Abstract
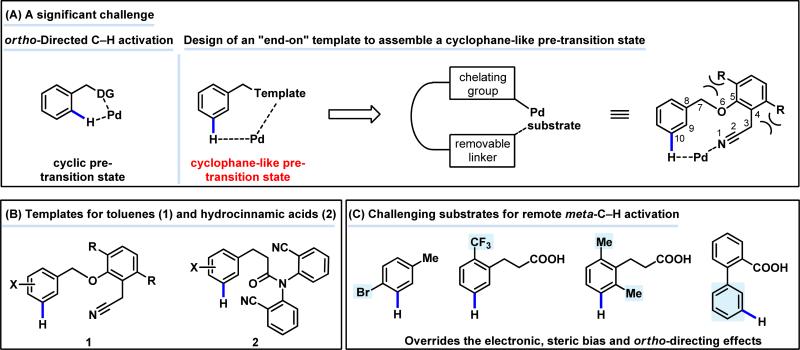
Controlling positional selectivity of C–H activation in molecules possessing multiple inequivalent C–H bonds is one of the most important challenges in developing synthetically useful C–H activation reactions. One widely used approach utilizes σ-chelating directing groups to achieve ortho-selectivity through conformational rigid five- or six-membered cyclic pre-transition states (TS).1–14 We envisioned that an “end-on” chelating template capable of delivering catalysts to previously inaccessible remote meta-C–H bonds via a macrocyclic cyclophane-like pre-TS could overcome the limitations imposed by traditional ortho-directing groups. Herein, we report a class of readily removable nitrile-containing templates that direct the activation of distal meta-C–H bonds (≥ 10 bonds away) of a tethered arene. We attribute this new mode of C–H activation to the weak “end-on” coordination of the linear nitrile group to metal center, as previously observed by Schwarz in the study of remote C–H activation of alkyl nitriles in gas phase.15, 16 The coordination geometry relieves the strain of the cyclophane-like pre-transition state of the meta-C–H activation event. Remarkably, this template overrides electronic and steric biases and ortho-directing effects with two broadly useful classes of arene substrates (toluene derivatives and hydrocinnamic acids), thus constituting a fundamentally new mode of directed C–H activation that is anticipated to be widely adopted.
The development of new transformations in which inert C–H bonds react as dormant functional groups holds great promise for expediting organic synthesis by providing unprecedented retrosynthetic disconnections. In this endeavor, controlling the positional selectivity of C–H cleavage in molecules that contain multiple C–H bonds is an outstanding challenge that must be addressed prior to widespread synthetic applications. To date, σ-chelation-directed metalation of C–H bonds has proven to be a powerful means of achieving ortho-selectivity.1–6 Diverse carbon–carbon and carbon–heteroatom bond– forming reactions with broadly useful substrates has recently been developed using Pd(II),7–9 Rh(III)10–13 and Ru(II)14 catalysts (Fig. 1). In directed C–H activation, assembly of a conformationally rigid cyclic pre-transition state is required, which introduces two inherent limitations. First, despite extensive and innovative efforts toward remote C–H functionalization reactions,15–19 the difficulties associated with forming macrocyclic pre-transition states larger than a six-membered ring generally precludes the activation of remote C–H bonds. Second, the high energy associated with well-defined cyclophane-like pre-transition states prevents meta- or para- C–H activation reactions (Fig. 1A). Rare examples of non-directed meta- and para- selective C–H functionalization reactions of mono-substituted arenes involving either electrophilic substitution (where the metal is not involved in the C–H cleavage step)20,21 or inner-sphere metalation22–27 have been reported in which the regioselectivity is governed by either the electronic or steric properties of the arene substrates. To date, a generally applicable approach for remote meta-C–H activation that overrides the electronic and steric properties of arene substrates remains to be developed (Fig. 1).
Figure 1.
A removable template for activation of distal meta-C–H bonds (10 bonds away).
Herein, we report the discovery of a class of novel nitrile-containing templates15,16 that deliver palladium to the vicinity of attached arene substrates to promote C–H olefination of distal meta-C–H bonds with exceedingly high selectivity. In theory, this linear “end-on” coordinating nitrile group is proposed to accommodate a cyclophane-like cyclic pre-transition state, thus overcoming the intrinsic limitations of traditional directed C–H activation. Remarkably, the template overrules the electronic and steric biases and ortho-directing effects of the arene substrates, consistently securing high meta-selectivity in most cases. meta-Selective C–H olefination of ortho,ortho-di-substituted arenes has also been achieved using this approach, which represents a unique method for constructing highly sophisticated tetra-substitution patterns. The generality of this template approach has been demonstrated with two categorically different classes of substrates (toluene and hydrocinnamic acid derivatives) (Fig. 1B). This new method has been also been applied to functionalize the natural amino acid phenylalanine and GABAB receptor agonist drug, Baclofen.
Results and discussion
Considering that the major limitations of directed C–H activation arise from the stringent requirement of assembling a rigid cyclic pre-transition state, we began to devise a removable chelating template that would recruit the Pd(II) catalyst through a less rigid assembly involving reversible weak coordination (Fig. 1A). We suspected that the weak binding would either make the cyclic pre-transition state less strained or would lead to release of the Pd(II) catalyst in the vicinity of the target C–H bond, thereby providing a high effective concentration. Although weak coordination is not as effective as strong coordination in terms of compensating for the entropic cost during the assembly of the pre-transition state, the Pd(II) center is more reactive due to higher electrophilicity which beneficial for C–H activation. We also anticipated that the linear coordination mode of the nitrile group would be more likely to anchor the Pd to the meta position rather than to the ortho position (Fig. 1A). In this putative structure, the interaction with the ortho-C–H bond would incur high levels of cyclophane ring strain due to the linear geometry of the nitrile group. Similarly, Schwarz's pioneering studies of “end-on” coordination of alkyl nitriles with Fe(II) in the gas phase showed that such coordination can guide the metal to perform remote C–H activation.15,16
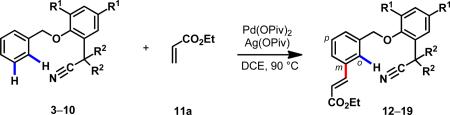
To reduce this hypothesis to practice, we engineered a nitrile-containing template in which the substrate was attached through a removable benzyl ether linkage (Fig. 1A). At the outset, we hypothesized that the flat arene would be beneficial for holding the substrate and nitrile group in a coplanar conformation. We further hypothesized that sterically bulky tBu groups at the ortho-positions of the arene template could serve as blocking groups to ensure that the pendant substrate and the nitrile group were kept in the same quadrant of the template to achieve high effective concentration of the Pd(II) catalyst. In our first design, we attached a nitrile-containing phenol as the template to arene substrate via benzylation to give benzyl ether 3 for testing meta-selective C–H activation reactions (Table 1).
Table 1.
Optimization of template
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| entry | template | R1 | R2 | yield (%) (mono)b | yield(%) (di)b | selectivity m:p:oc | entry | template | R1 | R2 | yield (%) (mono)b | yield (%) (di)b | selectivity m:p:oc |
| 1d | 3a | tBu | H | trace | 0 | — | 6 | 6 | tBu | —(CH2)4— | 50 | 11 | 88:7:5 |
| 2e,f | 3a | tBu | H | 4 | 0 | 56:26:18 | 7 | 7 | tBu | —(CH2)5— | 51 | 15 | 91:5:4 |
| 3 | 3a | tBu | H | 15 | 0 | 59:33:8 | 8 | 8a | tBu | iBu | 67 | 23 | >95:5(p+o) |
| 4 | 4 | tBu | Me | 60 | 17 | 91:7:2 | 9g | 9 | H | iBu | — | — | — |
| 5 | 5 | tBu | Et | 52 | 16 | 93:6:1 | 10 | 10 | Me | iBu | 39 | 10 | 91:8:1 |
a Unless otherwise noted, the reaction conditions were as follows: benzyl ethers 3–10 (0.05 mmol), olefin 11a (1.5 equiv.), Pd(OPiv)2 (10 mol%), Ag(OPiv) (2.1 equiv.), DCE (0.5 mL), 90 °C, 18 h.
Isolated yield after purification by silica gel column chromatography.
m:p:o denotes the ratio of meta:para:ortho mono-olefinated products, as determined by 1H NMR analysis of the unpurified reaction mixture.
O2 was used as the oxidant instead of Ag(OPiv).
Pd(OAc)2 and Ag(OAc) were used instead of Pd(OPiv)2 and Ag(OPiv) respectively.
NMR conversion was determined by using o-xylene as an internal standard.
C–H olefination occurred on both the arene substrate and on the template with a combined yield of approximately 20% yield.
Abbreviations: DCE, 1,2-dichloroethane; Me, methyl; Et, ethyl; tBu, tert-butyl; OPiv, pivalate.
Using olefination of 3 as the model reaction,29 we extensively screened catalysts, oxidants and solvent (see Supporting Information) and found that a combination of Pd(OPiv)2 as the catalyst and Ag(OPiv) as the oxidant gave 15% yield with encouraging levels of meta-selectivity (m:p:o = 59:33:8) (Table 1, entries 1–3). To further improve the reactivity via Thorpe–Ingold effect, we installed two alkyl groups at the α-position adjacent to the nitrile group. Both the yield and the meta-selectivity steadily improved with the increase of the size of the alkyls (entries 4–8). The i-butyl-substituted template gave a mixture of mono- and di-olefinated products in 90% combined yield (entry 8). Importantly, the meta-selectivity was found to be 97%. We also confirmed through control experiments that the presence of the bulky tBu blocking groups on the template was essential for reactivity (entries 9,10). Replacement of the nitrile by a methyl group resulted in complete loss of reactivity and selectivity (see supporting information).
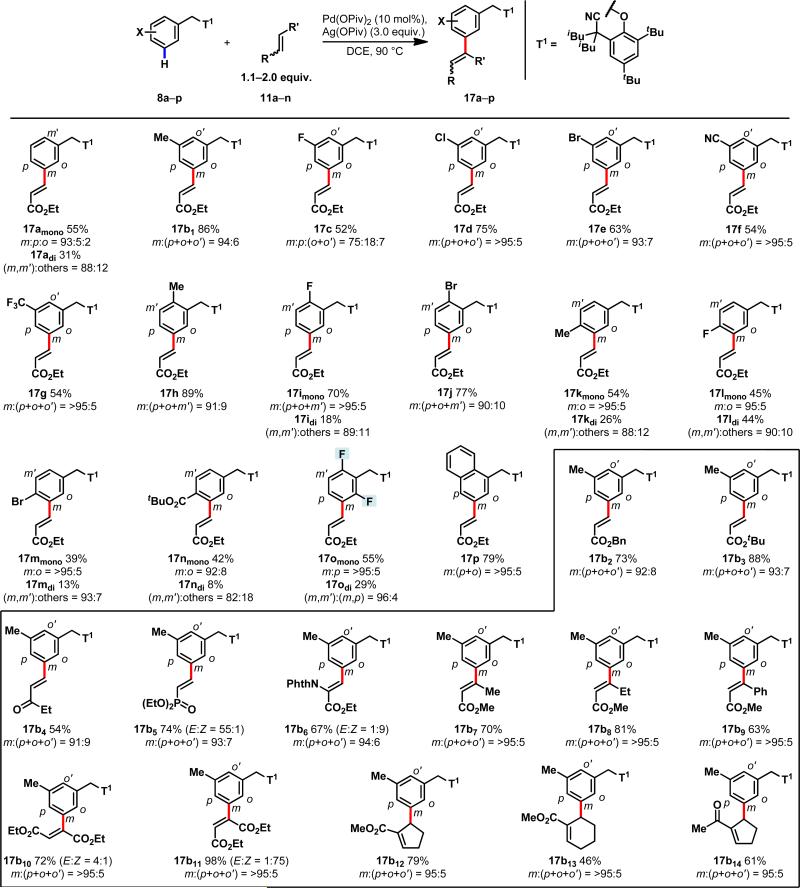
With this newly established meta-selective C–H olefination procedure in hand, we carried out olefination on a variety of substituted arenes (Fig. 2). meta-Substituted arenes were olefinated at the remaining meta position with excellent selectivity regardless the electronic properties of the substituents (17a–g). With ortho-substituted arene substrates, high meta-selectivity was also achieved with both an electron-donating methyl group and electron-withdrawing fluoro and bromo groups (17h–j). Notably, in the absence of the template, the para- and meta-C–H bonds of these 1,2-substituted arene substrates would be very difficult to distinguish, yet the template directed meta-C–H activation with high selectivity. For example, meta-selective olefination places the newly installed functional group at the position para to the bromo group, which is unprecedented and synthetically enabling (17j). Despite the steric hinderance, para-substituted arenes are also olefinated at the meta-position in excellent selectivity (17k–n). In sharp contrast to previously reported meta-selective C–H functionalization reactions,20,24 the template also effectively overrules the electronic effect of bromide or ester groups at the para position, affording predominantly the meta-olefinated products (17m, 17n). Finally, the template can guide the catalyst to reach and activate the meta-C–H bond in a selective manner in the presence of ortho,ortho-di-fluoro substitution, demonstrating another unique feature of this approach (17o). meta-C–H functionalization of ortho,ortho-di-substituted arenes provides an unprecedented disconnection for constructing 1,2,3,4-tetra-substituted arenes.
Figure 2. Template-directed meta-selective C–H olefination of toluene derivatives.
Arenes (8a–p) with a variety of substitution pattern undergo facile olefination. In the highlighted box, a myriad of election-deficient olefins, including vinyl ketones, vinyl phosphonate, α-imidoacrylate and various 1,2-disubstituted olefins were used. The isolated yield after purification by silica gel column chromatography is shown along with the selectivity. (The isolated yield of the di-olefinated product is also shown, when applicable). See Supplementary Information for experimental details. Selectivity of the mono product was determined by 1H NMR analysis and confirmed by NOESY experiments. Products 17b10 and 17b11 were prepared from diethyl maleate and diethyl fumerate respectively. Abbreviations: PhthN, N-phthaloyl.
We next extensively surveyed the scope of the olefin coupling partners. Olefination with commonly used electron-deficient α,β-unsaturated esters, ketones and phosphonates gave desired product in good yields (17b2–b6). Noting that the lack of reactivity with 1,2-di-substituted olefins in directed C–H olefination reactions is a significant challenge,28–30 we tested a series of representative di-substituted olefins. We were pleased to find that olefination with all of these 1,2-di-substituted olefins proceeded smoothly to give the desired products (17b7–b14) in moderate to excellent yields. Unlike in conventional directed ortho-C–H olefination reactions where the coordinated directing group could prevent the sterically hindered olefin from binding,28–30 in this case the nitrile group on the template is weakly coordinated to the [Pd(II)–Ar] intermediate and can be effectively displaced by hindered 1,2-di-substituted olefins, thereby allowing carbopalladation to proceed. Lastly, the template was readily removed through a Pd/C mediated hydrogenolysis to afford meta-alkylated toluenes in excellent yields (see Supporting Information). The structures of the meta-alkylated toluenes were confirmed by comparing to known standards.
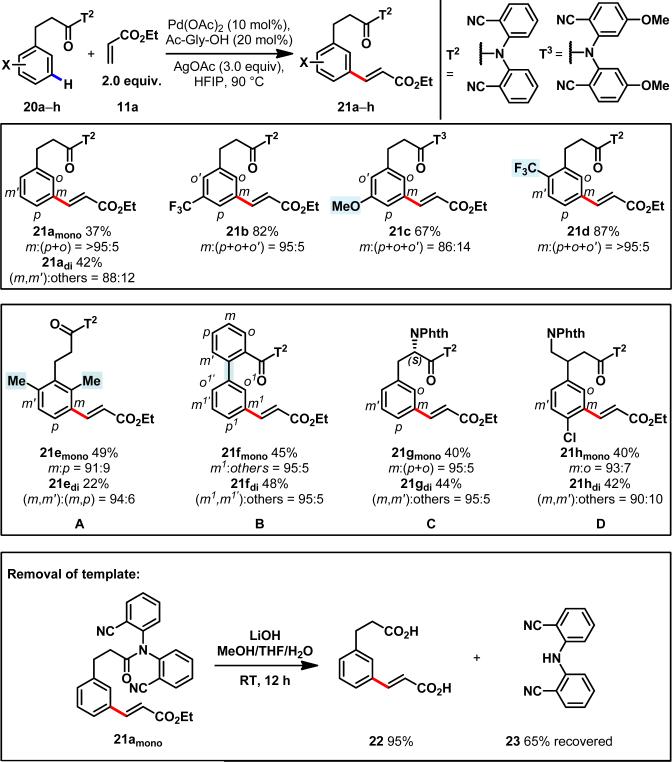
Having demonstrated the concept of using a nitrile-containing end-on template to activate meta-C–H bonds that are ten bonds away from the chelating atom, we moved forward to test if this approach would prove to be generally applicable to other broadly useful substrates, following appropriate tuning of the template. Thus, a readily cleavable amide linkage was employed to attach a benzonitrile template to hydrocinnamic acid to form 20a. Based on the established olefination protocol from above (Fig. 2), we screened various reaction parameters with substrate 20a and observed significant solvent effects, presumably due to the dramatic influence of the solvent medium on the assembly of the pre-transition state via weak coordination (see Supporting Information). Olefination of 20a in hexafluoroisopropanol gave mainly a m:p:o 79:18:3 mixture of mono-olefinated products in 73% conversion, as determined by 1H NMR analysis. To further improve the reactivity, we turned to mono-N-protected amino acid ligands which were recently found to accelerate C–H olefination reactions.30 We found through extensive ligand screening that N- acetyl-protected glycine was most effective, promoting the olefination to completion (see Supporting Information). meta-selectivity of the mono-olefinated product 21amono was also enhanced from 79% to 97% (Fig.3). Other regiosomers were formed in less than 5% yield (identified by 1H NMR in comparison with synthetic samples). Control experiments showed that replacement of the nitrile group on the template with a trifluoromethyl group resulted in poor reactivity and selectivity giving a m:p:o 1:1:2 mixture of mono-olefinated products in 38% conversion. In terms of practical considerations, the removal of the template in 21amono was readily accomplished by hydrolysis at room temperature using LiOH as a base to give diacid 22 in 95% yield (Fig.3). The hydrolysis conditions are routinely used in synthesis and the template can be recovered in 65% isolated yield.
Figure 3. Template-directed meta-selective C–H olefination of hydrocinnamic acid derivatives.
Four representative hydrocinnamic acid derivatives (top box). Regardless of electronic or steric factors, C–H olefination occurs at the meta position with high selectivity. Bottom box: (A) Use of the template allows meta-C–H olefination in the presence of ortho,ortho-disubstituents. (B) Out of the seven possible C–H bonds, only the meta C–H bond at the most remote position is activated using the template. (C) Biologically-active natural amino acid, N-protected phenylalanine, is olefinated at the meta position of the phenyl ring. (D) Baclofen, a blockbuster drug molecule, is directly modified using this methodology. Isolated yields after purification by silica gel column chromatography and selectivities are shown below each entry. (The isolated yield of the di-olefinated product is also shown, when applicable). See Supplementary Information for experimental details. Selectivity of the mono product was determined by 1H NMR analysis and confirmed by NOESY experiments.
The efficiency of this template was first demonstrated by the excellent observed reactivity with the electron-deficient meta-(trifluoromethyl)arene 20b. To showcase the power of the template in controlling the meta-selectivity, meta-methoxy and ortho-trifluoromethyl groups were introduced onto the arenes (20c and 20d respectively) to exert strong electronic effects against the observed meta-selectivity. Remarkably, excellent meta-selectivity was maintained in the olefination of both meta-methoxyarene 20c and ortho-trifluoromethylarene 20d to give 21c and 21d respectively, suggesting that the template effectively overrules the electronic effects of both electron-withdrawing and -donating groups. To further demonstrate the potential synthetic applications of this method, we first constructed a sterically encumbered tetra-substituted arene 21e via meta-selective olefination of the ortho,ortho-di-methylarene 20e in which case the steric hindrance was overcome by the template. Intriguingly, meta-selective C–H olefination of biphenyl substrate (20f) also proceeded at the remote aryl ring to afford synthetically useful biphenyl 21f with an unprecedented site-selectivity. Importantly, novel unnatural aminoacid 21g was also prepared via meta-selective olefination of N-phthaloyl-protected natural aminoacid 20g. Finally, N-phthaloyl protected Baclofen (20h), a GABA receptor agonist drug, was selectively olefinated at the meta-position to give 21h in 82% yield, providing access to a novel library of unique molecules of medicinal interest. It is worth noting that two previously reported catalytic systems for meta-selective C–H activation20,24 are not compatible with these substrates.
In summary, we have developed a template approach to activate remote meta-C–H bonds of two categorically distinct classes of substrates with high meta-selectivity, which are not possible with previous methods. The template-assisted meta-selective C–H activation overrides ortho-directing effects as well as electronic and steric biases on appended arene substrates. The template design is predicated on the weak interaction between the Pd(II) and nitrile group, where the nitrile group coordinates in an end on fashion and can overcome the difficulties associated with assembling a cyclophane-like pre-transition state. This new strategy for directing remote C–H activation provides a novel route for the preparation of toluene derivatives, hydrocinnamic acids, biphenyls, unnatural amino acids and drug molecules with sophisticated substitution pattern that are difficult to access using conventional C–H activation methods. We expect that this end-on nitrile-based template can be structurally modified to suit other classes of synthetically useful arene substrates.
Methods Summary
General procedure for template-directed meta-selective C–H olefination of toluene derivatives
A 15 mL sealed tube (with a Teflon cap) equipped with a magnetic stir bar was charged with substrate (46.1 mg, 0.10 mmol), Pd(OPiv)2 (3.0 mg, 0.010 mmol, 0.10 equiv.), and AgOPiv (62.7 mg, 0.30 mmol, 3.0 equiv.). Ethyl acrylate (16.5 μL, 0.15 mmol, 1.5 equiv.) was added, followed by DCE (1.0 mL). The tube was then capped and submerged into a pre-heated 90 °C oil bath. The reaction was stirred for a total of 42 h and cooled down to room temperature. The crude reaction mixture was filtered through a pad of Celite and washed with Et2O (2 mL × 3). The filtrate was concentrated in vacuo, and the resulting residue was purified by silica gel column chromatography using hexanes/EtOAc as the eluent. The positional selectivity was determined by 1H NMR of the unpurified reaction mixture. Full experimental details and characterization of new compounds can be found in the Supplementary Information.
Supplementary Material
Acknowledgements
We gratefully acknowledge The Scripps Research Institute, the NIH (NIGMS, 1 R01 GM084019-02) and the U. S. National Science Foundation (NSF CHE-0910014) for their financial support. We also thank the Agency for Science, Technology and Research (A*STAR) Singapore for a postdoctoral fellowship (D.L.).
Footnotes
The authors declare no competing financial interests.
Supplementary Information accompanies the paper on www.nature.com/nature
References
- 1.Snieckus V. Directed ortho metalation. Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics. Chem. Rev. 1990;90:879–933. [Google Scholar]
- 2.Flemming JP, Berry MB, Brown JM. Sequential ortho-lithiations; the sulfoxide group as a relay to enable meta-substitution. Org. Biomol. Chem. 2008;6:1215–1221. doi: 10.1039/b716954j. [DOI] [PubMed] [Google Scholar]
- 3.Ryabov AD. Cyclopalladated complexes in organic synthesis. Synthesis. 1985:233–252. [Google Scholar]
- 4.Kakiuchi F, et al. Catalytic addition of aromatic carbon–hydrogen bonds to olefins with the aid of ruthenium complexes. Bull. Chem. Soc. Jpn. 1995;68:62–83. [Google Scholar]
- 5.Jun C-H, Hong J-B, Lee D-Y. Chelation-assisted hydroacylation. Synlett. 1999:1–12. [Google Scholar]
- 6.Colby DA, Bergman RG, Ellman JA. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 2010;110:624–655. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daugulis O, Do H-Q, Shabashov D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc. Chem. Res. 2009;42:1074–1086. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engle KM, Mei T-S, Wasa M, Yu J-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc Chem Res. 2011 doi: 10.1021/ar200185g. DOI:10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh T, Miura M. Oxidative coupling of aromatic substrates with alkynes and alkenes under rhodium catalysis. Chem. Eur. J. 2010;16:11212–11222. doi: 10.1002/chem.201001363. [DOI] [PubMed] [Google Scholar]
- 11.Guimond N, Gorelsky SI, Fagnou K. Rhodium(III)-catalyzed heterocycle synthesis using an internal oxidant: improved reactivity and mechanistic studies. J. Am. Chem. Soc. 2011;133:6449–6457. doi: 10.1021/ja201143v. [DOI] [PubMed] [Google Scholar]
- 12.Rakshit S, Grohmann C, Besset T, Glorius F. Rh(III)-catalyzed directed C–H olefination using an oxidizing directing group: mild, efficient, and versatile. J. Am. Chem. Soc. 2011;133:2350–2353. doi: 10.1021/ja109676d. [DOI] [PubMed] [Google Scholar]
- 13.Park SH, Kim JY, Chang S. Rhodium-catalyzed selective olefination of arene esters via C–H bond activation. Org. Lett. 2011;13:2372–2375. doi: 10.1021/ol200600p. [DOI] [PubMed] [Google Scholar]
- 14.Ackermann L, Pospech J. Ruthenium-catalyzed oxidative C–H bond alkenylations in water: expedient synthesis of annulated lactones. Org. Lett. 2011;13:4153–4155. doi: 10.1021/ol201563r. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz H. Remote functionalization of C–H and C–C bonds by naked transition-metal ions (cosi fan tutte). Acc. Chem. Res. 1989;22:282–287. [Google Scholar]
- 16.Dietl N, Schlangen M, Schwarz H. Directed, remote gas-phase C–H and C–C bond activations by metal oxide cations anchored to a nitrile group. Chem.–Eur. J. 2011;17:1783–1788. doi: 10.1002/chem.201003041. [DOI] [PubMed] [Google Scholar]
- 17.Breslow R. Biomimetic control of chemical selectivity. Acc. Chem. Res. 1980;13:170–177. [Google Scholar]
- 18.Das S, Incarvito CD, Crabtree RH, Brudvig GW. Molecular recognition in the selective oxygenation of saturated C–H bonds by a dimanganese catalyst. Science. 2006;312:1941–1943. doi: 10.1126/science.1127899. [DOI] [PubMed] [Google Scholar]
- 19.Li J-J, Giri R, Yu J-Q. Remote C–H bond functionalization reveals the distance-dependent isotope effect. Tetrahedron. 2008;64:6979–6987. [Google Scholar]
- 20.Duong HA, Gilligan RE, Cooke ML, Phipps RJ, Gaunt MJ. Copper(II)-catalyzed meta-selective direct arylation of α-aryl carbonyl compounds. Angew. Chem. Int. Ed. 2011;50:463–466. doi: 10.1002/anie.201004704. [DOI] [PubMed] [Google Scholar]
- 21.Saidi O, et al. Ruthenium-catalyzed meta-sulfonation of 2-phenylpyridines. J. Am. Chem. Soc. 2011;133:19298–19301. doi: 10.1021/ja208286b. [DOI] [PubMed] [Google Scholar]
- 22.Cho J-Y, Tse MK, Holmes D, Maleczka RE, Jr., Smith MR., III Remarkably selective iridium catalysts for the elaboration of aromatic C–H bonds. Science. 2002;295:305–308. doi: 10.1126/science.1067074. [DOI] [PubMed] [Google Scholar]
- 23.Ishiyama T, et al. Mild iridium-catalyzed borylation of arenes. High turnover numbers, room temperature reactions, and isolation of a potential intermediate. J. Am. Chem. Soc. 2002;124:390–391. doi: 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y-H, Shi B-F, Yu J-Q. Pd(II)-catalyzed olefination of electron-deficient arenes using 2,6-dialkylpyridine ligands. J. Am. Chem. Soc. 2009;131:5072–5074. doi: 10.1021/ja900327e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueda K, Yanagisawa S, Yamaguchi J, Itami K. A general catalyst for the β-selective C–H bond arylation of thiophenes with iodoarenes. Angew. Chem. Int. Ed. 2010;49:8946–8949. doi: 10.1002/anie.201005082. [DOI] [PubMed] [Google Scholar]
- 26.Potavathri S, et al. Regioselective oxidative arylation of indoles bearing N–alkyl protecting groups: dual C–H functionalization via a concerted metalation−deprotonation mechanism. J. Am. Chem. Soc. 2010;132:14676–14681. doi: 10.1021/ja107159b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seregin IV, Gevorgyan V. Direct transition metal-catalyzed functionalization of heteroaromatic compounds. Chem. Soc. Rev. 2007;36:1173–1193. doi: 10.1039/b606984n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boele MDK, et al. Selective Pd-catalyzed oxidative coupling of anilides with olefins through C−H bond activation at room temperature. J. Am. Chem. Soc. 2002;124:1586–1587. doi: 10.1021/ja0176907. [DOI] [PubMed] [Google Scholar]
- 29.Wang D-H, Engle KM, Shi B-F, Yu J-Q. Ligand-enabled reactivity and selectivity in a synthetically versatile aryl C–H olefination. Science. 2010;327:315–319. doi: 10.1126/science.1182512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engle KM, Wang D-H, Yu J-Q. Ligand-accelerated C–H activation reactions: evidence for a switch of mechanism. J. Am. Chem. Soc. 2010;132:14137–14151. doi: 10.1021/ja105044s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.