Abstract
A fluorescent chemodosimeter for cysteine detection was developed based on a tandem conjugate addition and intramolecular cyclization reaction. The method exhibited an excellent selectivity for cysteine over other biothiols such as homocysteine and glutathione.
Cysteine (Cys) is an essential amino acid. It plays a key role in a variety of important cellular functions, such as protein synthesis, detoxification and metabolism, etc. Elevated levels of Cys have been associated with neurotoxicity.1 Cys deficiency is involved in a number of other disorders.2
In recent years, several fluorescent sensors and probes for biological thiols have been developed.3 Most are based on the inherent nucleophilicity of the sulfhydril group.3a Although these probes show high sensitivity towards mercapto biomolecules, the direct detection of Cys is highly challenging due to interference from other biothiols. In our previous work,4 we introduced fluorescein aldehydes as fluorescent probes for Cys and Hcy based on the cyclization of N-terminal Cys (or Hcy) residues to form the corresponding thiazolidines (or thiazinanes). Because both sulfhydryl and amino groups are responsible for the sensing mechanism, the selectivity of these types of probes is higher than traditional probes based mainly on non-specific thiol nucleophilicity.
Early on, during the development of selective aminothiol probes4b we observed that certain α,β-unsaturated aldehydes with decreasing electrophilicity exhibited predictable enhanced selectivity towards Cys. Research in this field has since been greatly extended and many probes containing aldehyde moieties have been reported,4a,5 with some exhibiting high selectivity for either Cys5c,d or Hcy.5b Although some have been developed for assaying either Cys or Hcy, there is still room for improvement in terms of selectivity, sensitivity, and performance with alternative reaction mechanisms.
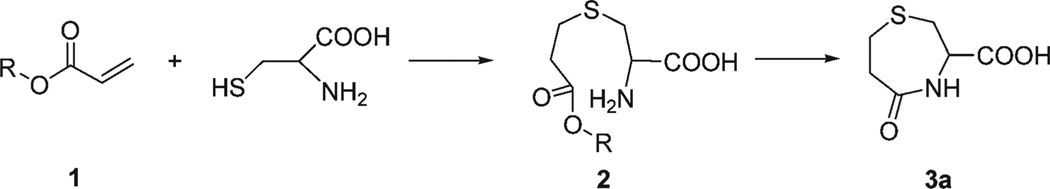
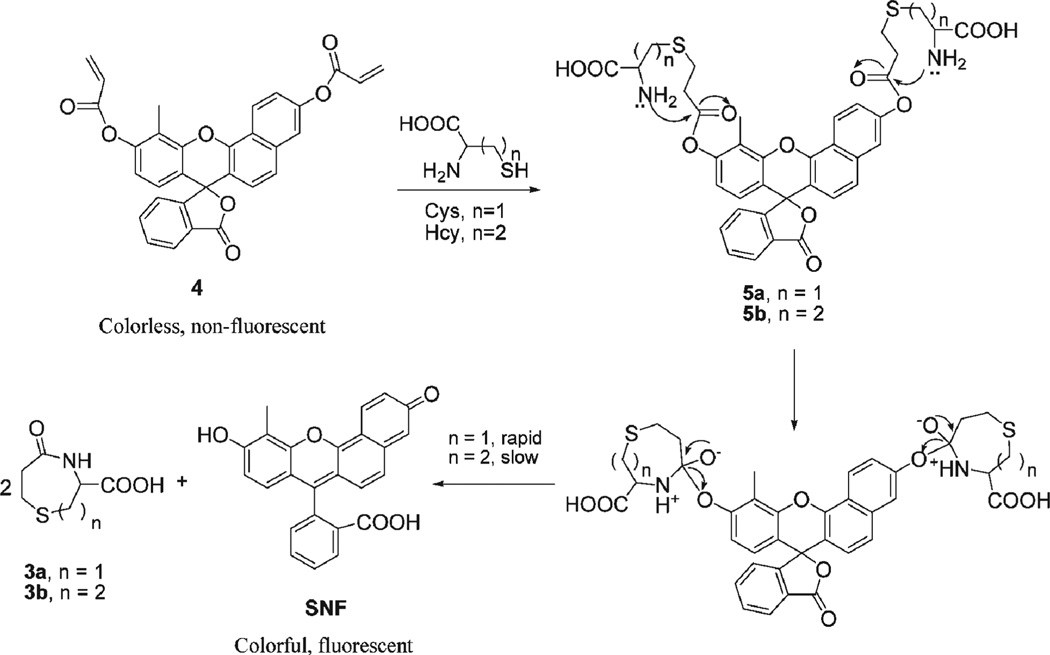
It has been known for several years that the conjugate addition of Cys to acrylates (1) will generate the corresponding thioether (2), which can further undergo an intramolecular cyclization to yield 3-carboxy-5-oxoperhydro-1,4-thiazepine (3a) (Scheme 1).6 Recently, a new design principle for a probe capable of distinguishing Cys and Hcy was developed in our labs. The (hydroxymethoxyphenyl) benzothiazole (HMBT)-based fluorescent probe functioned based on a combined PET and ESIPT mechanism.7 Since HMBT has a relatively low quantum yield8 and short excitation wavelength (304 nm) which may limit its application in biological samples, herein we report a long wavelength fluorescent chemodosimeter for Cys. It couples a conjugate addition/cyclization mechanism to a xanthene dye spirolactone-opening reaction. Seminaphthofluorescein (SNF) bis-acrylate 4 was synthesized by the condensation of SNF with acryloyl chloride (ESI, Scheme S1†). SNF was selected as a fluorescent reporter because of its long wavelength absorption and emission.9
Scheme 1.
Formation of 3-carboxy-5-oxoperhydro-1,4-thiazepine (3a) from the reaction of acrylates (R = alkyl, aryl) and Cys.
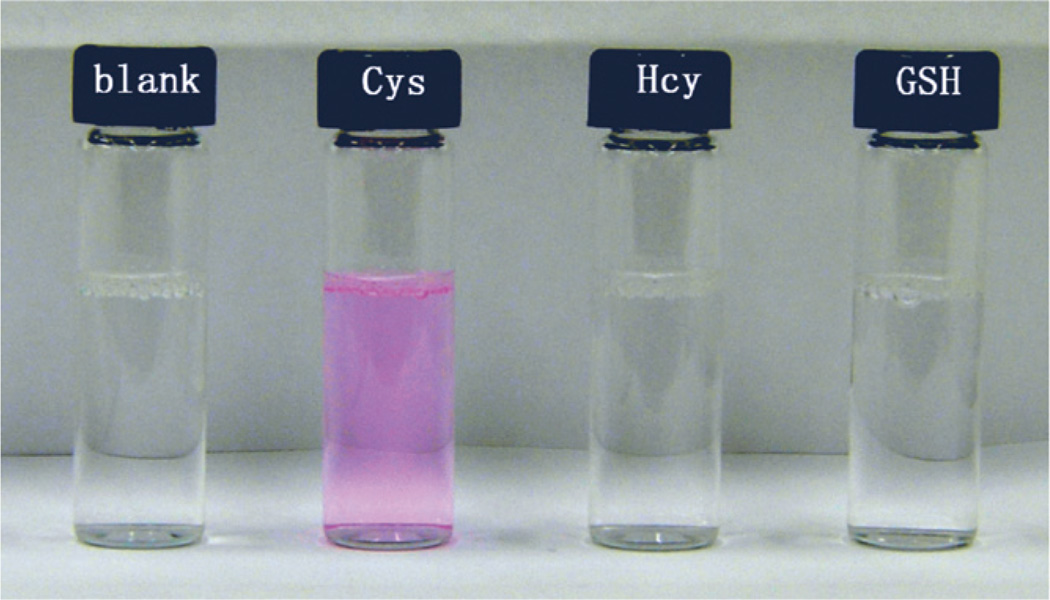
Interestingly, upon mixing Cys in colorless solution of 4 in 1.0 mM cetyltrimethylammonium bromide media (CTAB) buffered at pH 7.4, both a pink color and strong fluorescence appeared gradually, while solutions other amino acids and biothiols, such as Hcy and GSH afforded no obvious changes in the same conditions. This interesting feature indicates that 4 can serve as a selective “off-to-on” dosimeter for Cys (Fig. 1).
Fig. 1.
Color changes of the solution of 4 (10 µM) in the presence of different biothiols (2 equiv.) in 1.0 mM CTAB media buffered at pH 7.4 (Hepes buffer, 20 mM) after 25 min.
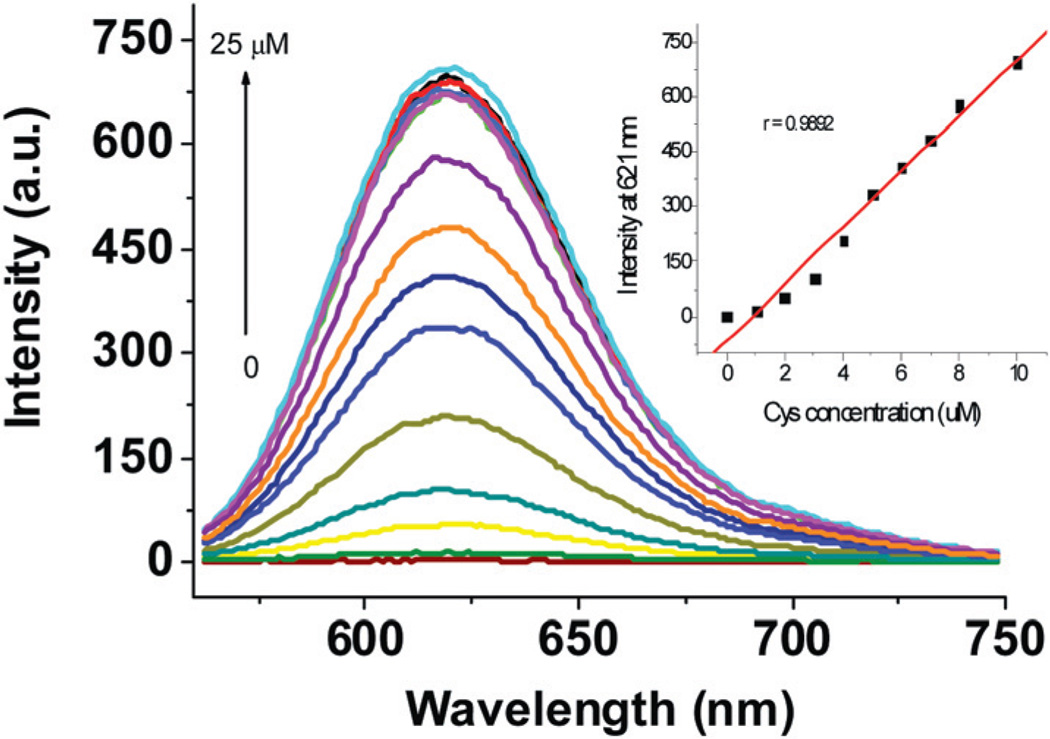
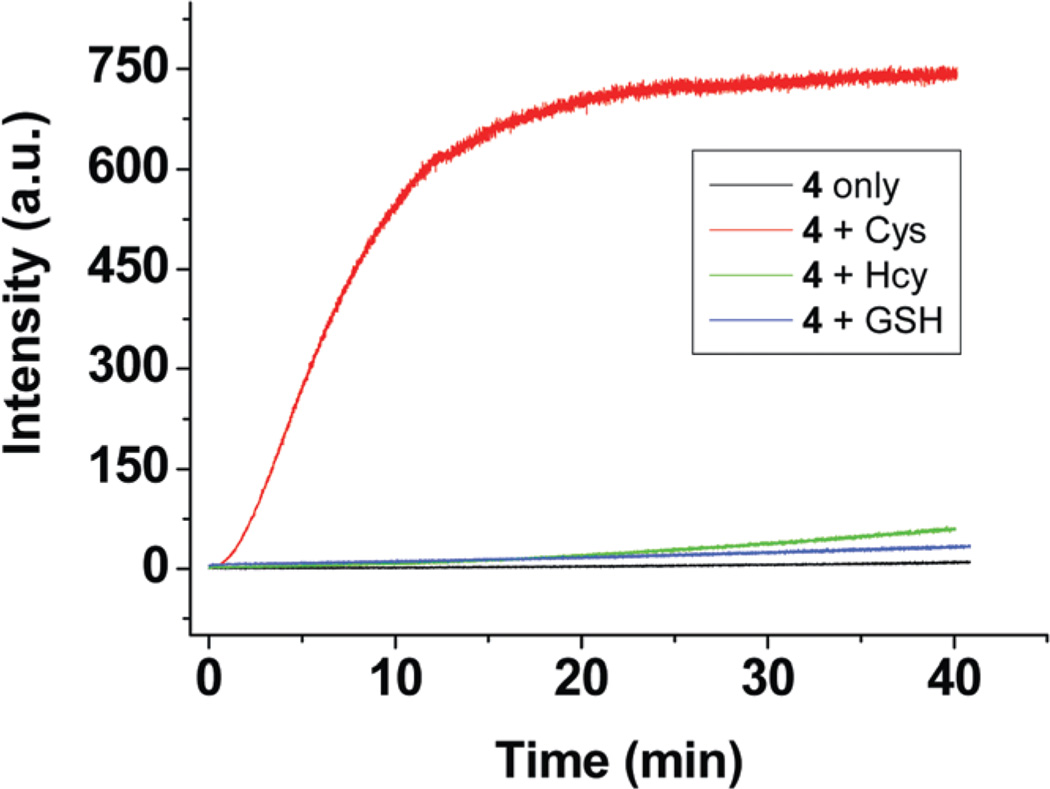
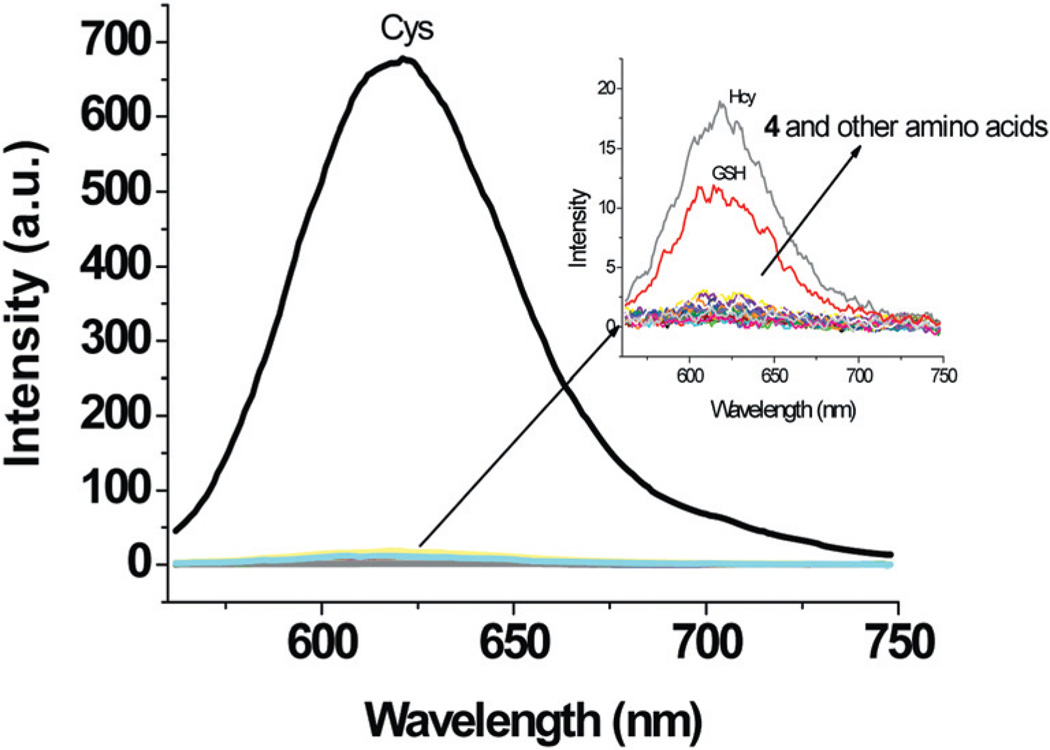
Fluorescence spectra of solutions containing 4 and increasing concentrations of Cys are shown in Fig. 2. The fluorescence intensity at 621 nm increases with increasing Cys concentration. The observed fluorescence intensity is nearly proportional to the Cys concentration up to 10 µM. Moreover, as low as 0.2 µM Cys can be readily detected by using the proposed dosimeter (ESI, Fig. S3†). The time course of the fluorescence assay is shown in Fig. 3 It can be seen that the fluorescence of the reaction with Cys is increasing with time and reaches a plateau at 20 min, whereas for Hcy and GSH, no significant fluorescence increase is observed during this period.
Fig. 2.
Fluorescence spectra (λex = 550 nm) of 4 (10 µM) with the addition of increasing concentrations of Cys in 1.0 mM CTAB media buffered at pH 7.4 (Hepes buffer, 20 mM), 25 min. The inset figure shows the changes in fluorescence intensity at 621 nm as a function of Cys concentration.
Fig. 3.
Time course study of as solution of 4 (10 µM) and 1 equiv. biothiols in 1 mM CTAB media buffered at pH 7.4. λex/λem = 562/621 nm.
To evaluate the selectivity of 4 for Cys, changes in the fluorescence intensity of 4 caused by other analytes were also measured. Fig. 4 shows the fluorescence spectra of the solution of 4 after the addition of these analytes after 25 min. It was observed that other amino acids and biothiols promote almost no fluorescence intensity changes under the same conditions, demonstrating the high selectivity of 4 for Cys over other analytes. To further test the Cys specificity of 4, competition experiments were conducted with added excess amounts of other amino acids. No significant variation in fluorescence intensity was found in comparison to solutions containing Cys only (ESI, Fig. S7†).
Fig. 4.
Fluorescence spectra (λex = 550 nm) of 4 (10 µM) with the addition of 1 equiv. of Cys, Hcy, GSH, leucine, proline, arginine, histidine, valine, methionine, threonine, glutamine, alanine, aspartic acid, norleucine, isoleucine, lysine, tryptophan, tyrosine, phenylalanine, cystine and homocystine for 25 min in 1 mM CTAB buffered at pH 7.4 (Hepes buffer, 20 mM).
The selectivity of 4 towards Cys over other biothiols was examined. Upon mixing Cys with 4, the conjugate addition product 5a is formed, which undergoes a rapid cyclization reaction to produce 3a while releasing the free SNF, thus strong fluorescent emission is recorded (Scheme 2). In the case of GSH, 1,4-addition of thiols to the α,β-unsaturated carbonyl moieties of 4 can occur. However, the ensuing intramolecular cyclization similar to that of Cys cannot proceed because the free amine does not attack the ester moiety in the 4–GSH adduct apparently due to entropic considerations involved in large ring formation. Thus, the dosimeter still exists in its colorless, non-fluorescent spirolactone form, even after the initial conjugate addition reaction. Mass spectrometric analysis of the product generated from the mixture of 4 with GSH in CH3OH–H2O (8 : 1, v/v) confirms the formation of the 4–GSH adduct, and a prominent peak at m/z 558.1390 corresponding to [4–GSH–2H]2− (calc. 558.1365 for C51H52N6O19S2) is clearly observed in the HRMS data (ESI, Fig. S13†). In the case of Hcy, the corresponding conjugate addition adduct 5b is produced, but the rate for the subsequent intramolecular cyclization is apparently too slow for 3b to be observed even after an extended incubation time (3 h) (the addition adduct 5b was confirmed by HRMS data, Fig. S14, see ESI†). This can be explained by the fact that for Cys, a 7-membered ring intermediate is formed, whereas for Hcy, because it has an additional methylene group in its side chain, a kinetically less favored 8-membered ring would form.10
Scheme 2.
Proposed mechanism of discrimination of Cys from Hcy when using 4.
Furthermore, experiments were carried out to prove the above sensing mechanism. Firstly, the product mixture of the reaction of cysteamine (cysteamine was selected instead of Cys because of its good solubility in organic solvents) with 4 in CH3OH was separated and 1,4-thiazepan-5-one (6) and the parent SNF were obtained, respectively. The structure of 6 was identified by 1H NMR, 13C NMR and HRMS (ESI, Fig. S15–S17†). The formation of SNF was confirmed by a major peak at m/z 395.0929, corresponding to [SNF–H]− (calc. 395.0919 for C25H15O5) was shown in the HRMS data (ESI, Fig. S18†). Secondly, cysteamine and 3-mercaptopropanoic acid (MPA) were introduced to the solution of 4, respectively, and it was observed that the former gives a prominent increase in fluorescence emission but the latter produces weak fluorescence increase at the same conditions. Lastly, N-acetyl-l-cysteine (NAC) was added to a solution of 4 and almost no fluorescence increase was observed under the same conditions (ESI, Fig. S8†). The above experiments serve as strong evidence that both sulfhydril and amino groups (the NAC amine is blocked) of Cys are responsible for the signal.
Compound 4 was used for the quantitative measurement of Cys content in a human plasma sample. 0.5 mL human plasma was reduced using triphenylphosphine (0.1 M, 80 µL) in the presence of HCl (0.2 M, 40 µL) for 15 min at rt.11 Proteins present in the sample after reduction were precipitated by the addition of acetonitrile (0.5 mL), followed by centrifugation (4000 rpm) of the sample for 20 min. The supernatant liquid was then added to a solution of 4 (10 µM) in pH 7.4 Hepes buffer solution (0.1 M, 5 mL) in the presence of 1.0 mM CTAB.7 As shown in Fig. S9,† the fluorescence emission shows a significant increase with the addition of reduced plasma. However, the fluorescence emission of the solution of 4 showed no obvious increase when controls of triphenylphosphine alone or deproteinized plasma (without reducing agent) were added, respectively, proving that the fluorescent increment of the solution of reduced plasma and 4 is indeed directly correlated to the presence of Cys. The amount of Cys in the plasma sample was determined by the standard addition method to be 172.8 ± 8.7 µM (n = 3), which is well within the reported Cys concentration range (135.8–266.5 µM) for human plasma samples from healthy individuals.12 These results prove that the proposed dosimeter may be useful for the quantitative detection of Cys in biological media.
In conclusion, we have developed a highly selective chemodosimeter for Cys over other biothiols including Hcy and GSH in aqueous solution. The extremely high selectivity of 4 towards Cys can be explained by the reaction of Cys with 4 to give the corresponding conjugate 5a, which undergoes intramolecular cyclization releasing free SNF, resulting in a dual chromo- and fluorogenic response. Due to its simplicity and selectivity, the proposed chemodosimeter can be used to detect Cys at physiological levels.
Supplementary Material
Acknowledgements
This work was support by the National Institutes of Health (RO1 EB002044). XF Yang acknowledges financial support from the China Scholarship Council.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ob25178g
Notes and references
- 1.(a) Wang XF, Cynader MS. J. Neurosci. 2001;21:3322–3331. doi: 10.1523/JNEUROSCI.21-10-03322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu J, Yeo HC, Overvik-Douki E, Hagen T, Doniger SJ, Chu DW, Brook GA, Ames BNJ. Appl. Physiol. 2000;89:21–28. doi: 10.1152/jappl.2000.89.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Shahrokhian S. Anal. Chem. 2001;73:5972–5978. doi: 10.1021/ac010541m. [DOI] [PubMed] [Google Scholar]
- 3.(a) Chen X, Zhou Y, Peng X, Yoon J. Chem. Soc. Rev. 2010;39:2120–2135. doi: 10.1039/b925092a. and references therein. [DOI] [PubMed] [Google Scholar]; (b) Lee JH, Lim CS, Tian YS, Han JH, Cho BR. J. Am. Chem. Soc. 2010;132:1216–1217. doi: 10.1021/ja9090676. [DOI] [PubMed] [Google Scholar]; (c) Zhang Y, Li Y, Yan XP. Anal. Chem. 2009;81:5001–5007. doi: 10.1021/ac900394e. [DOI] [PubMed] [Google Scholar]; (d) Kwon H, Lee K, Kim HJ. Chem. Commun. 2011;47:1773–1775. doi: 10.1039/c0cc04092d. [DOI] [PubMed] [Google Scholar]; (e) Jung HS, Ko KC, Kim GH, Lee AR, Na YC, Kang C, Lee JY, Kim JS. Org. Lett. 2011;13:1498–1501. doi: 10.1021/ol2001864. [DOI] [PubMed] [Google Scholar]; (f) Liu Y, Yu Y, Lam JWY, Hong YN, Faisal M, Yuan WZ, Tang BZ. Chem.–Eur. J. 2010;16:8433–8438. doi: 10.1002/chem.200902505. [DOI] [PubMed] [Google Scholar]; (g) Chen XQ, Ko S-K, Kim MJ, Shin I, Yoon J. Chem. Commun. 2010;46:2751–2753. doi: 10.1039/b925453f. [DOI] [PubMed] [Google Scholar]; (h) Sreejith S, Divya KP, Ajayaghosh A. Angew. Chem., Int. Ed. 2008;47:7883–7887. doi: 10.1002/anie.200803194. [DOI] [PubMed] [Google Scholar]; (i) Shiu HY, Chong HC, Leung YC, Wong MK, Che CM. Chem. –Eur. J. 2010;16:3308–3313. doi: 10.1002/chem.200903121. [DOI] [PubMed] [Google Scholar]; (j) Long LL, Lin WY, Chen BB, Gao WS, Yuan L. Chem. Commun. 2011;47:893–895. doi: 10.1039/c0cc03806g. [DOI] [PubMed] [Google Scholar]; (k) Shao JY, Guo HM, Ji SM, Zhao JZ. Biosens. Bioelectron. 2011;26:3012–3017. doi: 10.1016/j.bios.2010.12.004. [DOI] [PubMed] [Google Scholar]; (l) Yang Y-K, Shim S, Tae J. Chem. Commun. 2010;46:7766–7768. doi: 10.1039/c0cc02381g. [DOI] [PubMed] [Google Scholar]
- 4.(a) Rusin O, Luce NNS, Agbaria RA, Escobedo JO, Jiang S, Warner IM, Dawan FB, Lian K, Strongin RM. J. Am. Chem. Soc. 2004;126:438–439. doi: 10.1021/ja036297t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang WH, Rusin O, Xu XY, Kim KK, Escobedo JO, Fakayode SO, Fletcher KA, Lowry M, Schowalter CM, Lawrence CM, Fronczek FR, Warner IM, Strongin RM. J. Am. Chem. Soc. 2005;127:15949–15958. doi: 10.1021/ja054962n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Lim S, Escobedo JO, Lowry M, Xu XY, Strongin R. Chem. Commun. 2010;46:5707–5709. doi: 10.1039/c0cc01398f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chen HL, Zhao Q, Wu YB, Li FY, Yang H, Yi T, Huang CH. Inorg. Chem. 2007;46:11075–11081. doi: 10.1021/ic7010887. [DOI] [PubMed] [Google Scholar]; (c) Li HL, Fan JL, Wang JY, Tian MZ, Du JJ, Sun SG, Sun PP, Peng XJ. Chem. Commun. 2009:5904–5906. doi: 10.1039/b907511a. [DOI] [PubMed] [Google Scholar]; (d) Yuan L, Lin WY, Yang YT. Chem. Commun. 2011;47:6275–6277. doi: 10.1039/c1cc11316j. [DOI] [PubMed] [Google Scholar]
- 6.Blondeau P, Gauthier R, Berse C, Gravel D. Can. J. Chem. 1971;49:3866–3876. [Google Scholar]
- 7.Yang XF, Guo XY, Strongin RM. Angew. Chem., Int. Ed. 2011;50:10690–10693. doi: 10.1002/anie.201103759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henary MM, Fahrni CJ. J. Phys. Chem. A. 2002;106:5210–5220. [Google Scholar]
- 9.Chang CJ, Jaworskit J, Nolan EM, Sheng M, Lippard SJ. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1129–1134. doi: 10.1073/pnas.0308079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Illuminati G, Mandolini L. Acc. Chem. Res. 1981;14:95–102. [Google Scholar]
- 11.(a) Shang L, Yin JY, Li J, Jin LH, Dong SJ. Biosens. Bioelectron. 2009;25:269–274. doi: 10.1016/j.bios.2009.06.021. [DOI] [PubMed] [Google Scholar]; (b) Ivanov AR, Nazimov IV, Baratova L. J. Chromatogr., A. 2000;895:157–166. doi: 10.1016/s0021-9673(00)00713-5. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen DW, Gatautis VJ, Green R, Robinson K, Savon SR, Secic M, Ji J, Otto JM, Taylor LM., Jr Clin. Chem. 1994;40:873–881. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.