The MODY Hnf1α transcription factor activates endogenous MafA expression in pancreatic β-cells by binding within the Region 3 control domain.
Abstract
The expression pattern of genes important for pancreatic islet cell function requires the actions of cell-enriched transcription factors. Musculoaponeurotic fibrosarcoma homolog A (MafA) is a β-cell-specific transcriptional activator critical to adult islet β-cell function, with MafA mutant mice manifesting symptoms associated with human type 2 diabetes. Here, we describe that MafA expression is controlled by hepatocyte nuclear factor 1-α (Hnf1α), the transcription factor gene mutated in the most common monoallelic form of maturity onset diabetes of the young. There are six conserved sequence domains in the 5′-flanking MafA promoter, of which one, region 3 (R3) [base pair (bp) −8118/−7750] is principally involved in controlling the unique developmental and adult islet β-cell-specific expression pattern. Chromatin immunoprecipitation analysis demonstrated that Hnf1α bound specifically within R3. Furthermore, in vitro DNA-binding experiments localized an Hnf1α regulatory element between bp −7822 and −7793, an area previously associated with stimulation by the islet developmental regulator, Islet1. However, site-directed mutational studies showed that Hnf1α was essential to R3-driven reporter activation through bp −7816/−7811. Significantly, MafA levels were dramatically reduced in the insulin+ cell population remaining in embryonic and adult Hnf1α−/− pancreata. Our results demonstrate that Hnf1α regulates MafA in β-cells and suggests that compromised MafA expression contributes to β-cell dysfunction in maturity onset diabetes of the young.
Islet-enriched transcription factors play a critical role in controlling the embryonic and adult-specific expression pattern of genes of the endocrine and exocrine pancreas. For example, insulin gene expression in the β-cells of the islet of Langerhans is directed by the actions of musculoaponeurotic fibrosarcoma homolog A (MafA) (1–3), pancreatic and duodenal homeobox 1 (Pdx1) (4–6), paired box gene 6 (Pax6) (7), and neurogenic differentiation 1 (NeuroD1/β2) (8). MafA is unique among all other pancreas-enriched transcription factors in being produced very late in development and exclusively in hormone+ cells. Hence, MafA expression is first detected at embryonic day (E)13.5 within the insulin+ cells produced at the onset of the secondary and principal wave of islet β-cell formation (9). In contrast, insulin is also expressed in an earlier cell population during embryogenesis that lacks key proteins associated with β-cell function (e.g. glucose transporter 2 and glucokinase) (10), with closely related MafB mediating transcription in this minor and presumably dysfunctional cell population (11). In addition, MafB is present in glucagon+ (α)-cells and a very small number of Neurogenin3+ islet hormone− progenitors during development but then becomes restricted to islet α-cells soon after birth (12).
The significance of MafA in β-cell maturation and adult function was revealed upon analysis of MafA mutant mice, i.e. MafA−/− (13) and MafAΔPanc (14), because these animals manifested an adult type 2 diabetes (T2DM)-like phenotype, including defects in glucose sensing and insulin secretion. However, islet α, β, δ, ε, or PP cell formation was unaffected in MafA mutant mice, a distinction from most islet-enriched transcription factor knockouts (e.g. see Refs. 7, 15–18), including MafB−/− embryos, which have reduced insulin and glucagon expression (11). The unusual sensitivity of MafA to conditions that both stimulate [e.g. acute glucose treatment (1, 19, 20)] or reduce [e.g. palmitate (21)] islet β-cell activity further implicates this factor in adult islet β-cells. Notably, MafB+ insulin + cells generated during human embryonic stem cell differentiation were dysfunctional until becoming MafA+ insulin+ (22, 23). Moreover, reducing the levels of reactive oxygen species in β-cells by transgenic expression of glutathione peroxidase-1 in the T2DM db/db mouse model resulted in specific activation of MafA and drastically reduced blood glucose levels and improved islet β-cell volume and insulin granulation (24). Collectively, these results strongly suggest that a thorough understanding of MafA control could aid in the development of better diabetes diagnostic and treatment strategies.
The promoter region of mammalian MafA contains six areas of high sequence identity [termed regions (Rs) R1 through R6], with R3 [base pair (bp) −8118/−7750 relative to the transcription start site] critical to directing β-cell-specific transcription in vitro and in vivo (25, 26). R3 is also the only conserved domain found in the chicken MafA promoter, with roughly 88% identity to mouse or human (25). Several key transcription factors involved in β-cell development directly regulate MafA through R3, including Pdx1, NK homeobox (Nkx) 2.2, Forkhead box (Fox) A2, Islet1 (Isl1), and MafB (11, 18, 25). Given that MafA and hepatocyte nuclear factor 1-α (Hnf1α) are coexpressed in the developing and adult pancreas as well as cause islet β-cell dysfunction in knockout mice (13, 14, 27), we hypothesized that this important liver (28) and islet-enriched (29–31) transcriptional regulator also directly activates MafA expression. Here, we demonstrate that R3 and endogenous MafA expression is regulated by Hnf1α, which is also implicated in the most common type of human maturity onset diabetes of the young (termed MODY3), an autosomal dominant form of diabetes mellitus characterized by early onset (usually <25 yr) and defective insulin secretion (32). Hence, Hnf1α bound R3 in chromatin immunoprecipitation (ChIP) assays, with mobility shift assays localizing the core binding sequences to bp −7816/−7811 and mutational analysis illustrating a specific consequence to R3-driven reporter activation. In addition, Hnf1α−/− mice lacked MafA expression in insulin+ cells, lending in vivo relevance to Hnf1α binding and the MafA R3-driven reporter analyses. These studies demonstrate a functional and phenotypic link between this MODY3 factor and MafA gene expression.
Results
Hnf1α binds within MafA R3 in β-cells
Hnf1α (MODY3) and Hnf1β (MODY5) are homologous POU-homeodomain transcription factors expressed in the pancreas that bind an identical consensus sequence as homodimers or heterodimers (33). However, only Hnf1α is coexpressed with MafA during development and in adult β-cells, whereas Hnf1β is produced in endocrine progenitors earlier than Hnf1α and then exclusively in ductal cells in the adult pancreas (34–36).
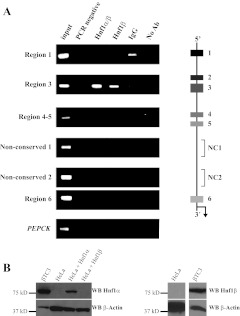
The ChIP assay was performed to determine whether Hnf1α occupied MafA 5′-flanking control region sequences (Fig. 1A). We employed chromatin isolated from the mouse β-cell line, βTC-3, which express both Hnf1α and Hnf1β (Fig. 1B). R3 DNA was selectively enriched with the Hnf1α/β recognizing antibody as well as with the Hnf1β-specific antibody (Fig. 1A, lanes 3 and 4). A commercial Hnf1α-specific antibody was unfortunately not usable for ChIP assays (data not shown). Importantly, little-to-no enrichment was observed in samples treated with IgG, without antibody, or in Hnf1 antibody precipitations over promoter sequences spanning an inactive gene in β-cells, phosphoenolpyruvate carboxykinase (PEPCK) (Fig. 1A). Moreover, R1, R4–5, R6, or intervening nonconserved sequences were not detected in the Hnf1α/β or Hnf1β antibody precipitates, further supporting the binding specificity to R3 (Fig. 1A). In contrast, the Pdx1 and Pax6 R3 activators also interacted with non-R3 sequences within the MafA promoter in ChIP assays (26).
Fig. 1.
Hnf1α/β uniquely bind to MafA R3 in βTC-3 cells. A, The schematic illustrates the approximately 10 kbp of MafA 5′-flanking promoter region analyzed for conserved and nonconserved (NC) sequence binding in ChIP with Hnf1α/β and Hnf1β antibodies. Immunoprecipitated DNA from βTC-3 cells was analyzed with MafA (R1, R3, R4–5, nonconserved 1, nonconserved 2, and R6) and PEPCK control specific primers. The PCRs were performed with total input DNA (1:100 dilution), PCR water control, α-Hnf1α/β, α-Hnf1β-specific, nonspecific goat IgG, or no antibody (no Ab). Hnf1α/β (which recognizes both Hnf1α and Hnf1β) and Hnf1β antibodies only precipitated R3. Each experiment was repeated with at least three independently isolated chromatin preparations. B, βTC-3 cells produce both Hnf1α and Hnf1β. Nuclear extracts from βTC-3, HeLa, and Hnf1α or Hnf1β transfected HeLa cells were separated by SDS-PAGE, and the transferred proteins probed with Western blot (WB) antibodies specific to Hnf1α or Hnf1β, with α-β-actin serving as control.
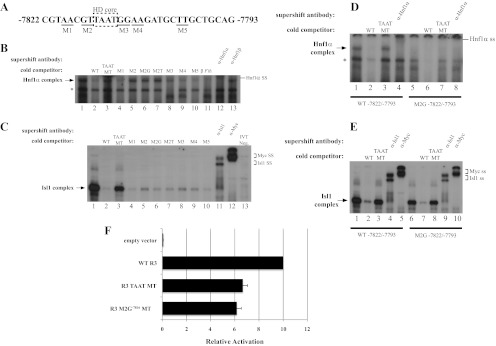
Fig. 3.
Hnf1α activates R3 through the bp −7816/−7811 element. A, The location of the M1 through M5 mutations within bp −7822/−7793 probe is illustrated, with the homeodomain (HD) core motif enclosed by the dashed box. B, The M2, M2G−7816, M2T−7815, and TAAT mutants failed to compete at a 50-fold molar excess for Hnf1α binding in βTC3 nuclear extracts, whereas M1, M3, M4, and M5 were effective competitors. C, In vitro translated Isl1-myc binding was reduced in the presence of 50-fold excess of the M2, M3, and M4 competitors, although less efficiently than wild type (WT), M2G−7816, M2T−7815, and M5. Isl1 and myc-epitope antibodies were used to localize the Isl1 binding complex, which was not detected with the in vitro translation reactions performed with the pcDNA3.1 vector (termed IVT neg). D and E, The bp −7822/−7793 M2G−7816 probe binds Isl1, but not Hnf1α. WT or M2G−7816 bp −7822/−7793 probes were incubated with βTC-3 nuclear extract (D) or Isl1-myc protein (E), WT or core TAAT MT competitor, and Hnf1α-, Isl1-, or myc-specific antibodies. F, The Hnf1α binding defective M2G−7816 mutation reduced transfected R3-driven reporter activity in βTC-3 cells. The reduced activity of M2G−7816 was similar to the TAAT mutant, which was deficient in both EMSA Hnf1α and Isl1 binding activity. *, Nonspecific complex; ss, supershift.
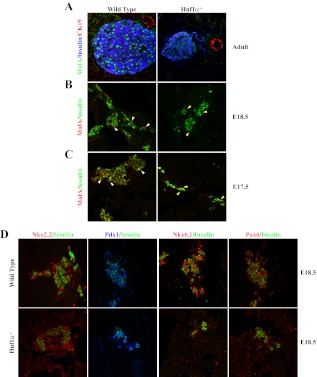
Fig. 4.
MafA is selectively reduced in the β-cells of Hnf1α−/− mice. A, MafA, insulin, and CK19 expression were examined by immunofluorescence in pancreatic sections from 3-month-old adult Hnf1α−/− and wild-type control mice. B and C, MafA expression was also compromised in E17.5 and E18.5 Hnf1α−/− insulin+ cells, whereas little-to-no apparent change was observed in insulin (B and C), Nkx2.2, Pdx1, Nkx6.1, and Pax6 (D) levels compared with control. White arrowheads point to MafA+ nuclei in the WT sections, whereas the yellow arrowheads illustrate the MafA− nuclei within Hnf1α−/− insulin+ cells. Representative images are shown for each staining condition. Notably, the reduction in MafA levels was observed in embryonic as well as adult Hnf1α−/− samples.
Identification of Hnf1α binding sites in R3
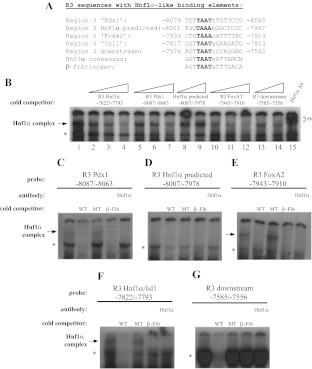
Our efforts next focused on precisely localizing the R3 cis-acting site(s) for Hnf1α in MafA+/insulin+ β-cells. We tested several potential A/T-rich homeodomain-like binding elements in R3 in EMSA assays (Fig. 2A) (18) with βTC-3 nuclear extract. Our analysis specifically focused on R3 elements ascribed to the Pdx1 homeodomain (bp −8078 to −8071), Isl1 LIM-homeodomain (bp −7816 to −7810), and FoxA2 forkhead (bp −7934 to −7920) transcription factors (25), as well as TRANSFAC-predicted Hnf1α/β sites within (bp −8000 to −7992) (25) and downstream of R3 (bp −7585 to −7556). The most robust sequence-specific Hnf1α binding appeared with the bp −7822/−7793 and bp −7943/−7910 probes (Fig. 2, B, E, and F). Specificity was determined using the wild-type oligonucleotide and the high affinity Hnf1-binding β-fibrinogen element (37) competitor, a mutated homeodomain core (TAAT to GCCG) competitor, and by addition of Hnf1α antibody (Fig. 2, C–G). Hnf1α binding was not detected using probes containing the in silico predicted Hnf1 binding sites (bp −8007/−7978, Fig. 2D; bp −7585/−7556, Fig. 2G).
Fig. 2.
Identification of R3 Hnf1α binding sites. A, Sequences of the MafA R3 sites and high affinity β-fibrinogen site used to test for Hnf1α binding, with the TAAT homeodomain highlighted. B, As a screen of putative R3 Hnf1α binding elements, the radiolabeled bp −7822/−7793 (Isl1) gel shift probe was used in reactions with βTC-3 nuclear extract and increasing amounts (5-, 10-, and 25-fold or 25- to 50-fold) of the R3 competitor oligonucleotides. The identity of the Hnf1α binding complex was determined by antibody supershift (ss) analysis. The Hnf1α/β-complex detected in βTC-3 nuclear extracts with the radiolabeled bp −8087/−8063 (C), bp −7943/−7910 (E), and bp −7822/−7793 (F) probes was sensitive to wild-type (WT) (100-fold molar excess) and β-fibrinogen Hnf1α/β site (50-fold) competition, α-Hnf1α/β antibody addition, but not by TAAT mutant (MT) competition (100-fold). In contrast, an Hnf1α/β-like complex was not observed with the bp −8007/−7978 (D) or bp −7585/−7556 (G) probes. *, Nonspecific complex.
Hnf1α stimulates R3-mediated activity
Although Hnf1α binding was principally observed at two R3 sites (Fig. 2, B, E, and F), we focused our studies on characterizing the cis-element within the −7822/−7793 probe, which was most similar to the Hnf1α/β consensus (Fig. 2A) and recently linked to activation by the Isl1 LIM-homeodomain factor (18). Dinucleotide transversional mutants were constructed surrounding the homeodomain core motif and used as cold EMSA competitors to identify distinguishing binding nucleotides between Hnf1α and Isl1 [termed dinucleotide transversional mutants (M)1–M5 in Fig. 3A]. M1, M3, M4, and M5 competed as effectively as the wild type for Hnf1α binding, whereas M2 was inefficient and behaved similarly to the homeodomain core mutant (Fig. 3B, compare lanes 3 and 5). Conversely, Isl1 binding was compromised to some extent by M2, M3, and M4 (Fig. 3C, lanes 5, 8, and 9).
R3 mutants at bp −7816 or −7815 in M2 were next analyzed for their impact on Hnf1α and Isl1 binding. Neither mutant oligonucleotide was able to compete for Hnf1α binding (Fig. 3B, lanes 6 and 7), whereas both the M2G−7816 and M2T−7815 mutant appeared as effective in reducing Isl1 binding as the wild type, M1, and M5 competitors (Fig. 3C). Hnf1α binding was also not observed with the M2G−7816 labeled probe, whereas Isl1 bound to this mutant effectively (Fig. 3, D and E). Collectively, these results demonstrated that bp −7816/−7811 were essential to Hnf1α binding, whereas Isl1 interactions were clearly distinguished by the individual M2G−7816 and M2T−7815 mutations.
The homeodomain core (TAAT MT) and M2G−7816 mutants were constructed in the R3-driven luciferase reporter to test whether the specific loss of Hnf1α binding affected activation. Both mutants reduced R3 stimulation by about 40% from wild type in βTC-3 cell transfections (Fig. 3F). These data strongly suggest that only Hnf1α stimulated R3 under these circumstances, because similar compromised activity levels were obtained under conditions permitting Isl1 binding (i.e. M2G−7816) as well as in the absence of both Hnf1α and Isl1 binding (TAAT MT).
MafA protein expression is reduced in developing and adult Hnf1α−/− β-cells
Hnf1α−/− mice have shared phenotypic characteristics with human MODY patients, including overt diabetes with reduced insulin secretion (27, 31). Immunofluorescence analysis was performed to determine the effect on MafA expression in Hnf1α−/− adult and E18.5 pancreata. MafA was greatly reduced in adult islet insulin+ cells in mutant mice, whereas CK19+ ductal staining was unchanged (Fig. 4A). Because hyperglycemic conditions associated with Hnf1α−/− mice can indirectly compromise insulin and MafA levels in adult islet β-cells (27, 31, 38), the study was extended to embryonic samples to more directly analyze the impact of the Hnf1α protein on MafA expression. Significantly, MafA staining was also barely detectable in the insulin+ cells found in E17.5 and E18.5 Hnf1α−/− pancreata (Fig. 4, B and C), whereas the expression levels of many other key islet-enriched transcription factors was unaffected in these cells (e.g. Nkx2.2, Pdx1, Nkx6.1, and Pax6) (Fig. 4D). Collectively, these data support a requirement for Hnf1α in MafA transcriptional activation through direct binding to R3 in β-cells in vivo.
Discussion
The linkage between transcriptional regulation, monogenic forms of diabetes, and the β-cell is clear, because many MODY subtypes are caused by mutations in critical islet-enriched transcription factors, including HNF4α (i.e. MODY1), transcription factor 1/HNF1α (MODY3), insulin promoter factor 1/PDX1 (MODY4), TCF2/HNF1β (MODY5), and NEUROD1 (MODY6) (39). Elucidating the key downstream targets of these MODY regulators provides insight into the underlying molecular mechanisms of β-cell dysfunction and T2DM, as well as aids in the search for additional susceptibility genes. Heterozygous mutations within the HNF1α/transcription factor 1 gene locus represents the most prevalent form of MODY, manifested in humans and Hnf1α−/− mice as overt diabetes resulting from a severe reduction in glucose and arginine-induced insulin secretion (13, 27, 31), whereas Hnf1α target function in the liver does not result in major phenotypic abnormalities in humans (30). The pleiotropic effects on β-cell activity in Hnf1α−/− mice result in part from effects on genes required for glucose metabolism (e.g. Slc2a2 encoding the Glut2 transporter) and notably the Hnf4α transcription factor and MODY1 gene (29, 40). We now demonstrate that Hnf1α is required for developmental and adult islet transcriptional activation of MafA, a transcription factor gene required in β-cell maturation and function. Importantly, Hnf1α and MafA deficiency models share similar adult islet β-cell phenotypes, with a notable dysfunction in stimulated insulin secretion (13, 31), mediated (in part) through common target genes like Slc2a2 (i.e. encoding glucose transporter 2) (13, 29). Although MafA presumably only impacts a subset of MODY3 affected genes, knowledge of this association could provide new perspectives into the onset and progression of β-cell dysfunction.
MafA expression is impacted by a variety of β-cell effectors, including glucose, fatty acids, and insulin promoter factor 1/PDX1 (causing MODY4) (20, 21, 25, 41). The presence of several potential Hnf1α/β binding sites within R3 (Fig. 2), the principal control domain driving islet β-cell-specific expression (25, 26), led us to consider the regulatory role of these closely related MODY factors. An Hnf1α/β activator site was in fact identified at bp −7816/−7811 through a combination of ChIP, gel shift, and MafA-driven reporter assays. Although our cell line data were compatible with Hnf1α and/or Hnf1β regulation, the expression of MafA and Hnf1β do not overlap in vivo, and, as a consequence, Hnf1β was eliminated as a MafA candidate. Thus, Hnf1β is produced in multipotent pancreatic progenitor cells before MafA expression and then exclusively in adult duct cells (35). Conversely, Hnf1α is contained within MafA+ insulin+ cells of the developing and adult pancreas. Hnf1α activation through the bp −7822/−7793 R3 element was described in depth here, but it is likely that homeodomain-containing proteins like Hnf1α and Isl1 also influence MafA through another R3 cis-element(s) because of their relatively simple core DNA binding motifs (e.g. the bp −8087/−8063 site associated with Pdx1 control) (Fig. 2).
MafA was specifically lost in insulin+ cells of the developing and adult Hnf1α−/− pancreas (Fig. 4). Importantly, the absence of MafA in forming β-cells is consistent with our biochemical and cell line based results, indicating a direct effect of Hnf1α in MafA R3-mediated stimulation. This contrasts with the expression pattern of many other key islet-enriched transcription factors, which were unaffected in this embryonic Hnf1α mutant cell population (Nkx2.2, Pdx1, Nkx6.1, and Pax6) (Fig. 4). These findings indicate that loss of MafA is not only impactful to β-cell dysfunction in Hnf1α−/− mice but also in human MODY3 patients.
Hnf1α was specifically bound to R3 within the endogenous MafA promoter (Fig. 1A), the control region directing β-cell-specific expression in vivo. The central role of R3 in MafA expression in the islet is based upon both cell line (25) and transgenic (26) experiments. For example, a transgenic reporter spanning R1–6 (i.e. bp −10428/+230) was uniquely active in MafA+ insulin+ cells during pancreas development and in adults, whereas β-cell expression was lost in lines lacking R3 (i.e. R1–6ΔR3), even though much of the nonpancreatic MafA expression pattern was maintained between R1–6 and R1–6ΔR3 mice (26). In contrast to Hnf1α, the Pdx1 and Pax6 transcription factors also occupied other non-R3 conserved sequence domains within the endogenous MafA promoter (26), potentially highlighting differences between these factors in pancreatic and nonpancreatic gene expression.
MafA is uniquely detected in the insulin+ cells produced during the secondary transition of pancreatic development, linking expression to β-cell maturation and function. An in-depth understanding of the mechanisms controlling transcription will likely impact future therapeutics, because many studies have illustrated the sensitivity of this factor to effectors of β-cell function and significance in the production of functional β-like cells from non-β-cells. For example, the reduction in MafA expression is associated with β-cell dysfunction under glucotoxic conditions (41), whereas human embryonic stem cells differentiated to produce insulin and many islet-enriched transcription factors were neither glucose responsive nor capable of protecting against streptozotocin-induced hyperglycemia until becoming MafA+ (23). We believe that efforts aimed at defining R3 activation will yield the greatest insight into how MafA expression, and β-cell function is controlled under normal and diabetic conditions.
Materials and Methods
Transient transfections and reporter gene assays
Mouse MafA R3 was cloned into the BamHI site of the pFox-Prl-Luc plasmid and cotransfected into βTC-3 cells with the phRL-TK Renilla luciferase plasmid using the Lipofectamine reagent (Invitrogen, Carlsbad, CA). The MafA R3 mutation at bp −7816 was produced using the Quick-Change Mutagenesis kit (Stratagene, La Jolla, CA) with the following oligonucleotide: 5′-CACGGCCGTAACTTTAATGGAAGATGCTTGCTGC-3′ (mutation is underlined). The oligonucleotide used to construct the −7814 TAAT −7811 homeodomain binding mutant has been described (18). Forty-eight hours after transfection, the cells were lysed and prepared for the dual-luciferase assay according to the manufacturer's protocol (Promega, Madison, WI). Each transfection was repeated at least three times using two independent plasmid preparations. Firefly luciferase measurements were normalized to the Renilla internal control.
Western blotting
βTC-3, HeLa, and pcDNA3.1-Hnf1α or pcDNA3.1-Hnf1β transfected HeLa nuclear extracts (42) were separated on 10% SDS-PAGE gels and electrophoretically transferred to Immobilon polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membranes were blocked with Tris-buffered saline Tween [10 mm Tris (pH 8.0), 150 mm NaCl, and 0.05% Tween 20] plus 5% nonfat dry milk before incubation with the primary antibody (dilution 1:2000, α-Hnf1α SC-6548 or α-Hnf1β SC-7411; Santa Cruz Biotechnology, Inc., Santa Cruz, CA; and 1:1000, α-β actin no. 4967; Cell Signaling Technology, Beverly, MA). α-Goat or α-rabbit IgG conjugated to horseradish peroxidase (1:2000) was used to detect the primary antibody. The membrane was washed with Tris-buffered saline Tween and the signal visualized using the Lumi-Light Western Blotting Substrate kit (Roche, Indianapolis, IN).
Electrophoretic mobility shift assays
DNA binding reactions (20 μl total) contained 10 μg of βTC-3 nuclear extract or 1 μl of myc epitope-tagged Isl1 prepared using the Promega Quick Coupled Transcription/Translation kit with pCS2 rat Isl1-myc (18). The sequence of the double-stranded 32P end-labeled probes (400 fmol) were: MafA R3 −8007 GGGGCTTGGTAAATGGCTCCACTCAGCCTT −7978, MafA R3 −7822 CGTAACGTTAATGGAAGATGCTTGCTGCAG −7793, MafA R3 −7585 GAACCCAGAAGTTAATCCCAGGAGGAAAG −7556, and β-fibrinogen 5′-TTTAGTTAATATTTGACAGTT-3′. The MafA R3 Pdx1 (bp −8087/−8063) and FoxA2 (bp −7943/−7910) site oligonucleotides were described previously (25). Binding reactions were conducted for 20 min at 30 C (Hnf1α) or 4 C (Isl1) in a buffer containing 10 mm HEPES (pH 7.9), 75 mm KCl, 2.5 mm MgCl2, 0.1 mm EDTA, 3% Ficoll, and 1 μg poly(dI-dC). Cold competitions contained a molar excess of the unlabeled wild-type or mutant labeled probe. Transversional mutants (G to T; C to A) within the bp −7822/−7793 probe at the TAAT homeodomain core and surrounding sequences were used in Fig. 3. Supershift analyses employed antibodies specific for Hnf1α/β (SC-8986), Hnf1α (SC-6548), Hnf1β (SC-7411), Isl1 [39.4D5-c; Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA], or the Myc peptide epitope (SC-40; Santa Cruz Biotechnology, Inc.) incubated with extract or Isl1-myc on ice for 15 min before probe or competitor addition. Binding reactions were separated on 6% nondenaturing acrylamide gels in 0.5× Tris-borate-EDTA buffer for 1.5 h at 150 V and the shifted complexes visualized by autoradiography.
ChIP assays
βTC-3 monolayer cells (∼4 × 106) cultured in 10-cm dishes for 72 h were cross-linked with 1% formaldehyde in DMEM for 5 min on ice, and protein-DNA chromatin fragmentation was performed as previously described (18). Chromatin (50 μg) was precleared with Protein A-agarose (Millipore, Temecula, CA) for 2 h at 4 C, then incubated overnight with α-Hnf1 or α-Hnf1β, species-matched preimmune IgG (Santa Cruz Biotechnology, Inc.) or no antibody. Bound complexes were precipitated with Protein A-agarose at 4 C for 4 h. After washing, complexes were eluted from beads, and the cross-links were reversed. Briefly, 5% of the immunoprecipitated DNA and 1:100 diluted input were used in a PCR with Taq polymerase Hotstart Mastermix (5 Prime, Gaithersburg, MD) and 15 pmol of primer. The following MafA primer sets were used: R1, −9471 TGGTGGGCAGTTTATAGGGTCAGT, −9124 GCCACCTACAGCTCACACAAACTT; R3, −8120 CACCCCAGCGAGGGCTGATTTAATT, −7750 AGCAAGCACTTCAGTGTGCTCAGTG; R4–5 −6348 TGTCCATTCCCTGTTCCTCTCCCT, −6041 TGTGTGGTAGTCAAGACAGGCCAA; −4661 ACCTCTTGCCCTATGGCTGATGAT, −4330 TGCACATTGATCTGGTGAGGTGGA; −1873 TGCCCAGACATGTAGCTCATCCTT, −1607 TGGTAGCCACAGCCATCAGTGTAA; and R6, −506 GAATTCCTGAACCCATCCCAACCA, −251 AGACCAAGTGGCAGATTCTGAGGT. PEPCK promoter analysis served as a negative control (−434 GAGTGACACCTCACAGCTGTGG, −96 GGCAGGCCTTTGGATCATAGCC) (18). PCR parameters were: 95 C for 2 min (1 cycle) and 95 C for 30 sec, 58 C for 30 sec, and 72 C for 30 sec (30 cycles). Reaction products were separated on 1.5% agarose gels using 1× Tris-acetate-EDTA buffer and visualized with ethidium bromide. Experiments were performed at least three times with independently prepared chromatin.
Hnf1α−/− mice and immunofluorescence analyses
Three-month-old littermate-matched wild-type and Hnf1α−/− (31) adult and staged embryos (E17.5 and E18.5) were paraffin embedded. Sections were cut to 6 μm, dewaxed, and incubated with primary antibodies overnight at 4 C after microwave antigen retrieval [insulin (1:1500; Linco, St. Charles, MO), MafA (1:2000–4000 BL1069; Bethyl Laboratories, Montgomery, TX), Nkx2.2 (1:200, DSHB 74.5A5), CK-19 (1:20, DSHB), Pdx-1 (1:10000; Chris Wright, Vanderbilt University Medical Center), Nkx6.1 (1:300; Beta Cell Biology Consortium), Pax6 (1:300; Covance Research, Princeton, NJ)], and then with Cy2-, Cy3-, or Cy5-conjugated donkey-α-guinea pig and α-chicken or α-rabbit IgG secondary antibodies (1:500; Jackson ImmunoResearch, West Grove, PA). The primary MafA antibody signal was amplified using TSA (PerkinElmer, Waltham, MA). Slides were imaged by confocal microscopy using a Zeiss LSM510 and images processed by LSM (Zeiss, Oberkochen, Germany) or ImageJ (National Institutes of Health, Bethesda, MD) software.
Acknowledgments
We thank technical assistance provided by Ms. Shilpy Dixit during the Western blotting and immunofluorescence studies. pFox-Luc was kindly provided by Dr. Raghavendra Mirmira and pCS2 rat Isl1-myc by Dr. Sam Pfaff. The monoclonal Isl1 and Nkx2.2 antibodies developed by Drs. Thomas M. Jessell and Susan Brenner-Morton were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biology (Iowa City, IA).
Address all correspondence and requests for reprints to: Roland Stein, Department of Molecular Physiology and Biophysics, Vanderbilt University Medical Center, 723 Light Hall, Tennessee 37232. E-mail: roland.stein@vanderbilt.edu.
Present address for J.C.R.: Department of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104.
This work was supported by the National Institutes of Health (NIH) Grant DK050203 (to R.S.) and the National Institute of Diabetes and Digestive and Kidney Diseases National Research Service Award Postdoctoral Fellowship F32 DK083160 (to C.S.H.). Confocal microscopy immunofluorescence images were collected in the Vanderbilt University Medical Center Cell Imaging Shared Resource Supported by NIH Grants CA68485, DK20593, DK58404, HD15052, DK59637, and EY08126. Partial support was provided to the Vanderbilt University Medical Molecular Biology laboratory by our NIH funded Diabetes Research and Training Center (Public Health Service Grant P60 DK20593).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- bp
- Base pair
- ChIP
- chromatin immunoprecipitation
- DSHB
- Developmental Studies Hybridoma Bank
- E
- embryonic day
- Fox
- Forkhead box
- Hnf1α
- hepatocyte nuclear factor 1-α
- Isl1
- Islet1
- M
- dinucleotide transversional mutant
- MafA
- musculoaponeurotic fibrosarcoma homolog A
- MODY3
- maturity onset diabetes of the young
- Nkx
- NK homeobox
- Pax6
- paired box gene 6
- Pdx1
- pancreatic and duodenal homeobox 1
- PEPCK
- phosphoenol-pyruvate carbo kinase
- R
- region
- T2DM
- type 2 diabetes.
References
- 1. Kataoka K, Han SI, Shioda S, Hirai M, Nishizawa M, Handa H. 2002. MafA is a glucose-regulated and pancreatic β-cell-specific transcriptional activator for the insulin gene. J Biol Chem 277:49903–49910 [DOI] [PubMed] [Google Scholar]
- 2. Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R. 2003. Members of the large Maf transcription family regulate insulin gene transcription in islet β cells. Mol Cell Biol 23:6049–6062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olbrot M, Rud J, Moss LG, Sharma A. 2002. Identification of β-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci USA 99:6737–6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohlsson H, Karlsson K, Edlund T. 1993. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J 12:4251–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peshavaria M, Gamer L, Henderson E, Teitelman G, Wright CV, Stein R. 1994. XIHbox 8, an endoderm-specific Xenopus homeodomain protein, is closely related to a mammalian insulin gene transcription factor. Mol Endocrinol 8:806–816 [DOI] [PubMed] [Google Scholar]
- 6. Petersen HV, Serup P, Leonard J, Michelsen BK, Madsen OD. 1994. Transcriptional regulation of the human insulin gene is dependent on the homeodomain protein STF1/IPF1 acting through the CT boxes. Proc Natl Acad Sci USA 91:10465–10469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sander M, Neubüser A, Kalamaras J, Ee HC, Martin GR, German MS. 1997. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev 11:1662–1673 [DOI] [PubMed] [Google Scholar]
- 8. Naya FJ, Stellrecht CM, Tsai MJ. 1995. Tissue-specific regulation of the insulin gene by a novel basic helix-loop-helix transcription factor. Genes Dev 9:1009–1019 [DOI] [PubMed] [Google Scholar]
- 9. Matsuoka TA, Artner I, Henderson E, Means A, Sander M, Stein R. 2004. The MafA transcription factor appears to be responsible for tissue-specific expression of insulin. Proc Natl Acad Sci USA 101:2930–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pang K, Mukonoweshuro C, Wong GG. 1994. β Cells arise from glucose transporter type 2 (Glut2)-expressing epithelial cells of the developing rat pancreas. Proc Natl Acad Sci USA 91:9559–9563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. 2007. MafB is required for islet β cell maturation. Proc Natl Acad Sci USA 104:3853–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A. 2006. A switch from MafB to MafA expression accompanies differentiation to pancreatic β-cells. Dev Biol 293:526–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. 2005. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol 25:4969–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. 2010. MafA and MafB regulate genes critical to β cells in a unique temporal manner. Diabetes 59:2530–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. 1997. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in β2/neuroD-deficient mice. Genes Dev 11:2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. 1997. Pax6 is required for differentiation of glucagon-producing α-cells in mouse pancreas. Nature 387:406–409 [DOI] [PubMed] [Google Scholar]
- 17. Ashery-Padan R, Zhou X, Marquardt T, Herrera P, Toube L, Berry A, Gruss P. 2004. Conditional inactivation of Pax6 in the pancreas causes early onset of diabetes. Dev Biol 269:479–488 [DOI] [PubMed] [Google Scholar]
- 18. Du A, Hunter CS, Murray J, Noble D, Cai CL, Evans SM, Stein R, May CL. 2009. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes 58: 2059–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vanderford NL, Andrali SS, Ozcan S. 2007. Glucose induces MafA expression in pancreatic β cell lines via the hexosamine biosynthetic pathway. J Biol Chem 282:1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao L, Guo M, Matsuoka TA, Hagman DK, Parazzoli SD, Poitout V, Stein R. 2005. The islet β cell-enriched MafA activator is a key regulator of insulin gene transcription. J Biol Chem 280:11887–11894 [DOI] [PubMed] [Google Scholar]
- 21. Hagman DK, Hays LB, Parazzoli SD, Poitout V. 2005. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem 280:32413–32418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. 2006. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24: 1392–1401 [DOI] [PubMed] [Google Scholar]
- 23. Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. 2008. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452 [DOI] [PubMed] [Google Scholar]
- 24. Harmon JS, Bogdani M, Parazzoli SD, Mak SS, Oseid EA, Berghmans M, Leboeuf RC, Robertson RP. 2009. β-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology 150:4855–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raum JC, Gerrish K, Artner I, Henderson E, Guo M, Sussel L, Schisler JC, Newgard CB, Stein R. 2006. FoxA2, Nkx2.2, and PDX-1 regulate islet β-cell-specific mafA expression through conserved sequences located between base pairs −8118 and −7750 upstream from the transcription start site. Mol Cell Biol 26:5735–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raum JC, Hunter CS, Artner I, Henderson E, Guo M, Elghazi L, Sosa-Pineda B, Ogihara T, Mirmira RG, Sussel L, Stein R. 2010. Islet β-cell-specific MafA transcription requires the 5′-flanking conserved region 3 control domain. Mol Cell Biol 30:4234–4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pontoglio M, Sreenan S, Roe M, Pugh W, Ostrega D, Doyen A, Pick AJ, Baldwin A, Velho G, Froguel P, Levisetti M, Bonner-Weir S, Bell GI, Yaniv M, Polonsky KS. 1998. Defective insulin secretion in hepatocyte nuclear factor 1α-deficient mice. J Clin Invest 101: 2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pontoglio M, Barra J, Hadchouel M, Doyen A, Kress C, Bach JP, Babinet C, Yaniv M. 1996. Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell 84:575–585 [DOI] [PubMed] [Google Scholar]
- 29. Párrizas M, Maestro MA, Boj SF, Paniagua A, Casamitjana R, Gomis R, Rivera F, Ferrer J. 2001. Hepatic nuclear factor 1-α directs nucleosomal hyperacetylation to its tissue-specific transcriptional targets. Mol Cell Biol 21:3234–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Servitja JM, Pignatelli M, Maestro MA, Cardalda C, Boj SF, Lozano J, Blanco E, Lafuente A, McCarthy MI, Sumoy L, Guigó R, Ferrer J. 2009. Hnf1α (MODY3) controls tissue-specific transcriptional programs and exerts opposed effects on cell growth in pancreatic islets and liver. Mol Cell Biol 29:2945–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee YH, Sauer B, Gonzalez FJ. 1998. Laron dwarfism and non-insulin-dependent diabetes mellitus in the Hnf-1α knockout mouse. Mol Cell Biol 18:3059–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox RD, Lathrop GM, Boriraj VV, Chen X, Cox NJ, Oda Y, Yano H, Le Beau MM, Yamada S, Nishigori H, Takeda J, Fajans SS, Hattersley AT, Iwasaki N, Hansen T, Pedersen O, Polonsky KS, Bell GI. 1996. Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3). Nature 384:455–458 [DOI] [PubMed] [Google Scholar]
- 33. Locker J, Ghosh D, Luc PV, Zheng J. 2002. Definition and prediction of the full range of transcription factor binding sites—the hepatocyte nuclear factor 1 dimeric site. Nucleic Acids Res 30:3809–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maestro MA, Boj SF, Luco RF, Pierreux CE, Cabedo J, Servitja JM, German MS, Rousseau GG, Lemaigre FP, Ferrer J. 2003. Hnf6 and Tcf2 (MODY5) are linked in a gene network operating in a precursor cell domain of the embryonic pancreas. Hum Mol Genet 12:3307–3314 [DOI] [PubMed] [Google Scholar]
- 35. Solar M, Cardalda C, Houbracken I, Martín M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. 2009. Pancreatic exocrine duct cells give rise to insulin-producing β cells during embryogenesis but not after birth. Dev Cell 17:849–860 [DOI] [PubMed] [Google Scholar]
- 36. Nammo T, Yamagata K, Tanaka T, Kodama T, Sladek FM, Fukui K, Katsube F, Sato Y, Miyagawa J, Shimomura I. 2008. Expression of HNF-4α (MODY1), HNF-1β (MODY5), and HNF-1α (MODY3) proteins in the developing mouse pancreas. Gene Expr Patterns 8:96–106 [DOI] [PubMed] [Google Scholar]
- 37. Courtois G, Morgan JG, Campbell LA, Fourel G, Crabtree GR. 1987. Interaction of a liver-specific nuclear factor with the fibrinogen and α 1-antitrypsin promoters. Science 238:688–692 [DOI] [PubMed] [Google Scholar]
- 38. Harmon JS, Stein R, Robertson RP. 2005. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic β cells. J Biol Chem 280:11107–11113 [DOI] [PubMed] [Google Scholar]
- 39. Hattersley AT. 1998. Maturity-onset diabetes of the young: clinical heterogeneity explained by genetic heterogeneity. Diabet Med 15:15–24 [DOI] [PubMed] [Google Scholar]
- 40. Boj SF, Parrizas M, Maestro MA, Ferrer J. 2001. A transcription factor regulatory circuit in differentiated pancreatic cells. Proc Natl Acad Sci USA 98:14481–14486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poitout V, Hagman D, Stein R, Artner I, Robertson RP, Harmon JS. 2006. Regulation of the insulin gene by glucose and fatty acids. J Nutr 136:873–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schreiber E, Matthias P, Müller MM, Schaffner W. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts,’ prepared from a small number of cells. Nucleic Acids Res 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]