FSH and testosterone regulate processes required for spermatogenesis and male fertility via a non-classical testosterone signaling pathway in Sertoli cells.
Abstract
Testosterone and FSH act in synergy to produce the factors required to maximize the production of spermatozoa and male fertility. However, the molecular mechanisms by which these hormones support spermatogenesis are not well established. Recently, we identified a nonclassical mechanism of testosterone signaling in cultured rat Sertoli cells. We found that testosterone binding to the androgen receptor recruits and activates Src tyrosine kinase. Src then causes the activation of the epidermal growth factor receptor, which results in the phosphorylation and activation of the ERK MAPK and the cAMP response element-binding protein transcription factor. In this report, we find that FSH inhibits testosterone-mediated activation of ERK and the MAPK pathway in Sertoli cells via the protein kinase A-mediated inhibition of Raf kinase. In addition, FSH, as well as inhibitors of Src and ERK kinase activity, reduced germ cell attachment to Sertoli cells in culture. Using pathway-specific androgen receptor mutants we found that the nonclassical pathway is required for testosterone-mediated increases in germ cell attachment to Sertoli cells. Studies of seminiferous tubule explants determined that Src kinase, but not ERK kinase, activity is required for the release of sperm from seminiferous tubule explants. These findings suggest the nonclassical testosterone-signaling pathway acts via Src and ERK kinases to facilitate the adhesion of immature germ cells to Sertoli cells and through Src to permit the release of mature spermatozoa. In contrast, FSH acts to limit testosterone-mediated ERK kinase activity and germ cell attachment.
Male fertility is regulated by a combination of hormonal and environmental signals. In the testis, the production of spermatozoa (spermatogenesis) is regulated by FSH and testosterone. These hormones signal somatic Sertoli cells to produce factors required to maintain the survival and maturation of developing spermatozoa (1).
Testosterone, which is essential for the maintenance of spermatogenesis, mediates its effects via the intracellular androgen receptor (AR). In the absence of testosterone or functional AR, spermatogenesis rarely proceeds beyond meiosis (2–4). In addition to supporting germ cell transit through meiosis, testosterone and AR have been found to be required for at least two essential spermatogenesis processes: maintaining the attachment of maturing spermatids to Sertoli cells and the release of mature spermatids/spermatozoa from the Sertoli cell. Withdrawal of testosterone results in the detachment of developing spermatids (step 8 through 19 spermatids) from Sertoli cells in the seminiferous epithelium and a subsequent total loss of spermatozoa production (5, 6). Studies of Sertoli cell-specific disruption of AR expression demonstrated that the loss of spermatids occurs during the transition from round to elongating stages of development and may involve a loss of adhesion of round spermatids to Sertoli cells (7). The release of mature spermatozoa from Sertoli cells (spermiation) requires testosterone because depletion of testosterone causes spermiation failure including the retention and degeneration of step 19 (mature) spermatids in rats (8). Testosterone depletion also causes spermiation failure in men (9–11). Furthermore, spermiation requires signaling through AR because this process was blocked in mice expressing a hypomorphic AR allele (7).
Testosterone has been shown to act via two mechanisms, the classical and nonclassical pathways. In the classical pathway, testosterone binds to the AR in the cytoplasm and causes AR to translocate to the nucleus where it binds to specific DNA sequences in gene promoter regions, recruits coregulator proteins, and regulates gene transcription (12). In the nonclassical pathway, testosterone binding to AR recruits Src kinase that then activates the epidermal growth factor receptor (EGFR) to initiate the activation of the MAPK cascade kinases [RAF, MAPK kinase (MEK), and ERK] and downstream kinase-dependent events including transcriptional regulation (13, 14). Thus far, the relative contributions of the two pathways toward maintaining spermatogenesis have not been investigated.
In this study, we demonstrate that FSH stimulation of cultured Sertoli cells blocks testosterone-mediated phosphorylation of ERK via the inhibition of Raf kinase activity. We also identify processes required for male fertility that are regulated by the nonclassical pathway of testosterone action. We find that inhibitors of Src, ERK, and the nonclassical pathway block testosterone-inducible attachment of germ cells to Sertoli cells. Finally, we show that testosterone-regulated Src kinase is required for the release of sperm from seminiferous tubule explants.
Results
FSH inhibits testosterone-induced ERK phosphorylation
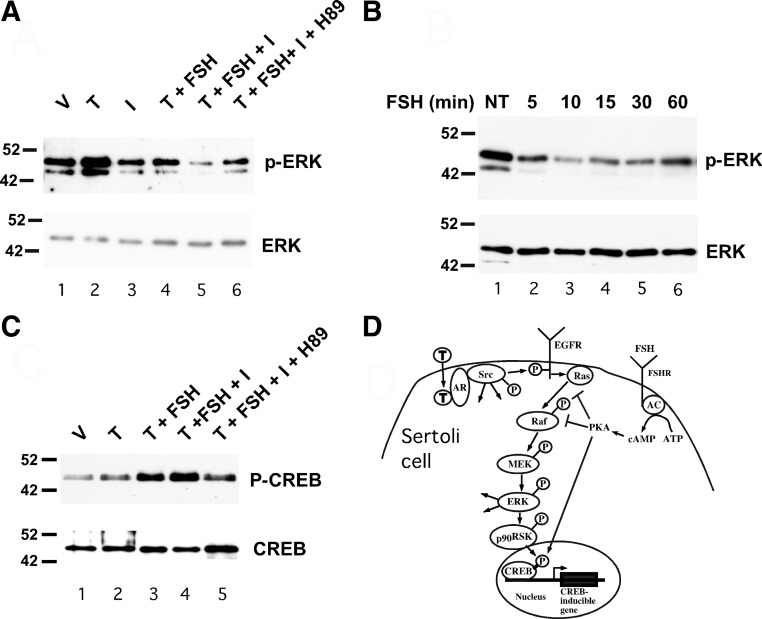
FSH has been shown to inhibit the MAPK cascade and ERK phosphorylation in mature Sertoli cells (15). Therefore, the potential for FSH to limit testosterone-mediated ERK activation was tested. As previously shown (13), stimulation of Sertoli cells from 20-d-old rats with testosterone alone for 10 min increased the levels of phosphorylated ERK (Fig. 1A). In contrast, pretreatment with FSH or the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX) for 20 min followed by stimulation with testosterone for 10 min reduced testosterone-mediated ERK phosphorylation to basal levels. FSH + IBMX reduced testosterone-mediated ERK phosphorylation further. To determine whether cAMP-dependent activation of protein kinase A (PKA) contributes to FSH-mediated inhibition of ERK activity, Sertoli cells were preincubated with the PKA inhibitor H89 for 30 min and then treated with testosterone + FSH + IBMX. Pretreatment with H89 allowed for rescue of testosterone-induced ERK phosphorylation to nearly basal levels. Similar results were obtained after replacing FSH with forskolin, a strong activator of adenylate cyclase and cAMP production (data not shown). Together, these studies indicate that FSH-induced increases in cAMP production and the subsequent activation of PKA blocks testosterone-mediated phosphorylation and activation of ERK. Further time course studies of FSH action revealed that in Sertoli cells isolated from 20-d-old rats, FSH stimulation decreased ERK phosphorylation within 5 min and that ERK phosphorylation remained diminished for at least 60 min (Fig. 1B).
Fig. 1.
FSH inhibits testosterone-mediated activation of ERK via PKA. A, Wild-type Sertoli cells were treated with EtOH-vehicle (V) 100 nm testosterone (T) alone for 10 min or T for 10 min after 20 min pretreatments with various combinations of IBMX (I, 0.5 mm), FSH (100 ng/ml), and H89 (10 μm) as indicated. Whole-cell extracts were assayed by Western blot using antiserum against phosphorylated ERK. The gels were repeated and probed for total ERK. B, Wild-type Sertoli cells were not treated (NT) or treated with FSH for 5–60 min and probed using antisera against phosphorylated ERK and total ERK (A). C, Wild-type Sertoli cells were treated with V, T alone for 10 min, or T for 10 min after 20 min pretreatments with various combinations of FSH (100 ng/ml), IBMX (I, 0.5 mm), and H89 (10 μm) as indicated. Whole-cell extracts were assayed by Western blot using antiserum against CREB phosphorylated at serine 133 or total CREB. The blots shown are representative of at least three independent experiments. D, The nonclassical testosterone signaling pathway initiates with testosterone diffusing through the plasma membrane and binding AR, which recruits and activates Src. The subsequent activation of EGFR, ERK, CREB, and intermediates is shown. Added arrows from Src and ERK represent additional potential activities of these kinases. FSH-mediated increases in adenylate cyclase (AC) activity result in increased conversion of ATP to cAMP, which activates PKA. Activation of PKA inhibits Ras activation of Raf and RAF kinase activity (as demonstrated in Figs. 2 and 3), which blocks phosphorylation and activation of ERK via the MAPK cascade. FSH-mediated activation of PKA also induces CREB phosphorylation and CREB-mediated transcription.
Parallel studies were performed to determine the effects of testosterone and FSH stimulation on phosphorylation and activation of the cAMP response element-binding protein (CREB) transcription factor. It has been established that stimulation of Sertoli cells with FSH results in the phosphorylation of the CREB transcription factor on serine 133 by PKA and the activation of CREB-mediated transcription (16). Previously, it was found that testosterone stimulation also resulted in increased CREB phosphorylation on serine 133 in Sertoli cells (13). In contrast to the observed testosterone-mediated decrease in ERK phosphorylation, costimulation with testosterone + FSH or testosterone + FSH + IBMX resulted in levels of phosphorylated CREB that were elevated over that of testosterone stimulation alone (Fig. 1C). Pretreatment of the cells with H89 reduced phosphorylation of CREB in contrast to what was observed for ERK. These results demonstrate that FSH stimulates the direct phosphorylation of CREB by PKA but blocks testosterone-mediated phosphorylation of ERK (Fig. 1D).
FSH blocks the MAPK cascade upstream of MEK
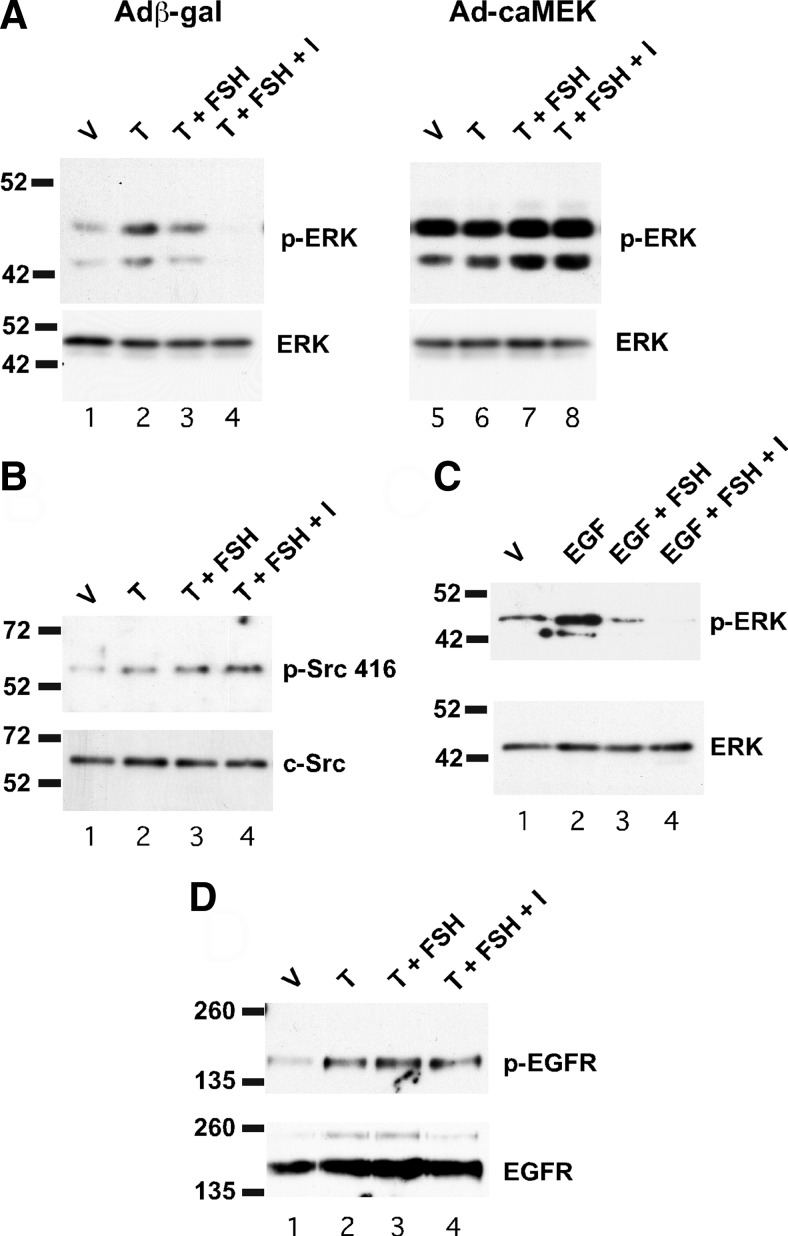
Further studies were performed to identify the point at which FSH acts on the nonclassical testosterone pathway to decrease ERK activity. ERK kinases are inhibited by dual specificity MAPK phosphatases that can be activated by cAMP-PKA-regulated pathways (17). To determine whether FSH and cAMP affected ERK phosphorylation directly by the activation of a phosphatase or some other mechanism, Sertoli cells were infected with adenovirus constructs expressing β-galactosidase as a control or a constitutively active form of MEK to activate ERK. In the presence of the constitutively active MEK, treatment with FSH or FSH + IBMX did not reduce ERK phosphorylation (Fig. 2A). These data suggest that ERK activity is not directly reduced by an FSH-inducible phosphatase or another mechanism that acts directly on ERK. The findings also suggest that FSH acts upstream of MEK to inhibit ERK activity, although it cannot be ruled out that ERK activation by the constitutively active MEK overcomes the actions of any downstream inhibitor.
FSH inhibits ERK phosphorylation downstream of Src and the EGF receptor
The nonclassical pathway of testosterone signaling is initiated by the activation of Src kinase that then causes the activation of the EGF receptor (14). To identify potential upstream sites at which FSH acts to block ERK activation, FSH regulation of Src kinase activity was investigated. Activation of Src occurs with the autophosphorylation of tyrosine 416 (18). FSH stimulation of Sertoli cells did not decrease testosterone-mediated phosphorylation of Src at tyrosine 416 (Fig. 2B). These data indicate that FSH does not affect testosterone-stimulated phosphorylation of Src and suggest that FSH acts downstream of Src to down-regulate ERK activity.
Fig. 2.
FSH acts upstream of MEK and downstream of the EGF receptor to inhibit ERK activity. A, Wild-type Sertoli cells were transfected with adenoviral constructs expressing either β-galactosidase (Adβ-gal) or constitutively active MEK (Ad-caMEK) for 2 d and then were stimulated with EtOH-vehicle (V), 100 nm testosterone (T), T + FSH (100 ng/ml), or T + FSH + IBMX (I, 0.5 mm) for 10 min. Whole-cell extracts were assayed for p-ERK and total ERK levels by Western blot. B, Wild-type Sertoli cells were treated with V, T, or T + various combinations of FSH or IBMX for 10 min. Whole-cell extracts were assayed by Western blot using antisera against Src phosphorylated at position 416 or total Src. C, Wild-type Sertoli cells starved of EGF for 16 h were stimulated with V, EGF (10 nm), or EGF + FSH, or EGF + FSH + IBMX (B). Whole-cell extracts were assayed for p-ERK, and total ERK levels were assayed by Western blot. D, Wild-type Sertoli cells were treated as in A and whole-cell extracts were immunoprecipitated with antiserum against total EGFR and then assayed for EGFR phosphorylated at position 1173 and total EGFR by Western blot. The blots shown are representative of three independent experiments.
To determine whether FSH alters EGF receptor activity or signaling downstream of the EGF receptor, FSH effects on EGF stimulation of Sertoli cells were assayed on Sertoli cells starved of EGF for 16 h. EGF stimulation for 10 min resulted in a dramatic induction of ERK phosphorylation (Fig. 2C). Costimulation with FSH decreased the EGF-mediated activation of ERK, and FSH + IBMX reduced ERK phosphorylation to basal levels. Together, these results indicate that elevated FSH levels can inhibit EGFR-mediated activation of the MAPK pathway in Sertoli cells. Additional studies of EGFR activation demonstrated that phosphorylation of EGFR was not blocked by FSH or FSH + IBMX (Fig. 2D). These results demonstrate that the inhibitory effects of FSH on ERK activity are effected downstream of the EGF receptor.
FSH inhibits testosterone-mediated phosphorylation of Raf-1
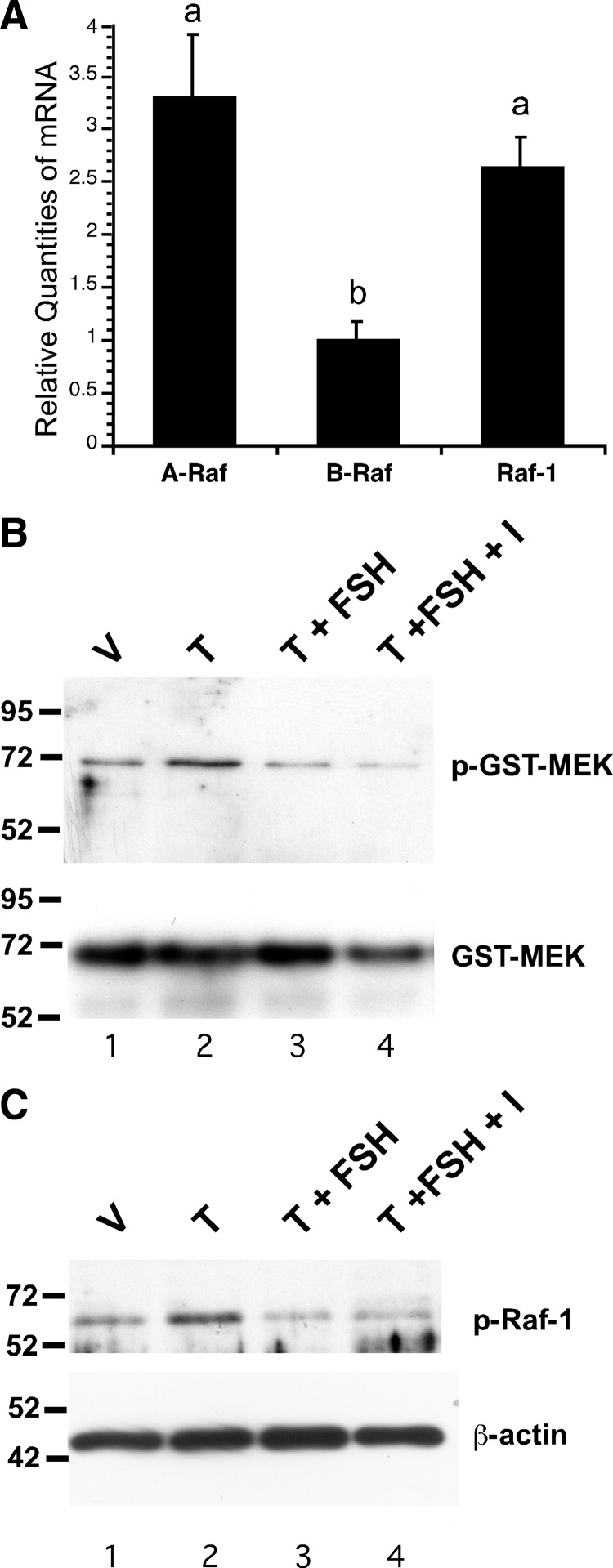
Because our results indicated that FSH acts downstream of EGFR and upstream of MEK, and because cAMP-regulated pathways and PKA have been shown previously to regulate Raf kinase activity by numerous mechanisms (reviewed in Refs 19–21), we focused on Raf kinases as potential targets of FSH in Sertoli cells. First, the relative expression levels of the three Raf isoforms were compared in Sertoli cells cultured from 15-d-old rats using quantitative PCR (qPCR). These assays determined that the relative mRNA levels of Raf-1 and A-Raf transcripts were similar and 3.3- or 2.6-fold greater than B-Raf, respectively (Fig. 3A). This result suggests that most signaling through the MAPK pathway in Sertoli cells would be expected to be transduced via Raf-1 and/or A-Raf, that have conserved phosphorylation sites and are regulated by similar mechanisms (22).
Fig. 3.
FSH inhibits Raf kinase activity. A, Total RNA from untreated wild-type Sertoli cells isolated from 15-d-old rats cultured for 5 d was assayed by quantitative real-time PCR. The levels of the mRNAs expressing Raf-1, A-Raf, and B-Raf are shown relative to B-Raf (=1). Values with different lowercase letters differ significantly (P < 0.05) (n = 3). B, Wild-type Sertoli cells were treated with EtOH-vehicle (V), 100 nm testosterone (T), T + FSH (1 μm), or T + FSH + IBMX (I, 0.5 mm) for 10 min. Whole-cell extracts were incubated with purified GST-MEK protein in the presence of ATP, and the relative levels of phosphorylated and total GST-MEK1 were determined by Western blot using antisera against phosphorylated or total MEK½. C, Whole-cell extracts from Sertoli cells treated (B) were assayed by Western blot using antiserum against phosphorylated Raf-1. The blots shown are representative of three independent experiments.
To determine whether FSH stimulation blocked the MAPK cascade by interfering with Raf activity, an assay of Raf kinase activity was performed after treating Sertoli cells with testosterone in the absence and presence of FSH. The assay quantifies the phosphorylation of glutathione-S-transferase (GST)-tagged MEK1 by Raf MEK kinase. Whole-cell extracts from treated Sertoli cells were incubated with purified GST-tagged MEK1 protein in the presence of ATP, and the relative levels of MEK1 phosphorylated by Raf were determined by Western blot. Extracts from cells stimulated with testosterone showed increased Raf-dependent phosphorylation of MEK (Fig. 3B). Pretreatment with FSH or FSH + IBMX reduced testosterone-stimulated MEK phosphorylation. These data confirm that FSH acts upstream of MEK to inhibit ERK activity and indicate that FSH inhibits Raf kinase activity in Sertoli cells. To further assess the FSH-mediated decrease in Raf-1 activity, Western blot assays were performed using an antiserum recognizing Raf-1 phosphorylated on serine 338, which is necessary for Raf-1 activation (23). These studies indicated that Raf-1 phosphorylation on serine 338 was increased after testosterone stimulation but was decreased by FSH and FSH + IBMX (Fig. 3C). Together, these data suggest that Raf-1 is the target through which FSH inhibits the MAPK cascade in Sertoli cells and testosterone-mediated activation of ERK.
FSH inhibits testosterone-mediated binding of germ cells to Sertoli cells
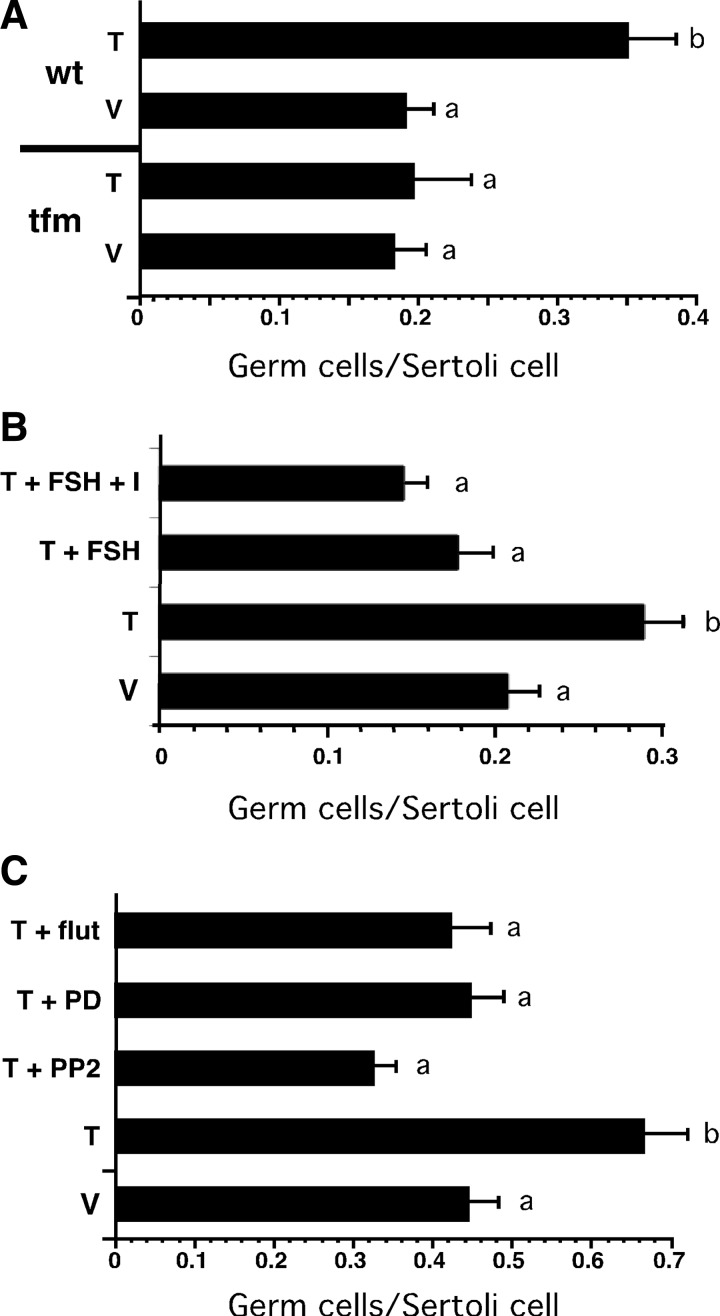
We next investigated FSH and nonclassical testosterone signaling in the regulation of testosterone-dependent spermatogenesis processes. To determine the extent to which testosterone and FSH facilitated the attachment of germ cells to Sertoli cells, coculture studies were established. Sertoli cells were isolated from either tfm rats lacking AR activity or from wild-type rats and then cocultured with germ cells isolated from wild-type adult rats. The cocultures were incubated with testosterone or EtOH (vehicle) for 48 h, and the number of germ cells associated with Sertoli cells was counted. Sertoli cells isolated from tfm rats incubated in the absence and presence of testosterone had similar numbers of associated germ cells (0.18 and 0.20 germ cells/Sertoli cell, respectively) (Fig. 4A). Germ cell attachment to wild-type Sertoli cells in the absence of testosterone was similar to that for tfm cells (0.19 germ cells/Sertoli cell) whereas germ cell attachment to wild-type Sertoli cells in the presence of testosterone was 80% greater (0.35 germ cells/Sertoli cell) than that of the tfm Sertoli cells. These studies indicate that testosterone acts through AR to increase germ cell attachment to Sertoli cells.
Fig. 4.
Inhibitors of Src and ERK kinases block testosterone-induced germ cell attachment. Sertoli cells were isolated from 20-d-old wild-type and tfm rats and placed in culture in the presence of FSH and testosterone for 5 d after which germ cells from adult wild-type rats were added to the culture. Staining with Sertoli cell-specific vimentin antiserum (data not shown) permitted identification of the germ cells located within the boundaries of Sertoli cells. A, The mean number (±se) of germ cells per Sertoli cell were determined for tfm and wild-type (wt) Sertoli cells treated with vehicle (V) or 100 nm testosterone (T) for 48 h. B, the mean (±se) number of germ cells attached per wild-type Sertoli cell were determined after 48 h treatment with V, T, T + FSH (100 ng/ml), or T + FSH + IBMX (I, 0.5 mm). C, the mean (±se) number of germ cells attached/wild-type Sertoli cell were determined after 48 h treatment with V, T, T + PP2 (10 nm), T + PD98059 (PD, 50 μm), or T + flutamide (flut, 1 μm). Cells were counted from at least five random fields from each of at least three experiments. Values with different lowercase letters differ significantly (P < 0.05) (A and C, n = 3; B, n = 4).
Cocultures of wild-type Sertoli cells and germ cells were assayed further to determine whether FSH stimulation interferes with maintaining Sertoli-germ cell attachment. Again incubation with testosterone for 48 h increased germ cell attachment to 70% over vehicle-treated cultures (0.20 to 0.29 germ cells/ Sertoli cell) (Fig. 4B). Treatment with testosterone + FSH or testosterone + FSH+ IBMX reduced the numbers of germ cells attached to below vehicle-treated levels (0.18 and 0.15 germ cells/Sertoli cell, respectively.
Src and ERK kinase activity is required for testosterone-mediated increases in germ cell binding to Sertoli cells
Germ cell attachment was then assayed for wild-type Sertoli cells in the presence of inhibitors of the nonclassical pathway. Stimulation with testosterone increased germ cell attachment over that observed for vehicle (0.67 vs. 0.44 germ cells/Sertoli cell) (Fig. 4C). In contrast, addition of the Src kinase inhibitor PP2 with testosterone decreased attachment efficiency by 50% (0.33 germ cells/Sertoli cell). Addition of the ERK kinase inhibitor PD98059 reduced germ cell attachment to that seen with vehicle treatment (0.46 germ cells/Sertoli cell). The decrease in germ cell attachment in the presence of PP2 or PD98059 was similar to or greater than that in the presence of the AR antagonist flutamide (0.42 germ cells/Sertoli cell). The mean number of Sertoli cells was not altered by the various treatments suggesting that there were no toxic effects of the inhibitors (data not shown). These data confirm that testosterone increases the efficiency of germ cell attachment to Sertoli cells and suggests that both Src and ERK kinases that are activated by the nonclassical pathway are required to facilitate Sertoli-germ cell attachment.
Regulation of classical vs. nonclassical pathways by pathway-specific AR mutants
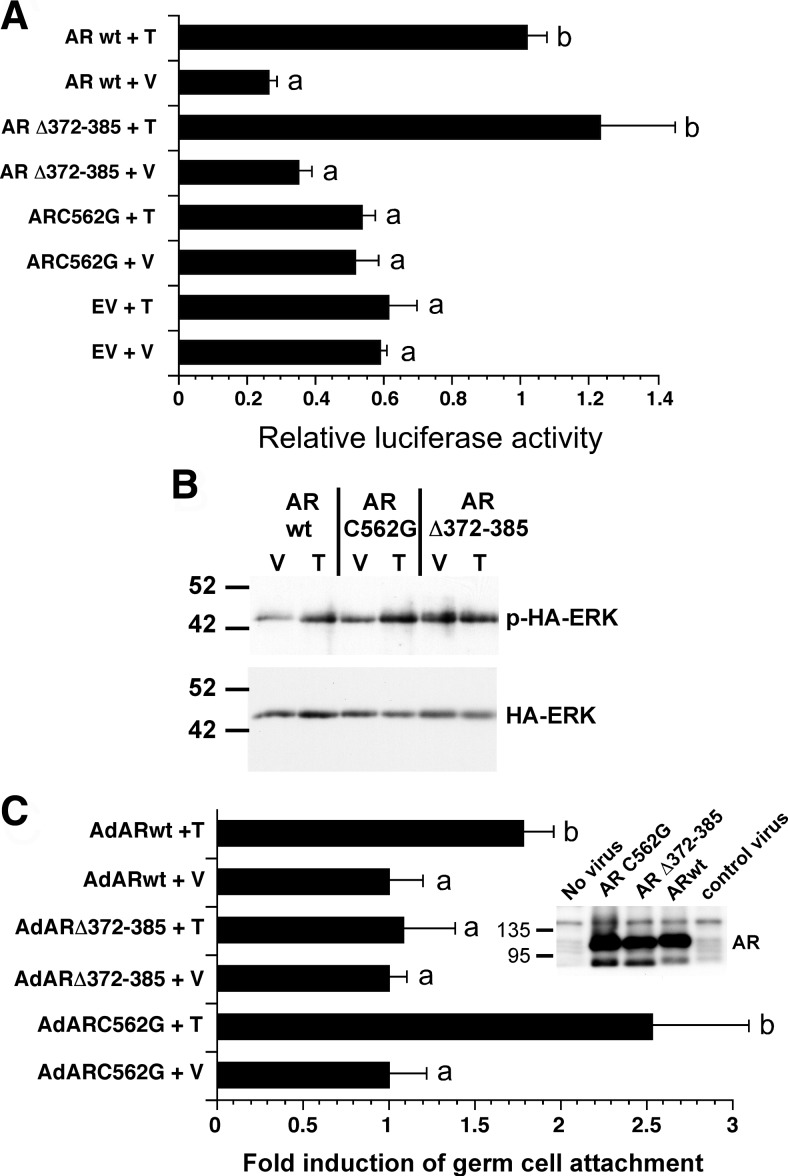
The finding that Src and ERK activity are required for testosterone-mediated increases in germ cell attachment suggested that the nonclassical testosterone-signaling pathway contributes to Sertoli-germ cell adhesion. To study the relative contributions of the classical and nonclassical pathways for maintaining Sertoli-germ cell attachment, the activity of AR mutants that selectively activate one of the pathways was assessed in Sertoli cells isolated from tfm rats (24). The ARC562G mutant having a one-amino acid change that disrupts the structure of a zinc finger has been shown to be incapable of binding to DNA and cannot increase transcription from an AR-inducible promoter in CV-1 cells (25). The ARΔ372–385 mutant was previously shown to retain the ability to activate AR-mediated transcription but lacks a praline-rich domain required to interact with c-Src and activate the nonclassical pathway (26). Figure 5A shows that transient transfection studies of Sertoli cells isolated from AR-defective tfm rats confirmed that ARC562G was incapable of activating the testosterone-inducible prostate-specific antigen (PSA) promoter in Sertoli cells. In contrast, the PSA promoter was induced after overexpression of wild-type AR or the ARΔ372–385 mutant.
Fig. 5.
The nonclassical pathway contributes to testosterone-mediated Sertoli-germ cell attachment. A, Sertoli cells isolated from 15-d-old tfm rats were transfected with empty vector (EV) or vectors expressing the ARC562G or ARΔ372–385 mutant forms of AR or the wild-type AR (AR wt) as well as a luciferase reporter plasmid driven by the AR-inducible PSA promoter. The cells were stimulated with EtOH vehicle (V) or 100 nm testosterone (T) for 24 h, and luciferase activity was determined after normalization for protein content. For each AR construct, the mean (±se) relative luciferase activity is normalized to that of ARΔ wt + T (=1). Values with different lowercase letters differ significantly (P < 0.05) (n = 3). B, Sertoli cells isolated from tfm rats were cotransfected with plasmids encoding wild-type AR, ARC562G, or ARΔ372–385 and a plasmid encoding HA epitope-tagged ERK. After stimulation with V or T for 10 min, whole-cell extracts were immunoprecipitated with antiserum against the HA epitope followed by Western blot analysis using an antiserum against phosphorylated ERK or total ERK. The image shown is representative of three experiments. C, Sertoli cells isolated from 15-d-old tfm rats were infected with adenovirus constructs expressing wild-type AR, ARΔ372–385, or ARC562G. germ cells were added 2 d later in the presence of vehicle (V) or 100 mm testosterone (T), and the number of germ cells attached per Sertoli cell was determined 48 h later. For each adenovirus construct, the mean (±se) percentage of germ cells bound per Sertoli cell was normalized to that of vehicle treatment (=1), and the results of testosterone treatment were expressed as fold induction over the paired vehicle levels. For comparisons of treatments for individual adenovirus constructs, values with different lowercase letters differ significantly (P < 0.05) (n = 4). Cells were counted from at least five random fields for each of three studies. The inset shows the results of a Western blot using extracts from COS-7 cells infected with no virus, AdARC562G, AdARΔ372–385, AdARwt, and AdRAP1DN (control virus).
Additional transient transfection studies were performed to assay the activation of the nonclassical pathway by the AR mutants in Sertoli cells isolated from tfm rats. The tfm Sertoli cells were transfected with plasmids encoding either wild-type AR, ARC562G, or ARΔ372–385 in combination with a plasmid encoding hemagglutinin (HA) epitope-tagged ERK to allow monitoring of ERK activation in transfected cells. The Sertoli cells were stimulated with testosterone, whole-cell extracts were prepared, and HA-ERK was immunoprecipitated followed by Western analysis using antisera against p-ERK and total ERK. Testosterone stimulation of tfm Sertoli cells expressing either wild-type AR or ARC562G resulted in increased phosphorylation of HA-ERK (Fig. 5B). HA-ERK phosphorylation was not increased by testosterone in cells expressing ARΔ372–385. Similar results were obtained in transfections of the MSC-1 Sertoli cell line that lacks AR (27) (data not shown). Together, the studies in Fig. 5, A and B, confirm that ARC562G is only capable of activating the nonclassical pathway and that ARΔ372–385 selectively activates the classical pathway in Sertoli cells.
Nonclassical signaling is required for testosterone-mediated increases in germ cell attachment
Sertoli cells isolated from tfm rats were infected with adenoviral constructs expressing wild-type AR or one of the pathway-specific AR mutants to determine the relative contributions of the classical and nonclassical pathways to Sertoli/germ cell adhesion. Sertoli cells infected with adenovirus constructs expressing wild-type AR or ARC562G that are capable of activating the nonclassical pathway displayed approximately a doubling of germ cells attached in the presence of testosterone (Fig. 5C). In contrast, the numbers of germ cells attached were not elevated after testosterone stimulation of Sertoli cells overexpressing the ARΔ372–385 mutant that is not able to activate the nonclassical pathway. Control Western blot studies indicated that the three adenovirus constructs expressed similar levels of wild-type or mutant AR proteins. (Fig. 5C, inset). These results indicate that the nonclassical pathway of testosterone action contributes to Sertoli-germ cell adhesion and that the DNA-binding activity of AR and the classical pathway is not sufficient to permit testosterone-induced increases in germ cell binding.
The Src inhibitor PP2 blocks the release of sperm from seminiferous tubule explants
Having established that FSH signaling inhibits testosterone-mediated activation of the MAPK cascade and the attachment of germ cells by Sertoli cells, we sought to determine whether FSH and/or activation of the kinases in the nonclassical testosterone-signaling pathway regulates the release of mature sperm. To study the regulation of sperm release, seminiferous tubule fragments corresponding to stage VII–VIII cell association stages that contain elongated spermatids/spermatozoa immediately before their release were collected as described previously (28).
Studies were performed to identify signaling pathways that participate in the release of mature spermatozoa from Sertoli cells. Pools of seminiferous tubule fragments containing only stages VII–VIII were incubated for 20 h with EtOH (vehicle), testosterone (100 nm), testosterone + various kinase inhibitors or the AR antagonist flutamide alone to assess differences in sperm release. Tubule fragments cultured under identical conditions were collected for histological analysis of structural integrity, cell viability, and maintenance of spermatogenesis. Analysis of tubule morphology indicated that the treatments had similar low levels of tissue damage with spermatogonia, spermatocytes, and round spermatids positioned correctly (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). It was observed that, after all treatments, the cycle of the seminiferous epithelium continued to progress based upon the finding that regions of the tubule that were originally in stage VIII with sperm poised to be released had advanced to stage IX in which the mature sperm had been released and round spermatid nuclei had begun to form ventral and dorsal surfaces characteristic to the stage. Cell viability was not affected by any of the conditions.
Quantitation of sperm release revealed that the mean percentage of sperm released from vehicle-treated tubule fragments was 39% (Fig. 6B), which was consistent with the results of a similar study performed by Chapin and colleagues (29). In comparison with the vehicle-treated tubules, testosterone stimulation alone did not alter sperm release. The finding that testosterone alone did not increase the percentage of sperm released from the tubules was unexpected because testosterone is required for the release of sperm in vivo (7). Therefore, we tested the hypothesis that the seminiferous tubule fragments retained sufficient testosterone in culture to maintain spermatogenesis. For this study, two stage VII–VIII tubule fragments were incubated for 20 h as for the sperm release assay. The tubule tissue or the media were then assayed for testosterone content by RIA. The two tubules were found to contain 169 ± 46 pg of testosterone. We estimated that the tubule fragments represented approximately 10 μl of volume or 10 mg wet weight. Assuming that 60% of the tubule weight is water, then the 169 pg of testosterone was contained in 6 mg (6 μl) of tubule wet weight, which corresponds to 94 nm. This concentration of testosterone is in excess of that required to maintain full fertility and activate the nonclassical testosterone-signaling pathway (13, 30). The concentration of testosterone found in the media after incubation of the tubules was found to be 0.64 nm ± 0.32 nm or a total of 193 ± 95 pg, which suggests that the original concentration of testosterone in the tubules immediately after isolation was approximately 201 nm, and thus 47% of the original testosterone remained in the tubule fragments at the end of the incubation period. Therefore, stimulation with additional testosterone would not be expected to increase the number of sperm released because testosterone levels in the seminiferous tubules were already sufficient for sperm release.
Fig. 6.
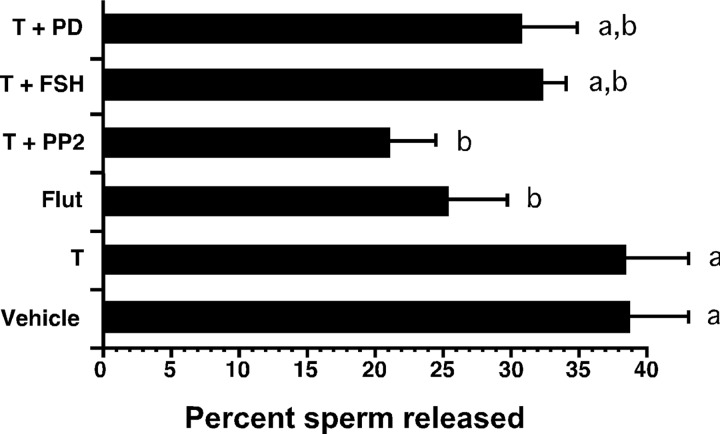
Src kinase activity is required for sperm release. Stage VII–VIII rat seminiferous tubule fragments were incubated for 20 h in the presence of EtOH-vehicle (V), testosterone (T, 100 nm), flutamide alone (Flut, 1 μm), T plus either PP2 (10 nm), FSH (100 ng/ml), or PD98059 (50 μm). The number of sperm or sperm heads present in the media and after sonication of the tubule tissue was counted, and the percent sperm released into the media was determined. Values with different lowercase letters differ significantly (P < 0.05) (n = 4 to 6).
Further analysis of factors that might regulate sperm release revealed that flutamide treatment alone reduced sperm release to 35% below control levels, confirming that testosterone and AR are required for sperm release (Fig. 6B). The inhibition of sperm release by PP2 was more effective than that observed with flutamide, because PP2 reduced the number of sperm released in the presence of added testosterone by 44%. Stimulation with FSH, the ERK inhibitor PD98059, or wortmannin in the presence of testosterone reduced the percentages of sperm released less dramatically (17%, 21%, and 9%, respectively), and these changes did not reach statistical significance. These results indicate that testosterone-mediated activation of Src kinase contributes to sperm release. In contrast, inhibition of ERK, either with a specific chemical inhibitor or with FSH, did not significantly affect sperm release.
Discussion
FSH acts synergistically with testosterone to increase the efficiency of spermatogenesis and fertility (1, 31). However, the periods of maximal effectiveness of FSH and testosterone signaling in Sertoli cells are temporally distinct. Specifically, during the 12.9-day, 14-stage (I–XIV) cycle of the seminiferous epithelium in the adult rat testis, FSH-mediated production of cAMP in Sertoli cells is highest in stages I–V but drops to its lowest levels in stages VI–VIII before rising again in stages IX–XIV (32). In contrast, the expression of AR and the extent of testosterone signaling is low or undetectable except in stages VI–VIII when AR levels peak (33). The out-of-phase nature of AR and FSH receptor expression raises the possibility that the two receptors may mediate complimentary and/or competing processes.
We found that FSH treatment inhibited testosterone-mediated phosphorylation of ERK in Sertoli cells. The addition of either forskolin, which elevates cAMP levels, or the phosphodiesterase inhibitor IBMX, which blocks cAMP degradation, also inhibited ERK phosphorylation by testosterone. These results, together with the finding that the PKA inhibitor H89 can rescue testosterone-mediated ERK activation, indicate that FSH acts via cAMP and PKA to inhibit the MAPK cascade. Because the rescue of ERK phosphorylation by H89 was not complete, it is possible that FSH may act via factors other than PKA to inhibit testosterone-mediated phosphorylation of ERK. However, it is also possible that the combined stimulation with FSH + IBMX elevates cAMP production and PKA activity to levels greater than can be inhibited with the standard H89 levels that were used.
In a previous study, we found that FSH stimulation of Sertoli cells from 15-d-old rats did not reduce ERK phosphorylation (13). We were unable to confirm this observation. The reason for this contradictory result is not yet known. However, one possibility is that the cells in the earlier study may have had characteristics of less mature Sertoli cells. Crepieux et al. (15) showed that FSH increased ERK phosphorylation markedly in immature Sertoli cells isolated from 5-d-old rats and ERK phosphorylation was elevated to a lesser extent by FSH in 12-d Sertoli cells. Our finding that FSH blocked testosterone-mediated induction of ERK in Sertoli cells isolated from 20-d-old rats is consistent with the results of Crepieux et al. (15) who found that FSH inhibited basal levels of ERK phosphorylation in mature Sertoli cells from 19-d-old rats.
In contrast to the inhibition of ERK activity by FSH, phosphorylation of the CREB transcription factor on serine 133 was increased by costimulation with FSH and testosterone. The increased CREB phosphorylation is a result of FSH-mediated activation of PKA that directly phosphorylates CREB. Thus, stimulation with either FSH or testosterone results in CREB phosphorylation. These findings suggest that the combined effects of FSH and testosterone signaling result in maintaining the activation of CREB in Sertoli cells throughout the cycle of the semininferous epithelium. This idea is in agreement with our earlier findings that phosphorylated CREB in Sertoli cells is required for the survival of spermatocytes and the subsequent production of spermatids (34).
Evaluation of FSH effects on the various components of the nonclassical testosterone pathway identified Raf-1 as the most likely target for inhibition by FSH. There are at least four pathways by which the FSH downstream effectors cAMP and PKA are known to down-regulate Raf-1. First, PKA can cause guanine nucleotide exchange factors to bind with Ras-related small guanosine triphosphatase Rap proteins and facilitate Rap protein binding to Raf kinases with a resulting down-regulation of Raf-1 activity (35). Second, Raf-1 activity can also be inhibited by cAMP in a PKA-independent mechanism in which the EPAC (exchange protein directly activated by cAMP) family of guanine nucleotide exchange factors activates Rap1 that then inhibits Raf-1 (36, 37). Third, when PKA is activated, Raf-1 is phosphorylated on serines 43, 233, 259, and 621 (38, 39). Phosphorylation of serine 43 blocks Raf-1 binding to Ras through steric hindrance, whereas phosphorylation of the remaining three sites stimulates Raf binding with 14-3-3 proteins, which restrains Raf-1 from adopting an active conformation and prevents Raf-1 recruitment to the plasma membrane (40, 41). Fourth, the phosphorylation of serine 238 that is required for activation of Raf-1 is decreased by PKA (38, 42). Additional studies will be required to determine the mechanism(s) used by FSH to down-regulate Raf-1 activity in Sertoli cells.
The finding that testosterone increased the number of immature germ cells bound to Sertoli cells in culture is consistent with earlier studies showing that purified fractions of round spermatids bind to Sertoli cells more efficiently in the presence of testosterone (43). The range of germ cell binding efficiency (from untreated control to testosterone treated) that we observed was nearly identical to an earlier report using the germ cell/Sertoli cell metric (44). After converting our data to germ cells per field, our binding efficiencies were within a factor of 2 of other studies using this alternative analysis strategy (data not shown) (43, 45, 46). Our coculture results also resemble those from in vivo studies in which depletion of testosterone caused round spermatids to disappear from the testis cross sections and accumulate in the cauda epididymis, indicating that round spermatids detached prematurely (5). Follow-up studies showed that the loss of spermatids was due to some defect in the adhesion function between Sertoli cells and germ cells (47). Studies of a transgenic mouse expressing a conditional hypomorphic AR also provide evidence that the lack of testosterone signaling causes detachment of less mature germ cells. In this model, germ cells are lost during the transition from round spermatids to elongated spermatids due to a lack of adhesion to Sertoli cells (7).
The number of germ cells attached to Sertoli cells was reduced after inhibiting ERK phosphorylation with the MEK inhibitor PD98059 and after FSH stimulation. These findings suggest that ERK activation is required to optimize germ cell attachment to Sertoli cells. This idea is consistent with an earlier report that ERK activity in Sertoli cells is required in cocultures for the progression of pachytene spermatocytes through meiosis to the round-spermatid stage of spermatogenesis (48). Inhibition of Src kinase by PP2 decreased the numbers of germ cells attached to Sertoli cells to a greater extent than FSH and PD98059, but it is not yet known whether Src activation is required to activate ERK or whether Src potentiates germ cell attachment via other ERK-independent mechanisms. In either case, these studies suggest that Src and ERK kinases, which are activated by the nonclassical testosterone signaling, are required for testosterone-mediated increases in germ cell attachment to Sertoli cells.
In a study by Perryman and colleagues (43), testosterone was only able to increase germ cell attachment in the presence of FSH. However, the Sertoli cells in the Perryman study were not pretreated with FSH, in contrast to the 3-d preincubations with FSH and testosterone that were performed before the addition of germ cells in the present study. The positive effect of FSH upon previously unstimulated cells may reflect the requirement for Sertoli cells to be preconditioned with FSH to induce junction-related germ cell binding competency as has been suggested previously (46). Furthermore, it is possible that the positive preconditioning attributes of FSH may cancel out negative effects of inhibiting ERK activity in regard to Sertoli-germ cell attachment.
The results of Sertoli-germ cell attachment assays employing pathway-specific AR mutants demonstrated that the classical pathway AR mutant ARC562G was capable of maintaining testosterone-inducible germ cell attachment to Sertoli cells. In contrast, the ARΔ372–385 AR mutation that does not interact with Src kinase or activate the nonclassical pathway was unable to support increased germ cell attachment after testosterone stimulation. Together, these studies indicate that the nonclassical signaling pathway (but not the classical pathway) is required for testosterone-mediated increases in germ cells attached to Sertoli cells.
Studies of seminiferous tubule explants revealed that structural integrity and cell viability were similarly well maintained under all conditions. The finding that neither FSH nor PD98059 significantly decreased sperm release suggests that neither FSH signaling nor ERK activation are major regulators of spermiation. The observation that FSH had little effect on sperm release agrees with results reported by Chapin and colleagues (29) and is consistent with FSH receptor levels and FSH signaling being reduced in vivo during the time of sperm release (32). In contrast, the Src kinase inhibitor PP2 reduced sperm release by 45% and was a more effective inhibitor than the AR antagonist flutamide. This result suggests that much of the testosterone-dependent release of sperm may be accounted for by the action of Src kinase and is consistent with the hypothesis that activation of Src by the nonclassical testosterone-signaling pathway is a major contributor to the initiation of sperm release.
The extent of the decrease in sperm release in response to incubation with PP2 and the blocking of the nonclassical pathway is likely to be physiologically meaningful. However, it must be noted that further in vivo studies need to be performed to confirm our ex vivo results. Although the culturing of seminiferous tubules is the best culture model available to observe the effects of blocking the components of the nonclassical pathway, there are limitations to the assay including possible nonspecific effects of the inhibitors. To develop an improved method to assay the importance of the nonclassical pathway for the release of sperm and other processes, we have initiated work to create transgenic mouse models. These mice will have endogenous AR expression replaced only in Sertoli cells with mutant ARs that selectively signal through only the classical or the nonclassical pathway. The results from these transgenic studies should identify the spermatogenic processes and factors that are regulated by each testosterone-signaling pathway.
Src appears to have different effects on Sertoli connections with less mature germ cells vs. more mature elongated spermatids and mature sperm. Cheng's group found that 3 d after injection of the Src inhibitor PP1 into the testis or into the jugular vein, spermatocytes and round spermatids were absent but elongating spermatids remained (49). These data suggest that activated Src is required to retain spermatocytes and round spermatids as well as to release elongated spermatids and mature spermatozoa.
An important question that remains is how does activation of Src result in the release of mature sperm but not less mature germ cells? The answer may lie in the changing structure of the Sertoli-germ cell adhesion complex and the timing of increased nonclassical testosterone signaling. Germ cells before the transition from round to elongated spermatids are attached to Sertoli cells via desmosome structures. Immediately before spermatid elongation (step 8 spermatids) the adhesion complex is remodeled such that desmosomes are replaced by the ectoplasmic specialization (ES) (reviewed in Ref. 50). Src is known to phosphorylate proteins in the ES including N-cadherin, β-catenin, and focal adhesion kinase (FAK) to facilitate disassociation of protein contacts between the Sertoli cell and mature spermatids (6, 51–53). Src is also associated with the α6β1-integrin/laminin γ3 linkages between Sertoli cells and germ cells at the ES (53). Neither the protein targets of Src nor activated Src has been found to be associated with the desmosome connections to less mature cells. Furthermore, AR expression required for activation of the nonclassical pathway and Src activation in Sertoli cells is limited to stages VII–VIII. The restricted timing of testosterone-mediated Src activation eliminates the problem of releasing elongated spermatids before full maturity because the only immature spermatids that are attached to Sertoli cells during the stages when Src is activated are round spermatids that have not yet transitioned to ES adhesion complexes.
Although it is entirely possible that regulation of sperm release by via nonclassical testosterone actions and Src kinase could occur at the ES, the results of our assays suggest that the release of sperm is also regulated at later stages of development. The ES junction is removed from elongated spermatids about 30 h before the final release of mature sperm (54). However, our results indicate that that differences in sperm release occur within 20 h of blocking testosterone or Src kinase activity in cultured tubule fragment, suggesting that adhesion events are altered after the removal of the ES. Two complexes are known to maintain connections between Sertoli cells and elongated spermatids after the ES is removed, the tubulobulbar complex and the focal adhesion-related disengagement complex. Previous studies from the O'Donnell group (54) suggest that only the disengagement complex remains associated with spermatids that failed to spermiate in the absence of testosterone. Notably, the disengagement complex retains α6β1 integrins and tyrosine-phosphorylated FAK from the ES (55). Furthermore, FAK is phosphorylated in response to nonclassical testosterone signaling, and FAK is a target of Src kinase (56–58). Thus, the lower levels of sperm release that occur after treatment of tubule fragments with PP2 may be due in part to blocking Src activation of FAK in the disengagement complex resulting in the inability to release sperm.
Based on the expression pattern of the FSH receptor and the effectiveness of FSH signaling in vivo throughout the cycle of the seminiferous epithelium, it would be expected that FSH would restrict the activation of ERK least during stages VII–VIII. It is possible that FSH signaling and inhibition of the MAPK cascade is limited during stages VII–VIII to permit the nonclassical actions of testosterone to activate ERK. In agreement with this hypothesis, phosphorylated ERK levels in Sertoli cells in vivo are only elevated in stages VII—VIII, and increased staining for phosphorylated ERK is absent by stage X (29). Interestingly, it is during stages VII–VIII when activated ERK levels are highest that the desmosome connections between Sertoli cells and round spermatids are replaced by the ES (59). Thus, bursts of ERK activation during stages VII–VIII may contribute to the remodeling of Sertoli-germ cell connections from desmosome to ES junctions.
In summary, this study identified FSH as an inhibitor of the nonclassical testosterone-signaling pathway at the level of Raf kinase. Also, Src and ERK kinases were found to be important regulators of spermatogenic processes. The finding that activated Src is required for the release of mature sperm and that testosterone-mediated attachment of less mature germ cells to Sertoli cells requires Src and ERK activation suggests that these kinases (and other factors in the nonclassical pathway) could be important targets for regulating male fertility. Further studies will be required to confirm the requirement for Src and ERK kinase activity to maintain spermatogenesis in vivo. After identifying the spermatogenic processes regulated by the nonclassical pathway in vivo, new strategies for male contraception could be developed that target specific factors of the pathway.
Materials and Methods
Animal care and use
Male Sprague Dawley rats were obtained from Charles River Laboratories (Boston, MA). Tfm rats that express a ligand binding-defective AR (24) were obtained from a breeding colony maintained at the University of Pittsburgh (13). Animals used in these studies were maintained and euthanized according to the principles and procedures described in the NIH Guide for the Care and Use of Laboratory Animals. These studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Reagents and antibodies
Ovine FSH from pituitary was obtained from Sigma Chemical Co. (St. Louis, MO). PP2, H89, and GGTI-298 were acquired from Calbiochem (San Diego, CA). All other chemicals were from Sigma unless stated otherwise.
Antisera employed included mouse monoclonals against β-actin (MAB1501, Chemicon International, Temecula, CA) and vimentin (V6630, Sigma) as well as a rat monoclonal against the HA epitope (sc-7392; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Rabbit polyclonal antisera against AR (sc-815), total Src (Sc 18), EGFR phosphorylated on Tyr 1173 (sc-12351), and total EGFR (sc-03) were obtained from Santa Cruz Biotechnology. Mouse monoclonal antiserum against total EGFR (M225) was a gift from J. Siegfried (60). Rabbit polyclonal antisera against phosphorylated MEK (07-461), total ERK (06-182), and CREB phosphorylated on Ser 133 (06-519) were obtained from Upstate Biotechnology, Inc. (Charlottesville, VA), whereas rabbit antisera against Raf-1 phosphorylated on Ser 338 (9427), Src phosphorylated on Tyr 416 (2101), ERK phosphorylated on Thr202/Tyr 204 (9101), and total CREB (9197) were obtained from Cell Signaling Technology (Danvers, MA).
Plasmids and adenovirus constructs
PSALuc (PSAEnh E4TATA-luc) containing a 496-bp enhancer region from the PSA gene in pGL3Basic was obtained from Elizabeth Wilson (University of North Carolina, NC) and has been described previously (61). The pSG5 expression vector, pSG5HA-AR (AR wild type), and pSG5-ARC562G having the DNA binding-defective rat AR were provided by J. Palvimo (University of Helsinki, Helsinki, Finland) (25). The pDC315hARΔ372–385 vector was constructed by excision of a 152-bp NruI-BstEII fragment including the deletion of the proline-rich domain of AR from pSG5Δ1hAR that was provided by A. deFalco (II University of Naples, Naples, Italy) (26) and insertion of the fragment into pDC315hAR digested with the same enzymes. pCDNA3HA-ERK encodes the HA epitope linked to the amino terminus of human ERK1.
The adenovirus construct AdEGFP, expressing enhanced green fluorescent protein, was obtained from Andrea Gambotto (University of Pittsburgh, Pittsburgh, PA). Ad-caMEK expressing constitutively active MEK was obtained from M. Tohyama (Osaka University, Osaka, Japan) and has been described previously (62). An adenovirus that directs the expression of a β-galactosidase (Ad-βgal) was provided by Dr. J. Alcorn (University of Texas Medical School, Dallas, TX). Adenoviruses expressing ARΔ372-385 (AdARΔ372-385) and ARC562G (AdARC562G) were constructed using the AdMax system (Microbix Biosystems, Inc., Toronto, Ontario, Canada). AdARΔ372-385 was constructed using the pDC315hARΔ372-385 shuttle vector. AdARC562G was produced from the pDC315ARC562G shuttle vector that was constructed by inserting the 2700-bp BamHI and BglII fragment from pSG5ARC562G into BamHI-digested pDC315. The adenoviral constructs were produced using the protocol described previously (63). Each adenovirus was amplified in 293T cells, and the virus concentrations were determined by measuring the OD at 260 nm using a ratio of 1.1 × 1012 viral particles per 1 OD unit (64).
Isolation of primary Sertoli cells
Sertoli cells were isolated from 15- or 20 d-old Sprague Dawley or tfm rats and cultured in serum-free media as described previously (14). Sertoli cells at this age have completed their proliferation period and are no longer dividing in vivo (65–67). Briefly, decapsulated testes were digested with collagenase (0.5 mg/ml, 33 C, 12 min) in enriched Krebs-Ringer bicarbonate buffer followed by three washes in enriched Krebs-Ringer bicarbonate medium to isolate seminiferous tubules. Tubules were digested with trypsin (0.5 mg/ml, 33 C, 12 min). An equal volume of DMEM containing 10% fetal calf serum was added to the Sertoli cells, which were then pelleted (100 × g, 5 min) and resuspended in serum-free medium containing 50% DMEM, 50% Ham's F-12, 5 mg/ml insulin, 5 mg/ml transferrin, 10 ng/ml epidermal growth factor, 1 mm sodium pyruvate, 200 U/ml penicillin, and 200 μg/ml streptomycin. Sertoli cells were cultured on Matrigel (Collaborative Research, Bedford, MA)-coated dishes (32 C, 5% CO2) or Matrigel-coated cover slips in dishes. The cells were washed with PBS on d 2 and cultured further in serum free media. The cultures were found to be routinely more than 95% pure as determined by phase microscopy and alkaline phosphatase staining.
Preparation of whole-cell extracts, membrane-associated protein extracts, Western blots, and immunoprecipitations
After 72 h of culture, Sertoli cells were incubated in the absence and presence of hormones and signaling pathway regulators for 10 min. In some cases, cells were pretreated for 30 min with H89. To prepare whole-cell extracts for direct analysis by Western immunoblot, cells were washed once with PBS and then lysed on the plates by using boiling Laemmli sample buffer to minimize phosphatase activity. Purified fractions of membrane-associated proteins were prepared as described previously (14). Cell lysates were fractionated by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and incubated with primary antibodies, followed by horseradish peroxidase-conjugated second antibody. The antigen-antibody complex was visualized with Millipore Immobilon Western Chemiluminescent horseradish peroxidase substrate (Millipore Corp., Billerica, MA).
For immunoprecipitations, Sertoli cells were lysed in enhanced lysis buffer and a cocktail of protease and phosphatase inhibitors, sonicated for 10 sec, and subjected to centrifugation (12,000 × g, 15 min). The supernatants (5–20 μg) were added to 500 μl of enhanced lysis buffer and incubated with monoclonal antibodies against the HA epitope or EGFR followed by incubation with protein G sepharose. Immuno-bound material was eluted by boiling in 2× Laemmli sample buffer for 5 min and fractionated on 8% SDS-PAGE gels. Detection of antigen-bound antibody was carried out by Western analysis as described above.
RNA isolation and quantitative real-time PCR
RNA was obtained from Sertoli cells using the method of Chomczynski and Sacchi (68). cDNAs were synthesized from Sertoli cell RNA by reverse transcription in the presence of 250 U Superscript II RNAse-H (Invitrogen, Carlsbad, CA), 4.5 mm MgCl2, 1 mm deoxynucleotide triphosphates, 2.25 μm random hexamer, 20 U SUPERase-In (Ambion, Inc., Austin, TX), and 1× PCR buffer. The reactants were incubated at 25 C for 10 min followed by 48 C for 30 min and then 95 C for 5 min. For qPCR, oligonucleotide primers (Table 1) were designed using Primer Express v. 1.5 (ABI Prism; Applied Biosystems, Foster City, CA). Primer sequences were compared by a BLAST search to ensure there was no homology with other rat genes to prevent cross detection.
Table 1.
Oligonucleotides used for qPCR
| Gene | Primers (5′ to 3′) |
|---|---|
| Raf-1 | CAGCAATGGTTTCGGACTGA |
| CGACGCTGATAGCCAAACTG | |
| B-Raf | CACGCCAAGTCAATCATCCA |
| CTAAACCAAAGTCACCTATTTTTACCG | |
| A-Raf | TCCCCACGGTCTGCGTTGACATG |
| TGTGGTAGAACTGTCGGCGGTTGGTACTC | |
| Ppia | ATGGTCAACCCCACCGTGT |
| TCTGCTGTCTTTGGAACTTTGTCT |
qPCR amplifications were performed in the ABI Prism 7900HT Sequence Detection System v 2.3 (Applied Biosystems) in a total volume of 20 μl, which included 2 μl of cDNA, 10 μl of Perfecta SYBR Green Fastmix ROX (Quanta Biosciences, Gaithersburg, MD) and 600 μm of each primer. Primers were independently validated (69) through the use of a standard curve derived from serial dilutions of the cDNA obtained from the reverse transcription reactions. The resulting Ct values for each sample were plotted vs. the log of the mRNA concentration present in each cDNA dilution. The slope of the line was used to calculate the efficiency of amplification [efficiency = 10(1/−slope)]. Primers with an efficiency of 2 ± 0.2 were considered acceptable. Ppia (peptidylprolyl isomerase A, commonly known as cyclophillin) was used as an endogenous control. The qPCR analysis initiated with melting of cDNA at 95 C for 15 min, followed by 40 amplification cycles (15 sec at 95 C and 1 min at 60 C). A dissociation curve was performed immediately after amplification to ensure there was only one (gene-specific) amplification peak.
The relative quantity of mRNA for each gene of interest was determined through the efficiency-corrected ΔCt method. The relative quantity is derived from the equation: quantity = (Efficiency)−Ct. For each sample, the calculated quantity was then normalized to the quantity found for Ppia. The means (±se) of three individual experiments were determined for each treatment group for each gene of interest.
Raf kinase assay
Whole-cell extracts from Sertoli cells or purified truncated (activated) Raf-1 protein were incubated with purified GST-tagged MEK1 protein for 30 min at 30 C using reagents from the Raf-1 Kinase Assay Kit (Upstate Biotechnology). An equal volume of SDS-PAGE 2× Laemmli sample buffer was added, the samples were boiled for 5 min, and 10% of the sample was assayed by Western blot using antiserum against phosphorylated MEK.
Sertoli-germ cell coculture
Sertoli cells from 20-d-old tfm or wild-type rats were cultured on cover slips in 60 mm dishes for 5 d in serum free media with the addition of testosterone (100 nm) and FSH (100 ng/ml) beginning on d 2. In some cases the Sertoli cell cultures were infected with adenovirus constructs (5 × 1010 particles/ml) on d 3. On d 5, germ cells were isolated from adult testes by mincing decapsulated testis tissue with a scalpel and twice pelleting Sertoli cells and tissue fragments at 100 × g. The germ cells were then filtered through 100 μm and 20 μm nylon mesh followed by filtering through glass wool to remove most mature elongated spermatids and spermatozoa. The purified germ cells (5 × 105 germ cells per 60 mm plate) were then added to the Sertoli cell cultures in the presence of testosterone and FSH. The cocultures were washed 24 h later and then cultured as above for an additional 24 h. The cocultures were then washed with PBS and placed into serum free media containing hormones and/or pathway inhibitors for 48 h. The cocultures were then fixed with 2% paraformaldehyde and the cells probed with antiserum against vimentin and secondary antiserum conjugated with Alexa 488 followed by Hoechst staining for nuclei. Sertoli cells and germ cells associated with Sertoli cells were counted within a defined field using a ×40 objective and fluorescent microscope. The mean (±se) number of germ cells attached to Sertoli cells was determined from at least five fields in each of three experiments for each treatment condition.
Transfections and luciferase assays
Sertoli cells were transfected 3 d after isolation using Fugene (Roche, Indianapolis, IN) according to the manufacturer's instructions with PSALuc and pSG5, pSG5-ARC562G, pDC315hARΔ372–385, or pSG5HA-AR. Twenty four hours after transfection the cells were treated for 24 h with testosterone or EtOH as a control. The cells were washed once with PBS and then lysed using Reporter Lysis Buffer (Promega Corp., Madison, WI). Relative light units of luciferase activity were detected using Luciferase Reagent (Perkin Elmer, Waltham, MA) on Wallac Victor2 1420 Multilabel Counter (PerkinElmer). Activity was then normalized to the protein concentration of the samples as detected using Protein Assay Reagent (Bio-Rad Laboratories, Richmond, CA).
To assay changes in ERK activity in response to specific protein expression, Sertoli cells were transfected with pcDNA3HA-ERK and AR expression vectors as described above. Alternatively, Sertoli cells were transfected with pcDNA3HA-ERK, washed after 4 h, and then infected with adenovirus constructs (5 × 1010 particles/ml). The cells were incubated for a further 48 or 72 h in serum free media before hormonal stimulation and the collection of whole cell extracts.
Seminiferous tubule microdissection, sperm release assay, and testosterone RIA
Testes were isolated from adult rats, the tunica albuginea was removed, and the seminiferous tubules were teased apart in PBS. Stage VII–VIII junctions were identified using a stereo microscope and transillumination as described by Parvinen (70). The tubules were cut at the border between stage VIII and IX and again 3 mm upstream within stage VII. Pools of tubule fragments containing only stages VII–VIII were incubated in 12-well plates with rocking at 33 C in 250 μl serum-free medium in the absence or presence of hormones and signaling pathway inhibitors. After 20 h, the media containing released sperm were then transferred to tubes, and the remaining tubule fragments were suspended in 250 μl serum free media and sonicated for 3 sec. The number of sperm heads released after sonication and the number of intact sperm present in the media were counted with a hemocytometer, and the percentage of sperm released was determined. Identically treated tubule fragments were fixed in Bouins fixative and embedded in paraffin, and 5-μm sections were stained with periodic acid Schiff and hematoxylin for analysis of tissue integrity and cell viability by light microscopy.
Total testosterone levels were measured using a RIA (catalog no. TKTT5; Diagnostic Product Corp., Los Angeles, CA). Duplicate wells containing two stage VII–VIII tubule fragments were incubated for 20 h as for the sperm release assay. The media were then removed, whole-cell extracts of the tubule tissue were prepared, and 12.5% of the tubule sample or 25% of the media sample was extracted with ether and assayed for testosterone content. The sensitivity of the testosterone assay was 0.03 ng/ml. The intra- and interassay coefficients of variation were less than 5.9% and less than 10.2%, respectively.
Statistical analysis
Results were analyzed by ANOVA with Newman-Keuls protected least significant difference at a 5% significance level utilizing GraphPad Prism 4.3 (GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgments
We thank Drs. Elizabeth Wilson (University of North Carolina), Jorma Palvimo (University of Kuopio), Antonietta de Falco (University of Naples), Andrea Gambotto (University of Pittsburgh), Masaya Tohyama (Osaka University), and Joeseph Alcorn (University of Texas Medical School, Dallas, TX) for plasmid and adenovirus constructs. We also thank Carolyn Phalin, Alyssa James, Alex Smith, and Cory Toocheck for technical assistance. We are also indebted to Dr. Tony Zeleznik (University of Pittsburgh) for assistance in constructing adenovirus and for editing of the manuscript.
Address all correspondence and requests for reprints to: Dr. William H. Walker, Department of Cell Biology and Physiology, Magee Women's Research Institute, University of Pittsburgh, 204 Craft Avenue, Room B305, Pittsburgh, Pennsylvania 15261. E-mail: walkerw@pitt.edu.
This work was supported by National Institutes of Health Grants 2R56 HD043143-06A1 and 1RO1 HD043143.
Disclosure Summary: The authors have nothing to disclose.
NURSA Molecule Pages:
Nuclear Receptors: AR;
Ligands: Testosterone | Flutamide.
Footnotes
- AR
- Androgen receptor
- CREB
- cAMP response element-binding protein
- EGFR
- epidermal growth factor receptor
- ES
- ectoplasmic specialization
- FAK
- focal adhesion kinase
- GST
- glutathione-S-transferase
- HA
- hemagglutinin
- IBMX
- isobutylmethylxanthine
- MEK
- MAPK kinase
- PKA
- protein kinase A
- PSA
- prostate-specific antigen
- qPCR
- quantitative PCR.
References
- 1. Sharpe RM. 1994. Regulation of spermatogenesis. In: Knobil E, Neil JD,eds. The physiology of reproduction. New York: Raven Press; 1363–1434 [Google Scholar]
- 2. Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM. 2003. Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology 144:509–517 [DOI] [PubMed] [Google Scholar]
- 3. Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. 2004. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA 101:6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. 2004. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. 1996. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod 55:895–901 [DOI] [PubMed] [Google Scholar]
- 6. Wong CH, Xia W, Lee NP, Mruk DD, Lee WM, Cheng CY. 2005. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology 146:1192–1204 [DOI] [PubMed] [Google Scholar]
- 7. Holdcraft RW, Braun RE. 2004. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131:459–467 [DOI] [PubMed] [Google Scholar]
- 8. Kerr JB, Millar M, Maddocks S, Sharpe RM. 1993. Stage-dependent changes in spermatogenesis and Sertoli cells in relation to the onset of spermatogenic failure following withdrawal of testosterone. Anat Rec 235:547–559 [DOI] [PubMed] [Google Scholar]
- 9. Zhengwei Y, Wreford NG, Royce P, de Kretser DM, McLachlan RI. 1998. Stereological evaluation of human spermatogenesis after suppression by testosterone treatment: heterogeneous pattern of spermatogenic impairment. J Clin Endocrinol Metab 83:1284–1291 [DOI] [PubMed] [Google Scholar]
- 10. McLachlan RI, O'Donnell L, Stanton PG, Balourdos G, Frydenberg M, de Kretser DM, Robertson DM. 2002. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones, and germ cell populations in normal young men. J Clin Endocrinol Metab 87:546–556 [DOI] [PubMed] [Google Scholar]
- 11. Matthiesson KL, Stanton PG, O'Donnell L, Meachem SJ, Amory JK, Berger R, Bremner WJ, McLachlan RI. 2005. Effects of testosterone and levonorgestrel combined with a 5α-reductase inhibitor or gonadotropin-releasing hormone antagonist on spermatogenesis and intratesticular steroid levels in normal men. J Clin Endocrinol Metab 90:5647–5655 [DOI] [PubMed] [Google Scholar]
- 12. Tsai MJ, O'Malley BW. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- 13. Fix C, Jordan C, Cano P, Walker WH. 2004. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci USA 101:10919–10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng J, Watkins SC, Walker WH. 2007. Testosterone activates MAP kinase via Src kinase and the EGF receptor in Sertoli cells. Endocrinology 148:2066–2074 [DOI] [PubMed] [Google Scholar]
- 15. Crépieux P, Marion S, Martinat N, Fafeur V, Vern YL, Kerboeuf D, Guillou F, Reiter E. 2001. The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation. Oncogene 20:4696–4709 [DOI] [PubMed] [Google Scholar]
- 16. Walker WH, Fucci L, Habener JF. 1995. Expression of the gene encoding transcription factor adenosine 3′,5′-monophosphate (cAMP) response element-binding protein: regulation by follicle-stimulating hormone-induced cAMP signaling in primary rat Sertoli cells. Endocrinology 136:3534–3545 [DOI] [PubMed] [Google Scholar]
- 17. Burgun C, Esteve L, Humblot N, Aunis D, Zwiller J. 2000. Cyclic AMP-elevating agents induce the expression of MAP kinase phosphatase-1 in PC12 cells. FEBS Lett 484:189–193 [DOI] [PubMed] [Google Scholar]
- 18. Superti-Furga G, Courtneidge SA. 1995. Structure-function relationships in Src family and related protein tyrosine kinases. Bioessays 17:321–330 [DOI] [PubMed] [Google Scholar]
- 19. Dhillon AS, von Kriegsheim A, Grindlay J, Kolch W. 2007. Phosphatase and feedback regulation of Raf-1 signaling. Cell Cycle 6:3–7 [DOI] [PubMed] [Google Scholar]
- 20. Dumaz N, Marais R. 2005. Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft fur Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J 272:3491–3504 [DOI] [PubMed] [Google Scholar]
- 21. Stork PJ, Schmitt JM. 2002. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12:258–266 [DOI] [PubMed] [Google Scholar]
- 22. Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. 1997. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem 272:4378–4383 [DOI] [PubMed] [Google Scholar]
- 23. Diaz B, Barnard D, Filson A, MacDonald S, King A, Marshall M. 1997. Phosphorylation of Raf-1 serine 338-serine 339 is an essential regulatory event for Ras-dependent activation and biological signaling. Mol Cell Biol 17:4509–4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. 1990. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem 265:8893–8900 [PubMed] [Google Scholar]
- 25. Karvonen U, Kallio PJ, Jänne OA, Palvimo JJ. 1997. Interaction of androgen receptors with androgen response element in intact cells. Roles of amino- and carboxyl-terminal regions and the ligand. J Biol Chem 272:15973–15979 [DOI] [PubMed] [Google Scholar]
- 26. Migliaccio A, Varricchio L, De Falco A, Castoria G, Arra C, Yamaguchi H, Ciociola A, Lombardi M, Di Stasio R, Barbieri A, Baldi A, Barone MV, Appella E, Auricchio F. 2007. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene 26:6619–6629 [DOI] [PubMed] [Google Scholar]
- 27. McGuinness MP, Linder CC, Morales CR, Heckert LL, Pikus J, Griswold MD. 1994. Relationship of a mouse Sertoli cell line (MSC-1) to normal Sertoli cells. Biol Reprod 51:116–124 [DOI] [PubMed] [Google Scholar]
- 28. Walker WH, Daniel PB, Habener JF. 1998. Inducible cAMP early repressor ICER down-regulation of CREB gene expression in Sertoli cells. Mol Cell Endocrinol 143:167–178 [DOI] [PubMed] [Google Scholar]
- 29. Chapin RE, Wine RN, Harris MW, Borchers CH, Haseman JK. 2001. Structure and control of a cell-cell adhesion complex associated with spermiation in rat seminiferous epithelium. J Androl 22:1030–1052 [DOI] [PubMed] [Google Scholar]
- 30. Zirkin BR, Santulli R, Awoniyi CA, Ewing LL. 1989. Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124:3043–3049 [DOI] [PubMed] [Google Scholar]
- 31. McLachlan RI, Wreford NG, O'Donnell L, de Kretser DM, Robertson DM. 1996. The endocrine regulation of spermatogenesis: independent roles for testosterone and FSH. J Endocrinol 148:1–9 [DOI] [PubMed] [Google Scholar]
- 32. Kangasniemi M, Kaipia A, Mali P, Toppari J, Huhtaniemi I, Parvinen M. 1990. Modulation of basal and FSH-dependent cyclic AMP production in rat seminiferous tubules staged by an improved transillumination technique. Anat Rec 227:62–76 [DOI] [PubMed] [Google Scholar]
- 33. Bremner WJ, Millar MR, Sharpe RM, Saunders PTK. 1994. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology 135:1227–1234 [DOI] [PubMed] [Google Scholar]
- 34. Scobey M, Bertera S, Somers J, Watkins S, Zeleznik A, Walker W. 2001. Delivery of a cyclic adenosine 3′, 5′-monophosphate response element binding protein (CREB) to seminiferous tubules results in impaired spermatogenesis. Endocrinology 142:948–954 [DOI] [PubMed] [Google Scholar]
- 35. Schmitt JM, Stork PJ. 2001. Cyclic AMP-mediated inhibition of cell growth requires the small G protein Rap1. Mol Cell Biol 21:3671–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL. 2002. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4:901–906 [DOI] [PubMed] [Google Scholar]
- 37. Wang Z, Dillon TJ, Pokala V, Mishra S, Labudda K, Hunter B, Stork PJ. 2006. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol 26:2130–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhillon AS, Pollock C, Steen H, Shaw PE, Mischak H, Kolch W. 2002. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol Cell Biol 22:3237–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mischak H, Seitz T, Janosch P, Eulitz M, Steen H, Schellerer M, Philipp A, Kolch W. 1996. Negative regulation of Raf-1 by phosphorylation of serine 621. Mol Cell Biol 16:5409–5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dumaz N, Marais R. 2003. Protein kinase A blocks Raf-1 activity by stimulating 14–3-3 binding and blocking Raf-1 interaction with Ras. J Biol Chem 278:29819–29823 [DOI] [PubMed] [Google Scholar]
- 41. Chong H, Guan KL. 2003. Regulation of Raf through phosphorylation and N terminus-C terminus interaction. J Biol Chem 278:36269–36276 [DOI] [PubMed] [Google Scholar]
- 42. Wu J, Dent P, Jelinek T, Wolfman A, Weber MJ, Sturgill TW. 1993. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science 262:1065–1069 [DOI] [PubMed] [Google Scholar]
- 43. Perryman KJ, Stanton PG, Loveland KL, McLachlan RI, Robertson DM. 1996. Hormonal dependency of neural cadherin in the binding of round spermatids to Sertoli cells in vitro. Endocrinology 137:3877–3883 [DOI] [PubMed] [Google Scholar]
- 44. Lampa J, Hoogerbrugge JW, Baarends WM, Stanton PG, Perryman KJ, Grootegoed JA, Robertson DM. 1999. Follicle-stimulating hormone and testosterone stimulation of immature and mature Sertoli cells in vitro: inhibin and N-cadherin levels and round spermatid binding. J Androl 20:399–406 [PubMed] [Google Scholar]
- 45. Enders GC, Millette CF. 1988. Pachytene spermatocyte and round spermatid binding to Sertoli cells in vitro. J Cell Sci 90:105–114 [DOI] [PubMed] [Google Scholar]
- 46. Cameron DF, Muffly KE. 1991. Hormonal regulation of spermatid binding. J Cell Sci 100:623–633 [DOI] [PubMed] [Google Scholar]
- 47. O'Donnell L, Stanton PG, Bartles JR, Robertson DM. 2000. Sertoli cell ectoplasmic specializations in the seminiferous epithelium of the testosterone-suppressed adult rat. Biol Reprod 63:99–108 [DOI] [PubMed] [Google Scholar]
- 48. Godet M, Sabido O, Gilleron J, Durand P. 2008. Meiotic progression of rat spermatocytes requires mitogen-activated protein kinases of Sertoli cells and close contacts between the germ cells and the Sertoli cells. Dev Biol 315:173–188 [DOI] [PubMed] [Google Scholar]
- 49. Lee NP, Cheng CY. 2005. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J Cell Physiol 202:344–360 [DOI] [PubMed] [Google Scholar]
- 50. Kopera IA, Bilinska B, Cheng CY, Mruk DD. 2010. Sertoli-germ cell junctions in the testis: a review of recent data. Philos Trans R Soc Lond B Biol Sci 365:1593–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Siu MK, Wong CH, Lee WM, Cheng CY. 2005. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem 280:25029–25047 [DOI] [PubMed] [Google Scholar]
- 52. Zhang J, Wong CH, Xia W, Mruk DD, Lee NP, Lee WM, Cheng CY. 2005. Regulation of Sertoli-germ cell adherens junction dynamics via changes in protein-protein interactions of the N-cadherin-β-catenin protein complex which are possibly mediated by c-Src and myotubularin-related protein 2: an in vivo study using an androgen suppression model. Endocrinology 146:1268–1284 [DOI] [PubMed] [Google Scholar]
- 53. Yan HH, Cheng CY. 2006. Laminin alpha 3 forms a complex with β3 and γ3 chains that serves as the ligand for α 6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem 281:17286–17303 [DOI] [PubMed] [Google Scholar]
- 54. Beardsley A, O'Donnell L. 2003. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol Reprod 68:1299–1307 [DOI] [PubMed] [Google Scholar]
- 55. Siu MK, Mruk DD, Lee WM, Cheng CY. 2003. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex-associated proteins. Endocrinology 144:2141–2163 [DOI] [PubMed] [Google Scholar]
- 56. Papakonstanti EA, Kampa M, Castanas E, Stournaras C. 2003. A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol Endocrinol 17:870–881 [DOI] [PubMed] [Google Scholar]
- 57. Cobb BS, Schaller MD, Leu TH, Parsons JT. 1994. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol 14:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. 1994. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol 14:1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Russell L. 1977. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat 148:313–328 [DOI] [PubMed] [Google Scholar]
- 60. Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM. 2005. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res 65:1459–1470 [DOI] [PubMed] [Google Scholar]
- 61. Huang W, Shostak Y, Tarr P, Sawyers C, Carey M. 1999. Cooperative assembly of androgen receptor into a nucleoprotein complex that regulates the prostate-specific antigen enhancer. J Biol Chem 274:25756–25768 [DOI] [PubMed] [Google Scholar]
- 62. Mitsuda N, Ohkubo N, Tamatani M, Lee YD, Taniguchi M, Namikawa K, Kiyama H, Yamaguchi A, Sato N, Sakata K, Ogihara T, Vitek MP, Tohyama M. 2001. Activated cAMP-response element-binding protein regulates neuronal expression of presenilin-1. J Biol Chem 276:9688–9698 [DOI] [PubMed] [Google Scholar]
- 63. Saxena D, Safi R, Little-Ihrig L, Zeleznik AJ. 2004. Liver receptor homolog-1 stimulates the progesterone biosynthetic pathway during follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology 145:3821–3829 [DOI] [PubMed] [Google Scholar]
- 64. Mittereder N, March KL, Trapnell BC. 1996. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 70:7498–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Orth JM. 1982. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec 203:485–492 [DOI] [PubMed] [Google Scholar]
- 66. Steinberger A, Steinberger E. 1971. Replication pattern of Sertoli cells in maturing rat testis in vivo and in organ culture. Biol Reprod 4:84–87 [DOI] [PubMed] [Google Scholar]
- 67. Bortolussi M, Zanchetta R, Belvedere P, Colombo L. 1990. Sertoli and Leydig cell numbers and gonadotropin receptors in rat testis from birth to puberty. Cell Tissue Res 260:185–191 [DOI] [PubMed] [Google Scholar]
- 68. Chomczynski P, Sacchi N. 1987. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- 69. Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF, eds 2006. High-throughput real-time quantitative reverse transcription PCR Current protocols in molecular biology. Chap 15, unit 15.18 New York: John Wiley, Sons, Inc. [DOI] [PubMed] [Google Scholar]
- 70. Parvinen M. 1982. Regulation of the seminiferous epithelium. Endocr Rev 3:404–417 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.