In vivo ablation of the interaction between NCoR and the TR leads to increased peripheral sensitivity to thyroid hormone and resets the HPT axis.
Abstract
The role of nuclear receptor corepressor (NCoR) in thyroid hormone (TH) action has been difficult to discern because global deletion of NCoR is embryonic lethal. To circumvent this, we developed mice that globally express a modified NCoR protein (NCoRΔID) that cannot be recruited to the thyroid hormone receptor (TR). These mice present with low serum T4 and T3 concentrations accompanied by normal TSH levels, suggesting central hypothyroidism. However, they grow normally and have increased energy expenditure and normal or elevated TR-target gene expression across multiple tissues, which is not consistent with hypothyroidism. Although these findings imply an increased peripheral sensitivity to TH, the hypothalamic-pituitary-thyroid axis is not more sensitive to acute changes in TH concentrations but appears to be reset to recognize the reduced TH levels as normal. Furthermore, the thyroid gland itself, although normal in size, has reduced levels of nonthyroglobulin-bound T4 and T3 and demonstrates decreased responsiveness to TSH. Thus, the TR-NCoR interaction controls systemic TH sensitivity as well as the set point at all levels of the hypothalamic-pituitary-thyroid axis. These findings suggest that NCoR levels could alter cell-specific TH action that would not be reflected by the serum TSH.
Undiagnosed hypo- or hyperthyroidism affects more than 10% of the American population including 5% of Americans who are on some form of thyroid hormone (TH) replacement (1). In all cases measurement of the serum TSH dictates both the diagnosis of hypo- and hyperthyroidism and serves as an indicator of the effectiveness of therapy. The utility of TSH as a unique biomarker that interprets systemic TH action is based on the ability of circulating TH to feed back at the level of both the TRH neurons in the hypothalamus and the thyrotrophs in the pituitary to regulate TSH production and secretion (2). It is presumed that in the vast majority of individuals, the cellular actions of TH at the level of the hypothalamus and pituitary are equivalent to its actions in all other cells and tissues, and thus, the TSH provides the best indication of systemic TH action. However, because TH controls TSH secretion by negatively regulating the trh and tsh subunit genes at the level of transcription it remains possible that this mechanism is separate from the one by which TH regulates positive target genes in other cell types. Thus, a strict understanding of the molecular mechanism by which TH controls the set point of TSH secretion will allow for a better understanding of its interpretation as a biomarker of TH action.
TH, principally through its active form T3, controls gene expression via the TH receptor isoforms (TRs) (3–5). On genes that are positively regulated by T3, the unliganded TRs are potent repressors of transcription due to their ability to recruit the nuclear corepressors, nuclear receptor corepressor (NCoR) and silencing mediator of retinoid and thyroid receptors (SMRTs), which in turn recruit a multiprotein complex that includes histone deacetylase 3 and allows for histone deacetylation (6–9). In the presence of T3, the corepressors are released and coactivators are recruited that allow for transcriptional activation (4, 10). In contrast, on negatively regulated genes, such as trh and the tsh subunit genes, the TR activates transcription in the absence of T3 and represses in the presence of the hormone. The mechanisms involved in ligand-independent activation by the TR are not known, although deletion of the steroid receptor coactivator (SRC)-1 as well as mutations in TRβ isoform that abolish coactivator binding in mice prevent normal negative regulation of TSH expression by T3, which suggests that coactivators may play a paradoxical role in T3-mediated repression (11–13).
To gain better insight into TH action and corepressor function, we recently developed a mouse model that expressed in a liver-specific manner a mutant NCoR protein (NCoRΔID) that could not interact with the TR. These mice demonstrated that NCoR plays a specific and sufficient role in transcriptional repression by the TR in the absence of T3 and established that NCoR also mediates T3 sensitivity on positive TR targets (14). The ability of NCoR to mediate ligand-dependent sensitivity in context of nuclear receptor signaling is supported by two other mouse models that also possess defective NCoR or SMRT function (15, 16).
To better understand the role of NCoR in global TH signaling, we have now developed mice that express the NCoRΔID in all tissues. These mice appear normal but have low circulating TH concentrations. Despite this, their serum TSH is not elevated compared with the wild-type controls, and markers of TH action in peripheral tissues do not indicate hypothyroidism. These results suggest that the loss of functional NCoR allows for the hypothalamic-pituitary-thyroid (HPT) axis to be reset in the face of increased peripheral sensitivity to TH. Thus, NCoR function mediates TH sensitivity in vivo and can alter the central set point of the thyroid axis.
Results
NCoRΔID mice develop normally but have altered thyroid function tests
To determine the function of NCoR in T3 signaling, we generated mice that express the NCoRΔID protein in all tissues. This was achieved by crossing NCoRlox/lox mice (14) with Zp3-Cre mice that express (Cre) recombinase under control of the zona pellucida gene regulatory elements in the developing oocyte (Supplemental Fig. 1A). Mice homozygous for the NCoRΔID allele (NCoRΔID mice) are born at expected Mendelian ratios and develop normally. The expression of NCoRΔID was confirmed in a variety of tissues by Western analysis using a specific C-terminal antibody that recognizes both NCoR and the smaller NCoRΔID (Supplemental Fig. 1B). Thus, whereas complete loss of NCoR leads to embryonic lethality, the region surrounding and including the third and second interacting domains (N3 and N2) of NCoR is not required for normal development.
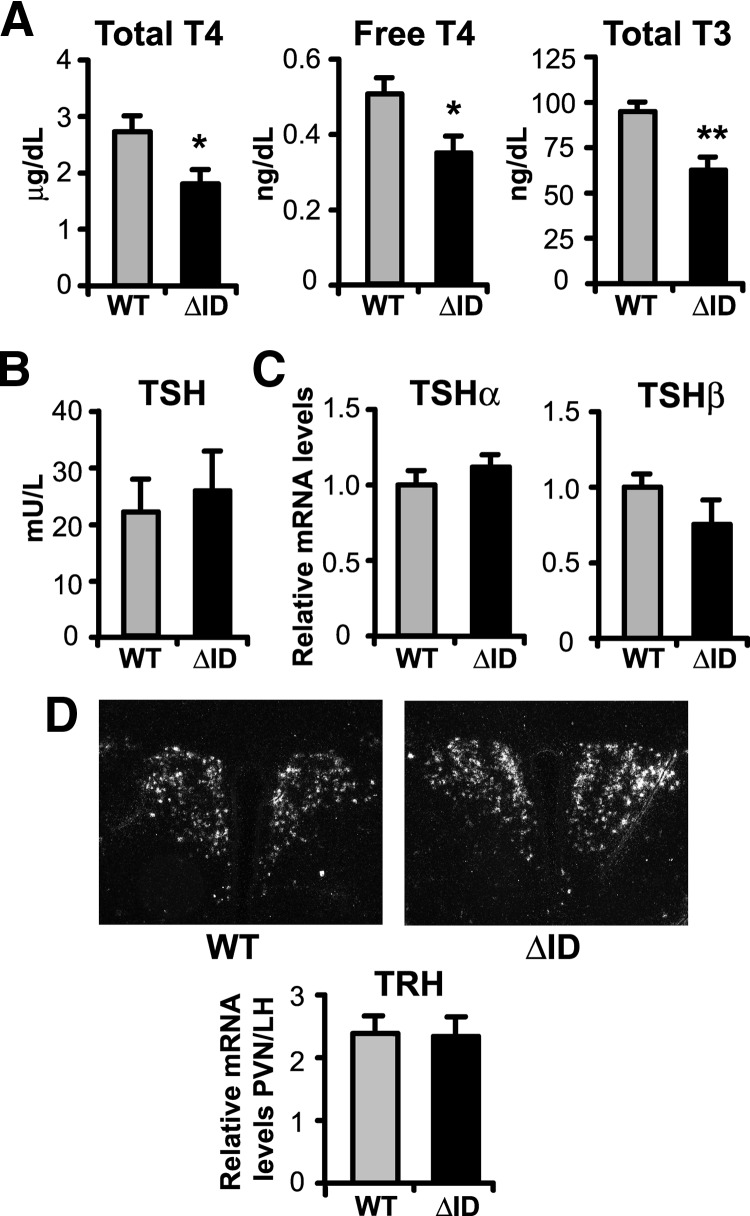
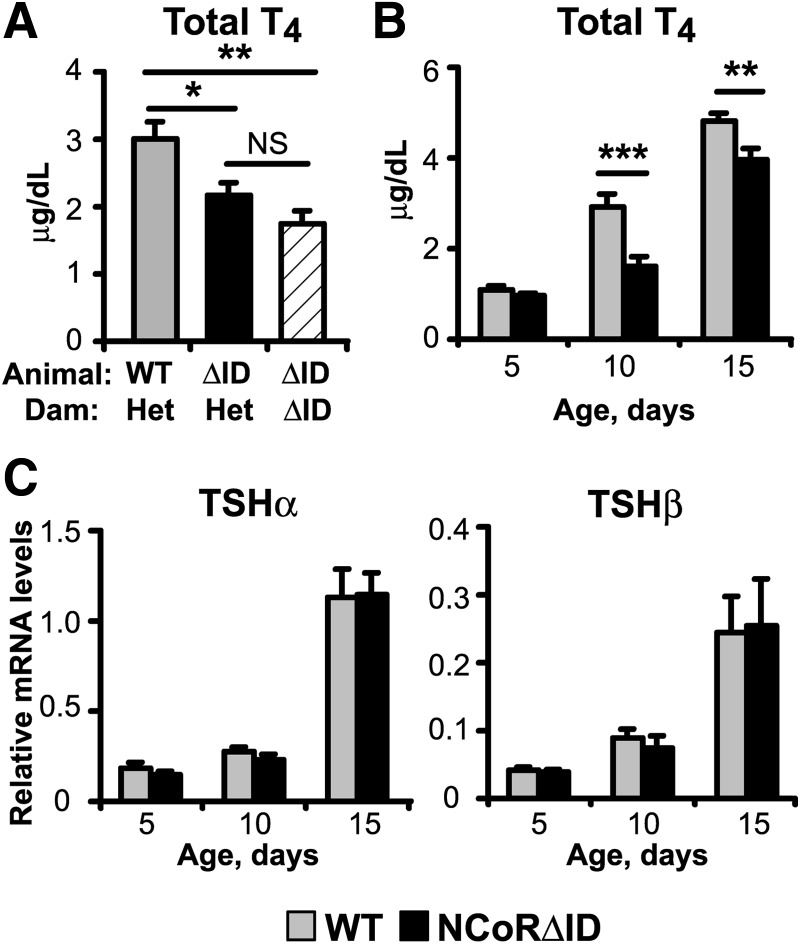
To assess whether the presence of NCoRΔID has a global effect on T3 signaling, we first measured thyroid function in both wild type (WT) and NCoRΔID mice. Remarkably, as shown in Fig. 1A, male NCoRΔID mice have 30% reductions in both their total T4 and total T3 levels compared with WT controls. To rule out a defect in serum TH binding proteins, we measured free T4 levels and found them to be low in NCoRΔID mice as well. Similarly, low TH levels are seen in female NCoRΔID mice (data not shown). Despite the low circulating TH concentrations, NCoRΔID mice have serum TSH levels that are identical to control animals (Fig. 1B). Similarly, NCoRΔID mice demonstrate unaltered expression of TSHα and TSHβ mRNA in the pituitary and TRH mRNA in hypophysiotrophic neurons in the paraventricular hypothalamus (PVH), as compared with WT mice (Fig. 1, C and D). Taken together, the thyroid function panel and analysis of gene expression in the pituitary and hypothalamus in NCoRΔID mice is most consistent with central hypothyroidism because normally such a decrease in circulating TH levels should lead to significant elevation of serum TSH and both tsh subunit and trh gene expression (17).
Fig. 1.
NCoRΔID mice have reduced circulating TH levels accompanied by normal serum TSH and normal TSH and TRH mRNA expression. A, Serum total T4, free T4, and total T3 were measured by RIA in male adult WT and NCoRΔID mice (n = 6–10 per group). B, Serum TSH was measured in the same groups of animals by high-affinity RIA (n = 6–7 per group). C, TSH subunit mRNA levels in pituitary of male adult mice were quantified by quantitative PCR. mRNA levels are expressed relative to WT group (n = 7 animals per group). D, ISH was performed on formalin-fixed brain sections from male mice with indicated genotypes using a 35S-labeled riboprobe against mouse TRH mRNA. Shown are representative images of the PVH at original magnification, ×10. The TRH mRNA expression was quantified using Image J software. Data are presented as relative pixel densities in PVH normalized to lateral hypothalamus (LH) (n = 5 animals per group). Data are presented as mean ± sem. *, P < 0.05; **, P < 0.01.
NCoRΔID mice have normal growth and enhanced energy expenditure
To determine whether NCoRΔID mice demonstrate a phenotype similar to other known mouse models of central hypothyroidism, we assessed their growth. NCoRΔID mice are identical in size to the littermate controls at birth and grow in a similar fashion (Fig. 2A), which is supported by pituitary GH mRNA levels that are equal to the WT controls (see Fig. 6C). In contrast, TRH, TRH receptor-1, and type 3 deiodinase knockout mice, well-described models of central hypothyroidism (with circulating TH levels similar to NCoRΔID mice but with elevated levels of TSH), demonstrate significant growth delay (18–20).
Fig. 2.
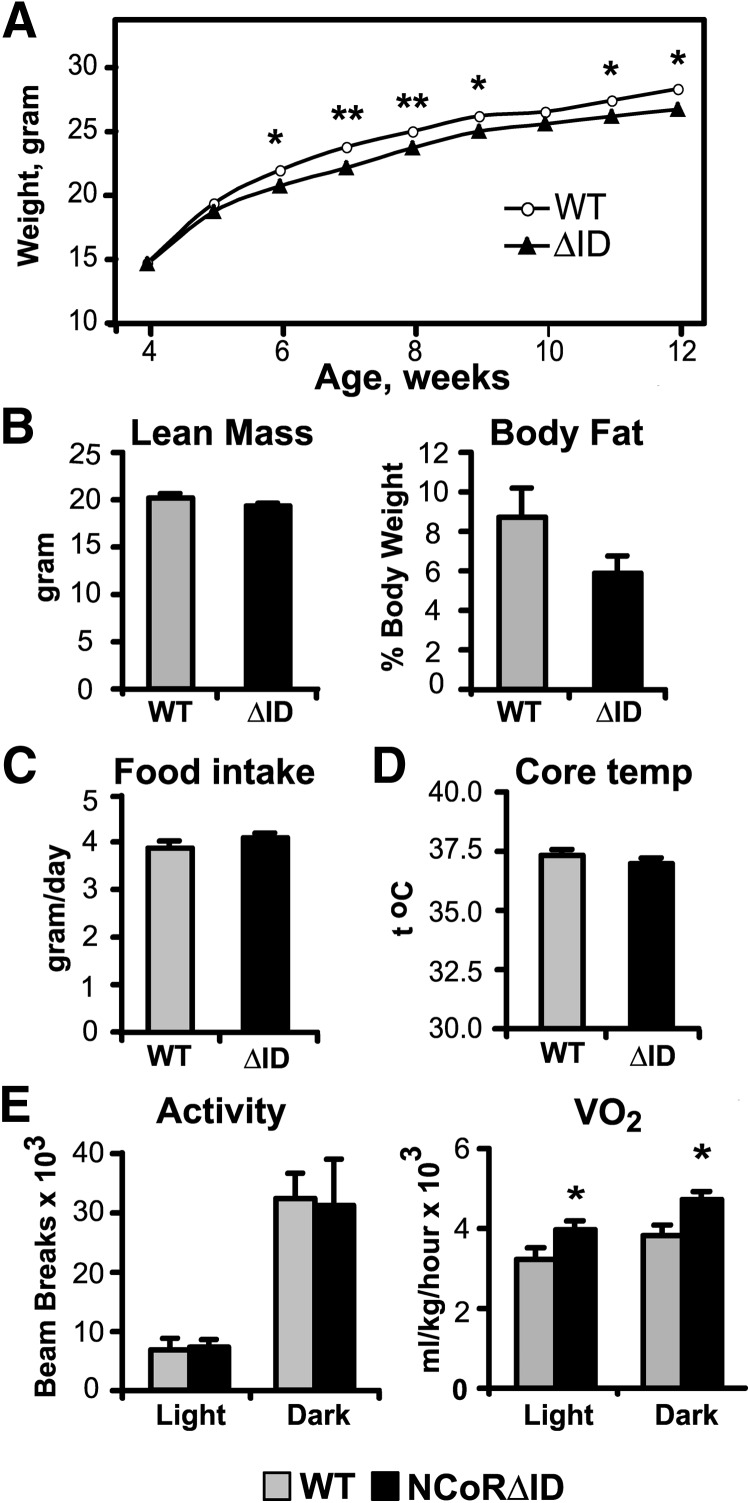
Energy homeostasis in NCoRΔID mice. A, BWs of male WT and NCoRΔID mice were measured weekly. Each point represents the mean ± sem of measurements in six to 20 animals. B, Body composition was measured in male mice of indicated genotypes by echo MRI at 17–19 wk of age. C, Food intake was recorded daily for 4 wk starting at 13–15 wk of age. D, Core body temperatures were measured using rectal probe at 1700 h for 3 consecutive days. E, Physical activity, oxygen consumption, and CO2 production were measured in 12- to 14-wk-old mice in a CLAMS apparatus at 14-h light, 10-h dark cycle. Shown are means of values collected over two cycles ± sem. Experiments in B–E were performed on the same groups of male mice (n = 6–8 animals per group).
Fig. 6.
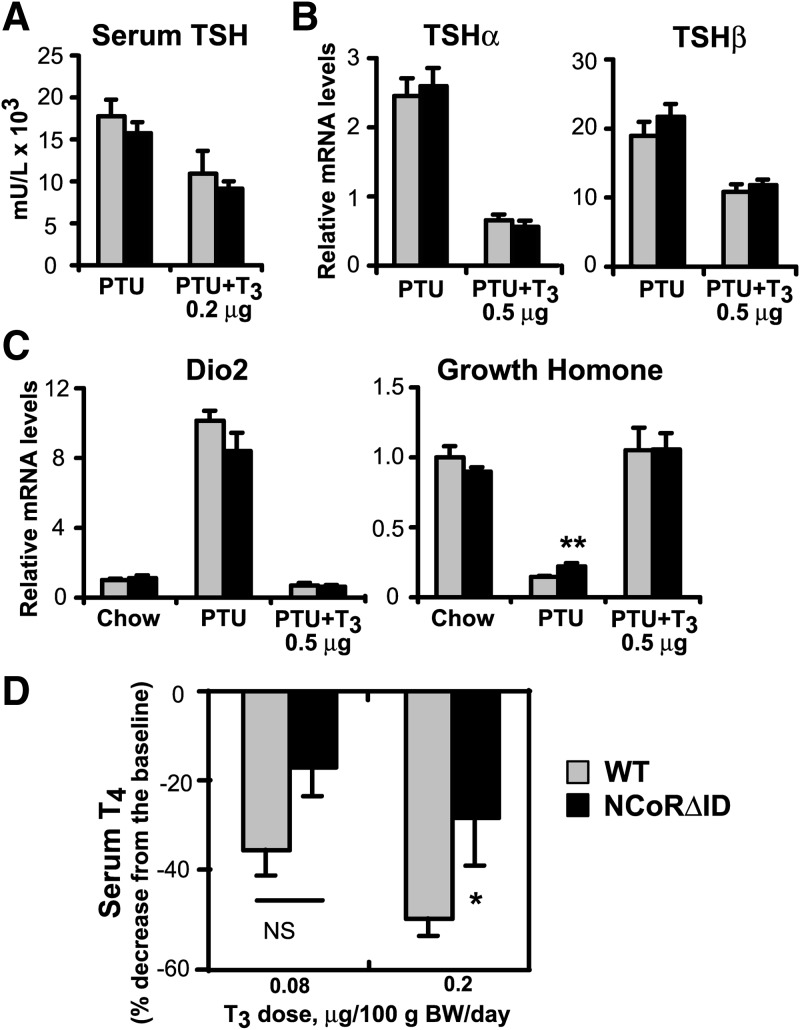
The HPT axis of NCoRΔID mice responds normally to changes in serum TH level. A, Serum TSH was measured by high-affinity RIA in hypothyroid (PTU) and low T3 replacement dose groups (n = 3–7 animals per group). B, TSH subunit mRNA levels were quantified by quantitative PCR in hypothyroid and physiological T3 replacement dose groups. mRNA levels are expressed relative to WT euthyroid animals (set to 1) (n = 5–7 animals per group). C, Expression of known TH targets in the pituitary of NCoRΔID mice at different circulating T3 levels. mRNA expression levels were quantified by quantitative PCR and presented relative to euthyroid WT group (set to 1) (n = 5–7 animals per group). D, Feedback suppression of thyroid function by exogenous T3. WT and NCoRΔID mice were given injections of two indicated incremental doses of T3 for 4 d each. Total serum T4 levels were measured at the end of each treatment period, and the decrease was expressed as a percentage of the baseline obtained in the same animals before the treatment (n = 7–9 animals per group). Data are presented as mean ± sem; * P < 0.05; **P < 0.01.
Beginning around 6 wk of age, NCoRΔID mice show a slight but significant reduction in body weight (BW) compared with WT controls (Fig. 2A). At the same time, the animals have normal lean body mass and a tendency for a lower body fat content accompanied by normal food intake and core body temperature (Fig. 2, B–D). Using indirect calorimetry in a comprehensive laboratory animal monitoring system (CLAMS), we found that NCoRΔID mice show significantly increased oxygen consumption and normal physical activity (Fig. 2E), demonstrating increased energy expenditure, which likely explains their leanness. This finding is in contrast with the low serum TH levels because hypothyroidism is known to be associated with decreased energy expenditure (21, 22).
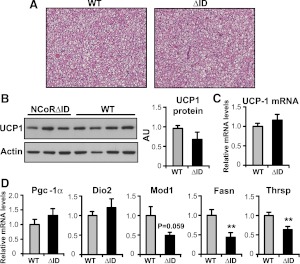
To further explore the changes in energy expenditure in NCoRΔID mice, we examined their brown adipose tissue (BAT), the major site of adaptive thermogenesis in rodents, a process in which T3 has been shown to play an important role. BAT is known to show signs of increased stimulation during cold exposure as well as in hypothyroidism and in animals that lack all TR isoforms (21, 23–25). Histological analysis of intrascapular BAT pads in NCoRΔID mice showed no signs of increased stimulation such as decreased lipid content or more acidophilic cytoplasm, compared with WT controls (Fig. 3A). Also, as shown in Fig. 3, B and C, protein and mRNA expression levels of uncoupling protein 1 (UCP-1), which is a known TRβ target gene and is essential for thermogenesis (26), were normal in NCoRΔID mice despite the low TH levels in these animals. Similarly, other signature genes that would indicate activation of BAT including pgc-1α and dio2 are also expressed normally in NCoRΔID mice (Fig. 3D). We also looked at expression of lipogenic genes because hypothyroid animals are known to activate lipolysis and lipogenesis in BAT (27, 28). Although lipogenesis and lipogenic enzymes such as fasn as well as thrsp, Spot 14 are known to be positively and directly regulated by TH in the liver and isolated brown adipocytes, they have been shown, in contrast, to be up-regulated in BAT of hypothyroid animals as a secondary effect of systemic hypothyroidism, presumably to activate adaptive thermogenesis to compensate for the decreased heat production in the face of a low metabolic rate (29, 30). In NCoRΔID mice mRNA expression of both thrsp and fasn were significantly reduced compared with WT controls, and expression of malic enzyme (mod1) trended in that direction (Fig. 3D). Thus, BAT of NCoRΔID animals does not show any signs of activation, which taken together with down-regulation of lipogenic enzymes likely reflects a reduced need for BAT-mediated thermogenesis due to the increased energy expenditure in other TH target tissues in these mice.
Fig. 3.
BAT of NCoRΔID mice does not show signs of activation. A, Representative images of hematoxylin and eosin staining of 5-μm sections of intrascapular BAT pads of WT and NCoRΔID mice. Original magnification ×20. B and C, Expression of UCP-1 protein and mRNA in BAT of NCoRΔID mice. B, Whole-cell protein extracts were isolated from BAT of NCoRΔID and WT mice and subjected to Western analysis with anti-UCP-1 and actin (loading control) antibodies. The images were quantified using ImageJ software and expressed relative to WT. C, UCP-1 mRNA expression levels in BAT were quantified by quantitative PCR, and expressed relative to the WT values. D, mRNA expression of other TH targets and lipogenic genes in BAT of NCoRΔID mice. mRNA levels were quantified by quantitative PCR and expressed relative to WT controls (n = 6 animals per group). All data are presented as mean ± sem. Significant differences compared with WT group: *, P < 0.05; **, P < 0.01.
NCoRΔID mice have increased peripheral sensitivity to TH
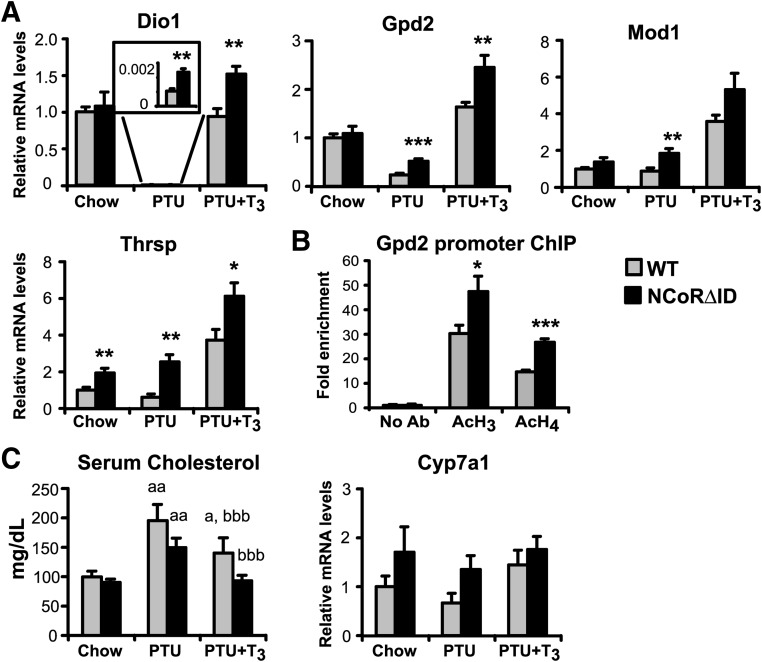
To further address tissue sensitivity to TH in NCoRΔID mice, we evaluated TH signaling in the liver and heart in cohorts of euthyroid (fed normal chow) and hypothyroid [fed low iodine/propylthiouracil (PTU) diet] animals, as well as a third cohort in which T3 levels were equalized between NCoRΔID and WT groups (see Materials and Methods) (Supplemental Fig. 2). As shown in Fig. 4, hepatic mRNA expression of known positive TH targets in the euthyroid state is either normal (dio1, gpd2, and mod1) or increased (thrsp) in NCoRΔID mice despite their low levels of circulating TH. In the hypothyroid state, each of the tested genes was activated or de-repressed in NCoRΔID mice, consistent with the obligate role of NCoR in the repression of positive targets in the hypothyroid state in liver. In the cohort with identical circulating T3 levels, most tested genes (except mod1 and cyp7a) showed elevated mRNA expression in NCoRΔID as compared with WT mice (Fig. 4A), consistent with enhanced hepatic sensitivity to TH. The inability of the TR to recruit NCoR to target promoters should prevent histone deacetylation and thus lead to activation of gene expression. Indeed, a chromatin immunoprecipitation (ChIP) assay (Fig. 4B) clearly demonstrates increased histone acetylation of the gpd2 promoter in the hypothyroid NCoRΔID mice as compared with the WT mice.
Fig. 4.
NCoRΔID mice demonstrate increased hepatic sensitivity to TH. A, mRNA expression of positive TR targets in the livers of WT and NCoRΔID mice in euthyroid (chow) and hypothyroid conditions (PTU) and with physiological T3 replacement (0.5 μg per 100 g of BW) (PTU + T3). mRNA expression levels were quantified by quantitative PCR and presented relative to euthyroid WT group (set to 1) (n = 5–7 animals per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001. B, ChIP analysis was performed using anti-acetyl histone H3 and anti-acetyl histone H4 antibodies on chromatin from livers of hypothyroid WT and NCoRΔID mice. The proximal gpd2 promoter was amplified using quantitative PCR. C, Effects of hypothyroidism and T3 replacement on serum cholesterol levels and Cyp7a1 mRNA expression in WT and NCoRΔID mice (n = 7–8 animals per group); a, significantly different from the same genotype on chow, b, significantly different from the same genotype on PTU diet. All values are presented as mean ± sem. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To confirm the enhanced sensitivity to TH at a physiological level, we also examined the response of serum cholesterol to T3 treatment in hypothyroid NCoRΔID and WT mice. Indeed, although T3 replacement significantly lowered serum cholesterol in both hypothyroid NCoRΔID and WT animals, the cholesterol levels were completely normalized only in NCoRΔID mice and remained elevated in WT animals. This effect, however, does not appear to be mediated by Cyp7a1 because we did not detect any differences in its mRNA expression between WT and NCoRΔID animals (Fig. 4C).
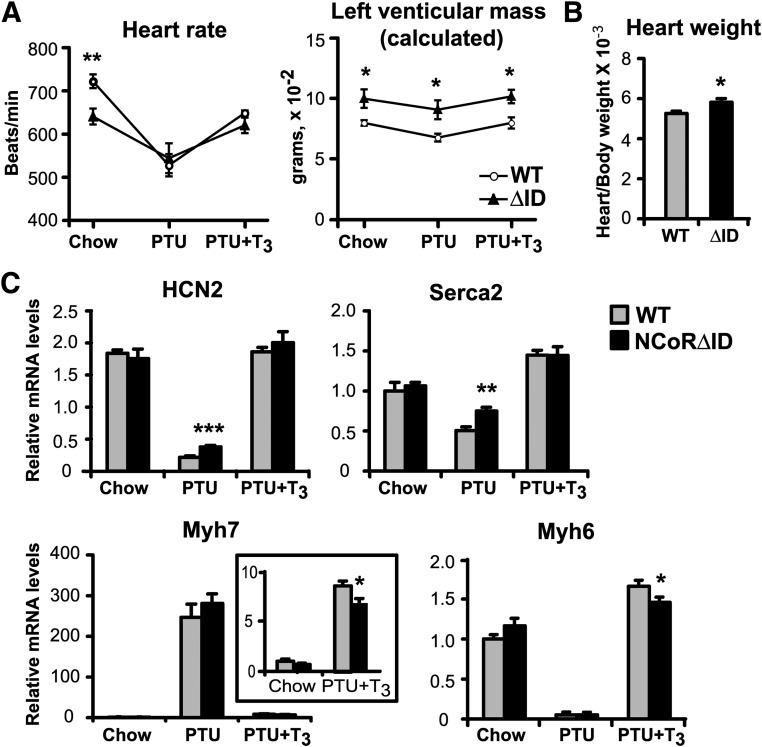
To evaluate TH sensitivity in the heart, we examined heart rate (HR) and left ventricular (LV) mass, well-known biomarkers of TH action (31, 32), by echocardiography in unanesthetized animals. Unlike the liver of NCoRΔID mice, which responded with enhanced sensitivity to T3 in all respects, the hearts of these animals showed significant bradycardia (610 vs. 710 bpm in WT mice) in the basal state (Fig. 5A), indicating that the hearts sense the low levels of circulating TH present in NCoRΔID mice. Furthermore, NCoRΔID animals responded appropriately to changes in TH levels with further bradycardia in hypothyroidism and appropriate normalization of HR with T3 treatment. In both the hypothyroid and replacement states, identical T3 levels in WT and NCoRΔID mice resulted in equal HR. Thus, NCoRΔID mice sense TH levels normally in the context of the genetic program mediating chronotropic effects of TH.
Fig. 5.
Hearts of NCoRΔID mice display mixed phenotype in terms of TH responsiveness. A, HR and LV were measured by echocardiography in WT and NCoRΔID mice in euthyroid (chow) and hypothyroid (PTU) conditions and at physiological T3 replacement dose (0.5 μg per 100 g) (PTU + T3) (n = 4–7 animals per group). B, Animals with indicated genotypes were killed after 4 d of physiological T3 replacement, and heart weights were measured and normalized to BWs (n = 7 per group). C, Expression of known positive and negative TH target genes at different circulating T3 levels in the hearts of WT NCoRΔID. mRNA expression levels were quantified by quantitative PCR and presented relative to euthyroid WT group (set to 1) (n = 5–7 animals per group). All values are presented as mean ± sem. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Surprisingly, whereas NCoRΔID mice appear to be hypothyroid in the context of HR, we found evidence of increased LV mass in NCoRΔID mice in all conditions by echocardiography (Fig. 5A). Indeed, an increase in LV mass in mice is known to be secondary to hyperthyroidism (33, 34), suggesting that even though low TH levels are being sensed by the mechanisms that control HR, the genetic program controlling LV mass senses these levels as elevated. The results of echocardiography were confirmed by direct measurements of the heart weights in T3-replaced mice (Fig. 5B). Thus, the heart in NCoRΔID mice has characteristics of both hypo- and hyperthyroidism. Interestingly, the TRα1 isoform is reported to control HR, whereas the TRβ1 isoform controls cardiac mass and hypertrophy (35, 36). This suggests that NCoR could have isoform-specific effects in context of the regulation of HR. Despite the physiological changes seen in HR and LV mass, we saw little change in cardiac-specific gene expression despite the low TH levels in NCoRΔID mice consistent with increased sensitivity to TH. Hcn2 has been reported to be important in setting HR and is a known T3 target (37) but is not different in the euthyroid setting and is slightly derepressed in the hypothyroid state. Similarly, serca-2 is derepressed in the hypothyroid state in NCoRΔID mice but not different in the euthyroid or T3-replaced states. There were no obvious changes in the regulation of the α and β myosin heavy-chain genes (myh6 and myh7 respectively) as well. (Fig. 5C).
The HPT axis is reset in NCoRΔID mice
Given that NCoRΔID mice have low circulating TH levels with evidence of peripheral hyperthyroidism, we next examined the HPT axis to determine why TSH levels remain normal in NCoRΔID mice. As shown in Fig. 6 (A and B), the induction of hypothyroidism leads to a dramatic rise of serum TSH and tsh subunit gene expression that is not different between NCoRΔID and WT mice. TRH mRNA was also similarly up-regulated in the PVH (data not shown). Thus, the N3 and N2 IDs of NCoR do not appear to be required for ligand-independent activation of these negative TR targets. Surprisingly, serum TSH and the tsh subunit genes also responded to T3 replacement in a similar fashion in NCoRΔID and WT mice, indicating that the hypothalamus and pituitary may not be more sensitive to T3 as opposed to peripheral tissues (Fig. 6, A and B). We also found that mRNA expression of two other pituitary TH target genes, dio2 and gh, is normal in NCoRΔID mice in all conditions, with gh being de-repressed in the hypothyroid state (Fig. 6C).
We next interrogated mRNA expression levels of TR isoforms and a number of coregulators in the pituitary of WT and NCoRΔID mice to determine whether the presence of NCoRΔID could affect tsh subunit gene expression indirectly by altering expression of these key regulators of transcription. Although we found the majority of coregulators to be regulated by the thyroid status, their expression did not vary between WT and NCoRΔID mice with the exception of SRC-3, which was slightly down-regulated in NCoRΔID animals (Table 1). Interestingly, we found that TRβ1 and TRβ2 mRNA levels were significantly reduced in NCoRΔID mice, which clearly did not affect their function. Importantly, it has been previously shown that mice with resistance to TH have increased mRNA levels of TRβ isoforms in the pituitary (13). Therefore, it appears that the changes in trb expression compensate for the changes in sensitivity to TH.
Table 1.
mRNA expression of coregulators and TR isoforms in the pituitary of WT and NCoRΔID mice with different TH levels
| mRNA | WT |
NCoRΔID |
||||
|---|---|---|---|---|---|---|
| Chow | PTU | PTU+T3 | Chow | PTU | PTU+T3 | |
| NCoR N-t | 100 ± 7.9 | 87 ± 6.6 | 115 ± 7.5 | 91 ± 5.8 | 61 ± 8.9 | 104 ± 5.6 |
| SMRT | 100 ± 2.8 | 50 ± 2.9 | 91 ± 5.3 | 89 ± 5.1 | 54 ± 2.1 | 91 ± 3.5 |
| SRC-1 | 100 ± 8.8 | 70 ± 3.4 | 120 ± 5.9 | 102 ± 6.0 | 54 ± 9.8 | 119 ± 6.2 |
| SRC-3 | 100 ± 5.5 | 59 ± 5.8 | 101 ± 6.4 | 78 ± 4.0* | 37 ± 5.5 | 85 ± 5.3 |
| MED1 | 100 ± 5.3 | 67 ± 7.1 | 95 ± 5.8 | 86 ± 4.3 | 56 ± 6.1 | 85 ± 5.2 |
| TRβ1 | 100 ± 14.4 | 45 ± 7.9 | 73 ± 7.8 | 58 ± 4.8** | 25 ± 1.1 | 74 ± 4.7 |
| TRβ2 | 100 ± 12.9 | 119 ± 22.5 | 89 ± 14.5 | 43 ± 4.6** | 57 ± 6.9* | 79 ± 9.3 |
| TRα | 100 ± 7.0 | 112 ± 8.8 | 117 ± 6.7 | 108 ± 5.5 | 94 ± 14.4 | 120 ± 6.3 |
Data are presented as mean ± sem (n = 5–7 animals per group). Significant differences compared with WT group on the same treatment:
, P < 0.05;
, P < 0.01.
To further investigate the central sensitivity to TH in NCoRΔID mice, we performed a T3-suppression test with low doses of T3 that should partially suppress TSH secretion and thus decrease serum T4 (38). As demonstrated in Fig. 6D, NCoRΔID mice, if anything, have a smaller drop in serum T4 in response to exogenous T3 and thus do not have increased sensitivity to TH at the level of the hypothalamus and the pituitary.
If the normal TSH present in NCoRΔID mice cannot be explained by increased sensitivity to circulating TH, we next asked whether maternal TH function could influence the set point of the thyroid axis. Indeed, it is well known that significant amount of maternal TH crosses the placenta and is required for fetal development. Because all of our NCoRΔID mice resulted from matings of heterozygote parents, we hypothesized that the homozygote NCoRΔID fetus would sense the normal TH levels in heterozygote dams (data not shown) as high and present with central suppression of the thyroid axis. Indeed, this does occur in WT mice born to mothers with resistance to TH (39). In contrast, NCoRΔID animals born to NCoRΔID dams would see low maternal T4 levels and avoid suppression of the axis. We thus generated NCoRΔID mice in parallel from dams heterozygote or homozygote for the NCoRΔID allele. As shown in Fig. 7A, at 9–11 wk of age, NCoRΔID mice born to NCoRΔID dams had T4 levels identical to NCoRΔID mice born to heterozygous dams. In both cases these T4 levels were significantly lower than in WT animals born to heterozygous dams. Thus, the central axis is not reset in utero due to increased sensitivity present in NCoRΔID mice during development. We next explored T4 levels in WT and NCoRΔID mice soon after birth to see when NCoRΔID animals developed low TH levels. We first measured T4 levels at postnatal d 5 because circulating T4 levels are very low in newborn mice. Interestingly, T4 levels in WT and NCoRΔID pups are identical at d 5 but diverge significantly by d 10 (Fig. 7B). At the same time, expression levels of TSH subunit mRNA was not different in NCoRΔID pups compared with WT in any of the age groups (Fig. 7C). Thus, the thyroid axis in NCoRΔID mice appears to be reset postnatally.
Fig. 7.
The HPT axis in NCoRΔID animals is reset during early neonatal development. A, Total T4 levels in NCoRΔID animals are not affected by maternal circulating TH concentrations. Total serum T4 was measured by RIA in 9 to 11 week old WT and NCoRΔID male mice born to euthyroid heterozygous dams, and NCoRΔID mice born to NCoRΔID dams with reduced serum TH concentrations; n = 4–5 animals per gr B, Total serum T4 levels, as measured by RIA, in WT and NCoRΔID animals of indicated ages; n = 7. C, TSH subunit mRNA levels were quantified by QPCR in mice of indicated ages; n = 4–11 animals per group. Data are presented as mean ± sem. *, P < 0.05; **, P < 0.01.
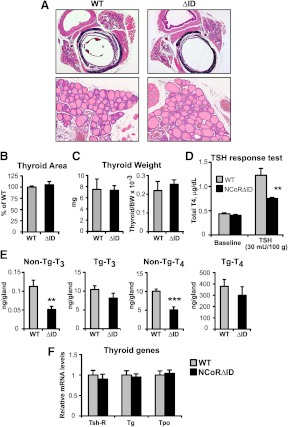
Whereas NCoRΔID mice have an inappropriate central response to the low circulating TH levels, their TSH levels are identical to those found in controls. Thus, it would be expected that the circulating T4 levels should not be different in NCoRΔID and WT animals unless the TSH produced in NCoRΔID mice was biologically less active or there was a defect in the response of the thyroid gland to stimulation by TSH. To answer this question, we first examined thyroid size and weight, sensitive measures of TSH bioactivity, and could find no difference between WT and NCoRΔID mice (Fig. 8, A–C). To test TSH responsiveness of the thyroid, we suppressed endogenous T4 and TSH production with T3 injections for 4 d and then administered to WT and NCoRΔID mice equal amounts of bovine TSH (bTSH) (30 mU per 100 g of BW). As shown in Fig. 8D, NCoRΔID and WT animals had a similarly suppressed T4 at baseline, whereas WT animals had a much greater elevation of T4 secretion in response to TSH than NCoRΔID mice. Thus, NCoRΔID mice also have a defect at the level of the thyroid. To determine the mechanism underlying the thyroidal defect, we evaluated T4 and T3 concentrations within the thyroid. As shown in Fig. 8E, intrathyroidal free non-thyroglobulin (Tg)-bound TH levels are decreased in NCoRΔID mice as compared with WT mice, suggesting that TH synthesis is compromised. To evaluate this further, we looked initially at a number of genes involved in TH synthesis including, tshr, tg, and tpo and found their expression to be similar in NCoRΔID and WT mice (Fig. 8F).
Fig. 8.
Thyroid histology and function in NCoRΔID mice. A, Representative images of hematoxylin and eosin staining of 5-μm cross-sections cut through the middle of thyroid/trachea/esophagus of WT and NCoRΔID mice. Original magnification, ×5 (upper panel) and ×20 (lower panel). B, The thyroid area was quantified using ImageJ software in the series of images taken at ×5 magnification, as shown in A. Shown are means of measurements taken from four animals per group, 13–28 images per animal. C, Thyroid glands of mice with indicated genotypes were excised and weighed. Shown are the mean weights ± sem as measured or normalized to BW (n = 6 animals per group). D, TSH response test. Animals were given T3 injections at 4 μg per 100 g of BW for 4 d to suppress endogenous TSH and TH production. T4 levels were measured on the next day after the last injection (baseline) and 3 h after an injection of bTSH at 30 mU per 100 g (n = 5–7 animals per group). E, Thyroidal non-Tg and Tg-bound T3 and T4 concentrations were measured in thyroid homogenates from WT and NCoRΔID mice. F, mRNA expression of TSH receptor (Tsh-R), Tg, and thyroid peroxidase (Tpo) in the thyroid glands of NCoRΔID mice. mRNA levels were quantified by quantitative PCR and expressed relative to WT controls (n = 6 animals per group). Data are presented as mean ± sem. **, P < 0.01, **, P < 0.001.
Discussion
The use of the TSH assay for the diagnosis and treatment of thyroid disorders is essential in clinical practice because the clinical signs of hypo- and hyperthyroidism are not always straightforward. Indeed, because circulating TH levels tightly regulate serum TSH through an elegant feedback system, TSH is felt to be the best biomarker of TH action. However, because the trh and tsh subunit genes are negatively regulated targets of the TR and T3, whereas many of the peripheral TR targets are positively regulated by T3, it remains possible that differences in the molecular mechanisms underlying positive and negative regulation may allow for discrepancies in hormone action on these targets and thus make the TSH unreliable in certain situations. Indeed, this is already the case in rare genetic syndromes such as resistance to TH and the Allan-Herndon Dudley syndrome (40–44).
Although it is clear from a variety of cell culture-based studies that the nuclear corepressors, NCoR and SMRT, play a critical role in TH action, only recently has it become possible to test their role in vivo. Previous studies from our laboratory and others have established that both NCoR and SMRT play an obligate role in TR-mediated repression in hypothyroidism and in addition appear to mediate sensitivity to T3 on positively regulated targets in the euthyroid state in the liver (14–16, 45). However, an understanding of the role of the corepressors in systemic TH signaling has not been appreciated in mice that express a SMRT protein that contains mutations in its nuclear receptor interacting domains and before this study could not be evaluated in mice that globally lack NCoR because of embryonic lethality (16, 46). A separate mouse model that expresses a NCoR molecule that cannot recruit histone deacetylase 3 (DADm mouse) has also been reported. These mice demonstrate increased energy expenditure similar to NCoRΔID animals but have normal circulating TH levels and slightly elevated TSH levels (15, 45). These and other differences between the DADm model and the one described in this report could be in part explained by the fact that in the DADm mice, the NCoR molecule can still be recruited to the TR and can therefore block coactivator binding and is thus a distinctly different model.
To circumvent the issue of embryonic lethality in NCoR knockout mice, we developed mice that globally express a NCoRΔID that lacks a region that contains the N3 and N2 interaction domains. We have shown previously that these domains are required for NCoR to recruit the TR. NCoRΔID mice are born at normal Mendelian ratios and have no developmental defects demonstrating that the region surrounding N3 and N2 is not required for normal development and suggests that the functions of domains remaining in NCoRΔID are essential for development. Remarkably when we examined thyroid function tests in NCoRΔID mice, we found that they possessed low TH levels but a normal TSH. Similar low levels of TH are observed in mice that lack either TRH or the TRH receptor and type 3 deiodinase knockout mice (18–20). All of these models had apparent central hypothyroidism with either characteristic impaired growth or diminished expression of TH-regulated genes in target tissues such as the liver. In addition, each of these models had slightly increased TSH. In contrast to each of these models and central hypothyroidism in general, NCoRΔID mice grow normally, have normal levels of serum TSH, and do not have evidence of diminished expression of TH target genes in any of the examined tissues. Taken together, NCoRΔID mice appear to have increased sensitivity to TH, which is further supported by their increased energy expenditure and slightly diminished BW as they age. These data further demonstrate that NCoR levels are critical for cellular sensitivity to TH.
Although increased sensitivity to TH is observed in different tissues of NCoRΔID mice, in context of gene expression, two notable physiological exceptions are present. The first is HR, which appears to respond appropriately to reduced TH levels in NCoRΔID animals. When TH levels are matched between NCoRΔID and WT mice, either in the hypothyroid state or during T3 replacement, the HR becomes identical between the two genotypes. Thus, the genetic program that governs HR is not influenced by the lack of NCoR. Because animal models that lack TRα are also bradycardic, demonstrating the importance of this isoform in regulation of HR, it remains possible that the actions of NCoR are either TR isoform specific or program specific (35, 37, 47). Importantly, the presence of bradycardia in NCoRΔID mice definitively establishes the relevance of the low circulating levels of TH present in these animals, making the lack of response in other tissues even more remarkable.
The second exception is the hypothalamic-pituitary axis. In all animal models of central hypothyroidism, the TSH is moderately elevated and there are deficits in the response of the HPT axis to hypothyroidism. Neither of these issues is present in NCoRΔID mice. Furthermore, in WT mice a reduction of TH levels by 30% would lead to a significant increase in serum TSH levels. In contrast, NCoRΔID mice have normal serum TSH and TRH mRNA levels despite low TH levels. This presentation led us to initially hypothesize that NCoRΔID mice were more sensitive to TH at the level of the pituitary and hypothalamus. However, a T3 suppression test does not support this hypothesis. Furthermore, the axis does not appear to be reset in utero, although low TH levels are present in NCoRΔID beginning very early in life. Further work will be required to understand how the hypothalamus and pituitary are reset in NCoRΔID mice.
Whereas the normal TSH in NCoRΔID mice contributes to the phenotype present, it does not fully explain the low TH levels seen. Indeed, because thyroid size is not different between genotypes, TSH bioactivity must be normal in NCoRΔID mice, and thus, these animals would have normal TH levels if they responded normally to TSH. The lack of a normal TSH response in NCoRΔID mice suggests that thyroidal function is altered. Indeed, this is supported by the reduced levels of non-Tg bound T4 and T3 within the thyroids of NCoRΔID mice. This finding is consistent with a defect in either TH synthesis or processing from Tg and is in contrast to the elevated levels of T4 and T3 found within the thyroids of monocarboxylate transporter-8 knockout mice who have a defect in TH secretion (48, 49). This finding raises the intriguing possibility that T3 signaling controls its own synthesis directly within the thyroid as well as at the level of the hypothalamus and pituitary.
In summary, the development of mice that express NCoRΔID in lieu of NCoR in all tissues has produced a model that shows enhanced sensitivity to TH across multiple tissues without affecting the serum TSH. This phenotype predicts that cellular NCoR levels could be key contributors to local TH action in vivo that would be missed by examining only the serum TSH. Thus, there is significant need to develop novel biomarkers of TH action across a variety of tissues to properly assess tissue specific effects of TH.
Materials and Methods
Generation of NCoRΔID mice
To obtain a mouse strain with global expression of NCoRΔID protein, we crossed NCoRlox/lox animals (14) with Zp3-Cre transgenic mouse line that expresses Cre recombinase under the control of zona pellucida gene regulatory elements, exclusively in the growing oocytes [C57BL/6-Tg(Zp3-cre)93Knw/J; Jackson Labs, Bar Harbor, ME]. After the germline transmission of the recombined NCoRΔID allele was confirmed by PCR, the NCoRΔID/+ were crossed to produce NCoRΔID/ΔID (NCoRΔID) and littermate control NCoR+/+ (WT) animals. Mice were maintained on a mixed B6-129S strain background.
Animal maintenance and sample collection
All experiments described were performed in male mice between 8 and 12 wk of age unless otherwise specified. Animals were housed in the Beth Israel Deaconess Medical Center animal facility on a 12-h light, 12-h dark cycle and given standard rodent chow (Harlan Teklad F6 Rodent Diet 8664; Indianapolis, IN) and water ad libitum. At the end of the experiments, the mice were killed by asphyxiation with CO2. Blood samples from adults and older neonates were taken by cardiac puncture, whereas trunk blood was collected from younger (5–10 d old) neonates. Serum was separated by centrifugation and stored at −80 C. Tissues were rapidly collected, flash frozen in liquid nitrogen, and stored at −80 C. For determination of heart weights, the hearts were rinsed with PBS, the liquid squeezed out, the atria removed, and the ventricles weighed and immediately frozen in liquid nitrogen and stored at −80 C. All experiments were approved by Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Histological analysis
Intrascapular BAT pads and thyroid glands attached to trachea were excised from adult male mice and fixed in formalin. Paraffin embedding, sectioning (5 μm), and hematoxylin and eosin staining of the sections were performed using standard techniques at AML Labs, Inc. (Baltimore, MD). For the assessment of the thyroid gland size, 13–28 images of midthyroid sections per animal were obtained using standard light-field microscopy, and thyroid gland size was estimated by measuring the thyroid area on the sections using ImageJ software (National Institutes Health, Bethesda, MD).
TH level manipulations
Hypothyroidism was induced by feeding the animals with a low-iodine diet supplemented with 0.15% PTU (LoI/PTU, Harlan Teklad TD.95125) for 3 wk. The T3 replacement groups were given T3 (Sigma, St. Louis, MO) as ip injections once a day at 0.2 μg (low dose) or 0.5 μg (physiological replacement) per 100 g of BW in PBS for the last 3 or 4 d of PTU feeding, respectively, and were killed 14–15 h after the last injection.
T3 suppression test
Groups of WT and NCoRΔID mice (nine and seven per group, respectively) were consecutively given incremental doses of T3 (0.08 μg per 100 g BW per day followed by 0.2 μg per 100 g BW per day) by ip injections once a day for 4 d each. Blood samples were collected from the submandibular vein before the treatment (baseline) and 14–15 h after the last injection for each T3 treatment. The total serum T4 levels were measured and the decrease after the treatment was expressed as a percentage of the baseline obtained in the same animal before the treatment.
TSH response test
To suppress endogenous production of T4 and TSH, WT and NCoRΔID mice (five and seven per group, respectively) were given ip injections of T3 at 4 μg per 100 g BW per day for 4 d. Fourteen to 15 h after the last injection, blood samples were collected from the submandibular vein to confirm that the T4 levels were suppressed and serve as a baseline. Animals were then given a single injection of highly purified bTSH (National Hormone and Peptide Program, Torrance, CA) at 30 mU per 100 g BW, and blood samples were taken 3 h later to assess the increase of total T4.
Indirect calorimetry
Metabolic rates were measured by indirect calorimetry in 12- to 14-wk-old male NCoRΔID and control single-housed mice (eight and six per group, respectively) within open-circuit Oxymax chambers of the CLAMS (Columbus Instruments, Columbus, OH). Ambulatory activity was evaluated at the same time by using an OPTO-M3 sensor system (Columbus Instruments). Consecutive adjacent photobeam breaks were scored as an ambulatory count. Cumulative ambulatory activity counts were recorded every 10 min. Mice were acclimated to monitoring cages for 72 h before data acquisition, weighed, and then underwent 72 h of monitoring. During this entire period, mice were maintained at approximately 22–24 C under a 14-h light, 10-h dark cycle. Food and water were available ad libitum.
Core body temperature
Body temperature was measured in the groups of mice used for indirect calorimetry, with a digital rectal thermometer (PhysiTemp ThermoAlert model TH-5; Physiotemp Instruments, Clifton, NJ) at 1700 h for 3 consecutive days. Data were calculated based on the means of the three measurements for each mouse.
Food intake
After the indirect calorimetry experiment, the mice were single housed, and food intake was measured daily for 4 wk. Data were calculated based on the means of all measurements obtained for each mouse.
Body composition
At the end of the food intake measurements, the mice were subjected to magnetic resonance imaging (MRI) using Echo MRI (Echo Medical Systems, Houston, TX) to determine the body composition.
Echocardiography studies
Echocardiography was performed on unanesthetized mice using a 13 L high-frequency linear (10 MHz) transducer (VingMed 5; GE Medical Services, Milwaukee, WI) with a depth set at 1 cm and 236 frames per second for two-dimensional images. M-mode images used for measurements were taken at the papillary muscle level. The measurements were taken on the same groups of mice in euthyroid state (8–9 wk of age), after induction of hypothyroidism (3 wk of PTU diet) and physiological T3 replacement (0.5 μg per 100 g BW for 4 d). LV mass was calculated using the M-mode (cubed) method, with the formula LV mass (grams) = 1.05[(septal thickness + LV cavity diameter + posterior wall thickness)3 − (LV cavity diameter)3] (50).
Blood chemistry and hormonal analysis
Total serum T4 and T3 as well as free T4 levels were measured by solid-phase RIA (Coat-a-Count; Diagnostic Products Corp., Los Angeles, CA) in 25 and 50 μl of serum, respectively. TSH was measured in 25 μl of serum using a sensitive, heterologous, disequilibrium, double-antibody precipitation RIA as previously described (51). Results are expressed in bioassayable TSH units. Enzymatic-colorimetric assay for total cholesterol was purchased from Stanbio Laboratory, Boerne, TX).
Determination of T4 and T3 content of the thyroid glands
Thyroidal content of T4 and T3 was measured as described by Trajkovic-Arsic et al. (48). Briefly, thyroid glands were homogenized in 500 μl of ice-cold barbital buffer (0.06 m, pH 8.6; Sigma) using the TissueLyser system (distributed by QIAGEN, Valencia, CA). The homogenates were sonicated 5 × 10 sec at power setting 5 and centrifuged. One hundred microliters of the supernatant were used for enzymatic degradation of Tg, whereas the rest was frozen and stored at −80 C. To degrade Tg, 100 μl of homogenate was mixed with 150 μl of protease solution (6.25 mg of Protease E; Sigma; 3.13.mg of 2-thiouracil; Sigma; in 10 ml of 0.75 m Tris HCl, pH 8.8) and 15 μl of toluol (Sigma) and incubated with shaking at 37 C for 48 h. After that the tubes were incubated at 98 C for 2 min, frozen, and stored at −80 C. T4 and T3 concentrations were measured by RIA in dilutions of untreated (non-Tg bound hormones) and digested with protease homogenates (Tg bound hormone), and total hormone content was calculated per gland.
Western blot analysis
Western blots were performed using whole-cell protein extracts from the tissues of animals with indicated genotypes. Approximately 30–50 mg of frozen tissues were homogenized in 1 ml of cell lysis buffer [20 mm Tris (pH7.5), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerolphosphate, 1 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, 1× protease inhibitor cocktail (Roche, Indianapolis, IN)[, sonicated four times for 5 sec at power level 4, and centrifuged for 20 min at maximum speed. Thirty micrograms of total protein were resolved on 3–8% gradient Tris-acetate or 10% Bis-Tris Novex gels (Invitrogen, Carlsbad, CA) and blotted with indicated primary antibodies, followed by appropriate horseradish peroxidase-conjugated secondary antibody, and developed using ECL Plus Western blot detection system (Amersham, Piscataway, NJ). The primary antibodies used were: an affinity-purified rabbit anti-NCoR antibody generated against the C-terminal portion of the NCoR molecule, anti-UCP-1 (M-17; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-actin (20–33; Sigma). The blots were scanned and quantified using ImageJ software (public domain, developed at the National Institute of Mental Health, Bethesda, MD).
In situ hybridization (ISH)
TRH mRNA expression was detected by ISH using 35S-radiolabeled antisense riboprobe on formalin-fixed brain slices as described previously (52). Dark-field digital images of PVH and LH were acquired with the same exposure time, brightness, and contrast on Zeiss Axioimager Z1 with Axiovision 4.5 software (Oberkochen, Germany). The ×10 magnification images were quantified using ImageJ as described (52). The pixel densities in the PVH were normalized to pixel densities in LH on the same section. TRH mRNA expression for each experimental condition (euthyroid, hypothyroid, and hyperthyroid) was tested in four to five animals per genotype. Data are presented as sample means ± sem. The statistical analysis of genotype variation was performed using the unpaired t test.
Real-time quantitative PCR
Total RNA was extracted from frozen tissues with STAT-60 reagent (Tel-Test, Friendswood, TX). Then 0.5 or 1 μg of total RNA was reverse transcribed using Advantage RT-for-PCR kit (CLONTECH, Mountain View, CA) with random hexamer primers. TaqMan gene expression assays for all mRNAs were purchased from Applied Biosystems (Carlsbad, CA). Each reaction contained 15–30 ng cDNA, 10 μl of TaqMan universal PCR master mix, No AmpErase UNG (Applied Biosystems), and 1 μl of TaqMan gene expression assay in a total volume of 20 μl. Quantitative PCR was performed in duplicates using the MX3000P real-time PCR System (Stratagene, Santa Clara, CA). Relative mRNA levels were calculated using the standard curve method and normalized to cyclophilin mRNA.
ChIP assays
ChIP assays were performed as described previously (53). Chromatin from livers of four different animals per each genotype was pooled, and immunoprecipitations were carried out in quadruplicate. Antibodies used were antiacetyl histone H3 and antiacetyl histone H4 (Millipore, Billerica, MA). Quantitative PCR was performed using SYBR green reaction chemistry (Dynamo SYBR green qPCR reagent; Finnzymes, Espoo, Finland) and a Stratagene Mx 3000 thermal cycler. Primer sequences for the mGpd2 promoter 5′-GGCTGAGTTGCCGGATCATCC-3′ and 5′-CACACACCTGAGATCGGTCGTC-3′. The results are shown as fold enrichment over no antibody control.
Statistical analysis
The differences between genotypes were tested using the unpaired Student's t test. The effectiveness of T3 treatments was tested using one-way or repeated-measures (when a parameter was tested in the same animals after different treatments, e.g. serum cholesterol) ANOVA with the Bonferroni post hoc test.
Supplementary Material
Acknowledgments
Address all correspondence and requests for reprints to: Anthony N. Hollenberg or Inna Astapova, 330 Brookline Avenue, CLS-738, Boston, Massachusetts 02215. E-mail: thollenb@bidmc.harvard.edu; or iastapov@bidmc.harvard.edu.
This work was supported by National Institutes of Health Grants DK078090 and DK056123 (to A.N.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- Brown adipose tissue
- bTSH
- bovine TSH
- BW
- body weight
- ChIP
- chromatin immunoprecipitation
- CLAMS
- comprehensive laboratory animal monitoring system
- HPT
- hypothalamic-pituitary-thyroid
- HR
- heart rate
- ISH
- in situ hybridization
- LV
- left ventricular
- MRI
- magnetic resonance imaging
- NCoR
- nuclear receptor corepressor
- NCoRΔID
- mutant NCoR protein
- PTU
- propylthiouracil
- PVH
- paraventricular hypothalamus
- SMRT
- silencing mediator of retinoid and thyroid receptor
- SRC
- steroid receptor coactivator
- Tg
- thyroglobulin
- TH
- thyroid hormone
- TR
- TH receptor isoform
- UCP-1
- uncoupling protein 1
- WT
- wild type.
References
- 1. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. 2000. The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- 2. Hollenberg AN. 2008. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid 18:131–139 [DOI] [PubMed] [Google Scholar]
- 3. Brent GA. 1994. The molecular basis of thyroid hormone action. N Engl J Med 331:847–853 [DOI] [PubMed] [Google Scholar]
- 4. Zhang J, Lazar MA. 2000. The mechanism of action of thyroid hormones. Annu Rev Physiol 62:439–466 [DOI] [PubMed] [Google Scholar]
- 5. Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- 6. Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho RA. 1997. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49–55 [DOI] [PubMed] [Google Scholar]
- 7. Heinzel T, Lavinsky RM, Mullen TM, Söderstrom M, Laherty CD, Torchia J, Yang WM, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG. 1997. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387:43–48 [DOI] [PubMed] [Google Scholar]
- 8. Nagy L, Kao HK, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM. 1997. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 89:373–380 [DOI] [PubMed] [Google Scholar]
- 9. Ishizuka T, Lazar MA. 2003. The N-CoR/histone deacetylase 3 complex is required for repression by thyroid hormone receptor. Mol Cell Biol 23:5122–5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee KC, Li J, Cole PA, Wong J, Kraus WL. 2003. Transcriptional activation by thyroid hormone receptor-β involves chromatin remodeling, histone acetylation, and synergistic stimulation by p300 and steroid receptor coactivators. Mol Endocrinol 17:908–922 [DOI] [PubMed] [Google Scholar]
- 11. Weiss RE, Xu J, Ning G, Pohlenz J, O'Malley BW, Refetoff S. 1999. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO J 18:1900–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ortiga-Carvalho TM, Shibusawa N, Nikrodhanond A, Oliveira KJ, Machado DS, Liao XH, Cohen RN, Refetoff S, Wondisford FE. 2005. Negative regulation by thyroid hormone receptor requires an intact coactivator-binding surface. J Clin Invest 115:2517–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alonso M, Goodwin C, Liao X, Ortiga-Carvalho T, Machado DS, Wondisford FE, Refetoff S, Weiss RE. 2009. In vivo interaction of steroid receptor coactivator (SRC)-1 and the activation function-2 domain of the thyroid hormone receptor (TR) beta in TRβ E457A knock-in and SRC-1 knockout mice. Endocrinology 150:3927–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Astapova I, Lee LJ, Morales C, Tauber S, Bilban M, Hollenberg AN. 2008. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc Natl Acad Sci USA 105:19544–19549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alenghat T, Meyers K, Mullican SE, Leitner K, Adeniji-Adele A, Avila J, Bu|$$|Aacan M, Ahima RS, Kaestner KH, Lazar MA. 2008. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature 456:997–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nofsinger RR, Li P, Hong SH, Jonker JW, Barish GD, Ying H, Cheng SY, Leblanc M, Xu W, Pei L, Kang YJ, Nelson M, Downes M, Yu RT, Olefsky JM, Lee CH, Evans RM. 2008. SMRT repression of nuclear receptors controls the adipogenic set point and metabolic homeostasis. Proc Natl Acad Sci USA 105:20021–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiss RE, Murata Y, Cua K, Hayashi Y, Seo H, Refetoff S. 1998. Thyroid hormone action on liver, heart, and energy expenditure in thyroid hormone receptor β-deficient mice. Endocrinology 139:4945–4952 [DOI] [PubMed] [Google Scholar]
- 18. Yamada M, Saga Y, Shibusawa N, Hirato J, Murakami M, Iwasaki T, Hashimoto K, Satoh T, Wakabayashi K, Taketo MM, Mori M. 1997. Tertiary hypothyroidism and hyperglycemia in mice with targeted disruption of the thyrotropin-releasing hormone gene. Proc Natl Acad Sci USA 94:10862–10867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rabeler R, Mittag J, Geffers L, Rüther U, Leitges M, Parlow AF, Visser TJ, Bauer K. 2004. Generation of thyrotropin-releasing hormone receptor 1-deficient mice as an animal model of central hypothyroidism. Mol Endocrinol 18:1450–1460 [DOI] [PubMed] [Google Scholar]
- 20. Hernandez A, Martinez ME, Fiering S, Galton VA, Germain D. 2006. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest 116:476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silva JE. 2006. Thermogenic mechanisms and their hormonal regulation. Physiol Rev 86:435–464 [DOI] [PubMed] [Google Scholar]
- 22. al-Adsani H, Hoffer LJ, Silva JE. 1997. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab 82:1118–1125 [DOI] [PubMed] [Google Scholar]
- 23. de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC. 2001. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108:1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mory G, Ricquier D, Pesquiés P, Hémon P. 1981. Effects of hypothyroidism on the brown adipose tissue of adult rats: comparison with the effects of adaptation to cold. J Endocrinol 91:515–524 [DOI] [PubMed] [Google Scholar]
- 25. Golozoubova V, Gullberg H, Matthias A, Cannon B, Vennström B, Nedergaard J. 2004. Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol Endocrinol 18:384–401 [DOI] [PubMed] [Google Scholar]
- 26. Ribeiro MO, Bianco SD, Kaneshige M, Schultz JJ, Cheng SY, Bianco AC, Brent GA. 2101. Expression of uncoupling protein 1 in mouse brown adipose tissue is thyroid hormone receptor-β isoform specific and required for adaptive thermogenesis. Endocrinology 151:432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freake HC, Oppenheimer JH. 1987. Stimulation of S14 mRNA and lipogenesis in brown fat by hypothyroidism, cold exposure, and cafeteria feeding: evidence supporting a general role for S14 in lipogenesis and lipogenesis in the maintenance of thermogenesis. Proc Natl Acad Sci USA 84:3070–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, Rabelo R, Curcio C, Martins L, Kimura ET, Bianco AC. 2004. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes 53:577–584 [DOI] [PubMed] [Google Scholar]
- 29. Freake HC, Moon YK. 2003. Hormonal and nutritional regulation of lipogenic enzyme mRNA levels in rat primary white and brown adipocytes. J Nutr Sci Vitaminol (Tokyo) 49:40–46 [DOI] [PubMed] [Google Scholar]
- 30. Blennemann B, Leahy P, Kim TS, Freake HC. 1995. Tissue-specific regulation of lipogenic mRNAs by thyroid hormone. Mol Cell Endocrinol 110:1–8 [DOI] [PubMed] [Google Scholar]
- 31. Klein I, Ojamaa K. 2001. Thyroid hormone and the cardiovascular system. N Engl J Med 344:501–509 [DOI] [PubMed] [Google Scholar]
- 32. Dillmann WH. 2002. Cellular action of thyroid hormone on the heart. Thyroid 12:447–452 [DOI] [PubMed] [Google Scholar]
- 33. Klein I. 1988. Thyroxine-induced cardiac hypertrophy: time course of development and inhibition by propranolol. Endocrinology 123:203–210 [DOI] [PubMed] [Google Scholar]
- 34. Lortet S, Zimmer HG, Rossi A. 1989. Inotropic response of the rat heart during development and regression of triiodothyronine-induced hypertrophy. J Cardiovasc Pharmacol 14:707–712 [DOI] [PubMed] [Google Scholar]
- 35. Weiss RE, Korcarz C, Chassande O, Cua K, Sadow PM, Koo E, Samarut J, Lang R. 2002. Thyroid hormone and cardiac function in mice deficient in thyroid hormone receptor-α or -β: an echocardiograph study. Am J Physiol Endocrinol Metab 283:E428–E35 [DOI] [PubMed] [Google Scholar]
- 36. Gassanov N, Er F, Endres-Becker J, Wolny M, Schramm C, Hoppe UC. 2009. Distinct regulation of cardiac I(f) current via thyroid receptors α1 and β1. Pflugers Arch 458:1061–1068 [DOI] [PubMed] [Google Scholar]
- 37. Gloss B, Trost S, Bluhm W, Swanson E, Clark R, Winkfein R, Janzen K, Giles W, Chassande O, Samarut J, Dillmann W. 2001. Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor α or β. Endocrinology 142:544–550 [DOI] [PubMed] [Google Scholar]
- 38. Macchia PE, Takeuchi Y, Kawai T, Cua K, Gauthier K, Chassande O, Seo H, Hayashi Y, Samarut J, Murata Y, Weiss RE, Refetoff S. 2001. Increased sensitivity to thyroid hormone in mice with complete deficiency of thyroid hormone receptor alpha. Proc Natl Acad Sci USA 98:349–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alonso M, Goodwin C, Liao X, Page D, Refetoff S, Weiss RE. 2007. Effects of maternal levels of thyroid hormone (TH) on the hypothalamus-pituitary-thyroid set point: studies in TH receptor β knockout mice. Endocrinology 148:5305–5312 [DOI] [PubMed] [Google Scholar]
- 40. Refetoff S, Weiss RE, Usala SJ. 1993. The syndromes of resistance to thyroid hormone. Endocr Rev 14:348–399 [DOI] [PubMed] [Google Scholar]
- 41. Beck-Peccoz P, Chatterjee VK. 1994. The variable clinical phenotype in thyroid hormone resistance syndrome. Thyroid 4:225–232 [DOI] [PubMed] [Google Scholar]
- 42. Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. 2004. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437 [DOI] [PubMed] [Google Scholar]
- 43. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. 2004. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, Echeverri R, Lubs HA, Voeller K, Simensen RJ, Stevenson RE. 2005. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet 77:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. You SH, Liao X, Weiss RE, Lazar MA. 2010. The interaction between nuclear receptor corepressor and histone deacetylase 3 regulates both positive and negative thyroid hormone action in vivo. Mol Endocrinol 24:1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jepsen K, Hermanson O, Onami TM, Gleiberman AS, Lunyak V, McEvilly RJ, Kurokawa R, Kumar V, Liu F, Seto E, Hedrick SM, Mandel G, Glass CK, Rose DW, Rosenfeld MG. 2000. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102:753–763 [DOI] [PubMed] [Google Scholar]
- 47. Wikström L, Johansson C, Saltó C, Barlow C, Campos Barros A, Baas F, Forrest D, Thorén P, Vennström B. 1998. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J 17:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trajkovic-Arsic M, Muller J, Darras VM, Groba C, Lee S, Weih D, Bauer K, Visser TJ, Heuer H. 2010. Impact of monocarboxylate transporter-8 deficiency on the hypothalamus-pituitary-thyroid axis in mice. Endocrinology 151:5053–5062 [DOI] [PubMed] [Google Scholar]
- 49. Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss Refetoff S. 2010. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest 120:3377–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. 1986. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57:450–458 [DOI] [PubMed] [Google Scholar]
- 51. Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. 1999. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9:1265–1271 [DOI] [PubMed] [Google Scholar]
- 52. Sugrue ML, Vella KR, Morales C, Lopez ME, Hollenberg AN. 2010. The thyrotropin-releasing hormone gene is regulated by thyroid hormone at the level of transcription in vivo. Endocrinology 151:793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ramadoss P, Unger-Smith NE, Lam FS, Hollenberg AN. 2009. STAT3 targets the regulatory regions of gluconeogenic genes in vivo. Mol Endocrinol 23:827–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.