The transcription factors C/EBPα/β are targets of ERK½ and control genes associated with luteinization and the formation of extensive vascular networks that sustain luteal cells.
Abstract
LH activation of the epidermal growth factor receptor/RAS/ERK½ pathway is essential for ovulation and luteinization because granulosa cell (GC) depletion of Erk½ (Erk½gc−/− mice) renders mice infertile. As mediators of ERK½-dependent GC differentiation, the CCAAT/enhancer-binding proteins, (C/EBP)α and C/EBPβ, were also disrupted. Female Cebpbgc−/− mutant mice, but not Cebpagc−/− mice, were subfertile whereas Cebpa/bgc−/− double-mutant females were sterile. Follicles failed to ovulate, ovaries were devoid of corpora lutea, luteal cell marker genes (Lhcgr, Prlr, Ptgfr, Cyp11a1, and Star) were absent, and serum progesterone levels were low. Microarray analyses identified numerous C/EBPα/β target genes in equine chorionic gonadotropin (eCG)-human (h)CG-treated mice. At 4 h post-hCG, a subset (19%) of genes altered in the Cebpa/b-depleted cells was also altered in Erk½-depleted cells; hence they are common effectors of ERK½. Additional genes down-regulated in the Cebpa/b-depleted cells at 8 and 24 h post-hCG include known (Akr1b7, Runx2, Star, Saa3) and novel (Abcb1b, Apln, Igfbp4, Prlr, Ptgfr Timp4) C/EBP targets and effectors of luteal and vascular cell development. Bhmt, a gene controlling methionine metabolism and thought to be expressed exclusively in liver and kidney, was high in wild-type luteal cells but totally absent in Cebpa/b mutant cells. Because numerous genes potentially associated with vascular development were suppressed in the mutant cells, C/EBPα/β appear to dictate the luteinization process by also controlling genes that regulate the formation of the extensive vascular network required to sustain luteal cells. Thus, C/EBPα/β mediate the terminal differentiation of GCs during the complex process of luteinization.
Ovulation and luteinization are obligatory for fertility in all mammals. Ovulation is triggered in by the preovulatory surge of LH and culminates in the release of a mature fertilizable oocyte and formation of the corpus luteum. The LH surge activates multiple signaling pathways in granulosa cells (GCs) of preovulatory follicles, including the cAMP/protein kinase A (PKA) and phosphatidylinositol 3-kinase/AKT pathways (1–5), p38MAPKα (MAPK14) (6) and the epidermal growth factor (EGF) receptor (EGFR)/RAS/ERK½ (MAPK3/1) signaling cascade (7–10). Substantial evidence has accumulated to indicate the essential role of the EGF-like factors (Areg, Btc, Ereg), EGFR, and ERK½ cascade in ovulation, including such critical events as cumulus cell-oocyte complex (COC) expansion (8, 9, 12, 13), oocyte maturation (12), and follicle rupture (14).
More recently, genetic studies of mice with targeted disruption of Areg or Ereg or mice with EGFR hypomorphic mutations have confirmed the obligatory role of EGF-like growth factors and ERK½ in ovulation and luteinization (15–17). In addition, when ERK½, key kinases in the EGFR pathway, are depleted in granulosa and cumulus cells, the Erk½gc−/− mice are sterile (18). Remarkably, most if not all, of the physiological effects of LH in the ovary, including oocyte meiotic maturation, cumulus expansion, follicle rupture, and luteinization, are completely abolished (18). Specifically, Erk½ null granulosa cells fail to terminally differentiate into progesterone-producing, Cyp11a1-expressing luteal cells. Rather, large antral, estradiol-producing, and Cyp19a1-expressing preovulatory follicles persist for an extended period of time with nonexpanded COCs locked inside. Collectively, these studies document that the EGFR/ERK½ cascade is an essential physiological switch that reprograms GCs and cumulus cells of preovulatory follicles (18). Although previous microarray analyses show that depletion of ERK½ alters the expression of a plethora of LH-regulated genes, the precise molecular targets, including transcription factors, that mediate the effects of ERK½ in the ovulatory process have not been completely identified (18).
Transcriptional regulators that impact the ovulation process include CCAAT/enhancer-binding protein (C/EBPβ), encoded by Cebpb) (19–22) and several members of the nuclear receptor family such as the progesterone receptor (Pgr) (23, 24) and liver receptor homolog-1 (Lrh1 or Nr5a2) (25, 26). C/EBPβ, a member of a family of basic leucine zipper proteins, has been shown to regulate proliferation and differentiation of multiple cell types in a diverse spectrum of biological processes, including adipogenesis (preadipocyte) (27), immune responses (monocyte and macrophage) (28), decidualization (uterine stoma cell) (29), and mammary gland epithelium differentiation (30, 31). In preovulatory follicles, C/EBPβ is increased by LH (18, 19) and activated in an ERK½-dependent manner (18). Targeted disruption of the Cebpb gene has been shown to cause reproductive defects in the ovary (ovulation and luteinization failure) (20) and uterus (decidualization and proliferation) (32). Because Cebpb knockout mice have defects in other tissues that compromise their health and viability, we recently generated GC-specific Cebpb knockout (Cebpbfl/fl;Cyp19-Cre) mice. These mice exhibit reduced, but not totally impaired, ovulation and luteinization, indicating that C/EBPβ is one but not the only target of LH and ERK½ in GCs and that other transcription factor(s), including members of the C/EBP family, might compensate (18).
C/EBPα is expressed in GCs of the rat ovary (21) and has been implicated in ovulation based on intrabursal injections of Cebpa small interfering RNA (22), whereas C/EBPδ is expressed in theca cells and is dispensable for fertility (33). In the studies presented herein, we document not only the central role of C/EBPα and C/EBPβ in controlling ovulation and luteinization but have identified novel targets that mediate the actions of C/EBPα and C/EBPβ in the formation and vascularization of corpora lutea (CL).
Results
The LH surge induces overlapping expression of C/EBPα and β in preovulatory follicles
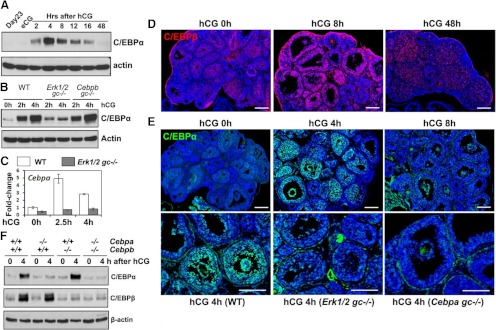
GC-specific Cebpb knockout (Cebpbfl/fl;Cyp19-Cre) mice exhibit reduced, but not totally impaired, ovulation and luteinization (18), suggesting other C/EBP family members might exert overlapping functions. C/EBPδ is expressed in theca and interstitial cells (33) but not GCs in the mouse ovary. However, the expression of C/EBPα has been detected in rat GCs (21) and is transiently induced by human chorionic gonadotropin (hCG) in the mouse ovary (Fig. 1A). Specifically, levels of C/EBPα protein were highest at 4 h after hCG and decline progressively thereafter to a basal level at 48 h post-hCG. This pattern is more transient than the expression of CEBPβ, which increases dramatically within 2 h, is highest at 12 and 16 h, and remains present at 48 h (18)(Supplemental Fig. 7A published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Moreover, the hCG-mediated increase in C/EBPα protein and mRNA is ERK½-dependent, as indicated by the reduced levels in the ovaries of the Erk½gc−/− mice at 2–4 h post-hCG (Fig. 1, B and C). However, C/EBPα protein was expressed at normal levels in the ovaries of Cebpbgc−/− mice (Fig. 1B).
Fig. 1.
Expression of C/EBPα and -β in preovulatory follicles. A, Western blots show the expression levels of C/EBPα protein in ovarian lysates of WT mice, before and after hCG treatment. B, Western blots show the expression levels of C/EBPα protein in ovarian lysates of WT, Erk½gc−/−, and Cebpa/bgc−/− mice, before and after hCG treatment. For each time point, ovarian lysates were made from three mice and pooled together. C, Real-time RT-PCR shows the expression levels of Cepba mRNA in granulosa cells collected from WT and Erk½gc−/− mice. D and E, Immunofluorescent staining shows the expression patterns of C/EBPβ (D) and C/EBPα (E) in ovaries of eCG-primed (44–48 h) 23-d-old immature mice before and after hCG treatment. (scale bar, 150 μm for all the images). F, Western blots show the expression levels of C/EBPα and -β protein in ovarian lysates of eCG-primed (44–48 h) WT, Cebpagc−/−, Cebpbgc−/−, and Cebpa/bgc−/− mice, before and after hCG treatment.
We further compared the expression patterns of C/EBPα and C/EBPβ in the mouse ovary by immunofluorescent labeling. Immunopositive C/EBPβ was low in GCs of equine (e)CG-primed immature mice (23 d old), but was specifically enhanced in preovulatory follicles at 4, 8, and 16 h after hCG injection (Fig. 1D)(Ref. 18 and Supplemental Fig. 7B). C/EBPβ levels decreased but remained present in the fully differentiated luteal cells at 48 h after hCG (Fig. 1D). C/EBPα, like C/EBPβ, was low in eCG-primed ovaries, but was specifically stimulated by hCG in GCs within 4 h (Fig. 1E). However unlike C/EBPβ, levels of C/EBPα decreased dramatically by 8 h after hCG injection and remained low thereafter. C/EBPα expression in ovary was also greatly impaired in the hCG-treated Erk½gc−/− mice (Fig. 1E), confirming the Western blot results (Fig. 1B).
Generation of GC-specific Cebpa knockout mouse strain
The ovarian function of C/EBPα has remained unclear because the Cebpa null mice die at birth of metabolic defects (34). To overcome the neonatal lethality caused by germline depletion of Cebpa and to study the ovarian function of C/EBPα, we generated a GC-specific, Cebpa knockout mouse strain (termed Cebpagc−/−) by mating Cebpafl/fl and Cyp19-Cre mice (7, 35). Western blot and immunofluorescent staining showed efficient depletion of C/EBPα, but not C/EBPβ, in the Cebpagc−/− GCs (Fig. 1, E and F).
When the reproductive phenotype of these mice was analyzed, the Cebpagc−/− females showed moderately reduced ovulation (∼30%) in superovulation studies (Fig. 2A) but were completely fertile in normal mating paradigms (Fig. 2B). Detailed characterization showed that the decreased ovulation in superovulation assay was caused by occasionally impaired follicle rupture. However, hCG-induced oocyte meiotic maturation, COC expansion, and GC luteinization were normal in the Cebpagc−/− females (Supplemental Fig. 1). Because the expression of C/EBPβ was not affected by C/EBPα depletion (Fig. 1F), we speculated that C/EBPβ might be able to compensate for the loss of C/EBPα in mediating the ovulation signal and hence, the double-knockout mice (Cebpafl/fl;Cebpbfl/fl;Cyp19-Cre, termed Cebpa/bgc−/−), were generated. In these mice, expression of C/EBPα and C/EBPβ was markedly depleted in the GCs (Fig. 1F).
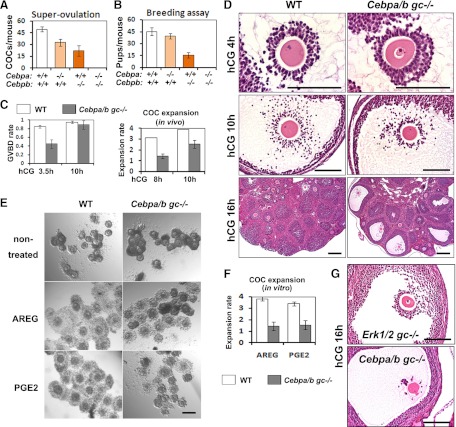
Fig. 2.
The Cebpa/bgc−/− mice cannot ovulate and are completely infertile. A and B, Fertility was examined by treating immature mice of each genotype with a superovulatory regimen of homones (A) and by breeding 6-wk-old females of indicated genotypes with fertile WT males for 6 months (n = 6 for each genotype) (B). Ovulated oocytes were collected from oviducts and counted at 16 h after hCG injections. (n = 8∼10 for each genotype.). C (left panel), The rate of oocyte GVBD was determined by counting the number of oocytes exhibiting GVBD in ovarian sections of WT and Cebpa/bgc−/− mice at 3.5 h and 10 h post-hCG (n = 6∼8 for each group); C (Right panel), The extent of COC expansion was determined as described in detail previously (74, 75) 0 = no expansion; 4 = maximum expansion. D, H&E staining shows the ovarian histology of 23-d-old WT and Cebpa/bgc−/− mice treated with eCG (48 h)+hCG (4 h, 10 h, and 16 h). Scale bar, 150 μm for all images. E, In vitro COC expansion assay. Fully grown COCs were isolated from WT and Cebpa/bgc−/− mice at 44–48 h after eCG treatment and cultured in COC medium (30 COCs/100 μl medium). Cumulus expansion was induced by an overnight treatment of AREG (100 ng/ml) or prostaglandin E2 (500 ng/ml). Scale bar, 150 μm. F, The extent of COC expansion in vitro was determined by quantification of expansion index. G, H&E staining shows the COC morphology of 23-d-old Erk½gc−/− and Cebpa/bgc−/− mice treated with eCG (48 h)+hCG (16 h). Scale bar, 70μm.
Ovulation is blocked in GC-specific Cebpa/b knockout mice
Although neither Cebpa nor Cebpb GC-specific knockout mice showed complete ovulation failure, we hypothesized that ovaries of Cebpa/bgc−/− double-mutant mice as with those of the Erk½gc−/− double-mutant mice would display more severe defects. Indeed, the Cebpa/bgc−/− double-mutant females failed to ovulate and were completely infertile (Fig. 2, A and B). When Cebpa/bgc−/− mice were treated with eCG and hCG, preovulatory follicular development was normal (Supplemental Fig. 2). However, oocyte germinal vesicle breakdown (GVBD) was delayed, and COC expansion was impaired slightly, as indicated by the rate of GVBD and the relative degree of expansion (termed the “expansion index”), respectively (Fig. 2, C and D). Furthermore, when COC expansion was tested in vitro, the physiological mediators of LH, amphiregulin (AREG) or prostaglandin E2 (8, 13), were less effective in inducing expansion of COCs collected from Cebpa/bgc−/− mice compared with wild-type (WT) controls (Fig. 2E).
More severely, LH induction of follicle rupture was completely blocked in the Cebpa/bgc−/− mice in vivo (Fig. 2D). Although oocytes resumed meiotic maturation and developed to the metaphase II stage (as indicated by the emission of first polar body), they remained trapped in follicles that failed to rupture (Fig. 2F). Most cumulus cells dissociated from the entrapped oocytes and formed clumps. This phenotype differs from that of the Erk½gc−/− mice, in which cumulus cells remained tightly packed around the immature oocyte, with no signs of expansion or dissociation even at16 h after hCG stimulation (Fig. 2F).
C/EBPα and -β appear dispensable for the early events of ovulation
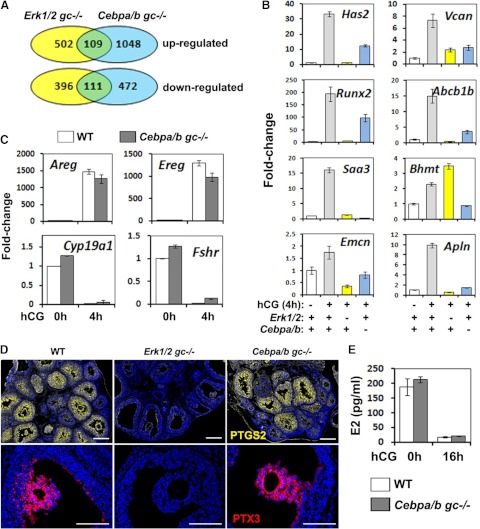
Previous studies have documented that ERK½ control the expression of many genes rapidly induced (within 4 h) by LH/hCG in GCs and cumulus cells of prevoulatory follicle (18). Because C/EBPα/β appear to be targets of ERK½, we sought to determine which genes regulated by ERK½ (18) were also targets of C/EBPα/β at this early time interval after the preovulatory LH surge. Furthermore, we sought to identify novel C/EBPα/β target genes. Therefore, microarray analyses were performed using RNA prepared from GCs isolated from eCG-primed WT and Cebpa/bgc−/− mice at 4, 8, and 24 h after hCG treatment. At the 4-h time interval, approximately 19% of the genes were similarly increased or decreased in the Erk½gc−/− and Cebpa/bgc−/− mutant GCs (Fig. 3, A and B). However, genes essential for early events of the ovulation process and that are immediate targets of the Erk½gc−/− were not affected by Cebpa/b depletion (Fig. 3C; Tables 1 and 2). For example, the genes encoding the EGF-like factors, Areg and Ereg, were dramatically induced by hCG within 4 h in both the WT and Cebpa/bgc−/− GCs, indicating C/EBPα/β function downstream of these factors (Fig. 3C). Likewise, FSH target genes such as Fshr and Cyp19a1 (Fig. 3C; and hence estradiol production, Fig. 3E) that are down-regulated rapidly by hCG in WT were also reduced in the Cebpa/bgc−/− ovaries (Fig. 3, C and E) but not in the Erk½gc−/− ovaries (18). Lastly, the expression of some essential LH target genes that control COC expansion, such as Ptgs2, Tnfaip6, Ptx3, and Il6 were completely blocked in the Erk½gc−/− but not in Cebpa/bgc−/− ovaries (Fig. 3D and data not shown).
Fig. 3.
Microarray analyses identified LH-target genes regulated by C/EBPα and -β at the early stages of ovulation. A, Microarray analyses identified the genes up- and down-regulated in GCs isolated from Erk½gc−/− and Cebpa/bgc−/− mice at 4 h post-hCG. Approximately 19% of the genes were regulated in a similar pattern in both Erk½gc−/− and Cebpa/bgc−/− GCs. B and C, Real-time RT-PCR shows the mRNA expression levels of selected genes in GCs of WT, Erk½gc−/−, and Cebpa/bgc−/− mice treated with eCG (48 h)+hCG (4 h). D, Immunofluorescent staining shows the expression of PTGS2 (yellow) and PTX3 (red) in preovulatory follicles of WT, Erk½gc−/−, and Cebpa/bgc−/− mice treated with eCG (48 h)+hCG (4 h). Scale bar, 150μm. E, Serum estradiol levels in 25-d-old WT and Cebpa/bgc−/− mice before and after hCG treatment (0 h and 16 h) (n = 5 for each treatment.)
Table 1.
Representative genes commonly regulated by ERK½ and CEBP α/β at hCG, 4h
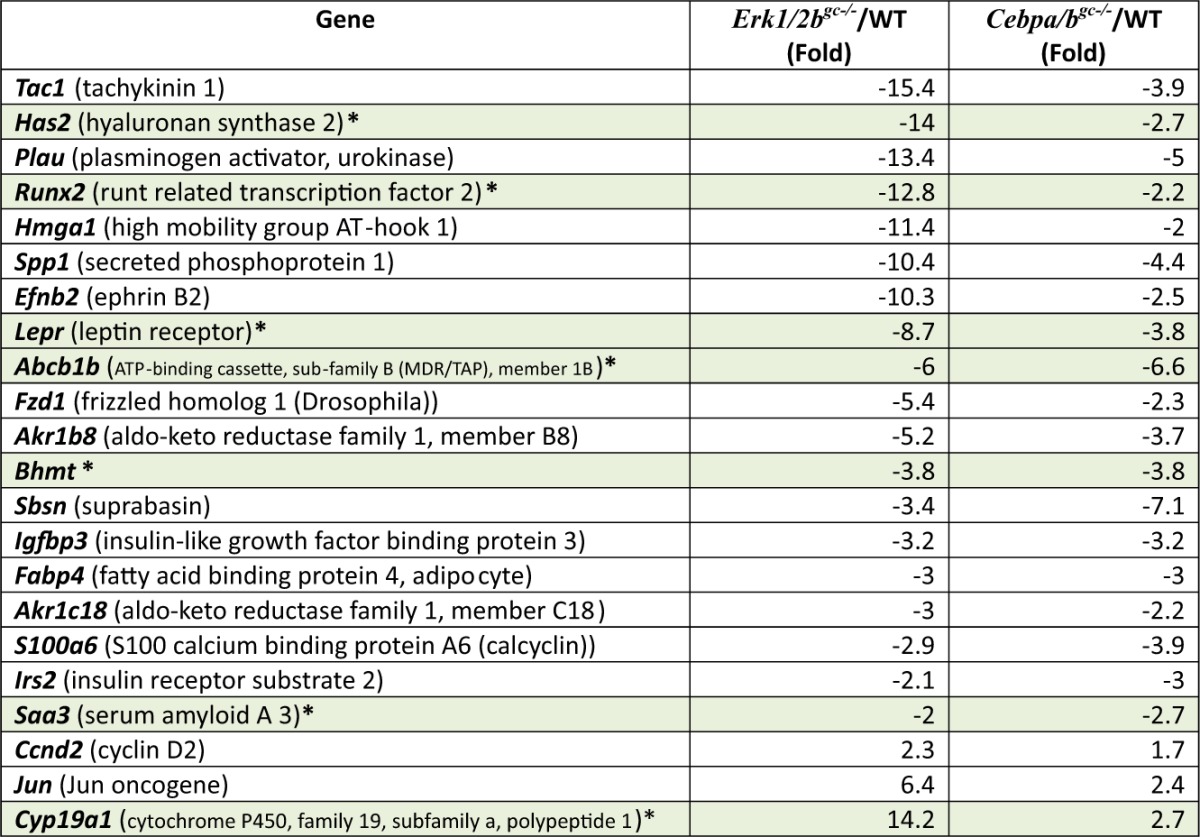
List of selected genes that were decreased markedly in both the Erk½gc−/− (19) and Cebpa/bgc−/− mutant granulosa cells at 4h post-hCG. Those varified by real-time RT-PCR are highlighted in gray.
Table 2.
Representative genes highly regulated by CEBPα/β at hCG, 24 h
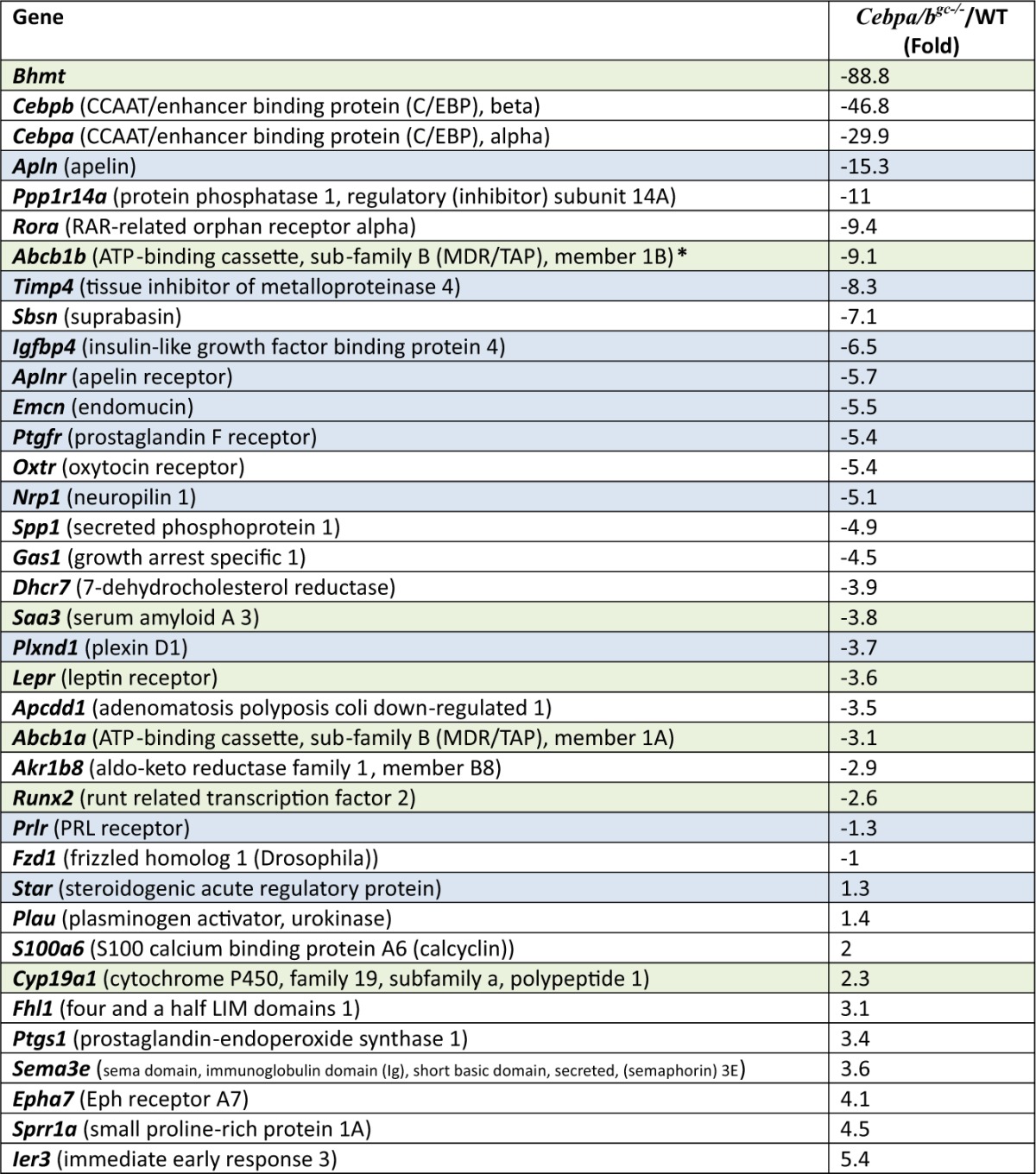
List of selected genes highly regulated in the Cebpa/bgc−/− mutant granulosa cells at 24 h post-hCG. Those verified by real-time RT-PCR are highlighted in light gray and gray. Those highlighted in green were regulated at 4, 8 and 24 h post-hCG.
Conversely, there were many genes selectively up- and down-regulated in the Cebpa/bgc−/− GCs that were not regulated in the Erk½gc−/− cells at 4 h post-hCG. These Cebpa/bgc−/−specific genes appear to be those that are downstream of these transcription factors and control events of ovulation and luteinization at the later time points that are never reached in follicles of the Erk½gc−/− mice where the ovulation process is aborted prematurely (Supplemental microarray data).
LH- and ERK½-regulated genes that are potential targets of C/EBPα/β
Although the earliest events regulated by LH and ERK½ appear to occur upstream of C/EBPα/β activation in this mouse model, gene profiling data revealed that a diverse subset of LH- and ERK½-induced genes are potential targets of C/EBPα/β in GCs of ovulating follicles at 4 h post-hCG (Table 1 and Fig. 3B). These include hyaluronan synthase 2 (Has2) that is known to be essential for the formation of extracellular matrix during COC expansion (36). The reduced expression of Has2 may explain the modest defects that are observed in COC expansion ovaries of the Cebpa/b mutant mice (Fig. 2,C–E). Additional noteworthy genes that are regulated by ERK½ and C/EBPα/β at 4 h post-hCG include those in lipid metabolism (Lepr, Igfbp3, Irs2), an acute phase-induced gene, Saa3, the transcription factor Runx2 expressed in bone and ovulating follicles, the multidrug-resistant gene (Abcb1b), aldo-keto reductases, (Akr1b8, also known as vas deferens protein; Akr1c18, also known as 20α-hydroxysteroid dehydrogenase), the urokinase protease (Plau), a neuronal G protein-coupled receptor (Tach1), a presumptive epidermal factor, suprabasin (Sbsn), and a regulator of methionine metabolism previously reported only in liver and kidney, betaine homocysteine methyltransferase (Bhmt) (Table 1; highlighted in green). Of these, Abcb1b, Bhmt, Runx2, Saa3, and Sbsn were also decreased in the Cebpa/b mutant cells at 8 h and 24 h post-hCG (Table 2), indicating that they are downstream of these two transcription factors. Akr1b7, Saa3, and Runx2 are known C/EBPβ target genes (37–39) but their specific roles in ovulation and luteinization are not entirely clear. The Akr1b7 knockout mice are fertile (39); Saa3 null mice have not been generated (38) whereas knockdown of Runx2 in cultured GCs reduces expression of Cyp11a1 (37).
GC-specific Cebpa/b knockout mice have severe defects of luteinization
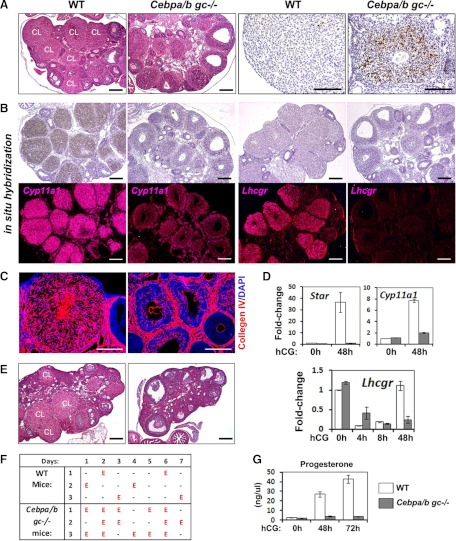
In addition to the ovulation failure, the luteinization process is impaired severely in Cebpa/bgc−/− ovaries, as shown by defective CL formation at 48 h after hCG injection (Fig. 4A). At the molecular level, reduced expression of known luteinization marker genes (Cyp11a1, Lhcgr, and Star) were detected by real-time RT-PCR and in situ hybridization (Fig. 4, B and D) and low serum progesterone levels at 48–72 h after hCG injection (Fig. 4G). The expression pattern of Lhcgr is particularly interesting because the FSH-induced expression in preovulatory follicles was not affected by Cebpα/β depletion, but the second peak of Lhcgr expression at hCG 48 h was totally blocked in the Cebpa/bgc−/− ovaries (Fig. 4, B and D). Whereas CL in the WT ovaries are highly vascularized, those in the Cebpa/bgc−/− ovaries exhibit defective vascular development, as indicated by the immunostaining of blood vesicle marker collagen IV (Fig. 4C and Supplemental Fig. 3). The poorly differentiated GCs in Cebpa/bgc−/− ovaries became apoptotic at 48 h after hCG as shown by terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) assays (Fig. 4A) and had completely disappeared within the next 24 h (data not shown). In the ovaries of adult Cebpa/bgc−/− females (n = 8), CL were completely absent (Fig. 4E and Supplemental Fig. 2). Vaginal cytology showed regular, normal 4 d of estrous cycles on consecutive days in control mice, whereas the Cebpa/bgc−/− females displayed an irregular pattern of estrous cycles indicative of lack of progesterone (Fig. 4F).
Fig. 4.
The Cebpa/bgc−/− mice exhibit severe defects in luteinization. A, Hematoxylin and eosin staining and TUNEL assay show the histology and apoptosis of luteal cells (48 h after hCG injection) in the ovaries of WT and Cebpa/bgc−/− mice. Scale bar, 150 μm. B, In situ hybridization shows the expression of mRNA encoding Cyp11a1 and Lhcgr in WT and Cebpa/bgc−/− ovaries at 48 h after hCG treatment. Histology of the ovaries is shown by hematoxylin staining (bright-field images); localization of Cyp11a1 and Lhcgr mRNAs is shown by dark-field images captured with Zeiss Axioplan microscope. Scale bar, 150μm. C, Immunostaining shows the presence of collgen IV, an endothelial cell marker, in the vascularized CL present in WT but not in Cebpa/bgc−/− mutant ovaries at 48 h after hCG. Scale bar, 150 μm. D, Real-time RT-PCR shows the expression of CL marker genes Star and Cyp11a1 in GCs and luteal cells of WT and Cebpa/bgc−/− mice before (0 h) and after (48 h) hCG treatment, respectively, and Lhcgr in cells isolated from WT and Cebpa/bgc−/− mice before (0 h) and after (8 h), and 48 h hCG treatment. (n = 3 for each group.) E, Hematoxylin and eosin staining shows the ovarian histology of 8-wk-old cycling WT and Cebpa/bgc−/− females. Scale bar, 150 μm. F, Days of estrus indicated by vaginal cytology in cycling WT and Cebpa/bgc−/− females (3 months old). G, Serum progesterone levels in 25-d-old WT and Cebpa/bgc−/− mice before and after hCG treatment (0 h, 48 h, and 72 h) (n = 5 for each treatment).
Microarray analyses identify novel C/EBP target genes that regulate luteinization
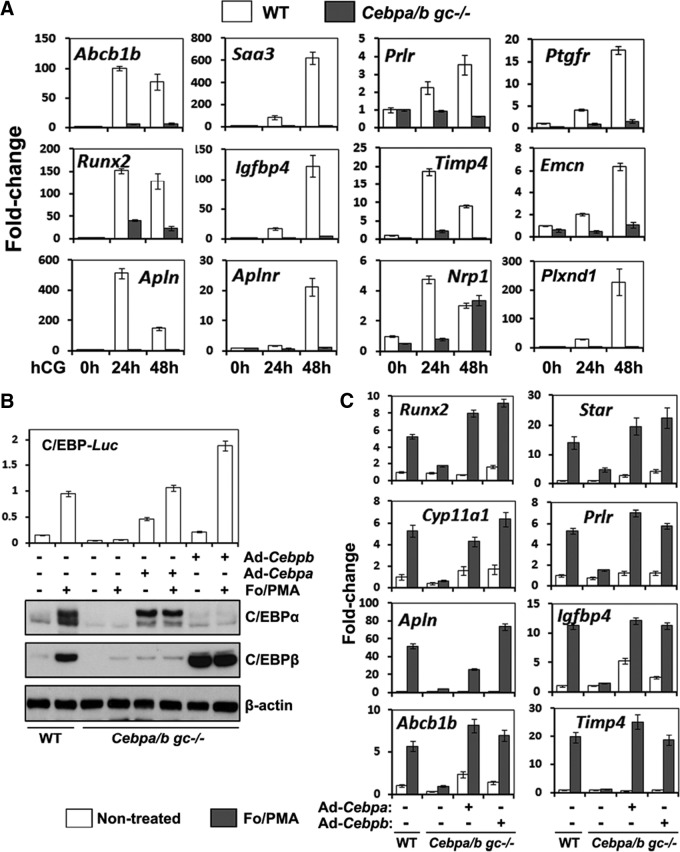
The gene profiling results showed that additional genes were selectively decreased in the Cebpa/bgc−/− ovaries at 8 h and 24 h post-hCG (Table 2; Fig. 5A, Fig. 6A; and Supplemental microarray data). These include genes regulated at 4 h (Abcb1b, Bhmt, Runx2, Saa3) (Table 2; highlighted in green) as well as the luteotropic prolactin (PRL) receptor (Prlr), IGF-binding protein 4 (Igfbp4), the prostaglandin F receptor (Ptgfr), tissue inhibitor of metalloproteinases 4 (Timp4), and vascular markers endomucin (Emcn), apelin (Apln), apelin receptor (Aplnr), neuropilin (Nrp1), and Plexin D1 (Plxnc1) (Table 2, highlighted in blue). The reduced expression of these genes appears to be related to impaired luteinization and vascular development. Some of these genes, such as the PRL receptor, are known to be essential for maintenance of luteal cell function (40, 41) but have not previously been linked to regulation by C/EBPα/β during CL formation (20). Genes associated with PRL action, such as Lhcgr, Sfrp4, and Cdkn1b were not observed on the array, most likely because of the reduced expression of the PRL receptor (42). Most intriguing is the novel subset of LH target genes that appear to be associated with neovascularization and endothelial cell functions (Emcn, Apl, Aplr, Nrp1, and Plxnd1). Real-time PCR experiments confirmed that these genes were induced by hCG during ovulation and luteinization and were down-regulated in Cebpa/bgc−/− mice (Fig. 5A).
Fig. 5.
Microarray analyses identify novel C/EBP target genes that regulate ovulation and luteinization. A, Granulosa/luteal cells were isolated from WT and Cebpa/bgc−/− mice before and after hCG treatment (0 h, 24 h, and 48 h) (n = 3 for each treatment). Total RNA was isolated, and the expression levels of indicated genes were determined by real-time RT-PCR. B, GCs were isolated from eCG primed (24 h) 23-d-old immature mice (WT and Cebpa/bgc−/−) and cultured for 24 h. At that time, Cebpa/bgc−/− GCs were washed and infected with adenoviral vectors encoding either C/EBPα or C/EBPβ. After 4 h, the cells were treated with For (10 μm) plus PMA (20 nm) for another 20 h. Western blots show the expression levels of C/EBPα and C/EBPβ proteins. C, GCs were cultured, and infected with adenoviral vectors as in panel B. Then the cells were treated with For/PMA for another 24 h. Expression of indicated C/EBP-target genes was detected by real-time RT-PCR.
Fig. 6.
Bhmt is a novel C/EBP-regulated gene that is required for the maintenance of luteal cell functions. A and B, Immunofluorescent staining (A) and real-time RT-PCR and Western blot (B) analyses show the expression of BHMT protein and mRNA in ovaries of WT and Cebpa/bgc−/− mice, before and after hCG treatment (48 h). Arrows in panel B indicate the extremely low expression levels of Bhmt mRNA in the ovaries of the Cebpa/bgc−/− mice. Scale bar, 150 μm. C, Quantitative RT-PCR shows the effects of BHMT-specific inhibitors, DMG or CBHcy, on the expression of selected luteal cell marker genes at 48 h post-hCG. D, Western blot shows the effect of DMG and CBHcy on the levels of BHMT protein in ovaries at 48 h post-hCG. E, Serum progesterone levels (48 h after hCG injection) of WT mice with or without treatment of BHMT inhibitors (n = 5 for each treatment). F and G, H&E staining (F) and TUNEL assay (G) show the histology and apoptosis of luteal cells (48 h after hCG injection) in WT mice treated with or without DMG treatment.
To further confirm the essential role of C/EBPα/β in the expression of these novel target genes, we employed a GC in vitro luteinization system. Undifferentiated GCs were collected from eCG-primed immature mice and cultured in serum-free medium. Forskolin and phorbol 12-myristate 13-acetate (PMA) (For/PMA) were used to mimic the effect of LH in inducing terminal differentiation (luteinization) (11). When a C/EBP promoter-luciferase reporter construct was transfected into the cultured GCs, For/PMA stimulated luciferase activity 10-fold. This was associated with increased expression of endogenous C/EBPα and -β protein in WT GCs. For/PMA did not activate the C/EBP luciferase reporter in GCs obtained from the Cebpa/bgc−/− mice where levels of endogenous C/EBPα/β protein were undetectable (Fig. 5B). However, transduction of Cebpa/bgc−/− GCs with adenoviral vectors expressing either Cebpa or Cebpb rescued the expression of C/EBPα and C/EBPβ protein, respectively, and restored For/PMA-mediated activation of the C/EBP luciferase reporter (Fig. 5B).
To extend these observations, real-time RT-PCR analyses were done to analyze the expression of selected ovarian C/EBP target genes that were identified by the array database. As shown, For/PMA induced the expression of ovulation and luteal marker genes (Runx2, Prlr, Star, Cyp11a1, Apln, Ifgbp4, Abcb1b, and Timp4) in WT GCs in culture but not in the Cebpa/b-depleted GCs. However, the induction of these genes by For/PMA was rescued in the mutant cells by the exogenous expression of C/EBPα or C/EBPβ (Fig. 5C). Collectively, these results indicate that expression and activation of either C/EBPα or C/EBPβ can induce for the expression of a subset of LH target genes.
Bhmt is a novel C/EBP-regulated gene that is required for the maintenance of luteal cell functions
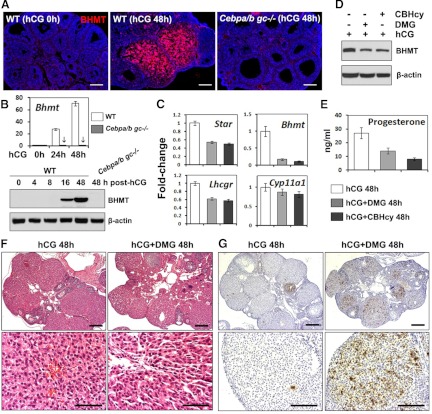
Among the novel C/EBP-regulated ovarian genes identified by our microarray analyses, Bhmt was one of the most highly induced and dramatically affected by Cebpa/b depletion at 8 h and 24 h post-hCG. The expression of Bhmt in the ovary is novel and completely unexpected because it is presumed to be a liver- and kidney-specific gene (44, 45). The Bhmt gene encodes a Zn2+-dependent thiolmethyltransferase that contributes to the regulation of homocysteine levels in the body and is essential for the generation of S-adenosylmethionine, the methyl donor for the methylation of DNA and histones. Although the methionine pathway has been known for many years, recent interest in BHMT has surfaced based on its potential link to atherosclerosis. The ovarian levels of Bhmt mRNA and protein were extremely low in preovulatory follicles before rupture but are dramatically up-regulated between 16–48 h after hCG injection (Fig. 6, A and B). BHMT protein was specifically localized to the fully differentiated luteal cells of WT mice and was totally absent in ovaries of Cebpa/b mice where luteinization is defective (Fig. 6A). Because the expression and function of BHMT in the ovary has not been analyzed previously, we further investigated the physiological role of this enzyme during ovulation and luteinization. When WT mice were treated with BHMT-specific inhibitors, either dimethalglycine (DMG) or (R,S)-carboxybutylhomocysteine (CBHcy), hCG-induced ovulation was not affected, but luteal cell marker genes (Lhcgr and Star) and serum progesterone levels were reduced, indicating that the function of luteal cells is impaired (Fig. 6, C and E). Interestingly, the Bhmt gene itself was dramatically down-regulated by inhibitor treatments, at both mRNA and protein levels (Fig. 6, C and D).
Histological analysis of the ovaries indicated that the CL in ovaries of the mice treated with BHMT inhibitors were smaller in size than the control mice and that the luteal cells showed signs of impaired terminal differentiation (Fig. 6F). Specifically, the luteal cells exhibited more condensed nuclei, less cytoplasm, and gaps between cells. These poorly differentiated luteal cells also showed significantly higher levels of apoptosis than the controls as indicated by TUNEL assays (Fig. 6G).
Discussion
Targeted disruption of ERK½ in GCs completely derails LH-mediated ovulation, luteinization, oocyte maturation, and COC expansion (18). These dramatic results led us to identify what specific transcription factors might serve as critical mediators of ERK½ in ovulating follicles. Herein we document that C/EBPα (22) and C/EBPβ (19, 20) mediate specific cell fate decisions initiated by their ERK½ activation and their transcriptional regulation of genes that control luteinization and the development of a vascular network essential for luteinization.
These cell fate decisions in GCs are distinct from the effects mediated by these transcription factors in other tissues. Specifically, the expression and functions of C/EBPα and/or C/EBPβ have been studied extensively in liver (34, 46), adipocytes (27), uterine cells (29, 32), and the mammary gland (30). In most tissues, one or the other of these transcription factors appears to exert predominance. In adipocytes, the expression of C/EBPβ, and its stimulation of proliferation precede that of C/EBPα whereas C/EBPα appears to be more critical for adipocyte differentiation (47). Although Cebpa-null mice are embryonic lethal, mutations in the Cebpa gene in humans are linked to leukemia and uncontrolled proliferation (48) whereas Cebpb appears to play a major role in mammary and uterine epithelial cell proliferation and differentiation (30, 32). Although important roles for these factors have also been identified in the ovary (20, 37, 38), the results presented herein provide the first comprehensive analysis of the physiological and molecular roles of C/EBPα and C/EBPβ in the mouse ovary in vivo.
We document that C/EBPα and -β are transiently induced by LH/hCG in GCs of mouse preovulatory follicles, in an LH- and ERK½-dependent manner. Although previous studies indicated that C/EBPβ might be required for ovulation (22), the GC-specific Cebpagc−/− mice exhibited normal follicular development and fertility, indicating that disrupting the Cebpa gene alone in this context is not sufficient to mimic the global effects of depleting Erk½. The phenotype of the Cebpbgc−/− mice was more severe (18) than that of depleting Cebpa alone, and depleting both the Cebpa and Cebpb genes in GCs caused Cebpa/bgc−/− mice to be completely infertile. These results document that these transcription factors have overlapping functions that control LH-mediated ovulation and luteinization. It is important to note, however, that this overlap occurs during a relatively short window of time after LH stimulation. C/EBPα was detected at its highest levels in GCs of preovulatory follicles at 4–8 h after hCG injection, whereas C/EBPβ was detected at high levels for as long as 16–24 h. Therefore, the dramatic impact of C/EBP α/β together on ovulation and the terminal differentiation of GCs to luteal cells appears to be initiated within the first 4 h and is maintained, amplified, or modified primarily by C/EBPβ at later time intervals. This hypothesis is supported by the evidence that GC luteinization can proceed in culture medium devoid of hormones, if the cells are exposed to the LH/hCG surge in vivo for as little a 5–7 h (49).
Unlike the Erk½gc−/− mice, the Cebpabgc−/− double-mutant mice do not exhibit major defects in LH-induced COC expansion or oocyte maturation. Thus, C/EBPα and C/EBPβ control a subset of genes regulated by ERK½ but not all genes. Indeed, gene-profiling analyses provided critical databases for determining which genes regulated by LH activation of ERK½ (18) were also targets of C/EBPα/β. Of particular relevance we identified a subset (∼19%) of genes that were up- and down-regulated similarly in GCs of the Erk½gc−/− and Cebpa/bgc−/− mice at 4 h post-hCG. The gene-profiling results also identified genes that are selectively regulated by either ERK½ or C/EBPα/β at this same early time interval after exposure to LH/hCG in vivo (Fig. 3A).
Because C/EBPβ (also known as NF-IL6 and IL6-DBP) has been linked functionally to the induction of acute-phase genes in liver such as Saa3 and α2-macrogobulin (50, 51) and specific cytokines, such as Il6, during inflammatory-like reactions such as ovulation (52) and in other tissues (53), we anticipated that depletion of Cebpa/b would reduce the expression of many inflammatory-related genes that are induced in GCs and cumulus cells by LH (54–56). In support of this and confirming that Saa3 is a C/EBPβ target gene in the ovary (38), the expression of Saa3 was markedly reduced in the Cebpab-depleted GCs. However, the Cebpa/b-depleted GCs exhibited marginal differences in the expression of inflammation/matrix genes Has2, Vcan, Ptgs2, Ptx3, Tnfaip6, and Il6 that are also induced in preovulatory follicles by LH (55). The lack of an effect on the expression of Ptgs2 and Il6 was surprising because the promoters of these two genes can bind and be activated by C/EBPβ in GCs in culture (19, 53). However, consistent with these data, depletion of Cebpa/b did not completely block COC expansion in vivo or in culture, a process that is critically dependent on the expression of the inflammatory- and matrix-related genes (55). Runx2 expression is reduced in the Cebpa/b-depleted cells (Fig. 4) as in the Cebpb-null mice (37). By contrast, Inha was up-regulated in the Cebpb-null mice (57) but not in our Cebpa/b mutant cells. Reasons for discrepancy of the latter may indicate that levels of Cebpa/b mRNA and protein are not sufficiently depleted at 2–4 h in the Cebpabgc−/− mice to prevent the very rapid suppression of some genes regulated by ERK½ (18). This notion seems plausible because Cyp19a1 and Ptgs2 (20) as well as Inha (57) remained elevated in ovaries of the Cebpb -null mice that are genetically and thus totally devoid of C/EBPβ.
The gene-profiling data revealed further that C/EBPα/β regulate a diverse subset of LH- and ERK½-induced genes in GCs of ovulating follicles. Those reduced in the Cepba/b-depleted cells at 4 h post-hCG include genes in lipid metabolism (Lepr, Saa3, Igfbp3, Irs2), drug resistance (Abcb1b), aldo-keto reductases (Akr1b7; Akr1c18), and a regulator of methionine metabolism previously reported to be expressed exclusively in liver and kidney (Bhmt). Expression of Abcb1b, Bhmt, Runx2, and Saa3 was also decreased in the Cebpa/b mutant cells at 8 h and 24 h post-hCG, indicating that they are likely to be specific C/EBPα/β target genes. Additional genes that were decreased at 24 h and that appeared of significance were the steroidogenic genes, such as Star, a known target of C/EBPβ (58), IGFBP4 (Igfbp4), a potent inhibitor of WNT signaling (59), as well as regulator of IGF signaling related to luteinization (60) and the tissue inhibitor of metalloproteases 4 (Timp4) (61).
Not only the gene-profiling data but also the histological results indicated that the most dramatic defects in the Cebpa/bgc−/− mice were the impaired formation of CL and the pronounced disruption of vascular development that is associated with luteinization. Because vascularization of the CL rivals that occurring during tumor growth (62, 63), C/EBPα/β appear to play a major role in this critical physiological process. In support of this, our gene-profiling data revealed that several genes linked to vascular development as well as luteinization were rapidly and dramatically decreased in the Cebpa/b mutant cells but could be restored in the presence of For/PMA and exogenous C/EBPα/β These include luteal cell (Abcb1b, Runx2, Prlr, Ptgfr, Star, Timp4, Igfbp4) and vascular cell (Apln) genes of which Abcb1b1, Apln, Timp4, Prlr, Ptgfr, and Igfpb4 represent novel targets of C/EBPα/β. Abcb1b encodes the multidrug-resistant transporter (also known as P-glycoprotein) that is acutely regulated by C/EBPα/β in GCs is highly expressed in liver and kidney (64), human CL (65), and some cancer cells (64). Although its function in luteal cells is not known, it is likely involved in the transport of cholesterol or progesterone. The functional roles of APLN and its receptor in the ovary have yet to be defined. However, APLN regulates many functions in numerous cell types, including angiogenesis in vascular endothelial cells (66). Other vascular-associated genes that appear to be regulated indirectly by C/EBPα/β include neuropilin 1 and plexin D1 that are involved in axon guidance but are also essential for vascular development and endothelial cell migration (67) and endomucin, a CD34-like sialoglycoprotein that is a marker of hematopoeitic stem cells (68) and functions as an antiadhesive molecule in vascular endothelial cells (69) and possibly leukemia cells (70).
The dramatic induction of Bhmt in luteal cells and the suppression of its expression in Cebpa/b mice as early as 4 h and greater at 8–24 h post-hCG provide additional unexpected and novel findings. Based on its potential role in vascular activity and the availability of specific inhibitors led us to determine whether it had a functional role in the ovary. BHMT is a Zn2+-dependent thiolmethyltransferase found most abundantly in liver and kidney and is essential for generating S-adenosylmethionine, the methyl donor for DNA and histones and for controlling the homeostatic levels of methionine and homocysteine in serum (44, 45). Using BHMT inhibitors in vivo we show that follicular development was normal but luteinization, luteal cell expression of specific genes, and progesterone production were impaired. The BHMT inhibitors also promoted luteal cell apoptosis. Thus, it appears that BHMT is important for luteal cell maintenance. Although the precise mechanisms by which BHMT is induced and regulates luteal cell functions remain to be determined, these results provide novel information on a factor that is highly expressed in these cells.
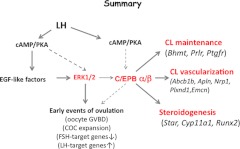
In summary, our studies document that the expression and functional activation of both C/EBPα and C/EBPβ in GCs are essential for LH-, cAMP/PKA- , and ERK½-mediated events associated with ovulation and luteinization in the mouse models analyzed (Fig. 7). Gene-expression profiles documented that C/EBPα and C/EBPβ are associated with approximately 19% of the genes regulated by ERK½ and the global reprogramming of GC functions during ovulation and luteinization. Although we anticipated that disruption of Cebpa/b would alter the expression of many inflammatory-related genes known to be expressed in GCs and required for ovulation, this class of genes was less prominent than genes associated with the formation and vascularization of CL. In addition, although Cebpa/b are often associated with proliferation and cancer, changes in cell cycle genes were not identified as key targets. Thus, the genes regulated in GCs by these two transcription factors appear to be highly specialized for the processes of ovulation, lutenization, and rapid vascular growth that are unique to the ovary.
Fig. 7.
A schematic of C/EBP α/β functions in GCs. LH induces ovulation and luteinization by activating the cAMP/PKA pathway leading to expression of the EGF-like factors, AREG, epiregulin, and betacellulin. These factors then bind the EGFR to activate the RAS/ERK½ signaling cascade (8, 76). The results presented herein show that C/EBPα and -β are essential for LH induction of ovulation and luteinzation because they mediate specific events downstream of LH, PKA, and ERK½ (8, 18, 20, 77). By generating the Cebpa/bgc−/− double-mutant mice, analyzing gene profiling datasets and verifying target genes by in vitro approaches, we have identified specific genes that are potential targets (direct or indirect) of C/EBPα/β. These genes are involved in the formation and maintenance of CL, steroidogensis, and the vascularization of ruptured follicles.
Materials and Methods
Animals
Immature C57BL/6 mice were obtained from Harlan, Inc. (Indianapolis, IN). Animals were housed under a 14-h light, 10-h dark schedule and were treated in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Mice lacking Cebpa or Cebpb in GCs were generated by crossing Cyp19-Cre mice (7) with previously reported Cebpafl/fl mice (35) or Cebpbfl/fl mice (71). GC-specific Erk½ knockout mouse strain (Erk½gc−/−) was reported as before (72, 73). All the mutant mouse strains are in the C57BL/6 background.
To study ovarian responses to exogenous gonadotropins, 21-d-old immature females were injected ip with 4 IU eCG (Calbiochem, La Jolla, CA) followed 48 h later with 5 IU hCG (human chorionic gonadotropin, American Pharmaceutical Partners, Schaumburg, IL) (18). BHMT inhibitors DMG (Sigma, St. Louis, MO; 10 μmol/mouse) or CBHcy (10 μmol/mouse, gifted by Dr. Jiri Jiracek (Academy of Sciences of the Czech Republic)) were dissolved in PBS and injected ip to the eCG-primed mice at 0 h and 24 h post-hCG.
Serum analysis
Mice were anesthetized and blood was collected by cardiac puncture. Progesterone and estradiol levels were analyzed by the University of Virginia Ligand Core Facility (Specialized Cooperative Centers Program in Reproduction and Infertility Research).
Immunofluorescence
Ovaries were fixed in 4% paraformaldehyde, embedded in Optional Cutting Temperature compound (Sakura Finetek USA, Inc.) and stored at −70 C before the preparation of 7-μm sections. Serial sections were sequentially probed with primary antibodies as indicated in the text and secondary Alexa Fluor 594- or 488-conjugated goat antirabbit IgG antibodies (Molecular Probes, Inc., Eugene, OR) as previously described (18). Slides were mounted using VectaShield with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Digital images were captured using a Zeiss Axioplan 2 microscope with ×5–×63 objectives (Carl Zeiss, Inc., Thornwood, NY). For all the experiments, exposure time was kept the same for control and mutant samples.
In situ hybridization
The riboprobe in vitro transcription systems kit (Promega Corp., Madison, WI) was used to make [35S]UTP-labeled antisense and sense probes of mouse Lhcgr and Cyp11a1 cDNAs as previously described (1). Ovaries were fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections were rehydrated, treated with 20 μg/ml proteinase K, and incubated with radiolabeled riboprobe overnight at 55 C. The next day, slides were washed and dipped in photographic NTB-2 emulsion, exposed at 4 C for an appropriate length of time, developed with D-19 developer and fixer (Eastman Kodak Co, Rochester, NY), and stained with hematoxylin. Tissue histology was observed by light-field illumination, and dark-field illumination was used to visualize the mRNA probe.
TUNEL assay
Analysis of apoptosis in WT and mutant mouse ovaries was carried out by TUNEL assay using the ApopTag Plus in situ apoptosis detection kit according to the manufacturer's instructions (Chemicon International, Temecula, CA) and as reported previously (1).
GC culture, adenoviral infection, and plasmid transfection
GCs were harvested from eCG-primed (24 h) 23-d-old mice and cultured as described previously (14). After overnight culture, cells were washed and cultured in serum-free medium and infected with an adenoviral vector expressing C/EBPα or C/EBPβ (gifted from Dr. Jianhua Shao and amplified by the Vector Development Laboratory, Baylor College of Medicine) at a multiplicity of infection of 4:1. At 24 h after infection, GCs were treated with For (10 μm) and PMA (20 nm) for 24 h. Firefly luciferase activity was normalized to Renilla luciferase activity (cotransfected as an internal control) as previously.
Western blot analyses
Cell extracts containing 30 μg protein were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA) and analyzed as previously (18) using primary antibodies C/EBPα, C/EBPβ, and BHMT (1:1000 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
RNA isolation, microarray analyses, and real-time RT-PCR
WT and Cebpa/bgc−/− immature mice (23 d old) were treated with eCG/hCG as described above. At 4, 8, and 24 h after hCG treatment, GCs were isolated from the antral follicles of four mice of each genotype and were pooled together. Total RNA was isolated using the RNeasy Mini kit (Qiagen Sciences, Germantown, MD). WT and Cebpa/bgc−/− riboprobes were then hybridized to Mouse 430.2 microarray chips (Affymetrix, Santa Clara, CA) by the Microarray Core Facility of the Baylor College of Medicine (GEO datasets; CSE 23084) as previously described (54).
Reverse transcription was done using the SuperScript One-Step RT-PCR system with Platinum Taq kit (Invitrogen, Carlsbad, CA). The real-time PCR was performed using the Rotor-Gene 3000 thermocycler (Corbett Research, Sydney, Australia). Relative levels of mRNAs were calculated using Rotor-Gene 6.0 software and normalized to the levels of endogenous β-actin in the same samples.
Statistical analyses
The data are represented as means ± sd. Data were analyzed by using GraphPad Prism Programs (ANOVA or t test; GraphPad Prism, San Diego, CA) to determine significance. Values were considered significantly different if P ≤ 0.05 or P ≤ 0.01.
Supplementary Material
Acknowledgments
We thank Dr. Michael Mancini and members of the Microscopy Core of Baylor College of Medicine (Houston, TX) for their assistance in microscopy. We thank Dr. Jianhua Shao for the Cebpa and Cebpb adenoviral constructs and Dr. Esta Sterneck for comments on the manuscript. The BHMT inhibitors were gifts from Dr. Jiri Jiracek (Academy of Sciences of the Czech Republic).
Address all correspondence and requests for reprints to: JoAnne S. Richards, PhD. Professor, Department of Molecular and Cellular Biology, Baylor College of Medicine (BCM:MS130), Houston, Texas 77030. E-mail: joanner@bcm.tmc.edu.
This work was supported by: National Institutes of Health (NIH) Grants NIH-HD16229, NIH-HD07495 (SCCPIR), Project II (to J.S.R.), the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (to P.F.J.), and National Research Service Award NIH-HD058429-01A1 (to H.Y.F.).
The authors have nothing to disclose.
Footnotes
- AREG
- Amphiregulin
- BHMT
- betaine homocysteine methyltransferase
- CBHcy
- (R,S)-carboxybutylhomocysteine
- C/EBP
- CCAAT/enhancer-binding protein
- CL
- corpora lutea
- COC
- cumulus cell-oocyte complex
- DMG
- dimethalglycine
- eCG
- equine chorionic gonadotropin
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- For
- forskolin
- GC
- granulosa cell
- GVBD
- germinal vesicle breakdown
- PMA
- phorbol 12-myristate 13-acetate
- PRL
- prolactin
- TUNEL
- terminal deoxynucleotide transferase-mediated dUTP nick-end labeling
- WT
- wild type.
References
- 1. Fan HY, Liu Z, Cahill N, Richards JS. 2008. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol 22:2128–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richards JS, Jonassen JA, Rolfes AI, Kersey K, Reichert LE., Jr 1979. Cyclic AMP, luteinizing hormone receptor, and progesterone during granulosa cell differentiation: effects of estradiol and follicle stimulating hormone. Endocrinology 104:765–773 [DOI] [PubMed] [Google Scholar]
- 3. Richards JS, Midgley AR., Jr 1976. Protein hormone action: a key to understanding ovarian follicular and luteal cell development. Biol Reprod 14:82–94 [DOI] [PubMed] [Google Scholar]
- 4. Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M. 2002. Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology 143:2986–2994 [DOI] [PubMed] [Google Scholar]
- 5. Sharma SC, Richards JS. 2000. Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells: relation of JunD and Fra2 to terminal differentiation. J Biol Chem 275:33718–33728 [DOI] [PubMed] [Google Scholar]
- 6. Liu Z, Fan HY, Wang Y, Richards JS. 2010. Targeted disruption of Mapk14 (p38MAPKa) in granulosa cells and cumulus cells causes cell-specific changes in gene expression profiles that rescue cell-oocyte complex expansion and maintain fertility. Mol Endocrinol 24:1794–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. 2008. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicular development and ovulation. Development 135:2127–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimada M, Gonzalez-Robayna I, Hernandez-Gonzalez I, Richards JS. 2006. Paracrine and autocrine regulation of EGF-like factors in cumulus oocyte complexes and granulosa cells: key role for prostaglandin synthase 2 (Ptgs2) and progesterone receptor (Pgr). Mol Endocrinol 20:348–361 [DOI] [PubMed] [Google Scholar]
- 9. Conti M, Hsieh M, Park JY, Su YQ. 2006. Role of the EGF network in ovarian follicles. Mol Endocrinol 20:715–723 [DOI] [PubMed] [Google Scholar]
- 10. Panigone S, Hsieh M, Fu M, Persani L, Conti M. 2008. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22:924–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris JK, Richards JS. 1993. Hormonal induction of luteinization and prostaglandin endoperoxide synthase-2 involves multiple cellular signaling pathways. Endocrinology 133:770–779 [DOI] [PubMed] [Google Scholar]
- 12. Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. 2002. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 143:2221–2232 [DOI] [PubMed] [Google Scholar]
- 13. Park JY, Su YQ, Ariga M, Law E, Jin SLC, Conti M. 2004. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 290:395–398 [DOI] [PubMed] [Google Scholar]
- 14. Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. 2006. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365 [DOI] [PubMed] [Google Scholar]
- 15. Hsieh M, Conti M. 2005. G-protein-coupled receptor signaling and the EGF network in endocrine systems. Trends Endocrinol Metab 16:320–326 [DOI] [PubMed] [Google Scholar]
- 16. Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. 2007. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andric N, Thomas M, Ascoli M. 2010. Transactivation of the epidermal growth factor receptor is involved in the lutropin receptor mediated down regulation of ovarian aromatase expression in vivo. Mol Endocrinol 24:552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. 2009. MAPK3/1 (ERK½) in ovarian granulosa cells are essential for female fertility. Science 324:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sirois J, Richards JS. 1993. Transcriptional regulation of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Evidence for a role of a cis acting C/EBP β promoter element. J Biol Chem 268:21931–21938 [PubMed] [Google Scholar]
- 20. Sterneck E, Tassarollo L, Johnson PF. 1997. An essential role for C/EBPb in female reproduction. Genes Dev 11:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piontkewitz Y, Enerbäck S, Hedin L. 1993. Expression and hormonal regulation of the CCAAT enhancer binding protein-α during differentiation of rat ovarian follicles. Endocrinology 133:2327–2333 [DOI] [PubMed] [Google Scholar]
- 22. Piontkewitz Y, Enerbäck S, Hedin L. 1996. Expression of CCAAT enhancer binding protein-a (C/EBPa) in the rat ovary: implications for follicular development and ovulation. Dev Biol 179:288–296 [DOI] [PubMed] [Google Scholar]
- 23. Lydon JP, DeMayo F, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW. 1995. Mice lacking progesterone receptor exhibit reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- 24. Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. 2000. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA 97:4689–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS. 2003. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology 144: 3598–3610 Hormone regulation of SF2 (LHR-1. NR5A2) in the rodent ovary. in submission [DOI] [PubMed] [Google Scholar]
- 26. Duggavathi R, Volle DH, Mataki C, Antal MC, Messeddeq N, Auwerx J, Murphy BD, Schoonjans K. 2008. Liver receptor homolog 1 is essential for ovulation. Genes Dev 22:1871–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X, Kim JW, Grønborg M, Urlaub H, Lane MD, Tang QQ. 2007. Role of cdk2 in the sequential phosphorylation/activation of C/EBPb during adipocyte differentiation. Proc Natl Acad Sci USA 104:11597–11602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakajima T, Kinoshita S, Sasagawa T, Sasaki K, Naruto M, Kishimoto T, Akira S. 1993. Phosphorylation of threonine-235 by ras-dependent mitogen activated protein kinase cascade is essential for transcription factor NF-Il6. Proc Natl Acad Sci USA 90:2207–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mantena SR, Kannan A, Cheon YP, Li Q, Johnson PF, Bagchi IC, Bagchi MK. 2006. C/EBPb is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci USA 103:1870–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grimm SL, Rosen JM. 2003. The role of C/EBPβ in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia 135:191–204 [DOI] [PubMed] [Google Scholar]
- 31. Robinson GW, Johnson PF, Hennighausen L, Sterneck E. 1998. The C/EBP β transcription factor regulates epithelial proliferation and differentiation in the mammary gland. Genes Dev 12:1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramanthal C, Bagchi IC, Bagchi MK. 2010. Lack of CCAAT enhancer binding protein β (C/EBPb) in uterine epithelial cells impairs estrogen-induced DNA replication, induces DNA damage response pathways, and promotes apoptosis. Mol Cell Biol 30:1607–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang AM, Rudelius M, Sharan S, McAllister JM, Raffeld M, Christenson LK, Sterneck E. 2007. The Cebpd (C/EBPδ) gene is induced by luteinizing hormone in ovarian theca and interstitial cells but is not essential for mouse ovary function. PLoS One 2:e1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. 1995. Impaired energy homeostasis in C/EBP α knockout mice. Science 269:1108–1112 [DOI] [PubMed] [Google Scholar]
- 35. Lee YH, Sauer B, Johnson PF, Gonzalez FJ. 1997. Disruption of the c/ebp α gene in adult mouse liver. Mol Cell Biol 17:6014–6022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sugiura K, Su YQ, Eppig JJ. 2009. Targeted disruption of Has2 mRNa in mouse cumulus cell-oocyte complexes by adenovirus-mediated short-hairpin RNA expression. Mol Reprod Dev 76:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park ES, Lind AK, Dahm-Kähler P, Brännström M, Carletti MZ, Christenson LK, Curry TE, Jr, Jo M. 2010. RUNX2 transcription factor regulates gene expression in luteinizing granulosa cells of rat ovaries. Mol Endocrinol 24:846–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Son DS, Terranova PF, Roby KF. 2010. Interaction of adenosine 3′5′-cyclic monophosphate and tumor necrosis factor-a on serum amyloid A3 expression in mouse granulosa cells: dependence on CCAAT-enhancer binding protein-b isoform. Endocrinology 151:3407–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baumann C, Davies B, Peters M, Kaufmann-Reiche U, Lessl M, Theuring F. 2007. AKR1B7 (mouse vas deferens protein) is dispensible for mouse development and reproductive success. Reproduction 134:97–109 [DOI] [PubMed] [Google Scholar]
- 40. Gibori G. 1993. The corpus luteum of pregnancy. New York: Raven Press [Google Scholar]
- 41. Stocco C, Telleria C, Gibori G. 2007. The molecular control of corpus luteum formation, function and regression. Endocr Rev 28:117–149 [DOI] [PubMed] [Google Scholar]
- 42. Bachelot A, Beaufaron J, Servel N, Kedzia C, Monget P, Kelly PA, Gibori G, Binart N. 2009. Prolactin independent rescue of mouse corpus luteum life span: identification of prolactin and luteinizing hormone target genes. Am J Physiol Endocrinol Metab 297:E676–E684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dayal S, Lentz SR. 2008. Murine models of hyperhomocysteinemia and their vascular phenotypes. Arterioscler Thromb Vasc Biol 28:1596–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pajares MA, Pérez-Sala D. 2006. Betain homocysteine S-Methyltransferase: just a regulator of homocysteine metabolism? Cell Mol Life Sci 63:2792–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Timchenko NA. 2009. Aging and liver regeneration, Trends Endocrinol Metab :171–176 [DOI] [PubMed] [Google Scholar]
- 47. Tang QQ, Otto TC, Lane MD. 2003. CCAAT/enhancer-binding protein β is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci USA 100:850–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pabst T, Mueller BU. 2007. Transcriptional dysregulation during myeloid transformation in AML. Oncogene 26:6829–6837 [DOI] [PubMed] [Google Scholar]
- 49. Oonk RB, Krasnow JS, Beattie WG, Richards JS. 1989. Cyclic AMP-dependent and -independent regulation of cholesterol side-chain cleavage P450 (P450scc) in rat ovarian granulosa cells and corpora lutea: cDNA and deduced amino acid sequence of rat P450scc. J Biol Chem 264:21934–21942 [PubMed] [Google Scholar]
- 50. Huang JH, Liao WS. 1994. Induction of the mouse serum amyloid A3 gene by cytokines requires both C/EBP family proteins and a novel constitutive nuclear factor. Mol Cell Biol 14:4475–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dajee M, Fey GH, Richards JS. 1998. Stat 5b and the orphan nuclear receptors regulate expression of the a2-macroglobulin (a2M) gene in rat ovarian granulosa cells. Mol Endocrinol 12:1393–1409 [DOI] [PubMed] [Google Scholar]
- 52. Liu Z, de Matos DG, Fan HY, Shimada M, Palmer S, Richards JS. 2009. IL6: an autocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinology 150:3360–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Poli V. 1998. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem 273:29279–29282 [DOI] [PubMed] [Google Scholar]
- 54. Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. 2006. Gene expression profiles of cumulus cell oocyte complexes (COCs) during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol 20:1300–1321 [DOI] [PubMed] [Google Scholar]
- 55. Richards JS. 2007. Genetics of ovulation. Semin Reprod Med 25:235–242 [DOI] [PubMed] [Google Scholar]
- 56. Richards JS, Pangas SA. 2010. The ovary: basic biology and clinical implications. J Clin Invest 120:963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Burkart AD, Mukherjee A, Sterneck E, Johnson PF, Mayo KE. 2005. Repression of the inhibin α-subunit gene by the transcription factor CCAAT/enhancer-binding protein-β. Endocrinology 146:1909–1921 [DOI] [PubMed] [Google Scholar]
- 58. Silverman E, Yivgi-Ohana N, Sher N, Bell M, Elmerl S, Orly J. 2006. Transcriptional activation of sterodogenic acute regulatory protein (StAR) gene: GATA4 and CCAAT/enhancer binding protein β confer synergistic responsiveness in hormone-treated rat granulosa cells and HEK293 cell models. Mol Cell Endocrinol 252:92–101 [DOI] [PubMed] [Google Scholar]
- 59. Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, Naito AT, Nishi J, Ueno H, Umezawa A, Minamino T, Nagai T, Kikuchi A, Asashima M, Komuro I. 2008. IGFBP-4 is an inhibitor of canonical WNT signaling required for cardiogenesis. Nature 454:345–349 [DOI] [PubMed] [Google Scholar]
- 60. Zhou J, Wang J, Penny D, Monget P, Arraztoa JA, Fogelson LJ, Bondy CA. 2003. Insulin-like growth factor binding protein 4 expression parallels luteinizing hormone receptor expression and follicular luteinization in the primate ovary. Biol Reprod 69:22–29 [DOI] [PubMed] [Google Scholar]
- 61. Rahkonen OP, Koskivirta IM, Oksjoki Sm, Jokinen E, Vuorio EI. 2002. Characterization of the murine Timp4 gene, localization within intron 5 of the synapsin 2 gene and tissue distribution of the mRNA. Biochim Biophy Acta 19:45–52 [DOI] [PubMed] [Google Scholar]
- 62. Robinson RS, Woad KJ, Hammon AJ, Laird M, Hunter MG, Mann GE. 2009. Angiogenesis and vascular function in the ovary. Reproduction 138:869–881 [DOI] [PubMed] [Google Scholar]
- 63. Fraser HM. 2006. Regulation of ovarian follicular vasculature. Reprod Biol Endocrinol 4:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Patel VA, Dunn MJ, Sorokin A. 2002. Regulation of MDR-1 (P-glycogprotein) by cyclooxygenase-2. J Biol Chem 277:38915–38920 [DOI] [PubMed] [Google Scholar]
- 65. Finstad CL, Saigo PE, Rubin SC, Federici MG, Provencher DM, Hoskins WJ, Lewis JL, Jr, Lloyd KO. 1990. Immunohistochemical localization of P-glycoprotein in adult human ovary and female genital tract patients with benign gynecological conditions. J Histochem Cytochem 38:1677–1681 [DOI] [PubMed] [Google Scholar]
- 66. Shimizu T, Kosaka N, Murayama C, Tetsuka M, Miyamoto A. 2009. Apelin and APJ receptor expression in granulosa cells and theca cells during different stages of follicular development in the bovine ovary: involvement of apoptosis and hormonal regulation. Anim Reprod Sci 116:28–37 [DOI] [PubMed] [Google Scholar]
- 67. Adams RH, Eichmann A. 2010. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol 2:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Matsubara A, Iwama A, Yamazaki S, Furuta C, Hirasawa R, Morita Y, Osawa M, Motohashi T, Eto K, Ema H, Kitamura T, Vestweber D, Nakauchi H. 2005. Endomucin, a CD-34 like sialomucin, marks hematopoietic stem cells throughout development. J Exp Med 202:1483–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kinoshita M, Nakamura T, Ihara M, Haraguchi T, Hiraoka Y, Tashiro K, Noda M. 2001. Identification of human endomucin-1 and-2 as membrane-bound O-sialoglycoproteins wit anti-adhesive activity. FEBS Lett 499:121–126 [DOI] [PubMed] [Google Scholar]
- 70. De Pittà C, Tombolan L, Campo Dell'Orto M, Accordi B, te Kronnie G, Romualdi C, Vitulo N, Basso G, Lanfranchi G. 2005. A leukemia-enriched cDNA microarray platform identifies new transcripts with relevance to the biology of pediatric acute lymphoblastic leukemia. Haematologica 90:890–898 [PubMed] [Google Scholar]
- 71. Sterneck E, Zhu S, Ramirez A, Jorcano JL, Smart RC. 2006. Conditional ablation of CEBPb demonstrates its keratinocyte-specific requirement for cell survival and mouse skin tumorigenesis. Oncogene 25:1272–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pagès G, Guèrin S, Grall D, Bonio F, Smith A, Anjuere F, Auberger P, Pouysségur J. 1999. Defective thymocyte maturaton in p44 MAP kinase (Erk1) knockout mice. Science 286:1374–1377 [DOI] [PubMed] [Google Scholar]
- 73. Fischer AM, Katayama CD, Pagès G, Pouysseg´ur J, Hedrick SM. 2005. The role of Erk1 and Erk2 in multiple stages of T cell development. Immunity 23:431–443 [DOI] [PubMed] [Google Scholar]
- 74. Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. 1990. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol 138:16–25 [DOI] [PubMed] [Google Scholar]
- 75. Fagbohun CF, Downs SM. 1990. Maturation of the mouse oocyte-cumulus cell comples: stimulation by lectins. Biol Reprod 42:413–423 [DOI] [PubMed] [Google Scholar]
- 76. Nautiyal J, Steel JH, Rosell MM, Nikolopoulou E, Lee K, Demayo FJ, White R, Richards JS, Parker MG. 2010. The nuclear receptor cofactor-interacting protein 140 is a positive regulator of amphiregulin expression and cumulus cell-oocyte expansion in the mouse ovary. Endocrinology 151:2923–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cottom J, Salvador LM, Maizels ET, Reierstad S, Park Y, Carr DW, Davare MA, Hell JW, Palmer SS, Dent P, Kawakatsu H, Ogata M, Hunzicker-Dunn M. 2003. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol Chem 278:7167–7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.