TNF is required for prostate regression and increases in a paracrine manner following castration; conversely, TRAIL and Fas are dispensable for this process.
Abstract
TNF, a proinflammatory and immune-regulatory cytokine, is a potent apoptotic stimulus in vitro. However, there have been few examples of a physiologic role for TNF-induced apoptosis in vivo. Here, we describe a novel role for TNF in prostate epithelial cell apoptosis after androgen withdrawal. Employing high-resolution serial magnetic resonance imaging to measure mouse prostate volume changes over time, we demonstrate that the extent of castration-induced prostate regression is significantly reduced in mice null for either the Tnf or Tnfr1 genes but not mice deficient for TNF-related apoptosis-inducing ligand or Fas signaling. Wild-type mice receiving soluble TNF (sTNF) receptor 2 (to bind TNF and block signaling) before castration exhibit an identical reduction of prostate regression. Together, these data indicate that uniquely among known extrinsic death signals, TNF is required for castration-induced prostate regression. Additionally, membrane-bound TNF protein and stromal cell specific TNF mRNA levels increase in rat prostate after castration. This is consistent with a paracrine role for TNF in prostate regression. When injected into the peritoneum of Tnf−/− mice at the time of castration, sTNF restores normal levels of prostate regression. However, wild-type mice receiving sTNF in the absence of castration do not exhibit prostate regression, indicating that TNF alone is not sufficient but acts in the context of additional castration-induced signals. These findings support a physiologic role for TNF in prostate regression after androgen withdrawal. Understanding this role may lead to novel therapies for prostate cancer.
Androgen withdrawal induces apoptosis of the secretory epithelium in normal prostate glands. Because prostate adenocarcinoma arises from the epithelial cell population in the prostate, androgen deprivation is used to treat both localized and metastatic disease (1). Metastatic cancers initially respond. However, the utility of androgen deprivation therapy (ADT) is limited, because it causes severe side effects (2–5) and because metastatic cancers usually become resistant to this therapy, developing into lethal androgen-deprivation resistant cancers (6). Thus, there is a need for novel targeted apoptotic therapies to overcome the limitations of ADT.
The mechanisms controlling androgen withdrawal-induced apoptosis (AWIA) in the normal rodent prostate, a model for ADT in human prostate cancers, are only partly understood. Castration affects expression of the apoptosis regulators Bcl-2 and Bcl-XL (7–9), as well as TGFβ (10). Direct administration of exogenous TGFβ induces partial prostate regression (11), suggesting that AWIA requires paracrine production of TGFβ by stromal cells (10, 12). The extrinsic apoptotic pathway, initiated by binding of death receptor ligands [Fas ligand (FasL), TNF-related apoptosis-inducing ligand (TRAIL), and TNF] to the corresponding death receptors, can be activated in prostate epithelial cell lines (13–15) and therefore may play a role in triggering AWIA in vivo. All three ligands stimulate the formation of protein complexes containing the adaptor molecule Fas-associated death domain (FADD) containing protein, initiating cleavage and activation of caspase-8, which in turn cleaves and activates effector caspases (e.g. caspase-3) (16). FADD-like IL-1β-converting enzyme-like inhibitory protein (FLIP) is a dominant negative inhibitor of caspase-8 (17). Previously, we demonstrated that FLIP mRNA and protein expression decline in the rat prostate after castration (18, 19), suggesting a role for death receptor signaling in this process. A critical question is which ligand is relevant in vivo.
An early investigation, relying on dissection and weighing, found that lpr mice (mutated in the Fas gene) were resistant to AWIA of the prostate, suggesting a role for FasL (20). However, others (21, 22) reexamined AWIA in lpr and gld (FasL deficient) mice histologically, using terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling (TUNEL) to quantitate apoptosis and concluded that these mutations do not affect AWIA. Underlying this conflicting data is the realization that it is difficult to accurately quantitate prostate regression in genetically engineered mice. Dissection of the mouse prostate is technically challenging, because the gland is quite small (∼20 mm3) and interdigitated with surrounding tissues. Histological determination of AWIA is also difficult, because the number of apoptotic cells is merely a few percent of the total, and these apoptotic cells are quite variably distributed among the glands. In addition, the appearance of pyknotic and TUNEL-staining cells is quite transitory, spanning a period of only 4 h in the rodent prostate (23). To reproducibly quantitate the volume of murine prostate glands, we developed a high-resolution magnetic resonance imaging (MRI) technique with chemical shift suppression (24) that allows discrimination of the dorsal-lateral prostate (DLP) lobes from the ventral prostate (VP) lobes in individual mice and, because it is noninvasive, can be repeated on the same mouse over multiple imaging sessions. Here, we use this technique to reproducibly measure small changes in prostate volume after castration in mice deficient in extrinsic death signaling.
Results
TRAIL and FasL signaling are not required for prostate regression
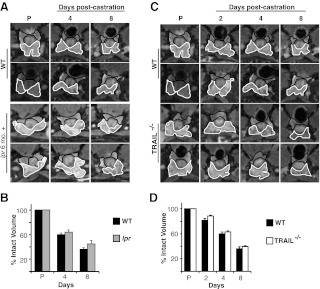
Unlike the human prostate, which is a singular organ, the rodent prostate consists of multiple lobes. The rodent VP contains up to 80% secretory epithelial cells. Because these cells are the target cell population for AWIA, the VP undergoes the greatest degree of regression after castration. Specifically, both rat and mouse VP regress 60–75%, within 8 d of castration (18, 24). The DLP contains a smaller fraction of secretory epithelium and undergoes correspondingly less regression after castration. To resolve conflicting data on the importance of Fas signaling in AWIA, we examined lpr mice for delayed or reduced regression of the prostate. Individual animals were examined sequentially using MRI, before as well as 4 and 8 d after castration, and prostate lobe volumes were determined from the images as described (24). There was no significant difference in the degree of regression between 6- to 8-month-old lpr and age-matched wild-type (WT) mice for the VP (Fig. 1, A and B). The lpr mice were near the reported age for onset of lymphoadenopathy; to eliminate any confounding effects, 3-month-old lpr mice were similarly examined. The starting total volume was 25% smaller than 6-month-old lpr mice (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org), but there was no significant difference in either the rate or total extent of regression after castration (Supplemental Fig. 2).
Fig. 1.
Neither FasL nor TRAIL is necessary for prostate regression after castration. A, Representative MR image series from two WT and 6-month-old lpr mice before castration (P) as well as 4 and 8 d after castration. The VP is outlined in white and the DLP in black. The genotype for each series is indicated to the left of each row. B, VP volume was calculated from MR images for WT and 6-month-old lpr animals (shown in A) before castration (P) as well as 4 and 8 d after castration. Data for each group are plotted as percentage of precastration volume (n = 4 for each genotype). Bars, sem. C, Representative MR image series from WT (from A) and two Trail−/− mice before castration (P) as well as 2, 4, and 8 d after castration. D, VP volume was calculated from MR images for WT and Trail−/− animals (shown in A) before castration (P) as well as 2, 4, and 8 d after castration. Data for each group are plotted as percentage of precastration volume (n = 4 for each genotype). Bars, sem.
Because there have been multiple reports that TRAIL induces apoptosis in prostate cell lines in vitro (14, 25, 26), the extent of castration-induced prostate regression was also examined in Trail−/− mice. Lack of TRAIL did not alter the volume of the prostate in intact (noncastrate) mice compared with WT mice (Supplemental Fig. 1). In addition, at 2, 4, and 8 d after castration, there was no difference in the extent of VP regression between Trail−/− and WT mice (Fig. 1, C and D).
TNF signaling is required for prostate regression
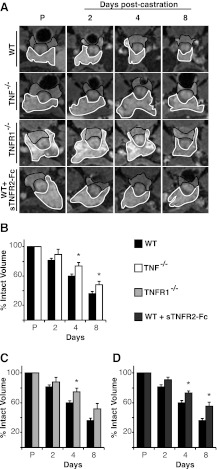
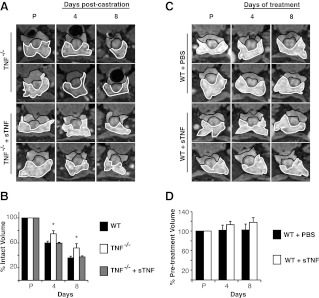
Because neither TRAIL nor Fas loss affected AWIA, we next examined castration-induced prostate regression in Tnf−/− mice. These mice are viable and develop normally but are defective in primary B-cell follicle formation and have increased susceptibility to bacterial infection (27). Mice were castrated, MR imaged, and prostate volume was determined after castration as above. Before castration, the VP volume of Tnf−/− mice did not differ significantly from WT (Supplemental Fig. 1). Four days after castration, WT mouse VP regressed 40 ± 3%, whereas Tnf−/− mouse VP regressed 25 ± 5% (P < 0.05) (Fig. 2, A and B). Eight days after castration, WT mouse VP regressed 64 ± 2%, whereas Tnf−/− mouse VP only regressed 48 ± 7% (P < 0.05) (Fig. 2, A and B). Thus, in the absence of TNF signaling, mice display a partial, but significant, inhibition of castration induced prostate regression.
Fig. 2.
TNF signaling is required for complete regression of mouse prostate after castration. A, Representative MR image series from one animal of each group before castration (P) and at 2, 4, and 8 d after castration. The VP is outlined in white and the DLP in black. The genotype or treatment for each series is indicated to the left of each row. Note that the WT animals shown are additional animals from the same group analyzed in Fig. 1. B–D, VP volume was determined for each group and is plotted as percent of precastration volume. The WT controls are the same animals for all three plots. *, P < 0.05 by ANOVA (n = 4 for each treatment or genotype). Bars, sem.
TNF binds two receptors [TNF receptor (TNFR)1 and TNFR2]. TNFR2 is expressed mainly in the immune system and lacks a functional death domain, preventing direct interaction with the adapter molecules TNFR1-associated DEATH domain and FADD. It is unlikely to activate caspase-8 and initiate apoptosis. In contrast, TNFR1 is ubiquitously expressed and binds TNFR1-associated DEATH domain to signal either through Nuclear Factor κB to activate inflammation and survival or through FADD to activate caspase-8 cleavage and apoptosis (28). As with Tnf−/− mice, Tnfr1−/− mice develop normally but exhibit increased susceptibility to certain bacterial pathogens (29). To confirm the reduced regression phenotype observed in Tnf−/− mice, Tnfr1−/− mice were castrated and MR imaged as above. Before castration, there was more variability in VP volume of Tnfr1−/− mice, but the volume did not differ significantly from WT (Supplemental Fig. 1). Tnfr1−/− mice exhibit a pattern of reduced prostate regression identical to that observed in Tnf−/− mice, regressing 26 ± 8% (P < 0.05) at 4 d and 50 ± 5% (P > 0.05) at 8 d (Fig. 2, A and C). These data are consistent with TNF acting through TNFR1 and confirms that TNF death signaling is required for normal prostate regression.
It is possible that these knockout mice have developed compensatory changes that account for the partial inhibition of regression observed in Fig. 2, B and C. To address this possibility, TNF signaling was blocked acutely in WT mice, by treating these animals with a soluble form of TNFR2 [sTNFR2-fragment crystalizable (Fc)] that binds to endogenous TNF, preventing activation of either TNFR1 or TNFR2. When sTNFR2-Fc was administered to WT mice before castration, at a dose previously shown to block TNF-induced inflammation in mice (30), MRI volume measurements revealed partial inhibition of prostate regression, very similar to that observed in Tnf−/− and Tnfr1−/− mice. Specifically, the VP of WT mice treated with sTNFR2-Fc regressed 27 ± 2% (P < 0.05) at 4 d and 45 ± 5% (P < 0.05) at 8 d after castration (Fig. 2D). This indicates that developmental compensation does not play a significant role in the prostate regression phenotype. Because sTNFR2-Fc blocks binding to either receptor, these experiments also show that TNFR2 is not required for AWIA. Taken together, the data in Fig. 2 demonstrate that TNF signaling is required for complete prostate regression after castration.
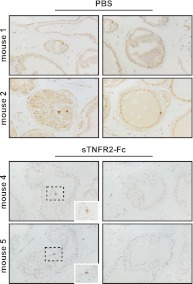
Castration-induced regression of the rodent prostate is an apoptotic event. To confirm that the observed reduction in prostate regression after TNF signaling ablation is detectable as a corresponding reduction in apoptosis, WT mice were treated with either PBS or sTNFR2-Fc before castration, and prostate tissues were immunohistochemically stained with antibody against the active (cleaved) form of caspase-3. There is a clear increase in epithelial cells positive for active caspase-3 when comparing intact vs. castrate (data not shown, DNS), and by 2 d, glands from PBS-treated castrate mice were atrophic and contained regions of diffuse cytoplasmic staining as well as occasional dark staining pyknotic cells indicative of activated caspase-3 (Fig. 3, top). However, although there was a trend toward a lower frequency of positive (apoptotic) epithelial cells in glands from sTNFR2-Fc-treated mice compared with PBS-treated mice (P < 0.3), there was no reduction in apparent atrophy, and the difference in darkly positive cells was not significant (Fig. 3 and DNS). Although TUNEL is a well-accepted histochemical indication of apoptosis, relative to active caspase-3 staining, the duration of TUNEL positivity in apoptotic cells is short (31). Using TUNEL to measure the apoptotic rate in VP epithelial cells, again, there is a significant increase in positive cells when comparing intact vs. castrate (P < 0.01, DNS). Confirming the observation in Fig. 3, there is no significant difference between sTNFR2-Fc and PBS-treated castrated mice (Supplemental Fig. 3, A and B). These histochemical results confirm numerous previous reports that castration induces apoptosis of prostate epithelial cells. However, we did not observe a castration-induced difference for these apoptosis biomarkers between mice treated with PBS vs. those receiving TNFR2-Fc. Using MRI, even small differences in the rate of apoptosis, when integrated over 4–8 d, produce a discernable effect on prostate volume. Additionally, variability of the histochemical measures due to sampling at a particular time point during the course of regression is reduced by the integration of the rates over many days. This suggests that MRI is a much more sensitive measure of the cumulative effect of apoptosis on prostate regression.
Fig. 3.
Active (cleaved) caspase-3 immunohistochemical staining of VP sections from mice pretreated with PBS or sTNFR2-Fc, 48 h after castration. Representative photomicrographs from two animals (of three in each group) per treatment (magnification, ×400) are shown. Insets (white box, ×1000) show positive cells from central portion of photomicrograph (outlined in black).
TNF signaling is not constitutive
TNF might trigger apoptosis in response to castration by a variety of signaling mechanisms. For example, androgen withdrawal might acutely induce TNF secretion and apoptosis or TNF might be produced constitutively. If constitutive, TNF might normally act as a homeostatic regulator of prostate size, and the occurrence of AWIA would depend on androgen regulation of downstream signaling events (e.g. receptor up-regulation or inhibitor down-regulation). Our previous observation that the caspase-8 inhibitor FLIP is down-regulated at the onset of AWIA (18, 19) is consistent with this latter hypothesis. If TNF were produced constitutively, then blockade of TNF in the absence of castration should lead to prostate hypertrophy. To test this, intact WT mice were treated with either sTNFR2-Fc or PBS for 24 d and MR imaged every 8 d. Blockade of TNF signaling in WT mice did not cause an increase in prostate volume (Supplemental Fig. 4), suggesting that castration-induced regulation of TNF signaling occurs acutely at the level of the ligand.
TNF expression increases after castration
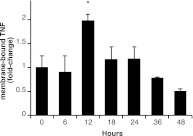
TNF is synthesized as a membrane-bound glycoprotein [membrane TNF (mTNF)] and can be cleaved by the protease TNFα-converting enzyme into a soluble, extracellular form [soluble TNF (sTNF)]. Although mTNF only acts locally, sTNF also activates TNFR1 or TNFR2 at distant sites from the TNF-producing cell. To test whether TNF increases after castration, we assayed TNF protein levels in rat VP 6–48 h after castration (Fig. 4 and Supplemental Fig. 5). Although sTNF was detected in some VP lysates, the expression levels were low and inconsistent between replicates. This could be due to rapid binding, internalization, and degradation of receptor-ligand complexes after activation (32). Conversely, mTNF levels increased 2.0-fold ± 0.1 (P < 0.05) in rat VP, 12 h after castration (Fig. 4). This increase is transient, with mTNF levels returning to baseline within 36 h after castration, perhaps due to rapid cleavage by TNFα-converting enzyme into the soluble form.
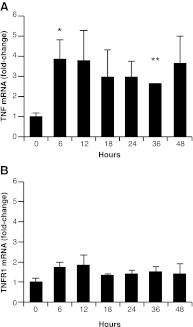
Fig. 4.
TNF protein in the rat VP increases within 12 h of castration. Quantification of mTNF by immunoblot (source data in Supplemental Fig. 5). Columns represent average normalized signal of three or four animals per time point, measured three times. Bars, sem. *, P < 0.05 vs. 0 h by Student's t test.
TNF mRNA increases in stromal cells after castration
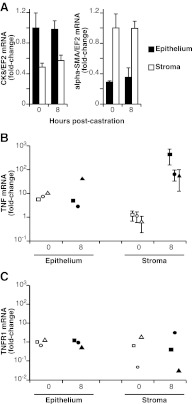
To determine whether the increase in TNF protein resulted from an increase in mRNA, steady-state levels of rat VP Tnf mRNA were measured after castration, using quantitative PCR (qPCR) (18). Figure 5A shows that Tnf mRNA levels increased 3.9-fold ± 1.0 (relative to β-actin) (P < 0.05) within 6 h after castration. Although there is variability throughout the time course, Tnf mRNA was elevated (2.6-fold ± 0.03) up to 36 h after castration (P < 0.01). Tnfr1 mRNA levels remained unchanged (Fig. 5B). To determine the site of Tnf mRNA synthesis, rat VP was collected 8 h after castration, and frozen tissue sections were laser-capture microdissected, to separate epithelial and stromal cell populations. As expected, epithelial cells were enriched for cytokeratin-8, whereas stroma was enriched for α-Sma mRNA (Fig. 6A). After castration, Tnf mRNA increased dramatically (50- to 500-fold) in stromal samples from each animal but in the epithelial compartment of only one animal (Fig. 6B; note logarithmic scale). Although there is variability among animals, increased expression observed in total VP (Fig. 5A) can be attributed to Tnf mRNA derived from the stromal compartment (Fig. 6B), which is a relatively small part of the total prostate mass. In contrast, Tnfr1 mRNA levels did not change in either total or microdissected samples (Figs. 5B and 6C).
Fig. 5.
Tnf mRNA is up-regulated in the rat prostate after castration. A, Tnf; and B, Tnfr1 mRNA levels in total rat VP of intact animals (0) or at the indicated times after castration. Columns represent average mRNA levels relative to intact animals from three animals per time point. Bars, sem. *, P < 0.05; **, P < 0.01 vs. 0 h by Student's t test.
Fig. 6.
Stromal specific Tnf mRNA is up-regulated in the rat prostate after castration. A, Marker gene mRNA levels confirm the separation of distinct cell populations using laser-capture microdissection. Columns represent average normalized signal of three animals per time point, measured three times. B, Tnf; and C, Tnfr1 mRNA levels in microdissected samples. Symbol shapes indicate mRNA from matched tissue samples derived from individual rats microdissected into indicated fractions. Bars, sem of triplicate samples run at least twice.
Soluble TNF restores prostate regression in Tnf−/− mice
The induction of Tnf mRNA and protein after castration suggests that administration of sTNF might restore castration-induced prostate regression in Tnf−/− mice. It has been shown that ip sTNF can rescue Tnf−/− mice from the lethal effects of cecal ligation and puncture (33). To test whether sTNF can restore prostate regression, Tnf−/−, mice were injected with 27 μg/kg sTNF ip at the time of castration as well as 24 h later and MR imaged as above. Human TNF was used because it binds mouse TNFR1 but not mouse TNFR2 (34). Upon castration, the VP of Tnf−/− mice treated with sTNF regressed 41 ± 1% by 4 d and 62 ± 2% by 8 d (Fig. 7, A and B). This regression rate is nearly identical to that of WT castrated mice, demonstrating that exogenous sTNF complemented the Tnf−/− defect (and that TNFR2 signaling is not required).
Fig. 7.
Soluble TNF rescues the prostate regression phenotype of Tnf−/− mice but is not sufficient to induce regression in the absence of castration. A, Representative MR image series of mice before castration (P) as well as 4 and 8 d after castration. MR images from two additional Tnf−/− animals from the group analyzed in Fig. 2B are included for comparison. B, VP regression after castration in WT, Tnf−/− and Tnf−/− plus sTNF. WT and Tnf−/− data (from Fig. 2) are included for comparison. C, Representative MR image series of mice before treatment (P) as well as 4 and 8 d after treatment as indicated. D, VP volume (percent of pretreatment volume) in WT noncastrated mice receiving either sTNF or PBS by injection. n = 4 for each set of animals. Bars, sem. *, P < 0.05 by ANOVA.
Soluble TNF is not sufficient to induce regression in the absence of castration
Because sTNF restores castration-induced prostate regression in Tnf−/− mice, we hypothesized that sTNF might be sufficient to induce prostate regression in the absence of castration. Therefore, intact WT mice were treated with sTNF as in Fig. 7A. The VP of sTNF-treated WT mice did not exhibit detectable regression (Fig. 7, C and D), indicating that sTNF is not sufficient to induce prostate regression in the absence of castration. Instead, sTNF must act in the context of additional castration-induced signals to induce VP apoptosis and regression.
Discussion
In this report, we employed serial high-field MRI technology to demonstrate that TNF is necessary, but not sufficient, for castration-induced regression of the murine prostate. Three key observations support a role for TNF signaling through TNFR1. First, prostate regression is diminished to an almost identical degree in Tnf−/−, Tnfr1−/−, and sTNFR2-Fc-treated WT mice, relative to untreated castrated WT mice. Second, administration of sTNF to Tnf−/− mice at the time of castration restores normal levels of prostate regression. Third, mTNF protein and stromal Tnf mRNA rapidly increase after castration, suggesting a paracrine model in which androgen withdrawal (castration) induces derepression of stromal Tnf gene transcription, triggering apoptosis of the secretory epithelium and regression of the gland. Further defining the mechanism of Tnf transcriptional (or posttranscriptional) regulation is an area for future study.
Despite many reports that TNF induces apoptosis in vitro (15, 35, 36), this is only the second demonstration of a physiologic role for TNF induced apoptosis in vivo. The other in vivo example is luteolysis, the regression of the corpus luteum that follows ovulation. Similar to our results, Tnfr1−/− mice and sTNFR2-Fc-treated WT mice exhibit a reduction in luteolysis (37). Additionally, TNF mRNA increases in the regressing bovine corpus luteum. In contrast to our findings, Tnfr1 and Tnfr2 mRNAs also increase (38). Collectively, the evidence in these studies bears striking similarity to our results, suggesting that luteolysis and AWIA are apoptotic processes that share a molecular mechanism in vivo.
Two of our experiments suggest a role for additional proapoptotic signals in AWIA. First, at a dose that is sufficient to restore castration-induced regression in Tnf−/− mice, sTNF is not sufficient to induce prostate regression in the absence of castration. This shows that additional castration-induced signals are necessary to sensitize the gland to TNF-induced apoptosis. Second, a partial regression phenotype is observed in Tnf−/− and Tnfr1−/− knockout animals and WT animals treated with sTNFR2-Fc. Further, Fig. 1 demonstrates that the other principal death ligands are not the additional signals needed to yield complete regression, because neither lpr nor Trail−/− mice showed any reduction in the extent of castration-induced regression. However, similar to TNF, TGFβ increases after castration (10, 12). Moreover, administration of exogenous TGFβ induces partial regression of rat VP in the absence of castration (11). Although the mechanism of TGFβ apoptosis is uncertain (39), these observations suggest that TGFβ is another death signal that induces partial regression in mice lacking TNF signaling.
Previously, our lab and others have reported that FLIP is transcriptionally regulated by androgens (19, 40, 41) and that FLIP mRNA and protein decline after castration but before the onset of apoptosis (18, 19). This suggests that the decline in FLIP is causal in sensitizing prostate epithelial cells to TNF-induced apoptosis during AWIA. For example, we have shown that in vitro silencing of FLIP in three prostate epithelial cell lines induces apoptosis, whereas FLIP overexpression blocks cell death (19, 42, 43). In the absence of proapoptotic TNF signaling, however, it is unlikely that FLIP down-regulation alone induces apoptosis, because FLIP acts downstream of the TNFR.
In conclusion, our data support a novel role for TNF as an inducer of apoptosis in normal prostate epithelium after androgen withdrawal. Because androgen withdrawal also induces apoptosis in prostate cancers, androgen deprivation is commonly used as a treatment (44). Thus, deciphering the molecular mechanisms of AWIA in normal glands should contribute to the design of more effective cancer therapies. TNF in combination with other apoptotic triggers (FLIP silencing, TGFβ, etc.) may therefore represent potential targeted therapies for prostate cancer.
Materials and Methods
Animals
Animal experiments were carried out according to the guidelines of the animal care and use committee of the University of California, Irvine. Mice (C57/Bl6), 6 months of age, were from the following sources: Trail−/− from Amgen (Thousand Oaks, CA); lpr, Tnf−/−, and Tnfr1−/− from The Jackson Laboratory (Bar Harbor, ME); and WT (C57/Bl6) from Charles River Laboratories (Wilmington, MA). Castration was performed as described previously (24). Some mice were given sTNFR2-Fc (Wyeth, New York, NY) at 4 mg/kg body weight by ip injection, 3 and 1 d before castration and every fourth day thereafter. Control mice received PBS on the same schedule. Soluble TNF (PeproTech, Rocky Hill, NJ) was injected (27 μg/kg) ip at the time of castration and again 24 h later. Rat tissues were from 90-d-old Sprague Dawley rats (Charles River Laboratories), castrated as above. No animals showed signs of distress, including weight loss, during any of the treatments.
In vivo imaging
High-field (7T) MRI, using a chemical signal suppression sequence to enhance lobe discrimination, was used to measure prostate lobe volumes, as previously described (24). Prostate volume was normalized to precastration (or pretreatment) volume for each animal, and the mean of the group at each time point (±sem), relative to precastration volume, is presented in each dataset. A selected subset of the data was quantitated by two independent observers blinded to genotype and analyzed according to Brodoefel et al. (45). Bland Altman analysis (Supplemental Fig. 1B) shows that the volume correlation for the VP was r = 0.94. Intraclass correlation coefficients for intraobserver assessment were 0.99 and 0.98, whereas interobserver correlation was r = 0.88. The corresponding intra- and interobserver variability (45) for regression of the VP was 2 and 10%, respectively, indicating that differences between the groups were not due to observer bias.
Quantitative PCR
Rat VP tissues were harvested after euthanasia, snap frozen in liquid nitrogen or frozen in OCT freezing compound (Tissue Tek), and stored at −80 C. Total RNA was extracted from pulverized tissue using TRIzol (Invitrogen, Carlsbad, CA). Five micrograms of total RNA was reverse transcribed using SuperScript III reverse transcriptase (Invitrogen) and random primers. The resulting cDNAs were subjected to quantitative real-time PCR with SYBR Green (Molecular Probes, Eugene, OR) using a Lightcycler (Idaho Technologies, Salt Lake City, UT). All samples were normalized to β-actin levels. The primers pairs spanned at least one intron for each gene measured and are as follows: for Tnf, 5′-ACCACGCTCTTCTGTCTA-3′ and 5′-TCCTGGTATGAAATGGCA-3′; for Tnfr1, 5′-ACTGGTTCCTTCTCTTGG-3′ and 5′-AATGCGTCTCACTCAGGT-3′; and for β-actin, 5′-CAGAGCAAGAGAGGCATC-3′ and 5′-CAGAGGCATACAGGGACAA-3′. For laser capture microdissected (LCM) tissues, tissue sections were cut (5 μm) on a Leica CM 3050 Cryostat at −20 C, thaw mounted on polyethylene naphthalate-membrane coated slides (Leica, Wetzlar, Germany), fixed in ribonuclease-free 100% ethanol, and laser-capture microdissected using a Leica LS-AMD system. Secretory epithelium or intervening stromal populations were isolated. Total RNA was extracted using TRIzol reagent (Invitrogen) and precipitated with 20 μg glycogen per sample. The concentration of each RNA sample was analyzed using a Nanodrop spectrophotometer (Thermo Scientific, Rockford, IL). For Ck8 and αSma quantification, each RNA sample (550 ng) was reverse transcribed directly using SuperScript III reverse transcriptase (Invitrogen) and subjected to qPCR using Sybr green and a Lightcycler (Idaho Technologies) instrument. All values were normalized to elongation factor 2 (Ef2) to normalize for total cDNA input. For Tnf, and Tnfr1 mRNA levels, portions of the same RNA samples (20 ng) as above were amplified and reverse transcribed using Ovation RNA Amplification System V2 (NuGEN, San Carlos, CA). The resulting cDNAs were subjected to qPCR using Sybr green and a StepOne Plus (ABI, Carlsbad, CA) instrument. All of the transcripts for LCM-qPCR were normalized to Ef2 mRNA levels. The primers used for LCM-qPCR were as follows, for transcripts measured using the Lightcycler instrument: for Ef2, 5′-TGCCTCTATGCCAGTGTG-3′ and 5′-GTCCAGGAAGTTGTCCAG-3′; for α-Sma, 5′-TGGTGTGTGACAATGGCTC-3′ and 5′-TCCGTTAGCAAGGTCGGAT-3′; and for Ck8, 5′-AGGTGGAGCTTGGCAACAT-3′ and 5′-TTGTCCATGGACAGCACCA-3′. For transcripts measured using the StepOne Plus instrument: for Ef2, 5′-TGGGCCACTAAGGAGGGTGCT-3′ and 5′-CCGCCCACCACTTGCTCAGG-3′; for Tnf, 5′-TCAGTTCCATGGCCCAGACCCTC-3′ and 5′-CGACGTGGGCTACGGGCTTG-3′; and for Tnfr1, 5′-GCTGTCACTGGTGCTCCTGGC-3′ and 5′-TCCTGCCCTGGGCTTGGACA-3′.
Immunoblotting
Tissue extracts were prepared by pulverizing frozen rat VP tissue in liquid nitrogen. The resulting tissue powder was lysed in sodium dodecyl sulfate-extraction buffer [50 mm Tris (pH 7.4), 100 mm NaCl, 5 mm EDTA, 0.1% Nonidet P-40, 1% sodium dodecyl sulfate, 400 μm phenylmethylsulfonyl fluoride, 1× protease inhibitor cocktail; Sigma, St. Louis, MO], homogenized, and sonicated. Insoluble proteins were removed via centrifugation. The soluble supernatant was collected, and the protein concentration was determined using bicinchoninic acid reagent (Pierce, Rockford, IL). Each sample (150 μg) was separated on SDS-PAGE and transferred to a nitrocellulose membrane. Primary antibodies were directed against TNF (1:1000, Calbiochem 654300; Calbiochem, San Diego, CA) or β-actin (1:5000, Sigma AC15). Goat antirabbit (TNF) or goat antimouse (β-actin) secondary horseradish peroxidase-conjugated antibodies were used. Chemiluminescence was captured using a cooled charge-coupled digital camera system (Kodak 4000R; Kodak, Rochester, NY) and quantitated using Kodak software. TNF signals were first normalized to β-actin and then to the internal standard run in duplicate on each gel. The values shown in Supplemental Fig. 5 are relative to the intact (0) animals.
Immunohistochemistry
Mouse urogenital blocks (bladder, all prostate lobes, and seminal vesicles) were harvested 2 d after castration from mice pretreated with either PBS or sTFR2-Fc. Tissues were formalin fixed, embedded in paraffin, sectioned, and processed for immunohistochemistry. For caspase-3 staining, antigen retrieval was performed by incubation in a pressure cooker for 10 min in tris-buffered saline, and slides were then incubated with anti-active (cleaved) caspase-3 antibody (AF835; R&D Systems, Minneapolis, MN) at a dilution of 1:200 and a biotinylated anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) at a dilution of 1:200. Slides were developed using 3,3′-diaminobenzidine tetrahydrochloride substrate as a chromogen and counterstained with hematoxylin. For TUNEL, antigen retrieval was performed as above, followed by labeling using tetramethyl rhodamine-2′-deoxyuridine, 5′-triphosphate, as directed by the kit manufacturer (Roche).
Supplementary Material
Acknowledgments
We thank O. Nalciouglu, M. Hamamura, and L. Qin of the University of California, Irvine Onco-imaging Center for performing MRI.
The University of California, Irvine Onco-imaging Center was supported by Grant P30-CA062203. J.S.D. was supported by the Department of Defense Grant PC073208.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADT
- Androgen deprivation therapy
- AWIA
- androgen withdrawal-induced apoptosis
- DLP
- dorsal-lateral prostate
- DNS
- data not shown
- Ef2
- elongation factor 2
- FADD
- Fas-associated death domain
- FasL
- Fas ligand
- Fc
- fragment crystalizable
- FLIP
- FADD-like IL-1β-converting enzyme-like inhibitory protein
- LCM
- laser capture microdissected
- MRI
- magnetic resonance imaging
- mTNF
- membrane TNF
- qPCR
- quantitative PCR
- sTNF
- soluble TNF
- sTNFR2-Fc
- soluble TNFR2-Fc
- TNFR
- TNF receptor
- TRAIL
- TNF-related apoptosis-inducing ligand
- TUNEL
- terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick end labeling
- VP
- ventral prostate
- WT
- wild type.
References
- 1. Denmeade SR, Isaacs JT. 2002. A history of prostate cancer treatment. Nat Rev Cancer 2:389–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. 2006. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med 166:465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM, Paszat LF. 2009. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 27:3452–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Higano CS. 2008. Androgen-deprivation-therapy-induced fractures in men with nonmetastatic prostate cancer: what do we really know? Nat Clin Pract Urol 5:24–34 [DOI] [PubMed] [Google Scholar]
- 5. Shah NL, Sanda M. 2004. Health-related quality of life in treatment for prostate cancer: looking beyond survival. Support Cancer Ther 1:230–236 [DOI] [PubMed] [Google Scholar]
- 6. Roy-Burman P, Tindall DJ, Robins DM, Greenberg NM, Hendrix MJ, Mohla S, Getzenberg RH, Isaacs JT, Pienta KJ. 2005. Androgens and prostate cancer: are the descriptors valid? Cancer Biol Ther 4:4–5 [DOI] [PubMed] [Google Scholar]
- 7. Perlman H, Zhang X, Chen MW, Walsh K, Buttyan R. 1999. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ 6:48–54 [DOI] [PubMed] [Google Scholar]
- 8. Bruckheimer EM, Cho S, Brisbay S, Johnson DJ, Gingrich JR, Greenberg N, McDonnell TJ. 2000. The impact of bcl-2 expression and bax deficiency on prostate homeostasis in vivo. Oncogene 19:2404–2412 [DOI] [PubMed] [Google Scholar]
- 9. Banerjee PP, Banerjee S, Brown TR. 2002. Bcl-2 protein expression correlates with cell survival and androgen independence in rat prostatic lobes. Endocrinology 143:1825–1832 [DOI] [PubMed] [Google Scholar]
- 10. Kyprianou N, Isaacs JT. 1989. Expression of transforming growth factor-β in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol 3:1515–1522 [DOI] [PubMed] [Google Scholar]
- 11. Martikainen P, Kyprianou N, Isaacs JT. 1990. Effect of transforming growth factor-β1 on proliferation and death of rat prostate cells. Endocrinology 127:2963–2968 [DOI] [PubMed] [Google Scholar]
- 12. Nemeth JA, Sensibar JA, White RR, Zelner DJ, Kim IY, Lee C. 1997. Prostatic ductal system in rats: tissue-specific expression and regional variation in stromal distribution of transforming growth factor-β 1. Prostate 33:64–71 [DOI] [PubMed] [Google Scholar]
- 13. Hedlund TE, Duke RC, Schleicher MS, Miller GJ. 1998. Fas-mediated apoptosis in seven human prostate cancer cell lines: correlation with tumor stage. Prostate 36:92–101 [DOI] [PubMed] [Google Scholar]
- 14. Nesterov A, Ivashchenko Y, Kraft AS. 2002. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) triggers apoptosis in normal prostate epithelial cells. Oncogene 21:1135–1140 [DOI] [PubMed] [Google Scholar]
- 15. Chopra DP, Menard RE, Januszewski J, Mattingly RR. 2004. TNF-α-mediated apoptosis in normal human prostate epithelial cells and tumor cell lines. Cancer Lett 203:145–154 [DOI] [PubMed] [Google Scholar]
- 16. Martin DA, Siegel RM, Zheng L, Lenardo MJ. 1998. Membrane oligomerization and cleavage activates the caspase-8 (FLICE/MACHα1) death signal. J Biol Chem 273:4345–4349 [DOI] [PubMed] [Google Scholar]
- 17. Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME, Yang X. 2002. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J 21:3704–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nastiuk KL, Kim JW, Mann M, Krolewski JJ. 2003. Androgen regulation of FLICE-like inhibitory protein gene expression in the rat prostate. J Cell Physiol 196:386–393 [DOI] [PubMed] [Google Scholar]
- 19. Cornforth AN, Davis JS, Khanifar E, Nastiuk KL, Krolewski JJ. 2008. FOXO3a mediates the androgen-dependent regulation of FLIP and contributes to TRAIL-induced apoptosis of LNCaP cells. Oncogene 27:4422–4433 [DOI] [PubMed] [Google Scholar]
- 20. Suzuki A, Matsuzawa A, Iguchi T. 1996. Down regulation of Bcl-2 is the first step on Fas-mediated apoptosis of male reproductive tract. Oncogene 13:31–37 [PubMed] [Google Scholar]
- 21. de la Taille A, Chen MW, Shabsigh A, Bagiella E, Kiss A, Buttyan R. 1999. Fas antigen/CD-95 upegulation and activation during castration-induced regression of the rat ventral prostate gland. Prostate 40:89–96 [DOI] [PubMed] [Google Scholar]
- 22. Sugihara A, Yamada N, Tsujimura T, Iwasaki T, Yamashita K, Takagi Y, Tsuji M, Terada N. 2001. Castration induces apoptosis in the male accessory sex organs of Fas- deficient lpr and Fas ligand-deficient gld mutant mice. In Vivo 15:385–390 [PubMed] [Google Scholar]
- 23. Berges RR, Furuya Y, Remington L, English HF, Jacks T, Isaacs JT. 1993. Cell proliferation, DNA repair, and p53 function are not required for programmed death of prostatic glandular cells induced by androgen ablation. Proc Natl Acad Sci USA 90:8910–8914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nastiuk KL, Liu H, Hamamura M, Muftuler LT, Nalcioglu O, Krolewski JJ. 2007. In vivo MRI volumetric measurement of prostate regression and growth in mice. BMC Urol 7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim Y, Suh N, Sporn M, Reed JC. 2002. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem 277:22320–22329 [DOI] [PubMed] [Google Scholar]
- 26. Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N, Srivastava RK. 2001. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene 20:6073–6083 [DOI] [PubMed] [Google Scholar]
- 27. Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. 1996. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med 184:1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Micheau O, Tschopp J. 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114:181–190 [DOI] [PubMed] [Google Scholar]
- 29. Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TW. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457–467 [DOI] [PubMed] [Google Scholar]
- 30. Grounds MD, Davies M, Torrisi J, Shavlakadze T, White J, Hodgetts S. 2005. Silencing TNFα activity by using Remicade or Enbrel blocks inflammation in whole muscle grafts: an in vivo bioassay to assess the efficacy of anti-cytokine drugs in mice. Cell Tissue Res 320:509–515 [DOI] [PubMed] [Google Scholar]
- 31. Glamoclija V, Vilović K, Saraga-Babić M, Baranović A, Sapunar D. 2005. Apoptosis and active caspase-3 expression in human granulosa cells. Fertil Steril 83:426–431 [DOI] [PubMed] [Google Scholar]
- 32. Mosselmans R, Hepburn A, Dumont JE, Fiers W, Galand P. 1988. Endocytic pathway of recombinant murine tumor necrosis factor in L-929 cells. J Immunol 141:3096–3100 [PubMed] [Google Scholar]
- 33. Echtenacher B, Urbaschek R, Weigl K, Freudenberg MA, Männel DN. 2003. Treatment of experimental sepsis-induced immunoparalysis with TNF. Immunobiology 208:381–389 [DOI] [PubMed] [Google Scholar]
- 34. Lewis M, Tartaglia LA, Lee A, Bennett GL, Rice GC, Wong GH, Chen EY, Goeddel DV. 1991. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci USA 88:2830–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kulik G, Carson JP, Vomastek T, Overman K, Gooch BD, Srinivasula S, Alnemri E, Nunez G, Weber MJ. 2001. Tumor necrosis factor α induces BID cleavage and bypasses antiapoptotic signals in prostate cancer LNCaP cells. Cancer Res 61:2713–2719 [PubMed] [Google Scholar]
- 36. Katdare M, Efimova EV, Labay E, Khodarev NN, Darga TE, Garofalo M, Nakamura S, Kufe DW, Posner MC, Weichselbaum RR. 2007. Diverse TNFα-induced death pathways are enhanced by inhibition of NF-κB. Int J Oncol 31:1519–1528 [PubMed] [Google Scholar]
- 37. Henkes LE, Sullivan BT, Lynch MP, Kolesnick R, Arsenault D, Puder M, Davis JS, Rueda BR. 2008. Acid sphingomyelinase involvement in tumor necrosis factor α-regulated vascular and steroid disruption during luteolysis in vivo. Proc Natl Acad Sci USA 105:7670–7675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korzekwa A, Murakami S, Wocławek-Potocka I, Bah MM, Okuda K, Skarzynski DJ. 2008. The influence of tumor necrosis factor α (TNF) on the secretory function of bovine corpus luteum: TNF and its receptors expression during the estrous cycle. Reprod Biol 8:245–262 [DOI] [PubMed] [Google Scholar]
- 39. Siegel PM, Massagué J. 2003. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer 3:807–821 [DOI] [PubMed] [Google Scholar]
- 40. Gao S, Lee P, Wang H, Gerald W, Adler M, Zhang L, Wang YF, Wang Z. 2005. The androgen receptor directly targets the cellular Fas/FasL-associated death domain protein-like inhibitory protein gene to promote the androgen-independent growth of prostate cancer cells. Mol Endocrinol 19:1792–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raclaw KA, Heemers HV, Kidd EM, Dehm SM, Tindall DJ. 2008. Induction of FLIP expression by androgens protects prostate cancer cells from TRAIL-mediated apoptosis. Prostate 68:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nastiuk KL, Yoo K, Lo K, Su K, Yeung P, Kutaka J, Danielpour D, Krolewski JJ. 2008. FLICE-like inhibitory protein blocks transforming growth factor β 1-induced caspase activation and apoptosis in prostate epithelial cells. Mol Cancer Res 6:231–242 [DOI] [PubMed] [Google Scholar]
- 43. Yoo KS, Nastiuk KL, Krolewski JJ. 2009. Transforming growth factor β1 induces apoptosis by suppressing FLICE-like inhibitory protein in DU145 prostate epithelial cells. Int J Cancer 124:834–842 [DOI] [PubMed] [Google Scholar]
- 44. Ohlson N, Wikström P, Stattin P, Bergh A. 2005. Cell proliferation and apoptosis in prostate tumors and adjacent non-malignant prostate tissue in patients at different time-points after castration treatment. Prostate 62:307–315 [DOI] [PubMed] [Google Scholar]
- 45. Brodoefel H, Burgstahler C, Sabir A, Yam CS, Khosa F, Claussen CD, Clouse ME. 2009. Coronary plaque quantification by voxel analysis: dual-source MDCT angiography versus intravascular sonography. AJR Am J Roentgenol 192:W84–W89 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.