Quantitative real-time PCR was performed as described earlier.8
Neutropenia
Polymorphonuclear neutrophils (PMNs) were depleted by intraperitoneal administration of rabbit anti-mouse PMN antibody (3 ml kg−1; Accurate Chemical and Scientific, Westbury, NY, USA) daily for 3 days, which induced neutropenia in the mice on the day of LPS injection.31,40,41 This resulted in a circulating absolute neutrophil count of <350 cells per μl by the day of injection.42 Additional doses of anti-PMN were given in the morning of injection. The control group received the same amount of normal rabbit anti-mouse IgG (Accurate Chemical and Scientific) as a negative control.31,40,41
Statistical analysis
Data are presented as mean±s.e.m. Differences in mean values were compared by ANOVA and were considered significant at P<0.05.
Results
LPS causes microglial activation and leukocyte infiltration
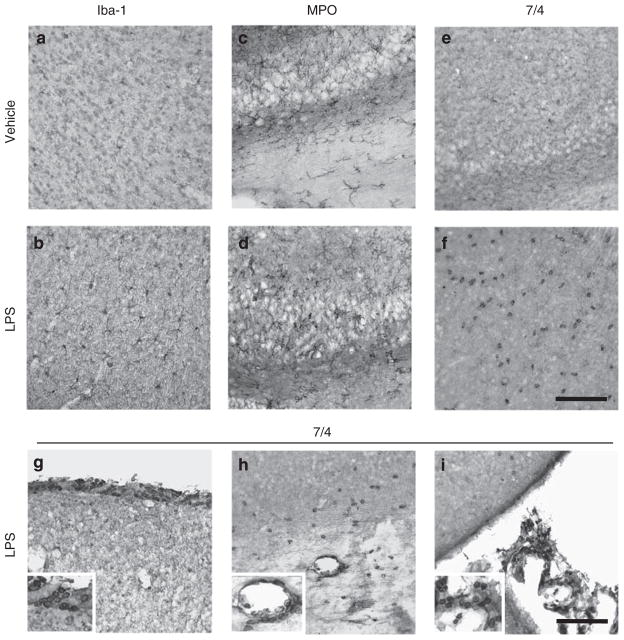
To assess LPS-induced microglial activation and leukocyte recruitment, we performed immunohistochemistry using Iba-1, a microglial marker, MPO, a key oxidative enzyme abundantly present in neutrophils,43 and 7/4, a neutrophil marker.44 Intense Iba-1+ microglia with enhanced staining intensity and hypertrophy appearance were observed in LPS-injected mice (Figure 1b). A number of MPO+ cells with morphology suggestive of reactive microglia and neutrophils were detected in brain of LPS-injected mice (Figure 1d). We also found a marked increase in 7/4+ neutrophils, with small and round appearance, in the parenchyma of LPS-injected mice (Figure 1f), whereas 7/4 immunostaining was low in the brain of vehicle-injected mice (Figure 1e). LPS injection induced microglial activation and strong neutrophils infiltration into the brain through potential entry routes including meninges (Figure 1g), parenchymal vessels (Figure 1h), and choroid plexuses (Figure 1i) as described earlier.45
Figure 1.
I.c.v. LPS induces microglial activation and leukocyte infiltration. (a, b) Iba-1 immunohistochemistry revealed highly ramified microglia in vehicle-injected mice (a) compared with increased microglial activation in LPS-injected mice whose cells have shorter and thicker processes and bigger cell bodies (b). (c–f) MPO and 7/4 immunostaining showed numerous neutrophils in LPS-injected mice (d, f), but not in vehicle-injected mice (c, e). (g–i) LPS led to the appearance of numerous 7/4+ neutrophils around meninges (g), parenchymal vessel (h), and choroid plexus (i). Scale bar, 100 μm.
COX-1 and COX-2 differentially regulate leukocyte infiltration
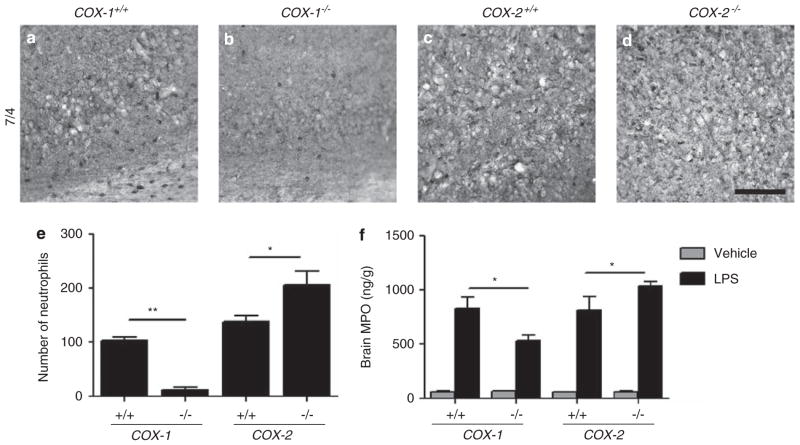
To examine the possibility that each COX isoform differentially regulates the inflammatory response after LPS7,8 through recruitment of peripheral leukocytes, we first performed immunohistochemistry using the 7/4 antibody and quantified the number of neutrophils in the hippocampus of COX-1−/−, COX-2−/−, and their WT mice 24 h after LPS injection. The number of 7/4+ neutrophils was increased in the hippocampus of COX-2−/− mice compared with WT mice (Figures 2d and e), whereas it was significantly decreased in COX-1−/− mice (Figures 2b and e). We further assessed whether deletion of COX-1 or -2 affects levels of brain MPO. Levels of brain MPO were decreased in LPS-injected COX-1−/− mice and increased in COX-2−/− mice compared with their respective WT mice (Figure 2f).
Figure 2.
Neutrophil infiltration in COX-1−/−, COX-2−/−, and their WT mice. (a–d) Immunohistochemistry of brain sections with 7/4 antibody. Scale bar, 100 μm. (e) Quantitation of 7/4+ cells showed a significant increase of infiltrated neutrophils in COX-2−/− mice, but not in COX-1−/− mice compared with their respective WT mice. (f) Brain MPO levels were decreased in COX-1−/− mice and increased in COX-2−/− mice compared with their WT controls. Mean±s.e.m. (n = 5–6 per group); *P<0.05, **P<0.01.
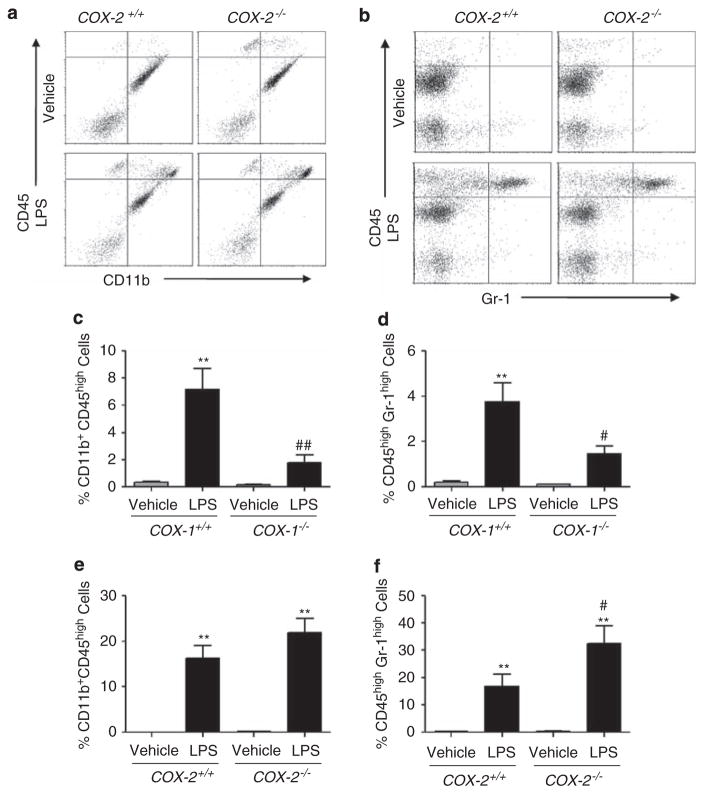
Using flow cytometry with antibodies to CD11b, CD45, and Gr-1 as described,36,38 we determined the number of infiltrated leukocytes in the brain of COX-1−/−, COX-2−/−, and their respective WT mice 24 h after LPS injection. Flow cytometry allows to distinguish cell types that possess the same antigen but in different amounts and thus, differentiates blood-derived monocytes from resident microglia in cellular isolates. Macrophages are identified as a CD11b+CD45high population of cells, whereas resident microglia are identified as a CD11b+CD45low population of cells.33 Neutrophils infiltrated from the peripheral blood are defined as a CD45highGr-1high population of cells.38 The dot plots resulted from the analysis of isolated whole brain including hippocampus from COX-2−/− mice showed a marked increase in the percentage of CD11b+CD45high cells compared to WT mice (top right quadrants; Figure 3a). Quantification of the proportion of Gr-1high cells (top right quadrants; Figure 3b) is displayed in bar graphs, after gating on CD11b+CD45+ cells only. The proportion of infiltrating neutrophils was significantly elevated in COX-2−/− mice (Figure 3f). In contrast, the increase in CD11b+CD45high and CD45highGr-1high population of cells was attenuated in COX-1−/− mice (Figures 3c and d), in accordance with what was observed with 7/4 immunohistochemistry.
Figure 3.
COX-2 deficiency exacerbates neutrophil infiltration. (a, b) Representative dot plots of flow cytometry of brain CD11b+ and CD45+ cells (a), and CD45+ and Gr-1+ cells (b) from WT and COX-2−/− mice injected with LPS or vehicle. (c, d) Quantitation of the percentage of brain CD11b+CD45high (c) and CD45highGr-1high cells (d) from COX-1−/− and WT mice. (e, f) Quantitation of the percentage of brain CD11b+CD45high (e) and CD45highGr-1high cells (f) from COX-2−/− and WT mice. n = 3–6 per group; **P<0.01 versus vehicle-injected WT mice; #P<0.05, ##P<0.01 versus LPS-injected WT mice.
Infiltrated neutrophils potentiate inflammation
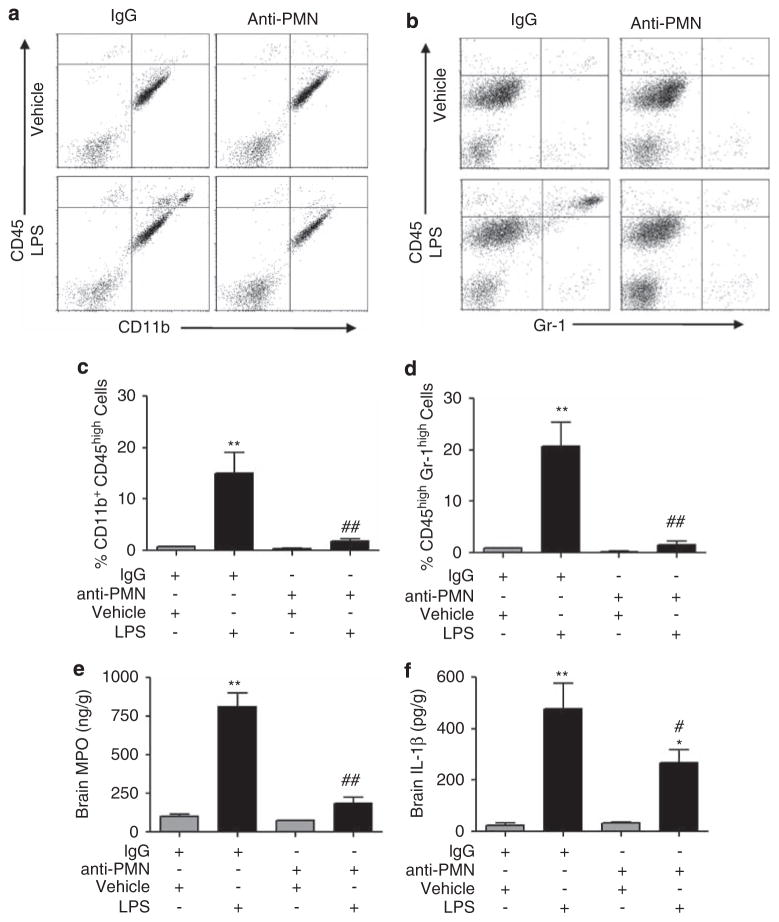
To determine whether neutrophils infiltrated into the brain from the periphery further potentiate the neuroinflammatory response, we depleted neutrophils using an anti-PMN antibody.31 The proportion of infiltrating macrophages (top right quadrants; Figure 4a) and neutrophils (top right quadrants; Figure 4b) was decreased in PMN-depleted mice (Figures 4c and d) compared with IgG-treated mice (Figures 4c and d) 24 h after LPS injection. We confirmed flow cytometric results by measuring MPO levels by ELISA. We found a similar decrease in the levels of MPO in the brains of PMN-depleted mice (Figure 4e), compared with IgG-treated mice (Figure 4e) after LPS.
Figure 4.
PMN depletion reduces neutrophil infiltration, MPO, and IL-1β levels. (a, b) Representative dot plots of flow cytometry of brain CD11b+ and CD45+ cells (a), and CD45+ and Gr-1+ cells (b) from IgG-treated or PMN-depleted mice injected with LPS or vehicle. (c, d) Quantitation of the percentage of brain CD11b+CD45high (c) and CD45highGr-1high cells (d) from PMN-depleted or IgG-treated mice. (e, f) Brain MPO (e) and IL-1β levels (f) by ELISA revealed a significant decrease in PMN-depleted mice compared with IgG-treated mice. Mean±s.e.m. (n = 5–6 per group); *P<0.05, **P<0.01 versus vehicle-injected mice with IgG treatment; #P<0.05, ##P<0.01 versus LPS-injected mice with IgG treatment.
The proinflammatory cytokine IL-1β is rapidly induced after inflammatory insults and has a pivotal role in the process of inflammation and leukocyte recruitment into the CNS.44 Thus, we assessed whether infiltrated peripheral leukocytes modulate IL-1β induction. Levels of brain IL-1β 24 h after LPS injection were significantly attenuated in PMN-depleted mice compared with IgG-treated mice (Figure 4f). These data indicate that neutrophils recruited to the brain from the periphery potentiate the inflammatory response.
COX-2 deletion exacerbates BBB permeability and plasma chemokine levels
As induction of inflammatory responses can cross-amplify disruption of BBB, which precedes leukocyte infiltration into the CNS,46 we determined whether COX-2 deficiency exacerbates BBB disruption using IgG immunohistochemistry as a marker of serum protein extravasation across the BBB.31 The leukocyte infiltration observed was accompanied by an increase in BBB leakage in LPS-injected COX-2−/− mice (Figure 5h). In contrast, marked decreases in the distribution and intensity of IgG immunoreactivity were evident in LPS-injected COX-1−/− compared with WT mice (Figure 5f). We further assessed whether COX-2 deletion affects plasma and brain levels of CCL2 and CXCL2, chemokines primarily involved in the mobilization and migration of neutrophils.31,47 Levels of CCL2 and CXCL2 were significantly increased in LPS-injected COX-2−/− mice compared with WT mice (Figures 5i–l).
Figure 5.
COX-2 deletion exacerbates BBB permeability and chemokine levels. (a–h) Representative brain sections from COX-1−/−, COX-2−/−, and their WT mice injected with LPS or vehicle and immunostained with IgG antibody. COX-1 and COX-2 deficiency differentially modulated the intensity and distribution of IgG immunoreactivity after LPS injection. Scale bar, 100 μm. (i–l) Plasma CCL2 (i), CXCL2 (j), brain CCL2 (k), and CXCL2 levels (l) by ELISA revealed a significant increase in COX-2−/− mice compared with WT mice. Mean±s.e.m. (n = 5–6 per group); *P<0.05, **P<0.01 versus vehicle-injected WT mice; #P<0.05, ##P<0.01 versus LPS-injected WT mice.
Discussion
Recent evidence highlights the distinct roles of COX-1 and -2 in neuroinflammation, and their possible contribution to the pathophysiology of several neurodegenerative diseases (reviewed in48). Microglia, the brain resident immune cells, are rapidly activated in response to inflammatory stimuli and neuronal damage,49 express COX-1, and can contribute to the modulation of leukocyte recruitment by releasing cytokines and chemokines. Infiltrated and activated neutrophils can also release a variety of proinflammatory mediators such as tumor necrosis factor-α, IL-1β, and matrix metallo-proteinases-9,50,51 and can contribute to oxidative stress and subsequently neuronal damage by producing superoxide anion and hypochlorous acid through nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) and MPO, respectively.52 In addition, neutrophils can also release several chemokines of the CC and CXC families that recruit distinct leukocytes into the inflamed tissue,53 potentially amplifying the cellular inflammatory response and further exacerbating brain injury. Although the mechanisms underlying the recruitment of leukocytes on CNS injury are not clearly defined, growing evidence suggests that activated leukocytes enter the brain parenchyma and initiate a chronic inflammatory process in many neurological diseases.54–56 In particular, considerable clinical data indicate that acute infiltration of neutrophils is risk factor for ischemic stroke, where it is associated with a worse clinical outcome.57,58 Using PMN depletion, our data provide evidence that i.c.v LPS worsens the inflammatory response by exacerbating the recruitment of neutrophils into the inflamed brain. Similarly, earlier studies in the absence of a systemic inflammatory stimulus, in which neutrophils have been depleted or their migration from the vasculature inhibited, have provided considerable evidence that neutrophils contribute to ischemic and excitotoxic brain damage.31,59,60
Our data indicate that COX-1 and COX-2 have distinct roles in the initiation of immune responses and differentially modulate microglial activation and recruitment of leukocytes, and particularly of neutrophils. We showed that the early infiltration of neutrophils was COX-1 dependent. Influx of neutrophils into the inflamed brain was decreased in COX-1−/− and increased in COX-2−/− mice, with parallel changes in BBB permeability and proinflammatory mediators. These results indicate that inhibition of COX-1 activity is anti-inflammatory, whereas suppression of COX-2 activity may worsen the neuroinflammatory response.
Although COX-1 has traditionally being considered as the isoform primarily responsible for homeostatic PG synthesis, it has recently been implicated as a critical player in neuroinflammation and neurodegenerative diseases.61 In rats, aging is associated with increased COX-1 expression in the hippocampus and COX-1-derived thromboxane B2, 62 which might increase brain susceptibility to inflammation. COX-1−/− mice also show reduced glial activation, oxidative damage, and neuronal death after i.c.v. injection of Aβ 1–42.61 We showed earlier that COX-1−/− mice or WT mice treated with a selective COX-1 inhibitor exhibit reduced microglial activation along with PGE2 production after i.c.v. LPS.8 On the other hand, COX-2 is rapidly induced by a variety of inflammatory stimuli and thus, traditionally has been considered as the most appropriate target for anti-inflammatory drugs. However, recent studies have pointed out a critical role of COX-2 in neuroprotection and in the resolution of inflammation,63 and we reported that COX-2−/− mice and WT mice treated with celecoxib, a COX-2 selective inhibitor, have increased neuroinflammatory response, neuronal damage, and oxidative stress after LPS.7 Similar observations using systemic immune stimuli provided evidence that inhibition of COX-2 resulted in an exacerbated early innate immune reaction.64 Although COX-2 activity could contribute to neurotoxicity in models of direct neuronal injury,65–67 its role in neuroinflammation could be linked to resolution through production of specific neuroprotective lipid mediators.63
Among COX-derived PGs, PGE2 is particularly important for the propagation of inflammation because it activates nuclear factor-κB,68 which, in turn, induces transcription of several proinflammatory cytokines and chemokines, including IL-1β, tumor necrosis factor-α, and CCL2. The production of these proinflammatory mediators leads to the influx and activation of neutrophils through modulation of matrix metalloproteinases and BBB permeability19,31 and to the subsequent leukocyte recruitment into the inflamed brain. Intracerebral injection of PGE2 produces marked BBB break-down16 and upregulation of matrix metalloproteinase-9 expression,69 which can be blocked by EP4 silencing and by a selective EP4 antagonist.70 Further studies are required to elucidate the individual roles of the different EP receptors and coupled upstream and downstream signaling pathways in pathological conditions involving the activation of the arachidonic acid cascade.
In summary, COX-2−/− mice showed more extensive leukocyte infiltration and inflammatory response than COX-1−/− mice. This influx of leukocytes was accompanied by a marked BBB compromise and specific induction of chemokines on inflamed brain. Our data suggest that the beneficial effects of COX-2 activity in the resolution of inflammation need to be considered when using COX-2 selective inhibitors, and that non-steroidal anti-inflammatory drugs with higher selectivity for COX-1 should be further evaluated as a therapeutic approach in diseases with a neuroinflammatory component, particularly when accompanied by BBB breakdown and infiltration of immune cells from the periphery.
Acknowledgments
We thank Dr Robert Langenbach (Laboratory of Molecular Carcinogenesis, National Institute of Environmental Health Sciences, National Institutes of Health) for providing COX-1−/−, COX-2−/−, and their WT mice. This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 2.Bosetti F. Arachidonic acid metabolism in brain physiology and pathology: lessons from genetically altered mouse models. J Neurochem. 2007;102:577–586. doi: 10.1111/j.1471-4159.2007.04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoozemans JJ, Rozemuller JM, van Haastert ES, Veerhuis R, Eikelenboom P. Cyclooxygenase-1 and -2 in the different stages of Alzheimer’s disease pathology. Curr Pharm Des. 2008;14:1419–1427. doi: 10.2174/138161208784480171. [DOI] [PubMed] [Google Scholar]
- 4.Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, et al. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci USA. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maihofner C, Probst-Cousin S, Bergmann M, Neuhuber W, Neundorfer B, Heuss D. Expression and localization of cyclooxygenase-1 and -2 in human sporadic amyotrophic lateral sclerosis. Eur J Neurosci. 2003;18:1527–1534. doi: 10.1046/j.1460-9568.2003.02879.x. [DOI] [PubMed] [Google Scholar]
- 6.Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3–35. doi: 10.1016/s0163-7827(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 7.Aid S, Langenbach R, Bosetti F. Neuroinflammatory response to lipopolysaccharide is exacerbated in mice genetically deficient in cyclooxygenase-2. J Neuroinflammation. 2008;5:17. doi: 10.1186/1742-2094-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Shaftel SS, Griffin WS, O’Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dormond O, Bezzi M, Mariotti A, Ruegg C. Prostaglandin E2 promotes integrin alpha Vbeta 3-dependent endothelial cell adhesion, rac-activation, and spreading through cAMP/PKA-dependent signaling. J Biol Chem. 2002;277:45838–45846. doi: 10.1074/jbc.M209213200. [DOI] [PubMed] [Google Scholar]
- 12.Scandella E, Men Y, Legler DF, Gillessen S, Prikler L, Ludewig B, et al. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood. 2004;103:1595–1601. doi: 10.1182/blood-2003-05-1643. [DOI] [PubMed] [Google Scholar]
- 13.Legler DF, Krause P, Scandella E, Singer E, Groettrup M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J Immunol. 2006;176:966–973. doi: 10.4049/jimmunol.176.2.966. [DOI] [PubMed] [Google Scholar]
- 14.Weller CL, Collington SJ, Hartnell A, Conroy DM, Kaise T, Barker JE, et al. Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc Natl Acad Sci USA. 2007;104:11712–11717. doi: 10.1073/pnas.0701700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tajima T, Murata T, Aritake K, Urade Y, Hirai H, Nakamura M, et al. Lipopolysaccharide induces macrophage migration via prostaglandin D(2) and prostaglandin E(2) J Pharmacol Exp Ther. 2008;326:493–501. doi: 10.1124/jpet.108.137992. [DOI] [PubMed] [Google Scholar]
- 16.Schmidley JW, Dadson J, Iyer RS, Salomon RG. Brain tissue injury and blood-brain barrier opening induced by injection of LGE2 or PGE2. Prostaglandins Leukot Essent Fatty Acids. 1992;47:105–110. doi: 10.1016/0952-3278(92)90145-9. [DOI] [PubMed] [Google Scholar]
- 17.Tuomanen E, Hengstler B, Rich R, Bray MA, Zak O, Tomasz A. Nonsteroidal anti-inflammatory agents in the therapy for experimental pneumococcal meningitis. J Infect Dis. 1987;155:985–990. doi: 10.1093/infdis/155.5.985. [DOI] [PubMed] [Google Scholar]
- 18.Kadurugamuwa JL, Hengstler B, Zak O. Cerebrospinal fluid protein profile in experimental pneumococcal meningitis and its alteration by ampicillin and anti-inflammatory agents. J Infect Dis. 1989;159:26–34. doi: 10.1093/infdis/159.1.26. [DOI] [PubMed] [Google Scholar]
- 19.Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, et al. Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther. 2007;323:488–498. doi: 10.1124/jpet.107.127035. [DOI] [PubMed] [Google Scholar]
- 20.Issekutz AC, Bhimji S. The effect of nonsteroidal anti-inflammatory agents on E. coli-induced inflammation. Immunopharmacology. 1982;4:11–22. doi: 10.1016/0162-3109(82)90022-4. [DOI] [PubMed] [Google Scholar]
- 21.Shimanuki T, Nakamura RM, diZerega GS. In vivo modulation of leukotaxis by non-steroidal anti-inflammatory drugs. Agents Actions. 1985;17:80–83. doi: 10.1007/BF01966687. [DOI] [PubMed] [Google Scholar]
- 22.Hirasawa N, Ohuchi K, Watanabe M, Tsurufuji S. Mechanism of the inhibitory action of cyclooxygenase inhibitors on leukocyte infiltration: involvement of endogenous histamine. Eur J Pharmacol. 1987;144:267–275. doi: 10.1016/0014-2999(87)90379-7. [DOI] [PubMed] [Google Scholar]
- 23.Montine TJ, Milatovic D, Gupta RC, Valyi-Nagy T, Morrow JD, Breyer RM. Neuronal oxidative damage from activated innate immunity is EP2 receptor-dependent. J Neurochem. 2002;83:463–470. doi: 10.1046/j.1471-4159.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- 24.Bonow RH, Aid S, Zhang Y, Becker KG, Bosetti F. The brain expression of genes involved in inflammatory response, the ribosome, and learning and memory is altered by centrally injected lipopolysaccharide in mice. Pharmacogenomics J. 2009;9:116–126. doi: 10.1038/tpj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 26.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 27.Delgado M, Ganea D. Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: potential therapeutic role in brain trauma. FASEB J. 2003;17:1922–1924. doi: 10.1096/fj.02-1029fje. [DOI] [PubMed] [Google Scholar]
- 28.Milatovic D, Zaja-Milatovic S, Montine KS, Shie FS, Montine TJ. Neuronal oxidative damage and dendritic degeneration following activation of CD14-dependent innate immune response in vivo. J Neuroinflammation. 2004;1:20. doi: 10.1186/1742-2094-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego, CA: 2001. p. xxv.p. 264. [Google Scholar]
- 31.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinoshita K, Kraydieh S, Alonso O, Hayashi N, Dietrich WD. Effect of posttraumatic hyperglycemia on contusion volume and neutrophil accumulation after moderate fluid-percussion brain injury in rats. J Neurotrauma. 2002;19:681–692. doi: 10.1089/08977150260139075. [DOI] [PubMed] [Google Scholar]
- 33.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 34.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- 38.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 39.D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blamire AM, Anthony DC, Rajagopalan B, Sibson NR, Perry VH, Styles P. Interleukin-1beta -induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J Neurosci. 2000;20:8153–8159. doi: 10.1523/JNEUROSCI.20-21-08153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, et al. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab. 2007;27:1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- 42.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, et al. Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112:232–240. doi: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 43.Choi DK, Pennathur S, Perier C, Tieu K, Teismann P, Wu DC, et al. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson’s disease in mice. J Neurosci. 2005;25:6594–6600. doi: 10.1523/JNEUROSCI.0970-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 46.Floris S, Blezer EL, Schreibelt G, Dopp E, van der Pol SM, Schadee-Eestermans IL, et al. Blood-brain barrier permeability and monocyte infiltration in experimental allergic encephalomyelitis: a quantitative MRI study. Brain. 2004;127:616–627. doi: 10.1093/brain/awh068. [DOI] [PubMed] [Google Scholar]
- 47.Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics. 2007;4:590–601. doi: 10.1016/j.nurt.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 50.Justicia C, Panes J, Sole S, Cervera A, Deulofeu R, Chamorro A, et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2003;23:1430–1440. doi: 10.1097/01.WCB.0000090680.07515.C8. [DOI] [PubMed] [Google Scholar]
- 51.Kantari C, Pederzoli-Ribeil M, Witko-Sarsat V. The role of neutrophils and monocytes in innate immunity. Contrib Microbiol. 2008;15:118–146. doi: 10.1159/000136335. [DOI] [PubMed] [Google Scholar]
- 52.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc Natl Acad Sci USA. 2005;102:431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 54.Wang PY, Kao CH, Mui MY, Wang SJ. Leukocyte infiltration in acute hemispheric ischemic stroke. Stroke. 1993;24:236–240. doi: 10.1161/01.str.24.2.236. [DOI] [PubMed] [Google Scholar]
- 55.McGeer EG, McGeer PL. Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 56.Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke. 1996;27:1739–1743. doi: 10.1161/01.str.27.10.1739. [DOI] [PubMed] [Google Scholar]
- 58.Price CJ, Menon DK, Peters AM, Ballinger JR, Barber RW, Balan KK, et al. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke. 2004;35:1659–1664. doi: 10.1161/01.STR.0000130592.71028.92. [DOI] [PubMed] [Google Scholar]
- 59.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 60.Ryu JK, Tran KC, McLarnon JG. Depletion of neutrophils reduces neuronal degeneration and inflammatory responses induced by quinolinic acid in vivo. Glia. 2007;55:439–451. doi: 10.1002/glia.20479. [DOI] [PubMed] [Google Scholar]
- 61.Choi SH, Bosetti F. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to β-amyloid. Aging. 2009;1:234–244. doi: 10.18632/aging.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aid S, Bosetti F. Gene expression of cyclooxygenase-1 and Ca(2+)-independent phospholipase A(2) is altered in rat hippocampus during normal aging. Brain Res Bull. 2007;73:108–113. doi: 10.1016/j.brainresbull.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(Suppl 1):S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blais V, Turrin NP, Rivest S. Cyclooxygenase 2 (COX-2) inhibition increases the inflammatory response in the brain during systemic immune stimuli. J Neurochem. 2005;95:1563–1574. doi: 10.1111/j.1471-4159.2005.03480.x. [DOI] [PubMed] [Google Scholar]
- 65.Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA. 2001;98:1294–1299. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Alvarez D, Al-Dalain S, Martinez G, et al. Assessment of the relative contribution of COX-1 and COX-2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia. J Neurochem. 2003;86:545–555. doi: 10.1046/j.1471-4159.2003.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sasaki T, Kitagawa K, Yamagata K, Takemiya T, Tanaka S, Omura-Matsuoka E, et al. Amelioration of hippocampal neuronal damage after transient forebrain ischemia in cyclooxygenase-2-deficient mice. J Cereb Blood Flow Metab. 2004;24:107–113. doi: 10.1097/01.WCB.0000100065.36077.4A. [DOI] [PubMed] [Google Scholar]
- 68.Poligone B, Baldwin AS. Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. J Biol Chem. 2001;276:38658–38664. doi: 10.1074/jbc.M106599200. [DOI] [PubMed] [Google Scholar]
- 69.Yen JH, Khayrullina T, Ganea D. PGE2-induced metalloproteinase-9 is essential for dendritic cell migration. Blood. 2008;111:260–270. doi: 10.1182/blood-2007-05-090613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pavlovic S, Du B, Sakamoto K, Khan KM, Natarajan C, Breyer RM, et al. Targeting prostaglandin E2 receptors as an alternative strategy to block cyclooxygenase-2-dependent extracellular matrix-induced matrix metalloproteinase-9 expression by macrophages. J Biol Chem. 2006;281:3321–3328. doi: 10.1074/jbc.M506846200. [DOI] [PubMed] [Google Scholar]