Reactive oxygen species mediate the effect of cAMP on Ras activation and ERK1/2 phosphorylation in Leydig cells.
Abstract
Activation of the LH receptor (LHR) in Leydig cells results in the phosphorylation of ERK1/2 by cAMP-dependent and cAMP-independent pathways. Here we examine the mechanisms by which cAMP stimulates ERK1/2 phosphorylation. We show that the stimulation of steroidogenesis is not necessary or sufficient to stimulate the phosphorylation of ERK1/2 but that other cAMP-dependent mitochondrial functions are involved. Using MA-10 cells as a model, we showed that cAMP analogs increase reactive oxygen species (ROS) formation and that an uncoupler of oxidative phosphorylation and a ROS scavenger prevent this increase. These two compounds also inhibit the increase in ERK1/2 phosphorylation provoked by cAMP analogs, thus suggesting that the cAMP-induced phosphorylation of ERK1/2 is mediated by mitochondrial ROS. In agreement with this hypothesis we also show that a reduction in glutathione levels, which alters the redox state of MA-10 cells, potentiates the effect of cAMP on ERK1/2 phosphorylation. Measurements of the dephosphorylation of ERK and the activation of Ras showed that the ROS scavenger prevents the cAMP-provoked activation of Ras and that cAMP, with or without a ROS scavenger, has little or no effect on the dephosphorylation of ERK. Lastly, we show that the uncoupler of oxidative phosphorylation and the ROS scavenger also prevent the ability of cAMP analogs to increase ERK1/2 phosphorylation in primary cultures of mouse Leydig cells. We conclude that, in Leydig cells, cAMP enhances the phosphorylation of ERK1/2 via a mitochondria-derived, ROS-dependent activation of Ras.
One of the consequences of the binding of LH/chorionic gonadotropin (CG) to the LH/CG receptor (LHR) in Leydig cells is the phosphorylation of ERK1/2 (1–4). This pathway has been implicated as a modulator of steroid synthesis (2, 3, 5) and as a critical component of the proliferation and survival of Leydig cells (4, 6).
Measurements of Ras and Rap1 activation, expression of dominant-negative mutants of Ras and Rap1, and addition of pharmacological inhibitors of MAPK kinase (MEK) have shown that the LHR-induced activation of the ERK1/2 cascade in MA-10 Leydig tumor cells occurs through the classical Ras/Raf/MEK pathway (1, 7). The LHR-induced activation of Ras is complex, however, and it involves at least two different pathways (8, 9). One is an intercellular pathway that uses arrestin-3 as the most proximal LHR-binding partner to activate Fyn, a member of the Src family of kinases (8, 10). Fyn, in turn, activates the release of soluble epidermal growth factor (EGF)-like growth factors (9) that bind to and activate the EGF receptor (EGFR) in an autocrine/paracrine fashion (8, 9). This transactivation of the EGFR results in the phosphorylation of Shc, the formation of Shc/Sos complexes, and the activation of Ras (7). The second pathway is intracellular and is mediated by cAMP and protein kinase A (PKA). Thus, expression of dominant-negative constructs, small interfering RNAs (siRNAs), and pharmacological inhibitors of PKA interfere with Ras activation and ERK1/2 phosphorylation (1–4, 9). Moreover, a cAMP analog that is selective for PKA activates Ras and phosphorylates ERK1/2, whereas a cAMP analog that is selective for the cAMP guanine nucleotide exchange factors does not elicit either of these effects (1, 4).
In the past few years we have concentrated on characterizing the intercellular pathway that mediates the effects of human (h)CG on ERK1/2 phosphorylation in MA-10 cells (1, 4, 7–10). In the studies presented here we return to the study of the cAMP-dependent, intracellular pathway. Our results show that mitochondria-derived reactive oxygen species (ROS) mediate the cAMP/PKA-induced activation of Ras and phosphorylation of ERK1/2.
Results
Abolishing cAMP accumulation reduces the hCG-induced phosphorylation of ERK1/2
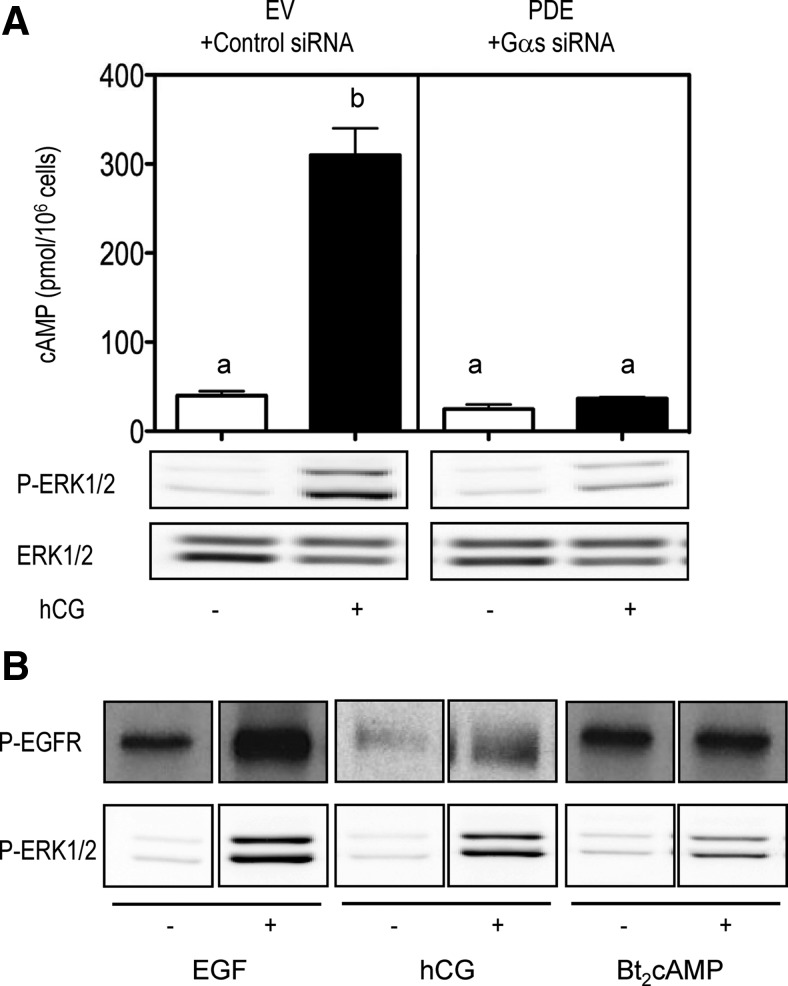
A quantitative decrease in the hCG-induced cAMP accumulation in MA-10 cells could be accomplished by cotransfection with an siRNA that targets Gαs, to inhibit the activation of adenylyl cyclase, and an expression vector coding for cAMP phosphodiesterase, to enhance the degradation of any residual cAMP synthesized (1, 8, 9, 11). This manipulation abolished the hCG-induced cAMP response and greatly inhibited, but did not abolish, the hCG-induced ERK1/2 phosphorylation (Fig. 1A).
Fig. 1.
cAMP does not transactivate the EGFR but is an important contributor to the hCG-induced phosphorylation of ERK1/2. A, MA-10 cells were cotransfected with an expression vector for the hLHR, an empty vector, and a control siRNA or with an empty vector for the hLHR, an expression vector for cAMP phosphodiesterase (PDE) and a Gαs siRNA as indicated. Cells were incubated with or without hCG for 15 min before measuring cAMP or for 30 min before measuring P-ERK1/2. ERK1/2 was subsequently measured in a different gel and blot. The bar graphs are the mean ± sem of three independent experiments. Different letters indicate statistically significant differences (P < 0.05) among the different groups. The Western blots are representative of three independent experiments. B, MA-10 cells were transfected with the hLHR and incubated with buffer only, EGF (10 ng/ml, 5 min), hCG (100 ng/ml, 30 min), or Bt2cAMP (1 mm, 30 min) before measuring the phosphorylation of the EGFR or ERK1/2 on different gels and blots. The Western blots shown are representative of at least three independent experiments. EV, Empty vector.
Addition of dibutyrl-cAMP (Bt2cAMP) to MA-10 cells did not increase the phosphorylation of the EGFR but still increased the phosphorylation of ERK1/2 (Fig. 1B) whereas addition of EGF or hCG increased the phosphorylation of the EGFR and ERK1/2 as expected (4, 7–9). Another group of investigators have previously reported that addition of forskolin to mouse (m)LTC-1 cells (a similar Leydig tumor cell line) results in the phosphorylation of the EGFR and that the forskolin-induced phosphorylation of ERK1/2 can be blocked with a pharmacological inhibitor of the EGFR kinase (3). We did not investigate the reasons for this discrepancy, but it could be due to the use of forskolin vs. Bt2cAMP or differences in the conditions used to culture MA-10 cells and mLTC-1 cells.
Because we wanted to characterize this cAMP-dependent but EGFR-independent component of ERK1/2 phosphorylation, all subsequent experiments were done using Bt2cAMP rather than hCG as the stimulus.
Steroid biosynthesis is not necessary or sufficient for ERK1/2 phosphorylation
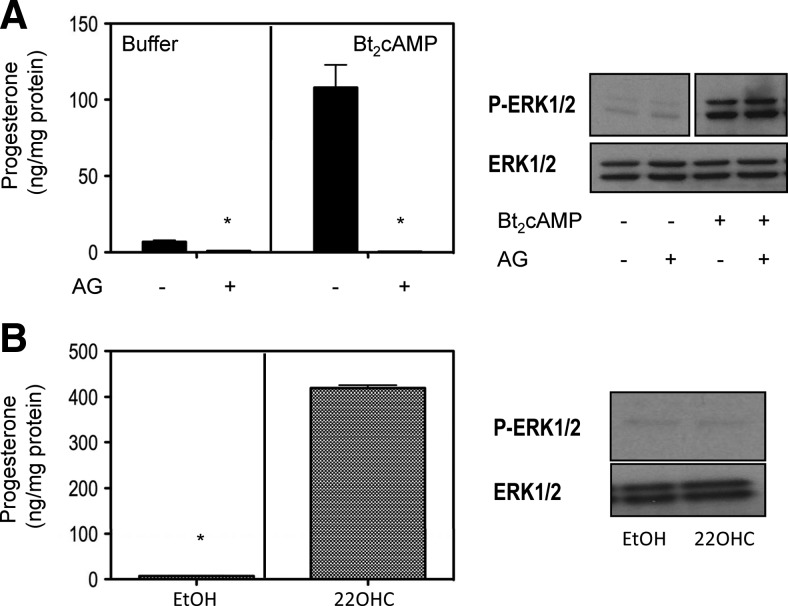
One of the most important and most extensively characterized actions of cAMP in MA-10 cells is the activation of steroid synthesis (12–14). To determine whether increased steroidogenesis results in the phosphorylation of ERK1/2, we first stimulated MA-10 cells with Bt2cAMP in the presence or absence of aminoglutethimide, an inhibitor of the cholesterol side chain cleavage enzyme (15). Figure 2A shows that aminoglutethimide completely inhibited the Bt2cAMP-stimulated progesterone synthesis as expected (16) but had no effect on ERK1/2 phosphorylation. Conversely, the addition of 22-hydroxycholesterol (22OHC), a substrate that bypasses the rate-limiting step in steroidogenesis and thus stimulates steroidogenesis in the absence of cAMP, does not provoke ERK1/2 phosphorylation (Fig. 2B).
Fig. 2.
Steroid biosynthesis is not necessary or sufficient for ERK1/2 phosphorylation. A, MA-10 cells were preincubated without or with 100 μm aminoglutethimide (AG) for 30 min (16) and then incubated without or with 1 mm Bt2cAMP for an additional 60 min before measuring P-ERK1/2 and progesterone. ERK1/2 was subsequently measured in a different gel and blot. B, MA-10 cells were preincubated for 30 min in fresh medium and then incubated with or without 10 μm 22OHC for an additional 60 min before measuring P-ERK1/2 and progesterone. ERK1/2 was subsequently measured in a different gel and blot. The bar graphs show the mean ± sem of three independent experiments, and the asterisks indicate statistically significant differences (P < 0.05) between the control and AG-treated groups (top) or the cells treated with or without 22OHC (bottom). The Western blots shown are representative of three independent experiments.
Mitochondria-derived ROS are necessary for the cAMP-provoked ERK1/2 phosphorylation
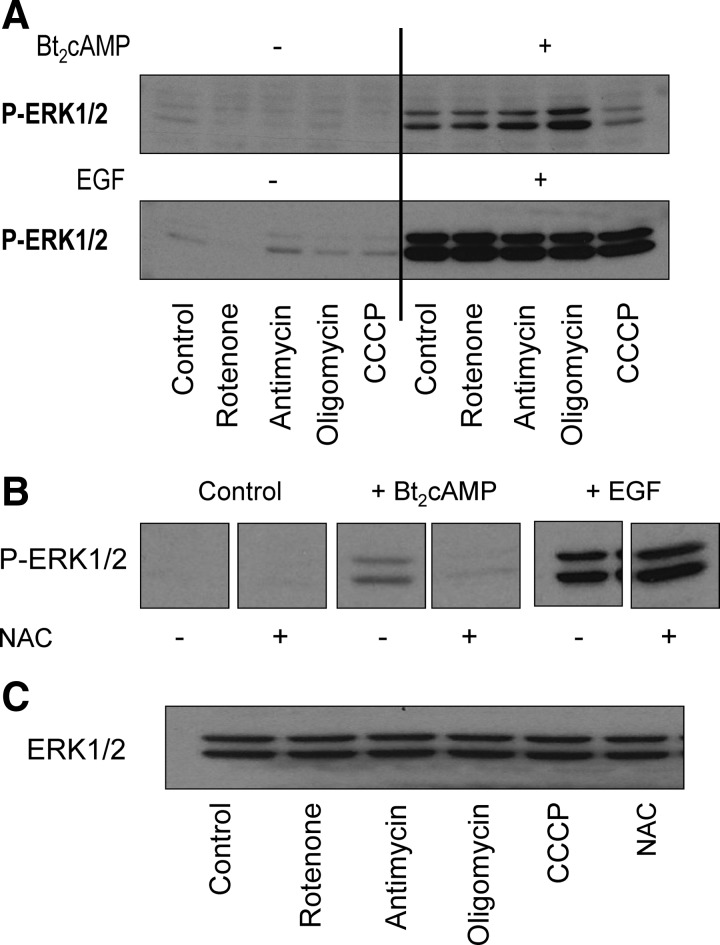
In addition to steroidogenesis, cAMP analogs also stimulate other mitochondrial functions in MA-10 cells such as oxygen consumption and electron transport (17). To determine whether these are involved in the cAMP-provoked phosphorylation of ERK1/2, we tested the effects of two inhibitors of electron transport (rotenone and antimycin), an inhibitor of mitochrondial ATP synthesis (oligomycin) and an uncoupler of oxidative phosphorylation, carbonyl cyanide m-chloro phenyl hydrazone (CCCP). Figure 3A shows that only CCCP inhibited the cAMP-provoked phosphorylation of ERK1/2. Oligomycin seemed to enhance the stimulatory effect of cAMP on ERK1/2 phosphorylation but we did not seek an explanation for this finding. The finding that none of these mitochondrial inhibitors influenced the EGF-induced phosphorylation of ERK1/2 (Fig. 3A) shows that mitochondria are not universally involved in ERK1/2 phosphorylation.
Fig. 3.
An uncoupler of oxidative phosphorylation and a ROS scavenger inhibit the cAMP-stimulated ERK1/2 phosphorylation. A, MA-10 cells were preincubated with vehicle only (dimethylsulfoxide, control), rotenone (10 μm), antimycin (10 μm), oligomycin (10 μm), or CCCP (5 μm) for 30 min. This was followed by a 30-min incubation with buffer only or 1 mm Bt2cAMP or a 5-min incubation with buffer only or 10 ng/ml EGF before measuring ERK1/2 phosphorylation. The concentrations of the different inhibitors used are based on previously published findings (17). B, MA-10 cells were preincubated with buffer only or with freshly made 5 mm NAC for 30 min. This was followed by a 30-min incubation with buffer only or 1 mm Bt2cAMP or a 5-min incubation with buffer only or 10 ng/ml EGF before measuring ERK1/2 phosphorylation. The concentration of NAC used was empirically chosen and is the minimal concentration that inhibits the cAMP effect. C, The Bt2cAMP-stimulated samples from panels A and B were subsequently run on a different gel and blot and used to assay for ERK1/2. In all panels the Western blots shown are representative of a minimum of three independent experiments.
Uncouplers of oxidative phosphorylation, such as CCCP, dissipate the mitochondrial membrane potential by collapsing the mitochondrial proton gradient (17–19). Because the mitochondrial membrane potential is involved in the generation of ROS (18–20), we next tested the effects of N-acetylcysteine (NAC), a ROS scavenger (20, 21) on the Bt2cAMP- and EGF-provoked phosphorylation of ERK1/2. Figure 3B shows that NAC inhibited the effect of Bt2cAMP but not that of EGF. The lack of effect of NAC on the EGF-stimulated ERK1/2 phosphorylation attests to the selectivity of inhibition.
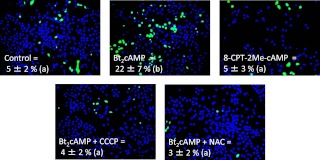
These experiments were complemented with measurements of ROS production in MA-10 cells. Using live cell imaging of MA-10 cells loaded with a fluorescent ROS indicator, we were able to show that Bt2cAMP enhances ROS accumulation and that CCCP and NAC inhibit this effect (Fig. 4). We also showed that 8-CPT-2Me-cAMP, a cAMP analog that does not activate PKA (22, 23) and does not stimulate Ras activation or ERK1/2 phosphorylation in Leydig cells (1, 4), does not enhance ROS accumulation in MA-10 cells (Fig. 4).
Fig. 4.
Effects of Bt2cAMP, 8-CPT-2Me-cAMP, CCP, and NAC on ROS levels in MA-10 cells. ROS production was quantitated in MA-10 cells as described in Materials and Methods. Each panel shows a representative merged image displaying nuclei (blue) and ROS (green) with the contrast adjusted to maximum. The numbers at the bottom of each panel are the mean ± sem of three to four independent experiments in which the percent of ROS-positive cells was quantitated as described in Materials and Methods. Different letters indicate statistically significant differences (P < 0.05) among the four different groups.
We also tested the possibility that the cytoplasmic reduced nicotinamide adenine dinucleotide phosphate oxidase complex, another major source of ROS, also contributes to the phosphorylation of ERK1/2 in MA-10 cells. This source is apparently not used by MA-10 cells, however, because diphenyleneiodonium, an inhibitor of this enzyme complex (24), does not prevent the stimulation of ERK1/2 phosphorylation by Bt2cAMP (data not shown).
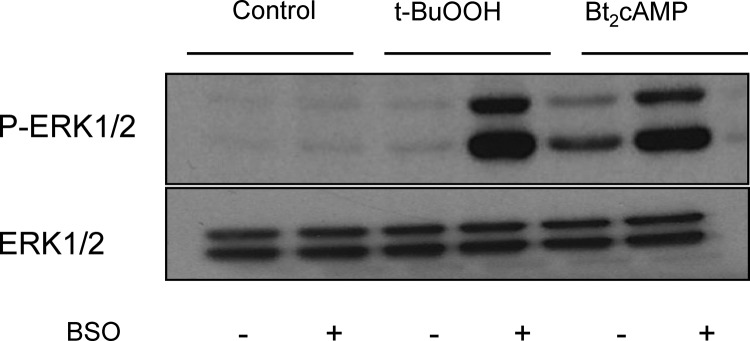
A recent report documented that enhancing ROS production by addition of tert-butyl hydroperoxide (t-BuOOH) to glutathione-depleted MA-10 cells leads to an increase in ERK1/2 phosphorylation (25). Because glutathione plays a critical role in the inactivation of ROS (21, 26), we predicted that, if Bt2cAMP uses ROS as an intermediate to enhance ERK1/2 phosphorylation, reducing glutathione levels in MA-10 cells should enhance this effect. The results presented in Fig. 5 show that this is indeed the case. These results also confirm those of Chen et al. (25), who showed that t-BuOOH stimulates the phosphorylation of ERK1/2 only in MA-10 cells depleted of glutathione.
Fig. 5.
Reducing glutathione levels enhances the effect of Bt2cAMP on ERK1/2 phosphorylation. MA-10 cells were depleted of glutathione by an overnight preincubation with 100 μm l-buthionine-sulfoximine (BSO) as described elsewhere (25). The medium with or without BSO was replaced, and the cells were incubated for an additional 30 min before the addition of 1 mm Bt2cAMP or 50 μm t-BuOOH. P-ERK1/2 was measured 30 min after addition of Bt2cAMP or 60 min after addition of t-BuOOH. The samples were subsequently run on a different gel and blot and used to assay for ERK1/2. The Western blot shown is representative of three independent experiments.
Altogether these results support the hypothesis that mitochondria-generated ROS are important intermediates in the Bt2cAMP-stimulated phosphorylation of ERK1/2 in MA-10 cells.
ROS stimulates ERK1/2 phosphorylation by activating Ras
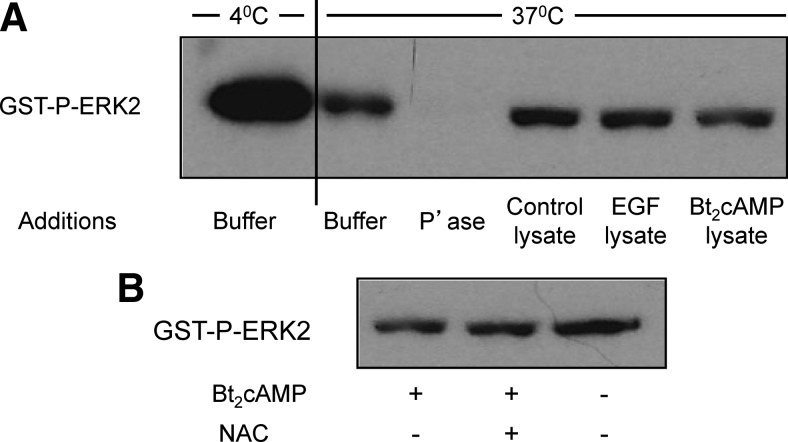
Although ROS can modulate the activity of a number of regulatory proteins in mammalian cells (27), tyrosine phosphatases are a group of enzymes that are particularly sensitive to inhibition by ROS (28, 29). Because ERK1/2 are dually phosphorylated on threonine and tyrosine residues, we considered the possibility that the Bt2cAMP/ROS-enhancement of ERK1/2 phosphorylation was due to the inhibition of one of the dual specificity phosphatases that dephosphorylate phospho-ERK (P-ERK)1/2. To address this possibility, we measured the dephosphorylation of P-ERK2 in the presence and absence of cell lysates obtained from control, Bt2cAMP-, or EGF-treated cells. Figure 6A shows that none of these cell lysates had much effect on the dephosphorylation of P-ERK2. We also found that lysates obtained from cells incubated with Bt2cAMP and NAC had little or no effect on the dephosphorylation of P-ERK2 (Fig. 6B). These findings show that changes in the dephosphorylation of P-ERK are unlikely to contribute to the ability of Bt2cAMP or EGF to enhance ERK1/2 phosphorylation. They also show that NAC has little or no effect on the dephosphorylation of ERK2.
Fig. 6.
Bt2cAMP does not affect the dephosphorylation of ERK2. A, A GST fusion protein of P-ERK2 (GST-P-ERK2) was incubated with buffer only for 40 min at 4 C or 37 C as indicated. Another sample was incubated for 40 min at 37 C with buffer containing 120 U of λ phosphatase (P'ase) and served as a positive control. Three additional samples were incubated with lysates prepared from control MA-10 cells or from MA-10 cells that had been incubated with EGF (10 ng/ml) for 5 min or Bt2cAMP (1 mm) for 30 min. These three lysates contained the same protein concentration and 10 μm UO126 to prevent ERK phosphorylation by the endogenous MEKs. At the end of the incubation the GST fusion protein was absorbed to glutathione magnetic beads, and the absorbed protein was eluted, resolved on an SDS gel, and eventually detected with a phospho-ERK1/2 antibody as described in Materials and Methods. A representative blot from three independent experiments is shown. B, MA-10 cells were incubated without or with freshly made 5 mm NAC for 30 min and then without or with 1 mm Bt2cAMP for 30 min. A fixed amount of a GST fusion protein of P-ERK2 (GST-P-ERK2) were then incubated for 40 min at 37 C with lysates prepared from the three groups of cells. These three lysates contained the same protein concentration and 10 μm UO126. At the end of the incubation the GST fusion protein was absorbed to glutathione magnetic beads, and the absorbed protein was eluted, resolved on an SDS gel, and eventually detected with a phospho-ERK1/2 antibody as described in Materials and Methods. Note that the sample from cells treated with Bt2cAMP but without NAC is the same as the sample from cells treated with Bt2cAMP in panel A. A representative blot from three independent experiments is shown.
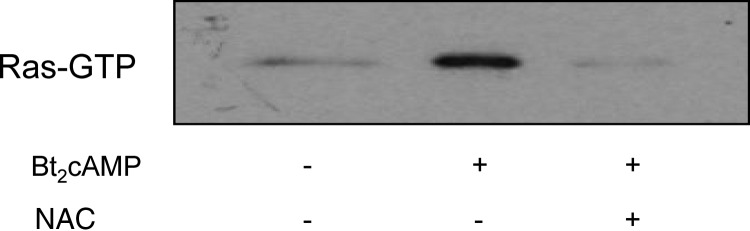
Because addition of cAMP analogs promotes the activation of Ras in MA-10 cells (1), we next tested the effect of NAC on this process. Figure 7 shows that NAC is an effective inhibitor of the Bt2cAMP-provoked activation of Ras. NAC did not inhibit the effects of EGF on Ras activation, however (data not shown).
Fig. 7.
NAC prevents the activation of Ras by Bt2cAMP. MA-10 cells were incubated without or with freshly made 5 mm NAC for 30 min and then without or with 1 mm Bt2cAMP for 30 min as indicated. Lysates containing the same amount of protein were then analyzed for GTP-bound Ras as described in Materials and Methods. A representative blot from three independent experiments is shown.
We conclude that ROS are intermediates in the Bt2cAMP-provoked phosphorylation of ERK1/2 because they participate in the activation of Ras rather than the inhibition of ERK1/2 phosphatases.
ROS also mediate the effects of cAMP on the phosphorylation of ERK1/2 in primary cultures of mouse Leydig cells
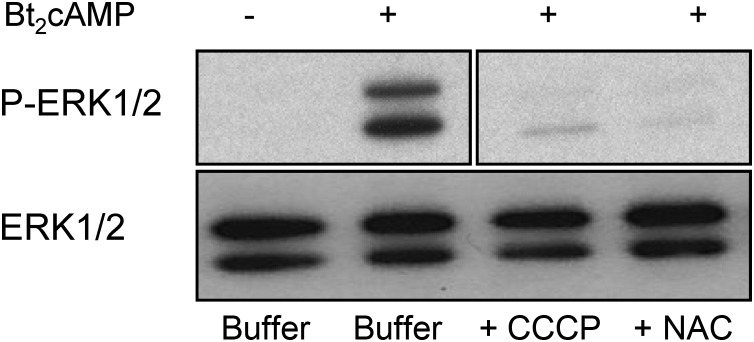
Although MA-10 cells are a widely accepted model in the study of Leydig cell physiology (12), it was also important to test the involvement of ROS in the phosphorylation of ERK1/2 in normal Leydig cells. We thus measured the phosphorylation of ERK1/2 in primary cultures of mouse Leydig cells incubated with Bt2cAMP alone or together with CCCP or NAC, the two compounds that effectively inhibit ERK1/2 phosphorylation in MA-10 cells. Figure 8 shows that these two compounds also inhibit ERK1/2 phosphorylation in primary cultures of mouse Leydig cells.
Fig. 8.
An uncoupler of oxidative phosphorylation and a ROS scavenger inhibit the Bt2cAMP-stimulated phosphorylation of ERK1/2 in primary cultures of mouse Leydig cells. Primary cultures of mouse Leydig cells were preincubated with buffer only, CCCP (5 μm), or freshly made 5 mm NAC for 30 min. This was followed by a 30-min incubation with buffer only or 1 mm Bt2cAMP before assaying for P-ERK1/2 by Western blotting. The samples were subsequently used to measure ERK1/2 in a different gel and blot. A representative blot from five different experiments is shown.
Discussion
There are at least two pathways leading to the phosphorylation of ERK1/2 that occur upon activation of the LHR in MA-10 cells (8, 9). The best characterized is a pathway that involves the transactivation of the EGFR. This pathway involves an arrestin-3-dependent activation of Fyn (8, 10) and the release of soluble EGF-like growth factors (9) that bind to and activate the EGFR (8, 9), thus resulting in the phosphorylation of Shc, the formation of Shc/Sos complexes, and the activation of Ras (7). The activated Ras then mediates the phosphorylation of ERK1/2 by the canonical Ras/Raf/Mek cascade. The second pathway, the focus of the experiments presented herein, involves a cAMP/PKA-mediated activation of Ras (1, 4, 9) that is poorly understood.
The data presented here allow us to make two novel conclusions. First, we can conclude that Bt2cAMP enhances the phosphorylation of ERK1/2 only through the activation of Ras. We have previously reported a cAMP/PKA-induced activation of Ras in MA-10 cells, and have shown that the activation of MEK, which is downstream of Ras, is necessary for ERK1/2 phosphorylation (1, 4). The data presented here, however, formally exclude the possibility that P-ERK1/2 phosphatases are also inactivated by cAMP/PKA.
Second, and more importantly, we can conclude that the cAMP/PKA-induced activation of Ras (and the phosphorylation of ERK1/2) is mediated by mitochondrial ROS. ROS levels in MA-10 cells are increased by Bt2cAMP, but not by 8-CPT-2Me-cAMP, a cAMP analog that is selective for guanine nucleotide exchange factors (22, 23) and cannot stimulate either Ras activation or ERK1/2 phosphorylation in MA-10 cells (1, 4). The involvement of ROS as a mediator of the cAMP/PKA effects on the ERK1/2 pathway is supported by the results obtained with the uncoupler of oxidative phosphorylation (CCCP) and NAC, a ROS scavenger. Our findings on the effects of ROS on ERK1/2 phosphorylation are also fully supported by a recent report showing that a pro-oxidant increases ERK1/2 phosphorylation in MA-10 cells that are depleted of glutathione (25). We reproduced this result here and also showed that glutathione depletion enhances the effect of Bt2cAMP on ERK1/2 phosphorylation. Pro-oxidants were also reported to enhance the phosphorylation of JNK and p38 in glutathione-depleted cells (25), and we were able to reproduce this finding for JNK (data not shown). We also found, however, that Bt2cAMP did not stimulate the phosphorylation of JNK or p38 in control or glutathione-depleted MA-10 cells (data not shown).
The mitochondrial electron transport chain is a major source of ROS in many mammalian cells (21, 30) and, according to the data presented here, it is also the major source of ROS in Bt2cAMP-stimulated MA-10 cells. AKAP121 is known to target PKA to mitochondria in MA-10 cells (31, 32). When activated by cAMP, this mitochondrial PKA enhances the translation and phosphorylation of StAR, two processes that are necessary for the activation of steroid synthesis (13, 32, 33). The AKAP-bound PKA can also phosphorylate several components of the cytochrome c oxidase complex (31, 34), thus affecting electron transport and the intramitochondrial membrane potential (17, 31). Intramitochondrial superoxide is generated upon reduction of molecular oxygen at complexes I and III and converted to hydrogen peroxide, a product that can escape the mitochondria and serve as a signaling molecule in other cellular compartments. Therefore one could easily predict an increase in mitochondrial ROS production upon addition of Bt2cAMP to MA-10 cells. Up until now, however, this was not demonstrated.
An important question that remains unresolved is how ROS can activate Ras and thus stimulate the phosphorylation of ERK1/2. The levels of active Ras (i.e. GTP-bound Ras) are regulated by promoting the exchange of the bound GDP for ambient GTP, a process that is facilitated by Ras guanine nucleotide exchange factors or by decreasing the hydrolysis of the Ras-bound GTP, a process that is facilitated by Ras GTPase-activating proteins (35, 36). Ras is also posttranslationally modified, and this process is important for its localization at the plasma membrane where activation occurs (36). Thus, Ras guanine nucleotide exchange factors, GTPase activating proteins, the localization of Ras at the plasma membrane, and even Ras itself could be ROS targets (27). We can, however, exclude Sos, the Ras guanine nucleotide exchange factor engaged by the EGFR (35, 36), as a potential ROS target because neither the activation of Ras nor the phosphorylation of ERK1/2 is influenced by ROS scavengers when they are engaged by EGF in MA-10 cells. This is important because in some cell types ROS have been shown to enhance the shedding of EGF-like growth factors leading to the phosphorylation of the EGFR and the activation of Ras (37). This pathway does not appear to be operative in MA-10 cells, however, because addition of Bt2cAMP does not increase the phosphorylation of the EGFR.
Lastly, up until now ROS has been considered to be an inhibitor of Leydig cell functions (25, 38), and an increase in ROS and/or a decrease in antioxidant levels (such as glutathione) have been proposed to contribute to the age-related decline in testosterone synthesis (25, 39, 40). The studies presented here show that Leydig cell ROS production is acutely increased by the cAMP-activated PKA and that it is a mediator of the activation of Ras and the ERK1/2 cascade. Because of the involvement of the ERK1/2 cascade on the proliferation and survival of Leydig cells (4, 6) and as a modulator of their steroidogenic properties (2, 3, 5), our data suggest that ROS may also serve as a positive signal for maintaining a functional adult Leydig cell population.
Materials and Methods
Cell culture
MA-10 cells (41) were maintained in gelatin-coated plastic ware as described earlier (42) except that we now use DMEM/F12 instead of Waymouth's MB752/1 as the growth and assay media.
The phosphorylation of ERK1/2 and the EGFR as well as the progesterone and cAMP assays were done using cells plated in six-well plates at a density of 1–2 × 105/well. Ras and ERK phosphatase assays were done in cells plated in 100-mm dishes at a density of 1–2 × 106/dish. ROS measurements were done using cells plated in 12-well plates at a density of 2–5 × 104/well. Two days after plating, all cells were washed three times with serum-free medium containing BSA (DMEM/F12 with 20 mm HEPES; 50 μg/ml gentamicin; and 1 mg/ml BSA, pH 7.4) and then incubated for 18–24 h in this serum-free medium before the experiment. The cells used for the ROS assays were washed with phenol red-free DMEM/F12 containing 20 mm HEPES and 50 μg/ml gentamicin and incubated in the same medium for 18–24 h before the experiment.
Primary cultures of mouse Leydig cells were prepared from the testes of 34- to 36-d-old C57Bl/6 mice. Interstitial cells were isolated basically as described by O'Shaughnessy et al. (43). The resulting cell suspension was filtered through a 70-μm filter, and the cells were recovered by centrifugation, resuspended in DMEM/F12 with 10 mm HEPES, 50 μg/ml gentamicin, insulin (10 μg/ml), transferrin (5.5 μg/ml), and selenium (5 ng/ml), pH 7.4, plated in fibronectin-coated 48-well plates at a ratio of two testes per four wells and maintained at 34 C. Residual germ cells were removed by vigorously washing the wells on the first and second day after plating. The cells were vigorously washed with the medium described above three times. After the washes on the second day the cells were cultured in DMEM/F12 with 20 mm HEPES, 50 μg/ml gentamicin, and 1 mg/ml BSA (pH 7.4) for 18–24 h before starting an experiment. Under these conditions the primary cultures contain Leydig cells (as judged by the presence of Cyp11a1 in Western blots) but are free of germ and Sertoli cells (as judged by absence of VASA and Dmrt in Western blots, respectively).
Transfections of MA-10 cells were done using Lipofectamine as described elsewhere (42). The expression vector for the hLHR and the sequences of the siRNA duplex targeting mouse Gαs and the scrambled siRNA used as control have been described elsewhere (8, 42). The expression vector for the rat cAMP phosphodiesterase 4D3 (44) was kindly donated by Dr. Marco Conti (University of California at San Francisco).
All animal procedures used were approved by the Institutional Animal Care and Use Committee for the University of Iowa.
ERK1/2 and EGFR phosphorylation
The concentrations and incubation times used for the ERK1/2 or EGFR phosphorylation assays were optimized to measure the desired effects and are based on previously published data (1, 7, 9, 10, 42). At the end of the experiment the medium was aspirated and the cells were washed twice with a buffer containing 150 mm NaCl and 20 mm HEPES (pH 7.4). After aspiration of the last wash, the monolayers were lysed with 50 μl of lysis buffer (150 mm NaCl; 50 mm Tris; 1 mm EDTA; 1% Nonidet P-40; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate (SDS); 1 mm Na3VO4; and 1 mm NaF, pH 7.4) supplemented with a commercial mixture of protease inhibitors (Roche, Indianapolis, IN). The plates were rocked at 4 C for 30 min, and the resulting lysates were clarified by centrifugation and assayed for protein content using BCA protein assay kit from Bio-Rad Laboratories, Inc. (Hercules, CA).
Equal amounts of solubilized protein (2–15 μg) were resolved on 7.5% or 12% SDS-polyacrylamide gels and electrophoretically transferred to polyvinylidene difluoride membranes. The blots were blocked for 2 h at room temperature with a solution containing 5% BSA in 10 mm Tris, 100 mm NaCl, 0.2% Tween 20 (pH 7.4), washed and then incubated overnight at 4 C in 1% BSA in 10 mm Tris, 100 mm NaCl, 0.2% Tween-20 (pH 7.4) containing either a 1:1,000 dilution of pTyr1068-EGFR antibody (Cell Signaling Technology, Inc., Danvers, MA; catalog no. 2234) a 1:2000 dilution of pERK1/2 antibody (Cell Signaling Technology; catalog no. 9122), or a 1:2,000 dilution of ERK1/2 antibody (Millipore Corp., Bedford, MA; catalog no. 06-182). After washing, the blots were incubated for 1 h with a 1:3000 dilution of a secondary antibody covalently coupled to horseradish peroxidase (Bio-Rad Laboratories, catalog no. 170-6515) in the same buffer described above. The immune complexes in the Western blots were eventually visualized using enhanced chemiluminescence (Pierce Chemical Co., Rockford, IL) and exposed to film.
For P-EGFR or P-ERK1/2, comparisons among different treatments were always done using the same amount of protein per lane applied to the same gel, transferred to the same blot, and developed for the same length of time. Some of the data presented are spliced from different parts of a given gel, as indicated by the borders on the blot pieces, however, because samples were not always run on the gels in the order in which they are presented in the figures. Total ERK on the same samples was always assayed later using separate gels from those used for the P-ERK1/2 experiments. Again, the same amount of protein per lane applied to the same gel, transferred to the same blot, and developed for the same length of time was used. These results are generally presented without splicing of lanes because they were run after the P-ERK1/2 blots when the order of sample application could be better tailored for publication purposes.
Ras activation assays
This assay is based on the ability of activated Ras to bind to a glutathione-S-transferase (GST)-fusion protein containing the Ras-binding domain of Raf-1 (45). After incubation of cell extracts with a fixed amount of the GST fusion protein, the latter is pulled down with glutathione beads, and the active Ras bound to it is visualized by Western blotting with an antibody to K-Ras (the most abundant Ras isoform in MA-10 cells). The assay was done as described elsewhere (1, 7) except that we used magnetic glutathione beads (instead of agarose glutathione beads) to isolate the complex formed by the endogenous activated Ras and the GST fusion protein.
ERK dephosphorylation assays
This assay is based on minor modifications of previously published procedures (46, 47). Briefly, cells were lysed with 150 μl of a buffer containing 1% Nonidet P-40, 0.3 m sucrose, 50 mm Tris, 100 mm KCl, 1 mm CaCl2, 2.5 mm MgCl2 (pH 7.5) containing protease inhibitors (see above), and the protein concentration of the lysates was adjusted to 1–2 mg/ml. Seventy five microliters of these lysates were mixed with 225 μl of a buffer containing 100 mm MgCl2, 100 mm HEPES (pH 7.4), 10 μm UO126, and 30 ng of a GST fusion protein of phosphorylated ERK2 (Sigma Chemical Co., St. Louis, MO; catalog no. E-1283). After a 40-min incubation at 37 C or 4 C the samples were diluted 2-fold with cold lysis buffer and then mixed with 50 μl of a suspension of glutathione coupled to magnetic beads (Pierce Chemical Co., catalog no.8821). After a 1-h incubation at 4 C the beads were isolated with the help of a magnet, washed three times with 500-μl aliquots of lysis buffer, and boiled in SDS sample buffer for 3 min to release the bound ERK2. The eluted samples were resolved on 7.5% SDS gels and analyzed for the presence of P-ERK2 by Western blotting as described above.
ROS assays
The generation of ROS was measured using 5, and 6-chloromethyl-2′,7-dichlorodihydrofluorescein diacetate ethyl ester (DCFH-DA) (Invitrogen, Carlsbad, CA; catalog no. C6827). This ester diffuses into cells where it is cleaved and trapped inside the cells as DCFH. DCFH is oxidized by ROS to 2′,7′-dichlorofluorescein, which can be easily detected by its strong fluorescence (48). This method was chosen because it has been successfully used to measure ROS generation in MA-10 cells (25, 49).
Cells (in 48-well plates) were preincubated with the indicated inhibitors for 30 min at 37 C in 250 μl of phenol red-free DMEM/F12 containing 20 mm HEPES, 50 μg/ml gentamicin. Each well then received 250 μl of the same medium (with or without the inhibitor) and 5 μm DCFH-DA. After a 30-min incubation at 37 C, the cells received buffer or Bt2cAMP (1 mm), and the incubation was continued for 25 min at 37 C. Each well then received 5 μl of a 1 mg/ml solution of Hoescht 33342 (to label the nuclei), and the incubation was continued for 5 min. Finally the wells were washed three times and examined at a 200-fold magnification using a Nikon TE-300 fluorescent microscope (Nikon Inc., Melville, NY). Digital images from random areas of each well were collected, and the blue fluorescent image was used to count the total number of cells (200–400 cells) with the help of Image J, a free image analysis software package (http://rsbweb.nih.gov/ij/). The green and blue images were merged using Image J and adjusted to maximal brightness and contrast. The cells with green fluorescence were then counted with the help of Image J and divided by the total cell counts (see above) to obtain the percentage of ROS positive cells.
Other assays
cAMP and progesterone were measured by RIA as described elsewhere (42).
Data presentation and analysis
All experiments were repeated at least three times and, when quantitative assays were done, the results were analyzed statistically either by a simple t test or by using ANOVA with Tukey's post test for multiple comparisons among groups. These analyses were performed using the InStat Software package from Graphpad Software (San Diego, CA). In all cases statistical significance was considered at P < 0.05.
Hormones and other supplies
Purified recombinant hCG was kindly provided by Ares Serono (Geneva, Switzerland). Cell culture medium was obtained from Invitrogen, and cell culture plastic ware was from Corning, Inc. (Corning, NY). The source of antibodies and other specialized reagents is specified in the different subheadings of this section. All other chemicals were obtained from commonly used suppliers.
Acknowledgments
We thank Colette Galet for performing the experiments presented in Fig. 1A.
This work was supported by Grant CA-40629 from the National Cancer Institute.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- Bt2cAMP
- Dibutyrl-cAMP
- CCCP
- carbonyl cyanide m-chloro phenyl hydrazone
- CG
- chorionic gonadotropin
- DCFH-DA
- 5, and 6-chloromethyl-2′,7-dichlorodihydrofluorescein diacetate ethyl ester
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- GST
- glutathione-S-transferase
- LHR
- LH receptor
- MEK
- MAPK kinase
- NAC
- N-acetylcysteine
- 22OHC
- 22-hydroxycholesterol
- P-ERK
- phospho-ERK
- PKA
- protein kinase A
- ROS
- reactive oxygen species
- SDS
- sodium dodecyl sulfate
- siRNA
- small interfering RNA
- t-BuOOH
- tert-butyl hydroperoxide.
References
- 1. Hirakawa T, Ascoli M. 2003. The lutropin/choriogonadotropin receptor (LHR)-induced phosphorylation of the extracellular signal regulated kinases (ERKs) in Leydig cells is mediated by a protein kinase A-dependent activation of Ras. Mol Endocrinol 17:2189–2200 [DOI] [PubMed] [Google Scholar]
- 2. Martinelle N, Holst M, Söder O, Svechnikov K. 2004. Extracellular signal-regulated kinases are involved in the acute activation of steroidogenesis in immature rat Leydig cells by human chorionic gonadotropin. Endocrinology 145:4629–4634 [DOI] [PubMed] [Google Scholar]
- 3. Evaul K, Hammes SR. 2008. Cross-talk between G protein-coupled and epidermal growth factor receptors regulates gonadotropin-mediated steroidogenesis in Leydig cells. J Biol Chem 283:27525–27533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiraishi K, Ascoli M. 2007. Lutropin/choriogonadotropin (LH/CG) stimulate the proliferation of primary cultures of rat Leydig cells through a pathway that involves activation of the ERK1/2 cascade. Endocrinology 148:3214–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manna PR, Huhtaniemi IT, Stocco DM. 2009. Mechanisms of protein kinase C signaling in the modulation of 3′,5′-cyclic adenosine monophosphate-mediated steroidogenesis in mouse gonadal cells. Endocrinology 150:3308–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tai P, Shiraishi K, Ascoli M. 2009. Activation of the lutropin/choriogonadotropin receptor (LHR) inhibits apoptosis of immature Leydig cells in primary culture. Endocrinology 150:3766–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shiraishi K, Ascoli M. 2006. Activation of the lutropin/choriogonadotropin receptor (LHR) in MA-10 cells stimulates tyrosine kinase cascades that activate Ras and the extracellular signal regulated kinases (ERK1/2). Endocrinology 147:3419–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galet C, Ascoli M. 2008. Arrestin-3 is essential for the activation of Fyn by the luteinizing hormone receptor (LHR) in MA-10 cells. Cell Signal 20:1822–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shiraishi K, Ascoli M. 2008. A co-culture system reveals the involvement of intercellular pathways as mediators of the lutropin receptor (LHR)-stimulated ERK1/2 phosphorylation in Leydig cells. Exp Cell Res 314:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mizutani T, Shiraishi K, Welsh T, Ascoli M. 2006. Activation of the lutropin/choriogonadotropin receptor (LHR) in MA-10 cells leads to the tyrosine phosphorylation of the focal adhesion kinase (FAK) by a pathway that involves Src family kinases. Mol Endocrinol 20:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swinnen JV, D'Souza B, Conti M, Ascoli M. 1991. Attenuation of cAMP-mediated responses in MA-10 Leydig tumor cells by genetic manipulation of a cAMP-phosphodiesterase. J Biol Chem 266:14383–14389 [PubMed] [Google Scholar]
- 12. Ascoli M. 2007. Immortalized Leydig cell lines as models for studying Leydig cell physiology. In: Payne AH, Hardy MP. eds. The Leydig cell in health and disease. New York: Humana Press; 373–392 [Google Scholar]
- 13. Dyson MT, Kowalewski MP, Manna PR, Stocco DM. 2009. The differential regulation of steroidogenic acute regulatory protein-mediated steroidogenesis by type I and type II PKA in MA-10 cells. Mol Cell Endocrinol 300:94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manna PR, Dyson MT, Stocco DM. 2009. Regulation of the steroidogenic acute regulatory protein gene expression: present and future perspectives. Mol Hum Reprod 15:321–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dexter RN, Fishman LM, Ney RL, Liddle GW. 1967. Inhibition of adrenal corticosteroid synthesis by aminoglutethimide: studies on the mechanims of action. J Clin Endocrinol Metab 27:473–480 [DOI] [PubMed] [Google Scholar]
- 16. Ascoli M, Freeman DA, Sheets JJ, Vickery LE. 1983. Inhibition of steroidogenesis in cultured Leydig tumor cells by 22-amino-23,24-Bisnor-5-Cholen-3β-Ol and (20R)20-phenyl-5-pregnene-3β,20-diol. Endocrinology 113:127–132 [DOI] [PubMed] [Google Scholar]
- 17. Allen JA, Shankara T, Janus P, Buck S, Diemer T, Hales KH, Hales DB. 2006. Energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology 147:3924–3935 [DOI] [PubMed] [Google Scholar]
- 18. Kadenbach B. 2003. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta 1604:77–94 [DOI] [PubMed] [Google Scholar]
- 19. Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. 2004. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 37:755–767 [DOI] [PubMed] [Google Scholar]
- 20. Storozhevykh TP, Senilova YE, Persiyantseva NA, Pinelis VG, Pomytkin IA. 2007. Mitochondrial respiratory chain is involved in insulin-stimulated hydrogen peroxide production and plays an integral role in insulin receptor autophosphorylation in neurons. BMC Neurosci 8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Forman HJ. 2010. Reactive oxygen species and α,β-unsaturated aldehydes as second messengers in signal transduction. Ann NY Acad Sci 1203:35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holz GG, Chepurny OG, Schwede F. 2008. Epac-selective cAMP analogs: New tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal 20:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gloerich M, Bos JL. 2010. Epac: Defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50:355–375 [DOI] [PubMed] [Google Scholar]
- 24. Bedard K, Krause KH. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 [DOI] [PubMed] [Google Scholar]
- 25. Chen H, Zhou L, Lin CY, Beattie MC, Liu J, Zirkin BR. 2010. Effect of glutathione redox state on Leydig cell susceptibility to acute oxidative stress. Mol Cell Endocrinol 323:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanukoglu I. 2006. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev 38:171–196 [DOI] [PubMed] [Google Scholar]
- 27. Veal EA, Day AM, Morgan BA. 2007. Hydrogen peroxide sensing and signaling. Mol Cell 26:1–14 [DOI] [PubMed] [Google Scholar]
- 28. Cho SH, Lee CH, Ahn Y, Kim H, Kim H, Ahn CY, Yang KS, Lee SR. 2004. Redox regulation of PTEN and protein tyrosine phosphatases in H2O2-mediated cell signaling. FEBS Lett 560:7–13 [DOI] [PubMed] [Google Scholar]
- 29. Tonks NK. 2005. Redox redux: revisiting PTPs and the control of cell signaling. Cell 121:667–670 [DOI] [PubMed] [Google Scholar]
- 30. Lambert AJ, Brand MD. 2009. Reactive oxygen species production by mitochondria. Methods Mol Biol 554:165–181 [DOI] [PubMed] [Google Scholar]
- 31. Livigni A, Scorziello A, Agnese S, Adornetto A, Carlucci A, Garbi C, Castaldo I, Annunziato L, Avvedimento EV, Feliciello A. 2006. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol Biol Cell 17:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dyson MT, Jones JK, Kowalewski MP, Manna PR, Alonso M, Gottesman ME, Stocco DM. 2008. Mitochondrial A-kinase anchoring protein 121 binds type II protein kinase A and enhances steroidogenic acute regulatory protein-mediated steroidogenesis in MA-10 mouse Leydig tumor cells. Biol Reprod 78:267–277 [DOI] [PubMed] [Google Scholar]
- 33. Stocco DM. 2007. The role of StAR in Leydig cell steroidogenesis. In: Payne AH, Hardy MP. eds. The Leydig cell in health and disease. Totowa, NJ: Humana Press; 149–155 [Google Scholar]
- 34. Carlucci A, Lignitto L, Feliciello A. 2008. Control of mitochondria dynamics and oxidative metabolism by cAMP, AKAPs and the proteasome. Trends Cell Biol 18:604–613 [DOI] [PubMed] [Google Scholar]
- 35. Cullen PJ, Lockyer PJ. 2002. Integration of calcium and Ras signaling. Nat Rev Mol Cell Biol 3:339–348 [DOI] [PubMed] [Google Scholar]
- 36. Buday L, Downward J. 2008. Many faces of Ras activation. Biochim Biophys Acta 1786:178–187 [DOI] [PubMed] [Google Scholar]
- 37. Myers TJ, Brennaman LH, Stevenson M, Higashiyama S, Russell WE, Lee DC, Sunnarborg SW. 2009. Mitochondrial reactive oxygen species mediate GPCR-induced TACE/ADAM17-dependent transforming growth factor-α shedding. Mol Biol Cell 20:5236–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Diemer T, Allen JA, Hales KH, Hales DB. 2003. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) Protein and steroidogenesis. Endocrinology 144:2882–2891 [DOI] [PubMed] [Google Scholar]
- 39. Chen H, Cangello D, Benson S, Folmer J, Zhu H, Trush MA, Zirkin BR. 2001. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol 36:1361–1373 [DOI] [PubMed] [Google Scholar]
- 40. Midzak AS, Chen H, Papadopoulos V, Zirkin BR. 2009. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol 299:23–31 [DOI] [PubMed] [Google Scholar]
- 41. Ascoli M. 1981. Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology 108:88–95 [DOI] [PubMed] [Google Scholar]
- 42. Hirakawa T, Galet C, Ascoli M. 2002. MA-10 cells transfected with the human lutropin/choriogonadotropin receptor (hLHR). A novel experimental paradigm to study the functional properties of the hLHR. Endocrinology 143:1026–1035 [DOI] [PubMed] [Google Scholar]
- 43. O'Shaughnessy PJ, Baker PJ, Heikkilä M, Vainio S, McMahon AP. 2000. Localization of 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase isoform expression in the developing mouse testis–androstenedione is the major androgen secreted by fetal/neonatal Leydig cells. Endocrinology 141:2631–2637 [DOI] [PubMed] [Google Scholar]
- 44. Swinnen JV, Joseph DR, Conti M. 1989. The mRNA encoding a high-affinity cAMP phosphodiesterase is regulated by hormones and cAMP. Proc Natl Acad Sci USA 86:8197–8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Triest M, Bos JL. 2004. Pull-down assays for guanoside 5′-triphosphate-bound Ras-like guanosine 5′-triphosphatases. Methods Mol Biol 250:97–102 [DOI] [PubMed] [Google Scholar]
- 46. Levinthal DJ, Defranco DB. 2005. Reversible oxidation of ERK-directed protein phosphatases drives oxidative toxicity in neurons. J Biol Chem 280:5875–5883 [DOI] [PubMed] [Google Scholar]
- 47. Laakko T, Juliano RL. 2003. Adhesion regulation of stromal cell-derived factor-1 activation of ERK in lymphocytes by phosphatases. J Biol Chem 278:31621–31628 [DOI] [PubMed] [Google Scholar]
- 48. Wardman P. 2007. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med 43:995–1022 [DOI] [PubMed] [Google Scholar]
- 49. Fan J, Traore K, Li W, Amri H, Huang H, Wu C, Chen H, Zirkin B, Papadopoulos V. 2010. Molecular mechanisms mediating the effect of mono-(2-ethylhexyl) phthalate on hormone-stimulated steroidogenesis in MA-10 mouse tumor Leydig Cells. Endocrinology 151:3348–3362 [DOI] [PMC free article] [PubMed] [Google Scholar]