Abstract
Pitx2 is a homeodomain transcription factor required in a dose-dependent manner for the development of multiple organs. Pitx2-null homozygotes (Pitx2−/−) have severe pituitary hypoplasia, whereas mice with reduced-function alleles (Pitx2neo/neo) exhibit modest hypoplasia and reduction in the developing gonadotroph and Pou1f1 lineages. PITX2 is expressed broadly in Rathke's pouch and the fetal pituitary gland. It predominates in adult thyrotrophs and gonadotrophs, although it is not necessary for gonadotroph function. To test the role of PITX2 in thyrotroph function, we developed thyrotroph-specific cre transgenic mice, Tg(Tshb-cre) with a recombineered Tshb bacterial artificial chromosome that ablates floxed genes in differentiated pituitary thyrotrophs. We used the best Tg(Tshb-Cre) strain to generate thyrotroph-specific Pitx2-deficient offspring, Pitx2flox/−;Tg(Tshb-cre). Double immunohistochemistry confirmed Pitx2 deletion. Pitx2flox/−;Tg(Tshb-cre) mice have a modest weight decrease. The thyroid glands are smaller, although circulating T4 and TSH levels are in the normal range. The pituitary levels of Pitx1 transcripts are significantly increased, suggesting a compensatory mechanism. Hypothyroidism induced by low-iodine diet and oral propylthiouracil revealed a blunted TSH response in Pitx2flox/−;Tg(Tshb-cre) mice. Pitx1 transcripts increased significantly in control mice with induced hypothyroidism, but they remained unchanged in Pitx2flox/−;Tg(Tshb-cre) mice, possibly because Pitx1 levels were already maximally elevated in untreated mutants. These results suggest that PITX2 and PITX1 have overlapping roles in thyrotroph function and response to hypothyroidism. The novel cre transgene that we report will be useful for studying the function of other genes in thyrotrophs.
The pituitary gland controls development and function of several important target organs. In mice, the anterior and intermediate lobes of the pituitary gland are derived from the oral ectoderm that invaginates to form Rathke's pouch, which gives rise to five anterior pituitary-specific cell lineages that produce GH, LH and FSH, TSH, prolactin, and ACTH (1). Pituitary organogenesis is a complex multistep process under the control of at least a dozen known transcription factors and likely several that remain unknown (2, 3). Mutations in the genes coding for these transcription factors lead to multiple pituitary hormone deficiencies. For instance, recessive or dominant mutations of POU1F1, a pituitary-specific POU homeodomain transcription factor, also known as PIT1, generally cause congenital somatotroph, lactotroph, and thyrotroph deficiencies (2). Mutations in the LIM (Lin11, Isl1, and Mec-3) domain transcription factor LHX4 cause somatotroph deficiency and variable thyrotroph, gonadotroph, and corticotroph insufficiencies (4). Despite the known transcription factor mutations, most cases of congenital hypopituitarism remain of unknown etiology. Conditional knockout mice represent an ideal model to improve our understanding of the genetic basis for hypopituitarism. Indeed, most of the genes known to cause hypopituitarism were predicted from mouse models.
Mutations in LHX3, LHX4, PROP1, and POU1F1 have been described in patients with combined pituitary hormone deficiency, which includes thyrotroph deficiency in the majority of cases (2). The mechanisms of thyrotroph cell specification remain poorly understood. Roles for pituitary transcription factors in thyrotroph ontogenesis and expansion have been proposed. Early acting transcription factors like LIM domain transcription factors LHX3 and LHX4 are crucial because mice with a homozygous inactivation of these transcription factors exhibit pituitary hypoplasia and decreased or absent thyrotroph cells (5–7). These LIM genes are not specific for thyrotrophs, however, as affected humans and mice have additional hormone deficiencies. Some later-acting transcription factors are also necessary for thyrotroph development. For example, Ames and Snell dwarf mice lack thyrotrophs due to spontaneous inactivation of the paired transcription factor PROP1 and the POU homeodomain transcription factor POU1F1 (or PIT1), respectively (8–10).
Gata2 was proposed to be critical for thyrotroph specification, but loss of function studies in mice suggest that it has a more modest role (11). Mice homozygous for pituitary-specific inactivation of Gata2 have transient growth insufficiency and hypogonadism. They are unable to respond to induced hypothyroidism with a robust elevation of TSH levels, but elevated Gata3 expression suggests that other members of the same gene family may compensate for the loss of Gata2 (11).
PITX2 is a homeodomain transcription factor involved in the early steps of pituitary organogenesis (12–14). PITX2 expression is first observed at embryonic d 8 (e8) in the stomodeum and at e10 in Rathke's pouch. It is still expressed in the adult pituitary gland (13, 15, 16). Mice with homozygous inactivation of Pitx2 (Pitx2−/−) present with severe pituitary hypoplasia due to reduced proliferation and enhanced cell death (13, 15, 16). It is difficult to evaluate the role of PITX2 in developing specific pituitary cell lineages because embyros die at e12.5 due to severe heart defects. Mice homozygous for a hypomorphic, or reduced function, allele of Pitx2 (Pitx2neo) survive until birth. Their pituitaries lack NR5A1 (also known as SF1) and gonadotropins and have reduced differentiation of the POU1F1 lineage, resulting in fewer somatotrophs and thyrotrophs (15). To characterize the role of PITX2 in specific pituitary lineages, it must be deleted specifically in that cell type. Deletion of PITX2 in gonadotrophs using an Lhb-cre transgene did not alter puberty or fertility, suggesting it is dispensable in differentiated gonadotrophs (17).
Several lines of evidence support the hypothesis that PITX2 plays a role in thyrotroph development, maintenance, and/or function. First, during embryogenesis, the expression of several transcription factors, including PROP1, POU1F1, and LHX4, is decreased in the pituitaries of mice with homozygous inactivation of Pitx2 (Pitx2−/−). This suggests that PITX2 is a master regulator of these downstream genes (15, 16). Second, PITX2 is able to transactivate Pou1f1 and Tshb promoters in cell culture, which is consistent with a direct role for PITX2 in the committed thyrotroph (18, 19). Third, more than 80% of adult thyrotroph cells express PITX2, implying a role in adult thyrotroph function and/or maintenance (20). Fourth, Pitx2neo/neo mice have reduced expression of POU1F1 and fewer thyrotroph cells, implicating PITX2 as a dosage-sensitive regulator of commitment or expansion of the thyrotroph lineage (15, 16).
Multiple lines of evidence support the idea of overlapping roles of PITX1 and PITX2 in the developing pituitary gland. PITX1 and PITX2 have nearly identical homeodomains and highly homologous carboxy termini. Both transcription factors can bind the same sites in cell culture, have similar trans-activation activity in cell culture (19), and are present in the majority of thyrotroph cells in adults (20). Double heterozygotes (Pitx1+/−, Pitx2+/−) have poor viability, but the double mutants (Pitx1−/−, Pitx2−/−) have arrested development of the pituitary primordium and lack LHX3 expression (20), which is unaffected in the single mutants. The pituitaries of Pitx1−/− embryos are not obviously altered in size (21) and Pitx2neo/neo mice have a modest reduction in pituitary volume (16), but the double Pitx1−/−, Pitx2neo/neo mutants have minute pituitary primordia similar to Pitx2−/− embryos (20), indicating overlapping function in growth of the primordium. This is consistent with the idea of overall gene dosage for genes with similar function (22). The LIM genes Lhx3 and Lhx4 also have overlapping function in establishing the pituitary primordium (23).
To test our hypothesis that PITX2 participates in thyrotroph maintenance and function, we generated a new cre recombinase driven by recombineering a Tshb bacterial artificial chromosome (BAC) (24). Previous studies with smaller portions of the Tshb gene were unsuccessful in directing thyrotroph-specific expression in transgenic mice (25). Pitx2flox/−;Tg(Tshb-cre) mice delete Pitx2 specifically in thyrotrophs once they are specified and begin to produce TSH. The Pitx2flox/−;Tg(Tshb-cre) mice produce TSH with striking elevation of pituitary Pitx1 expression, and they have slightly reduced growth. The affected mice have a blunted pituitary response to hypothyroidism, suggesting that PITX2 is important for the full range of thyrotroph function.
Results
Tshb-cre transgene is specific for pituitary thyrotroph cells
The Tshb-cre transgene construct was generated by using recombineering to insert a cre recombinase cassette at the transcription start site of a BAC from a C57BL6/J library that contains −144kb to +58kb of the Tshb gene. Four BAC transgenic founders contained the entire Tshb-cre transgene construct and were bred to a cre reporter strain (floxed LacZ) to characterize the specificity of cre activity for each line. Three of the Tg(Tshb-cre) lines had moderate levels of LacZ expression in the pituitary gland, but it was not specific to the thyrotrophs. One Tg(Tshb-cre) line exhibited higher levels of LacZ expression in the pituitary and greater specificity for thyrotrophs. This line was selected for further analysis of developmental regulation and tissue specificity.
Transgene expression was analyzed at e14.5 and e15.5, when endogenous TSHß is first detectable by immunohistochemistry in the caudo-medial aspect of the pituitary. As expected, X-gal staining is detectable in this area and in the POU1F1-independent, rostral tip thyrotrophs in transgenics and not in controls (Fig. 1, panel A). X-gal staining is strong in the anterior lobe of the adult pituitary gland and colocalizes with the majority of the thyrotrophs. A few somatotroph cells stained with X-gal. These may correspond to GH-TSH double-positive cells (26). No X-gal staining was observed in gonadotroph, lactotroph, or corticotroph cells identified by LH, prolactin, and ACTH immunostaining, respectively (Fig. 1B). This indicates efficient, developmentally regulated, and cell-specific expression of the Tshb-cre transgene.
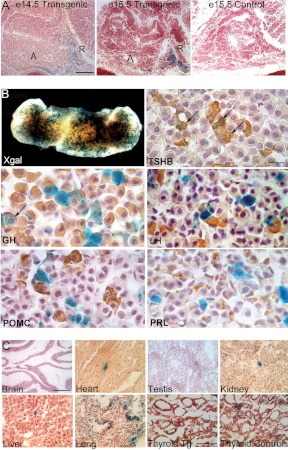
Fig. 1.
Thyrotroph-specific activity of Tshb-cre transgene. A, Transgene expression was analyzed in frozen sections from e14.5 and e15.5 fetuses carrying a Rosa26 cre reporter gene with a Tshb-cre transgene (transgenic) or without it (controls). X-gal staining (blue) was detected in the caudomedial aspect of the anterior lobe (A) and in the rostral tip (R) in Tg(Tshb-cre) mice but not in controls (×200). B, X-gal staining of intact adult pituitary glands (×50) was followed by fixation, paraffin embedding, sectioning, and hormone-specific antibody staining (×630). Black arrows indicate selected cells with X-gal stain and hormone. C, Frozen sections from adult nonpituitary tissues were stained with X-gal to detect ectopic transgene activity. The magnification bar in the e14.5 transgenic and the brain sections is equivalent to 50 μm and applies to the embryonic pituitary, brain, heart, testis, kidney, liver, and lung sections. The magnification bar for the thyroid sections is equivalent to 30 μm. The magnification bar in the TSHB immunostaining is equivalent to 10 μm and applies to all the pituitary hormone antibody staining. POMC, Proopiomelanocortin; PRL, prolactin.
Adult tissues were examined from Tg(Tshb-cre); floxed-LacZ mice to ascertain the degree of transgene leakiness in nonpituitary tissues. There was no evidence of transgene activity in the brain. Transgene activity was detected at very low levels in a few cells in the heart, the testis, the kidney, the liver, and at moderate levels in the lung (Fig. 1C). The faint X-gal staining detected in the thyroid of Tg(Tshb-cre);floxed-LacZ mice was indistinguishable from X-gal staining in the nontransgenic controls, consistent with a low background level of endogenous ß-galactosidase activity in the thyroid gland (Fig. 1C). We confirmed that there is no PITX2 expression in the thyroid glands of control mice (data not shown) (27). Thus, this Tshb-cre line is appropriate for examining gene function in pituitary thyrotrophs.
Pitx2flox/−;Tg(Tshb-cre) mice are smaller than wild-type littermates
The Tg(Tshb-cre) strain was mated with Pitx2+/− mice to create a stock, Pitx2+/−;Tg(Tshb-cre) for mating with Pitx2flox/flox mice. We obtained Pitx2flox/−;Tg(Tshb-cre) mice in the expected Mendelian ratios. Of 162 progeny 23% were Pitx2flox/−;Tg(Tshb-cre), 19% were Pitx2flox/+;Tg(Tshb-cre), and among those without the Tshb-cre transgene, 32% were Pitx2flox/+ and 19% were Pitx2flox/− mice (P = 0.304). Coimmunostaining with PITX2 and TSH antibodies confirmed that the majority of thyrotroph cells expressed PITX2 in controls, whereas there was no expression of PITX2 in most of transgenic thyrotroph cells (Fig. 2).
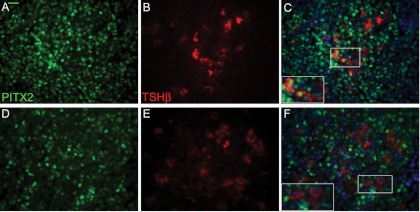
Fig. 2.
Efficient deletion of Pitx2 in Pitx2flox/−;Tg(Tshb-cre) mice. Paraformaldehyde-fixed, paraffin-embedded pituitary glands from 8-wk-old control (A–C) and Pitx2flox/−;Tg(Tshb-cre) mice (D–F) were sectioned and coimmunostained with PITX2 (green, panels A and D) and TSH (red, panels B and C), counterstained with DAPI (blue) and photographed, and images were merged (C and F) (×630). The majority of thyrotroph cells express PITX2 in controls (panel C) but not in Pitx2flox/−;Tg(Tshb-cre) mice (panel F). Boxed areas are magnified in the inset. The magnification bar in the TSHB and PITX2 immunostaining is equivalent to 10 μm and applies to all the pituitary hormone antibody staining.
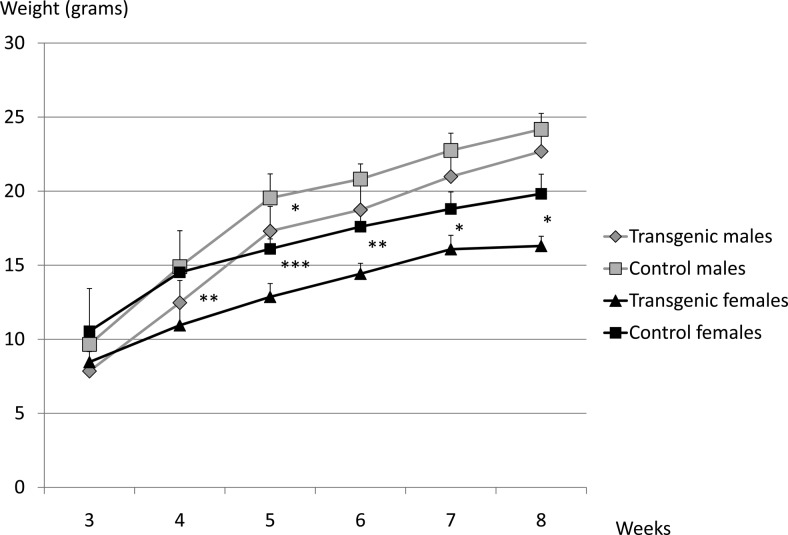
Male and female Pitx2flox/−;Tg(Tshb-cre) mice weighed less than their control littermates. The difference in weights was most significant in females at 5 wk (P = 0.001), but the mutants were smaller at other ages after weaning (P < 0.01 at 4 and 6 wk; P < 0.05 at 7 and 8 wk). Male mutants were smaller at 5 wk (P < 0.05). (Fig. 3). This growth insufficiency is modest compared with animals with severe hypothyroidism, such as the α-subunit knockout mice, CgatmSac, which are unable to produce biologically active TSH (28). To test for GH deficiency, we measured Igf1 mRNA levels in Pitx2flox/−;Tg(Tshb-cre) and control females (n =3 vs. 2 controls). There was no significant difference between these groups (data not shown).
Fig. 3.
Pitx2flox/−;Tg(Tshb-cre) mice have a moderate growth deficiency. Pitx2flox/−;Tg(Tshb-cre) and control male and female mice were weighed between 3 to 8 wk of age. Statistical significance: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Pitx2flox/−;Tg(Tshb-cre) mice have subtle alterations in the pituitary-thyroid axis
To assess the effect of Pitx2 deficiency on thyrotroph function, we performed TSH immunohistochemistry on sections from adult (8 wk) pituitary glands of normal and transgenic mice, quantified Tshb transcripts by RT-PCR, and measured circulating levels of TSH by RIA. Severe hypothyroidism increases the size of thyrotrophs, and chronic hypothyroidism increases the thyrotroph population (25). TSH immunostaining was performed systematically on every five slides throughout the pituitary gland in at least three controls and three transgenic pituitaries. We did not observe any significant differences in TSH immunostaining (Fig. 4, panel A). Pituitary Tshb and Cga mRNA levels (Fig. 4D) and circulating TSH levels (Fig. 5, panel C) were also indistinguishable in normal and mutant mice.
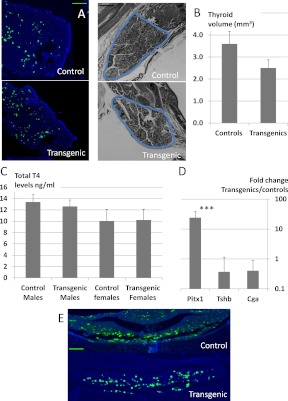
Fig. 4.
Subtle phenotypic changes in Pitx2flox/−;Tg(Tshb-cre) mice and dramatically increased Pitx1 transcripts. A, TSH immunostaining (green) and DAPI staining (blue) were done on pituitaries from 8-wk-old Pitx2flox/−;Tg(Tshb-cre) (transgenics) and controls. TSH staining was performed in three transgenics and three controls, in five pituitary sections at regular intervals for each mouse. The magnification bar for the pituitary sections is equivalent to 100 μm. B (left), Hematoxylin and eosin-stained thyroid gland sections from 8-wk-old control and Pitx2flox/−;Tg(Tshb-cre) transgenics were quantified for thyroid volume (blue line circumscribes the thyroid tissue). The magnification bar for the thyroid sections is equivalent to 200 μm. B (right), Thyroid volumes were averaged from four controls and four Pitx2flox/−;Tg(Tshb-cre) mice. C, Serum T4 levels were measured in control and Pitx2flox/−;Tg(Tshb-cre) transgenic mice by RIA. D, Pituitary mRNA samples from adult controls and Pitx2flox/−;Tg(Tshb-cre) transgenic mice were reverse transcribed, and levels of Pitx1, Tshb, and Cga cDNAs were quantified by PCR. Results are presented on a logarithmic scale as the fold change in levels in Pitx2flox/−;Tg(Tshb-cre) mice compared with controls. ***, P < 0.001. E, Newborn control and Pitx2flox/−;Tg(Tshb-cre) pituitaries were fixed, embedded, sectioned, and immunostained for TSH (green) and DAPI (blue). The magnification bar for the pituitary sections is equivalent to 100 μm.
Fig. 5.
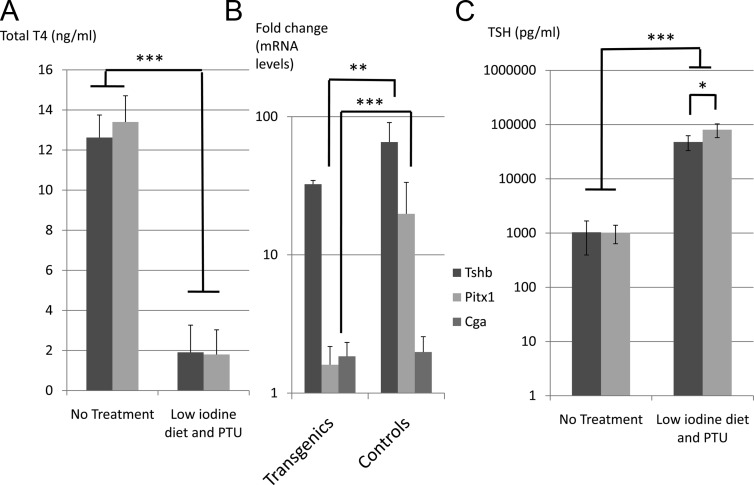
Pitx2flox/−;Tg(Tshb-cre) mice have blunted TSH response to hypothyroidism. Pitx2flox/−;Tg(Tshb-cre) (transgenics) mice and controls were treated for 4 wk with a low-iodine diet enriched in PTU (0.15%). A, Serum levels of total T4 were significantly decreased at the end of the treatment in transgenics (dark gray) and controls (light gray) relative to untreated mice. ***, P < 0.001. B, Tshb, Pitx1, and Cga transcripts quantified by RT-PCR in pituitaries from treated and untreated control and transgenic mice. Results are presented on a log scale depicting the effect of PTU treatment on each genotype group. ***, P < 0.001; **, P < 0.01. C, TSH levels in serum of transgenics (dark gray) and controls (light gray) were measured by RIA and presented on a logarithmic scale. *, P < 0.05.
Pituitary thyrotroph deficiency can cause the thyroid gland to develop and function poorly resulting in a smaller overall gland size, smaller follicle size, and thinner follicular epithelia in addition to reduced thyroid hormone production (28). The overall thyroid gland volume calculated for Pitx2flox/−;Tg(Tshb-cre) mice was smaller than control thyroid glands, although it is close to proportional (P = 0.067 after correction for weight) (Fig. 4B). The follicular size and height of the follicular epithelia were indistinguishable at high magnification (data not shown). Serum levels of total T4 were in the normal range in mutant males and females (Fig. 4C).
The hypothalamic-pituitary-thyroid axis becomes responsive to feedback regulation after birth (29). Newborn mice that lack TRH have a normal component of pituitary thyrotrophs at birth, but the population fails to expand after birth due to the TRH deficiency. A mild reduction in thyrotroph number at birth can be corrected by adulthood if the feedback system is functioning (11). To determine whether thyroid hormone feedback progressively corrected an earlier thyrotroph deficiency, we examined newborns and 4-wk-old mice. Immediately after birth, pituitary TSH immunostaining was similar in mutant and normal mice (Fig. 4E). At 4 wk of age the thyroid volumes of mutants were smaller, but the other parameters, including Tshb mRNA levels, TSH immunostaining, and serum T4 levels, were normal (data not shown).
PITX2 and PITX1 are two closely related homeodomain transcription factors: both are expressed in adult thyrotroph cells, and they are able to transactivate Pou1f1 and Tshb promoters. Interestingly, at 8 wk of age, the levels of Pitx1 mRNA were dramatically increased in transgenics compared with controls (24-fold increase, P < 0.001; Fig. 4D). This suggests that PITX1 might compensate for the deficiency of PITX2 in transgenic mice, allowing them to achieve a nearly euthyroid phenotype with a modest growth defect, smaller thyroid volume, but hormone levels in the normal range.
Pitx2flox/−;Tg(Tshb-cre) mice have a blunted TSH response to hypothyroidism
We hypothesized that mice lacking Pitx2 in thyrotrophs would have a blunted TSH response to low-thyroid hormone levels, similar to that observed in the pituitary-specific Gata2 knockout mice (11). We challenged transgenic mice and controls (n =6 vs. 8 controls) by inducing hypothyroidism with a low-iodine diet enriched in a goiterogen, 0.15% propylthiouracil (PTU), for 4 wk. As expected, control mice exhibited decreased circulating T4 levels (P < 0.001), causing a 65-fold increase in pituitary Tshb mRNA (P < 0.001), and an 80-fold increase in serum TSH levels (P < 0.001) (Fig. 5, A–C). Transgenic mice had a 32-fold increase in Tshb mRNA (P < 0.001) and 48-fold increase in serum TSH levels (P < 0.001) at a comparable level of total T4. These increases are inferior to those observed in controls. The hypothyroidism-induced pituitary response in serum TSH levels is significantly different in controls and transgenic mice: 80 ± 23 vs. 48 ± 15 pg/μl, respectively (P = 0.04).
To explore the mechanism of the blunted TSH response to hypothyroid challenge, we measured Pitx1 and Pitx2 mRNA levels. The hypothyroid challenge did not modify Pitx2 mRNA levels in controls (data not shown), but Pitx1 mRNA levels were increased 20-fold relative to untreated controls (P < 0.001). Pitx1 mRNA levels were the same in challenged and unchallenged transgenics; the apparent 1.6-fold elevation is not significant (Fig. 5B). The transgenics may have been unable to increase Pitx1 in response to the challenge because the levels were already elevated, presumably to compensate for the lack of Pitx2. These results suggest that PITX1 is a major factor in the pituitary's response to hypothyroidism with increased TSH transcription and secretion. This also implies that PITX2 is necessary to obtain an optimal response.
Discussion
Tshb-cre transgenic mice provide an effective tool for genetic engineering in pituitary thyrotrophs
Previous attempts to utilize the Tshb promoter, 5′-flanking region and large intron to drive transgene expression in thyrotrophs were not successful (25). We reasoned that essential regulatory sequences must be located at a distance from the gene and used a large Tshb BAC to drive expression. The cre recombinase is expressed when thyrotrophs initiate Tshb transcription at e14.5. This makes the transgenic line a useful tool for genetic modifications in mature thyrotrophs. It cannot provide any information about the process of thyrotroph development before the initiation of Tshb transcription. One in four Tshb-cre transgenic lines yielded developmentally regulated, efficient, thyrotroph-specific expression, with little or no activity in inappropriate sites. This suggests that the essential regulatory sequences lie within the BAC, which could be engineered to drive expression of other genes in thyrotrophs.
Pitx2 deletion in thyrotrophs leads to mild growth reduction and blunted TSH response to hypothyroidism challenge
Specific inactivation of Pitx2 in thyrotrophs leads to mild phenotypic changes in physiological conditions: Pitx2flox/−;Tg(Tshb-cre) mice have a modest growth insufficiency, but TSH and thyroid hormone levels are apparently in the normal range. It is possible that the TSH and T4 measurements missed small, but biologically relevant variations in hormone levels or a period of reduced hormone production, but it is clear that PITX2 is not essential for TSH production or survival of thyrotroph cells. The reduced growth is more obvious at 5 wk than 8 wk, suggesting that pituitary changes in gene expression, such as increased Pitx1 transcription, might progressively compensate for the lack of Pitx2. We considered the possibility that Pitx2flox/−;Tg(Tshb-cre) mice might have reduced GH production because a few somatotroph cells express the recombinase, and GH transcription is regulated by thyroid hormone (30). Although we cannot completely rule out an effect on GH production, it is unlikely to be a contributor because most GH cells do not express PITX2 or cre recombinase and liver Igf1 transcription is unaltered.
Hypothyroidism induced by low-iodine diet enriched in PTU, an antithyroid drug, leads to dramatically increased TSH levels. Pitx2flox/−;Tg(Tshb-cre) mice had a blunted TSH response, suggesting that PITX2-deficient thyrotrophs are only partially able to respond to low thyroid hormone levels. This observation supports the idea that PITX2-deficient thyrotrophs function less efficiently than normal thyrotrophs, possibly contributing to the modest growth deficiency. The phenotypic anomalies of Pitx2flox/−;Tg(Tshb-cre) mice are remarkably similar to those observed in the pituitary-specific knockout of Gata2 (11). The Gata2Pitko males have a transient growth deficiency around 5 wk of age, and newborns have reduced TSH immunostaining that is quickly corrected. Challenging Gata2 knockout mice with radioactive iodine-mediated thyroid ablation also produced a blunted TSH response, and Gata3 transcription was dramatically increased.
Hypothyroidism induces Pitx1 expression but not Pitx2
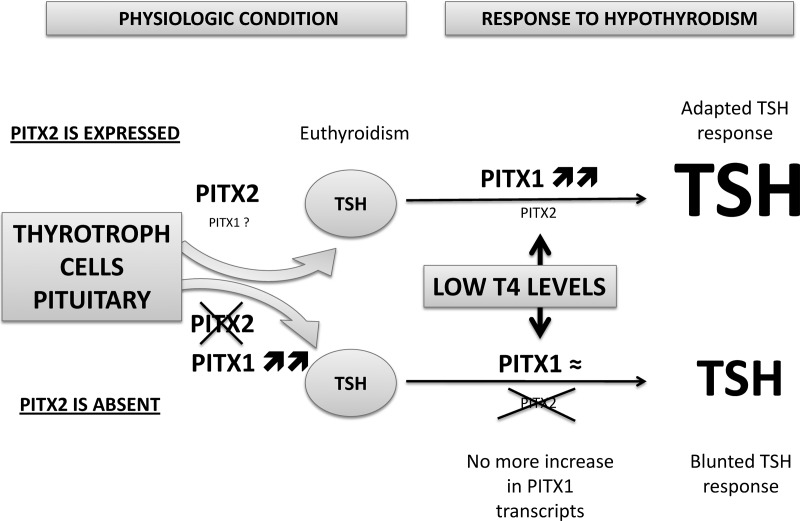
PITX1 and PITX2 have nearly identical homeodomains and conserved C termini, they are expressed in the majority of adult thyrotrophs, and they are able to transactivate the same promoters in cell culture (12, 16, 18). PITX1 appears to be a major contributor to pituitary-thyroid axis homeostasis because normal mice respond to hypothyroidism with elevated Pitx1 transcript levels, and there is no change in Pitx2 transcription. Pitx2flox/−;Tg(Tshb-cre) mice have dramatically increased Pitx1 transcripts under basal conditions, suggesting that Pitx2-deficient thyrotrophs have reduced function and compensate by elevating Pitx1 transcription. The level of Pitx1 transcripts in normal mice with induced hypothyroidism equals the basal levels of Pitx1 in transgenics. This implies that Pitx1 is maximally induced by either hypothyroidism or PITX2 deficiency, and that the low-iodine diet is unable to exert any further elevation of Pitx1 transcription in PITX2-deficient thyrotrophs, resulting in the blunted response (Fig. 6). Taken together, these results are consistent with the idea that the modest growth defect and smaller thyroid glands of Pitx2flox/−;Tg(Tshb-cre) mice under basal conditions are caused by the loss of PItx2 in thyrotrophs.
Fig. 6.
Model for thyrotroph function and response to hypothyroidism in the presence or absence of PITX2. Pituitary thyrotrophs secrete sufficient TSH in adult mice to maintain a euthyroid state in the presence or absence of Pitx2. Pitx1 transcript levels are elevated in the absence of Pitx2, suggesting the possibility of a compensatory role for Pitx1. The pituitary thyrotrophs of both normal and Pitx2-deficient mice produce additional TSH in response to hypothyroidism induced by PTU, but the response is blunted in Pitx2-deficient mice. Hypothyroidism causes a dramatic increase in Pitx1 transcription in normal mice, but Pitx1 transcript levels do not increase further in Pitx2-deficient mice, possibly because an additional increase in Pitx1 transcription is not achievable. These observations support the hypothesis that Pitx1 and Pitx2 have overlapping roles in thyrotrophs, and that Pitx1 is of major importance in response to hypothyroidism.
We expected that PITX2 would be important for gonadotroph function because Pitx2neo/neo mice lack gonadotrophs and have decreased expression of the gonadotroph transcription factors Gata2, Nr5a1, and Egr1. Moreover, PITX2 is expressed in the majority of adult gonadotrophs. We ablated Pitx2 in gonadotrophs using a specific Lhb-cre recombinase and found that PITX2 is dispensable for gonadotroph function after birth (17). PITX1 is expressed in adult gonadotrophs and could compensate for PITX2 deficiency.
Pitx1 mutant pituitaries are minimally affected just before birth, while Pitx2 null pituitaries are extremely underdeveloped. Thus, PITX2 is more important than PITX1 in pituitary development (20, 21). Our data suggests that PITX1 is an important contributor to adult pituitary function, especially in response to hypothyroidism, and that the overall dosage of PItx1 and Pitx2 contributes to pituitary regulation of homeostasis.
PITX2 mutations are rare in patients with combined pituitary hormone deficiencies
A variety of PITX2 mutations have been reported in patients with Axenfeld-Rieger syndrome. This is a genetically heterogenous condition that consists in underdevelopment of the anterior segment of the eye and associated tooth and craniofacial anomalies (14, 31–34). A few patients have morphological anomalies of the sella turcica (35). Only three patients with Axenfeld-Rieger syndrome have been reported with pituitary hypoplasia and GH deficiency, without thyrotroph deficiency. Our study of genetically modified mice suggests that PITX2-deficient patients may compensate by inducing elevated expression of PITX1. The stature of Axenfeld-Rieger patients is usually normal or rarely slightly reduced (33). Administering a TRH stimulation test for Axenfeld-Rieger patients could test this idea, but it is not clinically justifiable because these patients would only present partial thyrotroph deficiency if hypothyroid, which could be easily treated with thyroid hormone replacement. It is possible that some patients with isolated TSH deficiency could have mutations in PITX1 or PITX2 but the mutations would likely be in putative regulatory regions specific for thyrotrophs because systemic gene deletion in mice causes limb and cardiac anomalies in Pitx1- and Pitx2-deficient mice, respectively.
The overlapping roles of PITX1 and PITX2 in the pituitary gland probably explain the very low frequency of pituitary deficiencies in patients with Axenfeld-Rieger syndrome. The fact that doubly heterozygous mice have extremely poor viability points to the likelihood of overlapping roles in other critical organs like the head and the heart (20). Doubly heterozygous mutations of PITX1 and PITX2 could exist in patients with pituitary deficiencies and effects on a constellation of other organs. The phenomenon of digenic inheritance has been reported for patients with hypogonadotrophic hypogonadism and mutations in FGFR1, GnRH receptor, and NELF (36). Although humans doubly heterozygous for mutations in PITX1 and PITX2 might be not viable, the threshold for dosage-sensitive defects is often different in humans and mice (37).
In conclusion, we developed and characterized a new effective cre recombinase transgenic line that is specific for committed thyrotroph cells. This tool allowed us to test the importance of PITX2 in thyrotrope function. Mice with a conditional inactivation of Pitx2 in thyrotrophs are able to maintain TSH production and do not undergo obvious thyrotroph hypertrophy or hyperplasia. The affected mice have a moderate growth deficiency, and a blunted TSH response to hypothyroidism challenge, which supports the idea of a slight deficiency in thyrotroph function. The action of PITX2 may be partially compensated by PITX1 because Pitx1 is elevated in normal mice in response to induced hypothyroidism, and previous data show that these two transcription factors have overlapping roles in early pituitary development (20). These results also suggest that patients with Axenfeld-Rieger syndrome induced by PITX2 mutations could present a partial thyrotroph deficiency.
Materials and Methods
Generation of Tshb-cre transgene construct
The Tshb-cre transgene was generated by recombineering a mouse Tshb BAC from a male C57BL6 library (RP24-230F23, Roswell Park Institute) (24). The Tshb-containing BAC included: 144 kb of 5′-flanking region, all five exons, with intervening introns, and 40 kb of 3′-flanking region. In the murine Tshb gene, the first three exons are noncoding and contain an alternate splice site, whereas exons 4 and 5 encode the complete protein. In the recombineered BAC, the coding region of Tshb was substituted for nuclear localization signal (NLS)-cre-β-actin, using a NLS-cre-β-actin cassette, flanked by approximately 50 nucleotides 5′- and 3′-homology arms of Tshb immediately upstream and downstream of the initiation and termination codons, respectively. The final Tshb-cre BAC construct was confirmed by amplification and DNA sequencing of NLS-cre-β-actin, using primers outside of the recombination site (exons 4 and 5 of Tshb). The primers 5′-tgttcttgttattggctgtg-3′ and 5′-gcttcgtaagctctctaatg-3′ were used with the following conditions: 94 C for 3 min, followed by 35 cycles of 98 C for 20 sec, 57 C for 30 sec, and 72 C for 90 sec, and a final 72 C extension step for 10 min. In addition, the transgene-vector (pTARBAC) splice junctions were confirmed using T7 and SP6 primer sites in the vector.
Mice
All mice were maintained at the University of Michigan under the guidelines of the Unit for Laboratory Animal Medicine and the University Committee for Care and Use of Animals.
The transgenic founders carrying the Tshb-cre BAC were generated by microinjecting (C57BL/6 × SJL)F1 × F1 fertilized eggs and transferring to surrogate mothers. Founders were identified by amplification of genomic DNA from tail biopsies with primers designed to amplify the vector-genomic DNA junctions at the 5′- and 3′-ends of the vector and at the Tshb-cre fusion site in exons 4 and 5. The universal cre primers 5′-gcataaccagtgaaacagcattgctg-3′ and 5′-ggacatgttcagggatcgccaggcg-3′ were used with the following conditions: 94 C for 3 min, followed by 32 cycles of 94 C for 30 sec, 60 C for 60 sec, and 72 C for 90 sec, and a final 10-min extension at 72 C.
Tg(Tshb-cre) mice were maintained by crossing transgenic founders with C57BL/6J mice from The Jackson Laboratory (Bar Harbor, ME). B6;129-Gt(ROSA)26Sortm2Sho/J cre-reporter mice were obtained from The Jackson Laboratory and maintained as homozygotes. These mice, referred to here as “floxed-LacZ” mice, were genotyped by PCR using primers 5′-ggcttaaaggctaacctgatgtg-3′, 5′-gcgaagagtttgtcctcaacc-3′, and 5′-ggagcgggagaaatggatatg-3′ under the following conditions: 94 C for 3 min, followed by 35 cycles of 94 C for 30 sec, 64 C for 60 sec, and 72 C for 60 sec, and a final 10-min extension at 72 C. The B6;129-Gt(ROSA)26-Sortm2Sho/J band is 1146 bp and wild-type band is 374 bp.
For transgene analysis, experimental animals carried one allele of the cre transgene and one allele of the reporter gene, whereas controls were negative for the cre transgene but positive for a reporter gene.
Pitx2flox/−;Tg(Tshb-cre) mice were generated by mating B6-Pitx2+/− mice with Tg(Tshb-cre) positive mice. The Pitx2+/−;Tg(Tshb-cre) offspring were mated to B6-Pitx2flox/flox mice, and genotyping was performed as previously described (15).
Tissue preparation and histology
X-gal staining
X-gal staining was performed as previously described (38). Pituitaries were fixed in 4% formaldehyde overnight, rinsed in PBS, dehydrated, and embedded in a Citadel 1000 (Thermo Electric, Chesire, UK) paraffin-embedding machine, and sectioned coronally at 5 μm thickness. Immunohistochemical detection of the pituitary hormones was performed as previously described (39). Embryos and adult organs were dissected, frozen on dry ice, and stored at −80 C. Embryos were embedded in OCT (Sakura Finetek, Torrance, CA) and cryosectioned at 16 μm thickness. After X-gal staining, sections were counterstained for 2 min with 1% neutral red stain plus 4% sodium acetate-glacial acetic acid. Sections were dehydrated and mounted with xylene-permount 1:2 (Fisher Scientific, Pittsburgh, PA) mounting media. Whole-mount capture of freshly dissected Tg(Tshb-creSac1 x B6;129-Gt(ROSA)26Sortm2Sho/J progeny was achieved using a Leica MZFL III stereo/dissecting fluorescent microscope (Leica Corp., Deerfield, IL).
Pituitaries
Adult pituitaries were collected at 4 and 8 wk of age and fixed for 1 h in 4% formaldehyde in PBS. Newborn heads (d 2 after birth) were also collected and fixed overnight in 4% formaldehyde in PBS. Pituitaries and heads were rinsed in PBS, dehydrated, and embedded in a Citadel 1000 (Thermo Electric, Chesire, UK) paraffin-embedding machine, and sectioned coronally at 5 μm thickness.
Immunohistochemistry for PITX2 and TSHß was performed on pituitary sections from 4- and 8-wk-old mice. Epitopes were unmasked by boiling in citric acid (10 mm) for 5 min. After 10 min recovery at room temperature, endogenous peroxidases were quenched in 3% H202 for 20 min. After 1 h of Tyramide signal amplification (TSA) blocking (PerkinElmer, Boston, MA,), TSHß antibody (from the National Hormone Pituitary Program, Torrance, CA) was diluted 1:500, and 100 μl were placed on each slide overnight at 4 C. Slides were washed three times for 5 min using 0.5% Triton-X100 in 1× PBS. Washing with 0.5% Triton-X100 in PBS was followed by a 1-h incubation with biotinylated antirabbit secondary antibody and 0.5% Triton-X100/PBS washes. Streptavidin-horseradish peroxidase (PerkinElmer) was added for 1 h at 1:200 dilution, followed by three washes in 1× PBS. TSA-tetramethylrhodamine isothiocyanate (PerkinElmer) was diluted 1:50, and 100 μl were placed on each slide for 10 min. Two blockings were then performed: antirabbit IgG diluted 1:100 was placed on each slide for 3 h. After three washing steps in 0.5% Triton-X100 in PBS, another blocking step was performed with 5% normal goat serum, 10% avidin, 10% biotin, in TSA block for 1 h. Rabbit-anti-PITX2 antibody, a gift from J. Drouin (Montreal, Canada), was diluted 1:300 in the same block described earlier, after which 100 μl were placed on each slide overnight at 4 C. Secondary detection was performed as described earlier using biotinylated antirabbit antibody (Vector Laboratories, Inc., Burlingame, CA) and then washed in PBS/Triton. Streptavidin-horseradish peroxidase was added for 1 h at 1:200 dilution, followed by three washes in 1× PBS. TSA-fluorescein isothiocyanate (PerkinElmer) was diluted 1:50, and 100 μl were placed on each slide for 10 min. 4′,6-diamidino-2-phenyl indole (DAPI) was diluted 1:600 in PBS and placed on each slide for 5 min. After three washes in PBS, slides were mounted with fluorescent mounting media, and images were captured using a Leica DMRB fluorescent microscope.
TSHß staining was performed identically in newborns and 4- to 8-wk-old mice. After TSA-tetramethylrhodamine isothiocyanate, nuclear staining with DAPI was performed, and slides were mounted and captured using a fluorescent microscope. Staining was performed systematically every five slides in at least three controls and three transgenic pituitaries.
Thyroid volumetrics
Adult thyroids were collected at 4 and 8 wk of age or later and fixed for 1 h in 4% formaldehyde in PBS. Hematoxylin and eosin staining was carried out for 20 sec each on 5-μm sections. Thyroid area was measured every 10 sections with Image J software, and the whole thyroid volume (mm3) was determined by multiplying by the section thickness (0.005 mm). PITX2 staining was performed as previously described in the thyroids of 8-wk-old control and Pitx2flox/−;Tg(Tshb-cre) mice.
Hypothyroidism challenge
Low-iodine diet enriched in PTU (0.15%) (Harlan Laboratories, Madison, WI) was given to 4- to 8-wk-old Pitx2flox/−;Tg(Tshb-cre) and control mice for 4 wk (40).
Hormone and mRNA evaluations
Blood was collected from 4- to 8-wk-old mice fed regular chow or low-iodine diet by cardiac puncture after the mice were euthanized and their hearts were still beating. After collection, the blood clotted at 4 C for 24 h and was centrifuged at 8000 × g for 10 min. The serum was analyzed for total T4 (5 μl) concentration (MP Biomedicals, Solon, OH), and TSH levels (Millipore Corp., Bedford, MA) (25 μl of serum diluted in Serum Assay Buffer included in the Millipore TSH Multiplex kit. Serum from euthyroid animals was diluted 1:5. Serum from mice placed on low-iodine diet and PTU was diluted 1:50). Each T4 measure was performed in triplicate, whereas TSH level was measured in single samples.
Tshb (Mm00437190_m1), Pitx1 (Mn00440824_m1), and Pitx2 (Mm00660192_g1) mRNA levels were evaluated by quantitative real-time PCR (Applied Biosystems, Foster City, CA) in cDNA prepared from pituitaries of 4- to 8-wk-old transgenic and control mice with or without low-iodine diet enriched in PTU. Gapdh (4308313) levels were used to normalize the results, and Cga (Mm00438189_m1) mRNA levels were used as a positive control. Igf1 (Mm00439559_m1) mRNA levels were evaluated by real-time PCR in liver cDNA from 8-wk-old transgenic and control mice.
Statistical analysis
Data are given as mean ± sd. Student's t test or ANOVA (for continuous data) was used for statistical comparisons. Data were analyzed with SPSS version 17.0. P < 0.001 was considered significant.
Acknowledgments
We thank Dr. Jun Z. Li and his laboratory, and Dr. James Harper and the Miller Laboratory for their help. We thank the following individuals for reagents: Neal Copeland, National Cancer Institute for recombinogenic bacterial strains and DNA plasmids, Mark Lewandoski, NIH, for pML78 containing the NLS-cre-β-actin cassette, and Al Parlow and the National Hormone and Pituitary Program for antibodies. The transgene was generated by D.F.G., J.M.K., and E.C.R., Tshb-cre transgenic mice were bred and characterized by M.L.B. and A.H.M., Pitx2flox/−;Tg(Tshb-cre) mice were bred and analyzed by F.C., serum TSH was measured by K.R.V. and A.H., and the major contributors to writing the manuscript were F.C., M.L.B., and S.A.C.
This work was supported by grants from Novo-Nordisk, Societe Francaise d'endocrinologie (to F.C. and T.B.); Novartis, Ipsen, ADEREM, and the Center for Genetics in Health and Medicine, University of Michigan (all for F.C.); the National Institutes of Health (R37HD30428 and R01HD34283 to S.A.C.); and NIH grants (CA46592, AR20557, DK34933, DK20572, P30DK08194) to the University of Michigan Transgenic Animal Model Core facility.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAC
- Bacterial artificial chromosome
- DAPI
- 4′,6-diamidino-2-phenyl indole
- e8
- embryonic d 8
- LIM
- Lin11, Isl1, and Mec-3
- NLS
- nuclear localization signal
- PTU
- propylthiouracil
- TSA
- Tyramide signal amplification.
References
- 1. Burrows HL, Douglas KR, Seasholtz AF, Camper SA. 1999. Genealogy of the anterior pituitary gland: tracing a family tree. Trends Endocrinol Metab 10:343–352 [DOI] [PubMed] [Google Scholar]
- 2. Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. 2009. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30:790–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis SW, Castinetti F, Carvalho LR, Ellsworth BS, Potok MA, Lyons RH, Brinkmeier ML, Raetzman LT, Carninci P, Mortensen AH, Hayashizaki Y, Arnhold IJ, Mendonça BB, Brue T, Camper SA. 2010. Molecular mechanisms of pituitary organogenesis: in search of novel regulatory genes. Mol Cell Endocrinol 323:4–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castinetti F, Saveanu A, Reynaud R, Quentien MH, Buffin A, Brauner R, Kaffel N, Albarel F, Guedj AM, El Kholy M, Amin M, Enjalbert A, Barlier A, Brue T. 2008. A novel dysfunctional LHX4 mutation with high phenotypical variability in patients with hypopituitarism. J Clin Endocrinol Metab 93:2790–2799 [DOI] [PubMed] [Google Scholar]
- 5. Ellsworth BS, Butts DL, Camper SA. 2008. Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Dev Biol 313:118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raetzman LT, Ward R, Camper SA. 2002. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development 129:4229–4239 [DOI] [PubMed] [Google Scholar]
- 7. Sheng HZ, Zhadanov AB, Mosinger B, Jr, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H. 1996. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science 272:1004–1007 [DOI] [PubMed] [Google Scholar]
- 8. Li S, Crenshaw EB, III, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. 1990. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347:528–533 [DOI] [PubMed] [Google Scholar]
- 9. Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carrière C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. 1996. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384:327–333 [DOI] [PubMed] [Google Scholar]
- 10. Camper SA, Saunders TL, Katz RW, Reeves RH. 1990. The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics 8:586–590 [DOI] [PubMed] [Google Scholar]
- 11. Charles MA, Saunders TL, Wood WM, Owens K, Parlow AF, Camper SA, Ridgway EC, Gordon DF. 2006. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol Endocrinol 20:1366–1377 [DOI] [PubMed] [Google Scholar]
- 12. Gage PJ, Camper SA. 1997. Pituitary homeobox 2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Hum Mol Genet 6:457–464 [DOI] [PubMed] [Google Scholar]
- 13. Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu F, Izpisúa-Belmonte JC, Rosenfeld MG. 1999. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 401:279–282 [DOI] [PubMed] [Google Scholar]
- 14. Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC. 1996. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet 14:392–399 [DOI] [PubMed] [Google Scholar]
- 15. Gage PJ, Suh H, Camper SA. 1999. Dosage requirement of Pitx2 for development of multiple organs. Development 126:4643–4651 [DOI] [PubMed] [Google Scholar]
- 16. Suh H, Gage PJ, Drouin J, Camper SA. 2002. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development 129:329–337 [DOI] [PubMed] [Google Scholar]
- 17. Charles MA, Mortensen AH, Potok MA, Camper SA. 2008. Pitx2 deletion in pituitary gonadotropes is compatible with gonadal development, puberty, and fertility. Genesis 46:507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quentien MH, Pitoia F, Gunz G, Guillet MP, Enjalbert A, Pellegrini I. 2002. Regulation of prolactin, GH, and Pit-1 gene expression in anterior pituitary by Pitx2: An approach using Pitx2 mutants. Endocrinology 143:2839–2851 [DOI] [PubMed] [Google Scholar]
- 19. Tremblay JJ, Goodyer CG, Drouin J. 2000. Transcriptional properties of Ptx1 and Ptx2 isoforms. Neuroendocrinology 71:277–286 [DOI] [PubMed] [Google Scholar]
- 20. Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ. 2005. PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol 19:1893–1903 [DOI] [PubMed] [Google Scholar]
- 21. Szeto DP, Ryan AK, O'Connell SM, Rosenfeld MG. 1996. P-OTX: a PIT-1-interacting homeodomain factor expressed during anterior pituitary gland development. Proc Natl Acad Sci USA 93:7706–7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McIntyre DC, Rakshit S, Yallowitz AR, Loken L, Jeannotte L, Capecchi MR, Wellik DM. 2007. Hox patterning of the vertebrate rib cage. Development 134:2981–2989 [DOI] [PubMed] [Google Scholar]
- 23. Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H. 1997. Multistep control of pituitary organogenesis. Science 278:1809–1812 [DOI] [PubMed] [Google Scholar]
- 24. Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camper SA, Saunders TL, Kendall SK, Keri RA, Seasholtz AF, Gordon DF, Birkmeier TS, Keegan CE, Karolyi IJ, Roller ML, et al. 1995. Implementing transgenic and embryonic stem cell technology to study gene expression, cell-cell interactions and gene function. Biol Reprod 52:246–257 [DOI] [PubMed] [Google Scholar]
- 26. Westlund KN, Chmielowiec S, Childs GV. 1983. Somatostatin fibers and their relationship to specific cell types (GH and TSH) in the rat anterior pituitary. Peptides 4:557–562 [DOI] [PubMed] [Google Scholar]
- 27. Huang Y, Guigon CJ, Fan J, Cheng SY, Zhu GZ. 2010. Pituitary homeobox 2 (PITX2) promotes thyroid carcinogenesis by activation of cyclin D2. Cell Cycle 9:1333–1341 [DOI] [PubMed] [Google Scholar]
- 28. Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA. 1995. Targeted disruption of the pituitary glycoprotein hormone α-subunit produces hypogonadal and hypothyroid mice. Genes Dev 9:2007–2019 [DOI] [PubMed] [Google Scholar]
- 29. Yamada M, Satoh T, Mori M. 2003. Mice lacking the thyrotropin-releasing hormone gene: what do they tell us? Thyroid 13:1111–1121 [DOI] [PubMed] [Google Scholar]
- 30. Jones PM, Burrin JM, Ghatei MA, O'Halloran DJ, Legon S, Bloom SR. 1990. The influence of thyroid hormone status on the hypothalamo-hypophyseal growth hormone axis. Endocrinology 126:1374–1379 [DOI] [PubMed] [Google Scholar]
- 31. Phillips JC. 2002. Four novel mutations in the PITX2 gene in patients with Axenfeld-Rieger syndrome. Ophthalmic Res 34:324–326 [DOI] [PubMed] [Google Scholar]
- 32. Saadi I, Toro R, Kuburas A, Semina E, Murray JC, Russo AF. 2006. An unusual class of PITX2 mutations in Axenfeld-Rieger syndrome. Birth Defects Res A Clin Mol Teratol 76:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tümer Z, Bach-Holm D. 2009. Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet 17:1527–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weisschuh N, Dressler P, Schuettauf F, Wolf C, Wissinger B, Gramer E. 2006. Novel mutations of FOXC1 and PITX2 in patients with Axenfeld-Rieger malformations. Invest Ophthalmol Vis Sci 47:3846–3852 [DOI] [PubMed] [Google Scholar]
- 35. Meyer-Marcotty P, Weisschuh N, Dressler P, Hartmann J, Stellzig-Eisenhauer A. 2008. Morphology of the sella turcica in Axenfeld-Rieger syndrome with PITX2 mutation. J Oral Pathol Med 37:504–510 [DOI] [PubMed] [Google Scholar]
- 36. Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W. 2007. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 117:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strachan T, Read AP. 1994. PAX genes. Curr Opin Genet Dev 4:427–438 [DOI] [PubMed] [Google Scholar]
- 38. Brinkmeier ML, Gordon DF, Dowding JM, Saunders TL, Kendall SK, Sarapura VD, Wood WM, Ridgway EC, Camper SA. 1998. Cell-specific expression of the mouse glycoprotein hormone α-subunit gene requires multiple interacting DNA elements in transgenic mice and cultured cells. Mol Endocrinol 12:622–633 [DOI] [PubMed] [Google Scholar]
- 39. Kendall SK, Saunders TL, Jin L, Lloyd RV, Glode LM, Nett TM, Keri RA, Nilson JH, Camper SA. 1991. Targeted ablation of pituitary gonadotropes in transgenic mice. Mol Endocrinol 5:2025-2036 [DOI] [PubMed] [Google Scholar]
- 40. Weiss RE, Murata Y, Cua K, Hayashi Y, Seo H, Refetoff S. 1998. Thyroid hormone action on liver, heart, and energy expenditure in thyroid hormone receptor beta-deficient mice. Endocrinology 139:4945–4952 [DOI] [PubMed] [Google Scholar]