Summary
Purpose
P450 enzymes (CYPs) play a major role in hepatic drug metabolism. It is unclear whether these enzymes are functionally expressed by the diseased human blood–brain barrier (BBB) and are involved in local drug metabolism or response. We have evaluated the cerebrovascular CYP expression and function, hypothesizing possible implication in drug-resistant epilepsy.
Methods
CYP P450 transcript levels were assessed by cDNA microarray in primary endothelial cultures established from a cohort of brain resections (n = 12, drug-resistant epilepsy EPI-EC and aneurism domes ANE-EC). A human brain endothelial cell line (HBMEC) and non-brain endothelial cell line (HUVEC) were used as controls. The effect of exposure to shear stress on CYP expression was evaluated. Results were confirmed by Western blot and immunohistochemistry on brain specimens. Endothelial drug metabolism was assessed by high performance liquid chromatography (HPLC-UV).
Results
cDNA microarray showed the presence of CYP enzymes in isolated human primary brain endothelial cells. Using EPI-EC and HBMEC we found that CYP mRNA levels were significantly affected by exposure to shear stress. CYP3A4 protein was overexpressed in EPI-EC (290 ± 30%) compared to HBMEC and further upregulated by shear stress exposure. CYP3A4 was increased in the vascular compartment at regions of reactive gliosis in the drug-resistant epileptic brain. Metabolism of carbamazepine was significantly elevated in EPI-EC compared to HBMEC.
Discussion
These results support the hypothesis of local drug metabolism at the diseased BBB. The direct association between BBB CYP enzymes and the drug-resistant phenotype needs to be further investigated.
Keywords: Blood–brain, barrier, Endothelial drug metabolism, Shear stress
The blood–brain barrier (BBB) represents one of the main obstacles to central nervous system (CNS) drug delivery (Abbott, 2002). Understanding the mechanisms regulating drug passage across the BBB may lead to the development of novel therapies based on the manipulation of specific BBB targets. This can overcome mechanisms of drug resistance in CNS diseases such as drug-refractory epilepsy. The mechanisms regulating the bioavailability of drugs across the BBB are similar to those used by other organ systems (e.g., gut and liver). These functions are performed by transporter proteins and metabolic enzymes (CYPs) that dictate the extent and chemical form of xenobiotics available (Dauchy et al., 2008, 2009).
Most studies related to the diseased BBB (e.g., drug resistant epilepsy) have focused on “transport” rather than “metabolic” barrier mechanisms. In vitro studies have demonstrated CYP expression by neurons and glia (Walther et al., 1986). General CNS expression of CYP1A1, 1B1, epoxide hydrolase, and UDP-glucuronosyltransferase was confirmed using rodent models (Ghersi-Egea et al., 1987) and human brain tissue (Ghersi-Egea et al., 1993; Volk et al., 1995). Recently, the presence of CYP enzymes was evaluated in an immortalized human-derived brain endothelial cell line (Dauchy et al., 2008, 2009).
In the present study, we evaluated the expression of CYP enzymes in primary endothelial cells established from a cohort of brain resections. We specifically investigated the effect of exposures to shear stress in regulating CYP gene levels using a dynamic in vitro system (DIV) capable of generating laminar flow recapitulating physiologic conditions (Stanness et al., 1996; Cucullo et al., 2008). Previous evidence demonstrated that exposure to laminar flow affects the regulation of transcription and protein synthesis by BBB endothelial cells (Desai et al., 2002). We investigated the expression of the CYP3A4 enzyme, involved in the metabolism of antiepileptic drugs (AEDs), using EPI-EC and matched drug-resistant epileptic brain specimens. We assessed the functional relevance of CYP endothelial expression in carbamazepine (CBZ) metabolism by high performance liquid chromatography (HPLC-UV). A human hepatocyte cell line was used as positive control.
Materials and Methods
Endothelial cells
We used the following: (1) primary endothelial cells derived from brain specimens resected from drug-resistant epileptic patients (EPI-EC, Table S1); (2) primary endothelial cells derived from brain specimens resected from aneurism domes (ANE-EC, Table S1); (3) commercially available control human brain microvascular cerebral endothelial cells (ScienCell, cat. num. 1000, HBMEC); and (4) human-derived umbilical vein endothelial cells (HUVEC, ScienCell, cat num. 8000).
EPI-EC and ANE-EC
The investigation conforms to the principles outlined in the Declaration of Helsinki. Patient consent was obtained as per the institutional review board instructions before collection of the specimens. Cells were isolated from secondary branches of the middle cerebral arteries of brain specimens from patients undergoing temporal lobectomies (EPI-EC, Table S1) to relieve medically intractable seizures or remove aneurism domes (ANE-EC) (Dombrowski et al., 2001; Desai et al., 2002). Briefly, surgical specimens were incubated in collagenase type II (2 mg/ml, Worthington chemicals) at 37°C for 20 min to dissociate the endothelial cells. Collagenase was then washed off with medium (1.5 g/100 ml, MCDB 105 supplemented with endothelial cell growth supplement 15 mg/100 ml, heparin 800 units/100 ml, 10% fetal bovine serum, and penicillin/streptomycin 1%). Cells stained positive for Von Willebrand factor (vWF) and were negative for glial fibrillary acidic protein (GFAP). EPI-EC were initially expanded in 75 cm2 flasks precoated with fibronectin (3 μg/cm2) (Cucullo et al., 2007). HBMEC and HUVEC were cultured under the same conditions. Human astrocytes (HAs, cat. no. 1800) were purchased from ScienCell Research Laboratories (San Diego, CA, U.S.A).
Hepatocytes
A human immortalized human hepatocyte cell line (cat. CRL-11233) was purchased from American Type Culture Collection (ATCC, Rockville, MD, U.S.A.). Cells were grown as recommended (CC3170; Clonetics Corporation, Walkersville, MD, U.S.A).
cDNA arrays
Gene analysis (total number of genes 4,319) was performed in EPI-EC, ANE-EC, HBMEC, and HUVEC (Fig. S1 and Table S2). Patients’ data (Table S1) were analyzed individually: EPI-EC (n = 4 patients GeneFilter, Research Genetics Inc., Huntsville, AL, U.S.A.), ANE-EC (n = 4 patients GeneFilter Research Genetics Inc.), HUVEC (GeneFilter Research Genetics Inc.), and HBMEC (Illumina bead-array, San Diego, CA, U.S.A.). Transcription changes were analyzed using Ingenuity Pathway Analysis (Ingenuity Systems, Mountain View, CA, U.S.A). GenBank/UniGene IDs were used. Among the genes screened, we analyzed the mRNA levels of different CYPs and drug transporter proteins. cDNA arrays were also performed on HBMEC and EPI-EC cultured under shear stress conditions.
Briefly, cells were purged from the culture dishes by enzymatic dissociation (collagenase) and total RNA was extracted with Trizol reagent (Gibco Invitrogen, Carlsbad, CA, U.S.A.). EPI-EC used for the present study were passaged no more than two times after isolation (Dombrowski et al., 2001; Desai et al., 2002). For gene expression analysis, human Genefilters (Research Genetics Inc.) were used. 33P-dCTP was used to label probes used for hybridization to produce clean and sharp signals. To produce first strand cDNA probes, total RNA (1 μg) isolated from cells was mixed with oligos (1 μg/μl, 10–20 bases; Research Genetics), denatured for 10 min at 70°C and chilled on ice. Synthesis mixture consisting of first strand buffer, DTT, dNTPs mixture (dATP, dGTP, and dTTP at 20 mM, Pharmacia), Reverse Transcriptase (Superscript II, Life Technologies, Carlsbad, CA, U.S.A.) and 50 μCi 33P-dCTP (ICN Radiochemicals, Irvine, CA, U.S.A.) were then added. The mixture was incubated at 37°C for 90 min and purified by gel filtration column chromatography. Filters were prehybridized with 1 μg/ml of poly dA and 1 μg/ml of Cot1 DNA for 2 h at 42°C. The probe was denatured at 90 °C for 3 min and added to the prehybridization mixture. Hybridization was performed overnight (12–18 h) at 42°C.
Dynamic in vitro model (DIV) (Stanness et al., 1996)
DIV modules were purchased from Spectrum (cat. no. 400-025, Spectrum Lab, CA, U.S.A) or Flocel Inc. (Cleveland, OH). Each module consists of hollow polypropylene capillaries embedded in a clear plastic chamber. The capillaries are connected with a medium reservoir and a pulsatile pump apparatus (Cellmax QUAD, Spectrum Lab, CA, U.S.A). Media is pumped in the luminal side. We used a flow rate of 4–5 ml/min corresponding to a shear stress of 3 dyne/cm2. Flow was applied to endothelial cells for 1 week using a dynamic in vitro (DIV) system. Control brain endothelial cells and EPI-EC (4 × 106/DIV) were seeded in the luminal side as described previously (Cucullo et al., 2007). Cells were also seeded in the extraluminal side of the DIV and, therefore, not exposed to shear stress. Astrocytes (4 × 106) were cocultured in the abluminal side of the DIV.
Protein isolation and Western blot analysis
Total proteins were extracted from HBMEC and EPI-EC cultured in the DIV (n = 4) or from HBMEC and EPI-EC not exposed to shear stress (n = 4) or hepatocytes (n = 4) as previously described (Marchi et al., 2006). The membranes incubated with the primary antibody (rabbit polyclonal Cytochrome P450 3A4, 1:1,000, Abcam Inc, Cambridge, MA, U.S.A) and mouse monoclonal β-Actin 1:500 dilution (Sigma, St Louis, MO, U.S.A) overnight at 4°C.
Histologic and immunocytochemical staining
Histologic and immunohistochemical (IHC) staining were performed from blocks of neocortical epileptic tissues obtained during surgery (Table S1). Human drug-resistant epileptic brain (n = 3) were evaluated for the study. For histologic studies, five sections (30–35 μm) from the temporal cortex were collected and stained with 1% Cresyl violet (CV) for cytoarchitectural analysis (dyslamination, abnormal neuronal morphology, ectopias, and vascular malformations. Free floating sections were stained with Cyp3A4, GFAP, and vWF (Marchi et al., 2004, 2006). We used: rabbit polyclonal anti-human Cyp3A4 (AB1254) (1:1,000, Chemi-Con, now Millipore, U.S.A.); mouse monoclonal anti-GFAP (G 3893, 1:100; Sigma, St Louis, MO, U.S.A.); mouse monoclonal anti-vWF (3H3126, 1:200; Santa Cruz, U.S.A.). Secondary antibodies: Texas red affinipure donkey anti-mouse IgG (1:100; Jackson Laboratories Inc., West Grove, PA, U.S.A.), and fluorescein isothiocyanate (FITC)–conjugated affinipure donkey anti-rabbit IgG (1:100; Jackson Laboratories Inc., West Grove, PA, U.S.A.). Autofluorescence was blocked with Sudan black B. Sections were analyzed by fluorescent microscopy.
CYP3A4 expression was quantified by measuring the green fluorescent signal in nine sections (n = 3 patients, Table S1). For quantification of CYP3A4 and GFAP expression, green and red fluorochromes were excited by a laser beam at 488 and 575 nm, respectively. All sections were scanned in the 1,600 × 1,200 pixel format in the x–y direction and the acquired images were processed using QCapture-Pro Software and Photoshop CS2. These measurements represent the “volume” of fluorescence (Marchi et al., 2006). Pixel number and intensity were measured using a green (or red) channel only and adjustment of the background signal to zero.
HPLC-UV analysis
Carbamazepine was added (CBZ, Sigma-Aldrich) to obtain a final concentration of 40–60 μg/ml, as assessed by HPLC at time zero in each experiment (Table 1). CBZ metabolism was estimated at different time points (0, 24, 48, and 72 h, Table 1 and Fig. 5) from flow and no-flow conditions (n = 4 per experiment) by reverse-phase high-performance liquid chromatography (HPLC) UV detection (Agilent 1100 Series) (Patil & Bodhankar, 2005). HPLC was performed using a Zorbax Eclipse Plus C18 stainless steel column (4.6 × 150 mm, 3.5 μm; Agilent Technologies, Santa Clara, U.S.A.).
Table 1.
CBZ metabolized with time as determined by HPLC-UV
| 0 h | 24 h | 48 h | 72 h | % Metabolized (0–72 h) | |
|---|---|---|---|---|---|
| Media only Hepatocytes | 45.42 ± 0.01 | 48.86 ± 0.55 | 49.95 ± 1.79 | 45.77 ± 0.46 | 0 |
| Media only HBMEC | 45.22 ± 0.20 | 46.91 ± 0.68 | 46.40 ± 1.19 | 44.43 ± 0.63 | 1.7 |
| No-shear HBMEC | 47.04 ± 0.02 | 39.36 ± 0.05 | 39.18 ± 0.09 | 37.56 ± 0.10 | 20.2 |
| Shear HBMEC | 44.85 ± 0.40 | 40.77 ± 0.41 | 35.80 ± 1.13 | 31.95 ± 1.33 | 28.7 |
| Hepatocytes | 43.25 ± 0.01 | 35.65 ± 0.54 | 29.77 ± 1.54 | 26.24 ± 1.09 | 39.3 |
| EPI-EC | 64.91 ± 0.35 | 56.27 ± 1.95 | 47.15 ± 1.07 | 37.92 ± 1.84 | 41.6 |
Data are expressed as mean values (μg/ml) ± SE (n = 4 experiments/condition). Data are relative to sampling performed at indicated time points.
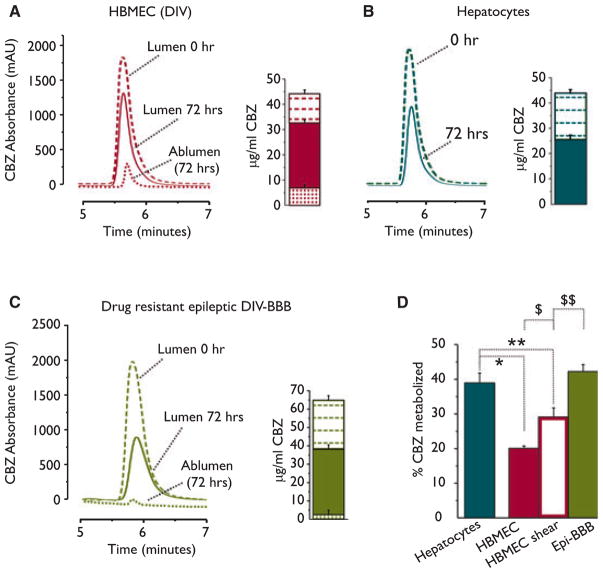
Figure 5.
Metabolism of carbamazepine (CBZ) at the blood–brain barrier. (A) CBZ was significantly metabolized within 72 h by dynamically grown control endothelial cells. An example of high-performance liquid chromatography (HPLC) traces demonstrated the decrease of CBZ, whereas bar-graph indicates the absolute values (μg/ml) of CBZ at time zero and 72 h after (standard curve: y = −396.5 + 567 X, r2 = 0.98). Panel (B) displays the metabolic potency of hepatocytes evaluated in the same time frame. (C) Human drug-resistant epileptic blood–brain barrier (BBB) was established using EPI-EC cocultured with astrocytes (DIV-BBB). Note the reduction of CBZ in the luminal side and the near-zero levels of CBZ in the abluminal side (dotted line), suggesting poor penetration across the drug-resistant epileptic BBB. The amount of CBZ penetrating the BBB was higher in the control endothelium (dotted line in A). The relative percentage of CBZ metabolized by hepatocytes, HBMEC shear, and no-shear and epileptic is summarized in (D) and in Table 1 (One-way ANOVA, *p < 0.05 EC-shear vs. Epi-BBB; **p < 0.05 hepatocytes vs. EC-shear; $p < 0.05 HBMEC vs. HBMEC shear; $$p < 0.05 HBMEC shear vs. EPI-EC.) Results are expressed as mean ± SEM.
Preparation of standard solutions
A stock solution containing 1 mg/ml of CBZ was prepared in methanol. The calibration standards (0.5, 5, 10, 20, 40, and 60 μg/ml) were prepared by further dilution of stock solution with drug-free media. All solutions were stored at −20°C.
Chromatographic conditions
The mobile phase consisted of phosphate buffer (10 mM)-methanol-acetonitrile-acetone (55:22:12:11, v/v/v/v) at pH adjusted to 7.0 with 0.5 M NaOH. 10 mM phosphate buffer was prepared by dissolving 1.36 g of potassium dihydrogen phosphate (KH2PO4) in 1 L of doubly distilled water. Chromatography was performed at room temperature (flow rate of 1.2 ml/min at 210 nm).
Sampling and extraction procedure
Two hundred microliters of endothelial and hepatocyte media samples were collected and centrifuged at 4,900 g for 10 min. The supernatant was filtered through 0.2 μm membrane filter and 50 μl of filtrate was injected onto the column.
Specificity and precision
The method was evaluated for specificity by analyzing different batches of drug-free media to check the interference of peaks of endogenous components of media. Stability: CBZ is stable for at least 4 weeks when stored at −20°C.
Statistical analysis
We used Origin 7.0 (Origin Lab, Northampton, MA, U.S.A) and JMP 7.0 (SAS) software. Shapiro-Wilk test was used to evaluate the normal distribution of the data (CYPs and drug transporters). Data are indicated as mean ± standard error of the mean (SEM). Student t test was used for direct comparison of two populations of data. One-way analysis of variance (ANOVA) (and t test) was used on paired populations (e.g., HBMEC vs. EPI-EC, HBMEC vs. ANE-EC, and ANE-EC vs. EPI-EC). Multiple comparisons were executed using the Tukey-Kramer test. Two-way ANOVA was used in cases of two independent variables (e.g., cells type and exposure to shear). p < 0.05 was considered statistically significant.
Results
CYP mRNAs in primary human brain endothelial cells
Using cDNA microarray we evaluated the levels of CYP mRNA levels in EPI-EC, ANE-EC (see Table S1), HUVEC, and HBMEC. The primary cells EPI-EC and ANE-EC used for the study were passaged no more than two times after isolation. Previous study demonstrated that EPI-EC and ANE-EC retain their original phenotype, up to three passages (Desai et al., 2002; Cucullo et al., 2007).
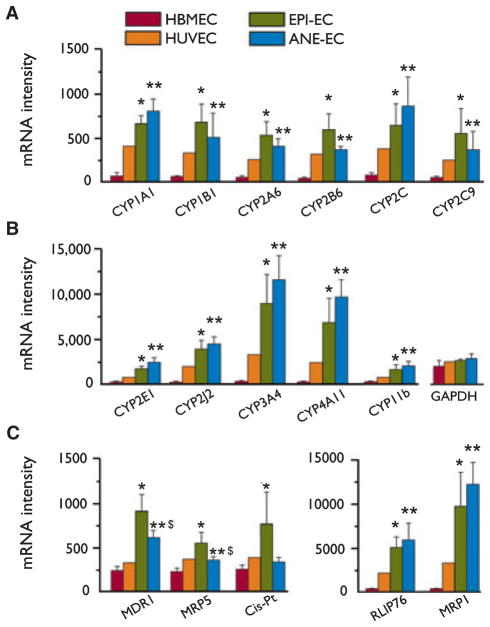
The complete list of the genes analyzed (GenBank/Uni-Gene IDs) is provided as a Table S2. We found levels of CYP enzymes in freshly isolated human brain endothelial cells (EPI-EC and ANE-EC). Of a total of 16 CYP isoforms examined, 11 were significantly increased in EPI-EC and ANE-EC compared to the controls used (Fig. 1A, B). Differences between EPI-EC and ANE-EC were not statistically significant. The levels of the housekeeping gene GAPDH did not vary among the endothelial cells used. We did not find significant changes in the mRNA levels of CYP3A5, CYP4B1, CYPC1, CYP21A, and CYP51A1. Other genes that did not change significantly were: tight junction (ZO1), tropomyosin alpha chain, potassium channel β-subunit, e-selectin, ubiquitin-like protein, glucose 6-phosphate, and glutathione peroxidase I. Other CYP enzymes (Dauchy et al., 2008) were not included in the analysis performed. Levels of drug transporter proteins (MDR1, MRP1-5, RLIP76, and cis-Pt, Fig. 1C) were increased as previously reported (Dombrowski et al., 2001; Marchi et al., 2004; Awasthi et al., 2005; Loscher, 2007).
Figure 1.
Levels of CYP mRNAs in primary human brain endothelial cells. (A–B) Primary endothelial cell cultures (EPI-EC and ANE-EC) were established from brain resections (Table S1). cDNA analysis shows the presence of CYP enzymes in primary brain endothelial cells. CYP mRNA levels were increased compared to the controls (HBMEC and HUVEC). Levels of drug transporter proteins were also assessed (C). The levels of GAPDH mRNA were used as an internal control. Results are expressed as mean ± standard error of the mean (SEM) (one-way ANOVA, *p < 0.05 HBMEC vs. EPI-EC, **p < 0.05 HBMEC vs. ANE-EC, $p < 0.05 EPI-EC vs. ANE-EC). See also Fig. S1 and Table S2.
CYP mRNA levels are affected by exposure to shear stress
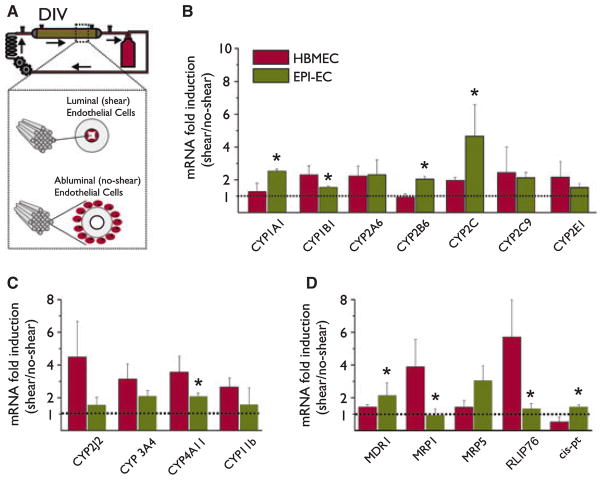
We evaluated the mRNA level of CYPs and drug transporter proteins specifically in HBMEC and EPI-EC, exposed or not to shear stress (1 week, 3 dyne/cm2). Cells were seeded either in the luminal side of the capillaries (exposed to shear) or in the extraluminal side (not exposed to shear) of a dynamic in vitro model (Fig. 2A and Desai et al., 2002). For HBMEC and EPI-EC, we calculated the ratio (mRNA)shear/(mRNA)no-shear for each CYP and drug transporter. Mean ± SEM was then calculated (n = 4, Fig. 2). Ratio of 1 indicates no change, whereas ratio >1 indicates the positive effect on transcription exerted by shear stress. Exposure to shear stress significantly affected the overall gene expression (Fig. S1B and Desai et al., 2002). Exposure to shear stress significantly increased the transcript levels of CYPs and drug transporters (ratio > 1, Fig. 2B, D). The following genes did not change significantly in response to shear stress: CYP 2J2, 11b, MRP1, and RLIP76 in EPI-EC; CYP1A1, 2C9, and 2B6 in control endothelium.
Figure 2.
CYP mRNAs are under the control of shear stress. (A) Schematic representation of the experimental design adopted. In the dynamic in vitro (DIV) system, media is pumped in the luminal side of polypropylene fibers, which determines shear forces. Control endothelial cells or EPI-EC (Table S1) were seeded either in the luminal side (exposed to shear stress) or in the abluminal space (no shear) of the DIV. (B–D) Exposure to shear stress significantly increased the transcript levels of the CYPs and drug transporters [(mRNA)shear/(mRNA)no-shear], suggesting a positive effect of shear stress on transcription. The comparison between mRNA levels in EPI-EC and HBMEC unveiled cell-specific differences to flow response (one-way ANOVA, *p < 0.05 HBMEC vs. EPI-EC). Results are expressed as mean ± SEM. See text for details. See also Fig. S1.
Figure 2 shows the differential response to shear stress of EPI-EC and control endothelium. Note that CYP1A1, 1B1, 2B6, 2C, 4A11, MDR1, MRP1, RLIP, and Cis-Pt were differentially affected by exposure to shear stress in EPI-EC vs. HBMEC (*p < 0.05). The mRNA levels of enzymes involved in the metabolic conversion of antiepileptic drugs (Cyp3A4 and Cyp2A6) (Levy, 1995; Pearce et al., 2002) were also augmented by flow.
Effects of shear stress and drug exposure on CYP3A4 protein expression
CYP3A4 is known to be involved in the metabolism of AEDs (Levy, 1995; Pearce et al., 2002). We have, therefore, selected this specific enzyme and assessed its protein expression and functional relevance to drug metabolism. Fig. 3A, B shows that CYP3A4 protein, as assessed by western blot, was significantly higher (p < 0.05) in EPI-EC (n = 4 patients, Table S1) compared to HBMEC and that exposure to shear stress further augmented this protein. Results in Fig. 3B represent the mean values of CYP3A4 expression as evaluated in EPI-EC. Fig. 3A depicts a representative western blot. We also used a human-derived hepatocyte cell line as positive control for CYP3A4 expression. Hepatocytes demonstrated CYP3A4 protein levels higher than HBMEC but similar to EPI-EC (280 ± 20% in hepatocyte and 290 ± 30% in EPI-EC, Fig. 3B, D). Hepatocyte CYP3A4 expression and metabolic potency were not significantly affected by exposure to shear stress (Fig. S3). We confirmed that endothelial and hepatocytic levels of CYP3A4 are induced by exposure to drugs (carbamazepine, CBZ; Fig. 3C, D, (Luo et al., 2002). However, exposure to CBZ did not have a significant effect on CYP3A4 levels in shear-exposed endothelial cells (HBMEC shear vs. HBMEC no-shear, Fig. 3D). Both CBZ and shear stress increased CYP3A4 protein expression, but the initial exposure of endothelial cells to shear stress precludes further modulation provoked by drug exposure.
Figure 3.
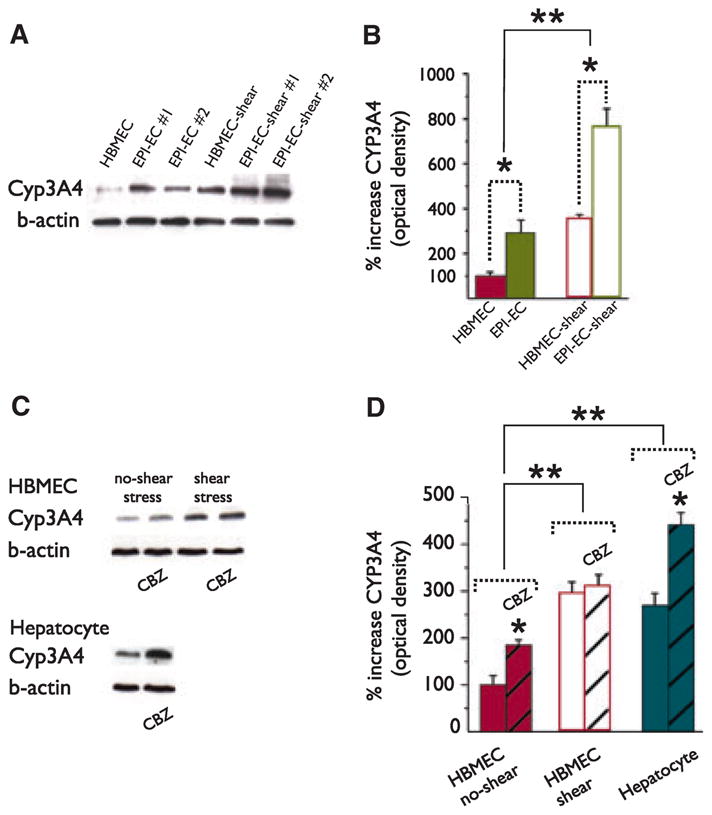
Pattern of CYP3A4 protein expression in HBMEC and EPI-EC exposed to shear stress. (A–B) Western blot analysis shows that the CYP3A4 expression is significantly increased (p < 0.05) in EPI-EC (n = 4 patients, Table S1) compared to HBMEC (*). CYP3A4 expression was significantly increased by shear stress in both cell types (**). Expression of CYP3A4 in EPI-EC was comparable to hepatocytes. (C–D) Exposures to carbamazepine (CBZ) induced the expression of CYP3A4 in endothelial cells and hepatocytes (*). However, CBZ exposure did not exert significant effects on shear-stress preexposed endothelial cells (HBMEC-shear). In HBMEC with no-shear exposure CYP expression was significantly different compared to HBMEC shear and hepatocytes (**). The intensity of the bands obtained by western blot was normalized by β-actin value. Results are expressed as mean ± SEM [two-way analysis of variance (ANOVA), p < 0.05].
Immunohistochemical evaluation of CYP3A4 expression at the BBB of drug resistant epileptic patients
Sections of temporal cortex were collected from drug-resistant epileptic brain tissue (Table S1) and stained with cresyl violet for cytoarchitectural analysis (Fig. S2). Cortical abnormalities included the presence of ectopic neurons, disrupted columnar organization, and vascular dysplasia adjacent to relatively normal cortex. Adjacent sections were stained for CYP3A4, GFAP, and vWF (Fig. 4). The montage image in Fig. 4A shows CYP3A4 expression in the dysplastic temporal cortex of epileptic human brain. Both penetrating and small caliber vessels were positive for CYP3A4 staining. CYP3A4 staining was more pronounced in gliotic brain regions (Fig. 4B, D, and E). Panels A1 and A2 shows details of CYP3A4 staining in a non-gliotic region compared to a gliotic one. Glial cells in the proximity of blood vessels were positive for GFAP (Fig. 4D, E). CYP3A4 also colocalized with vWF (Fig. 4F, G).
Figure 4.
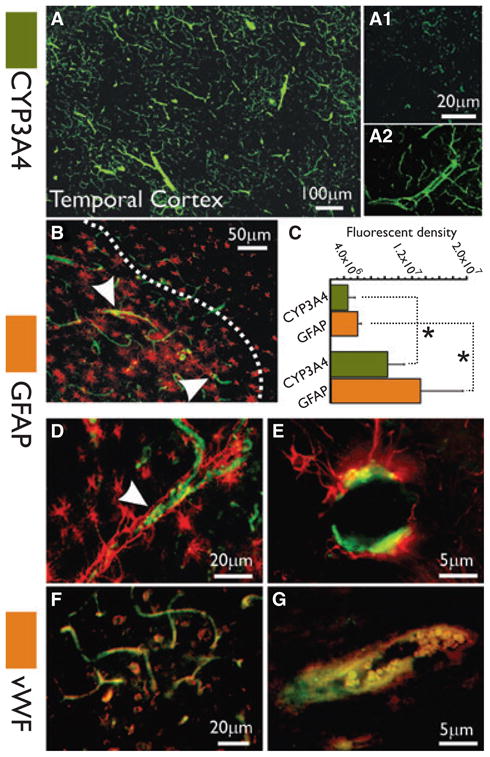
CYP3A4 expression at the blood–brain barrier of patients with drug-resistant epilepsy. (A) Montage reconstruction illustrates CYP3A4 expression in the temporal cortex of epileptic human brain. Both penetrating and small caliber vessels were positive for CYP3A4 staining (green). (B) CYP3A4 expression was more pronounced in regions of reactive gliosis, delimited by the dotted line. (D–E) Note the perivascular GFAP staining delimiting the vascular bed. (F–G) Endothelial expression of CYP3A4 colocalized with vWF. (C) CYPA4 expression (green bars) is significantly increased (*p < 0.05) in brain areas associated with reactive gliosis (bar graph n = 4 patients, three slices each). One-way analysis of variance (ANOVA) was used. Results are expressed as mean ± standard error of the mean (SEM).
We quantified the vascular CYP3A4 signal in brain regions associated with reactive gliosis (Fig. 4C). Values in the bar graph represent the fluorescence density values relative to the green or red signals. CYP signal was 25 × 105 ± 1.3 × 105 in nongliotic brain regions (GFAP signal 39.3 × 105 ± 3.1 × 105), whereas the signal was significantly increased to 8.37 × 106 ± 2.5 × 106 in gliotic regions (GFAP signal 13.2 × 106 ± 6.2 × 106).
HPLC-UV analysis of carbamazepine metabolism by brain endothelial cells
We evaluated the functional significance of CYP upregulation in EPI-EC by measuring the levels of drug (CBZ) metabolized (HPLC-UV). CBZ was chosen due to the known metabolic transformation exerted by CYP3A4 (Levy, 1995; Pearce et al., 2002). A summary of the results is shown in Table 1 and Fig. 5. Endothelial and hepatocyte media alone did not provoke or facilitate the spontaneous degradation of CBZ (Table 1). Endothelial cells exposed to shear stress determined a significantly higher decay of CBZ compared to no-shear conditions within 72 h (28.7% and 20.2%, respectively; Table 1). This represents a relative increase in ~70% of metabolism between the two conditions. The results in Fig. 5A, B emphasize the reduction in CBZ levels after 72 h in culture. The histograms in the Fig. 5A, B show the average results obtained from three experiments under identical conditions. EPI-EC displayed levels of CBZ metabolism that was equal to hepatocytes and larger than the metabolic potency of control endothelium (Fig. 5C and Table 1). Interestingly, small levels of CBZ were detected in the abluminal side of the epileptic BBB (small dotted line in chromatogram and bar graph, Fig. 5C) throughout the duration of the experiments (0–72 h) compared to the amount of CBZ permeate in the abluminal space when using control endothelium in the DIV (small dotted line chromatogram and bar-graph, Fig. 5A). These results support the presence of metabolic and transporter mechanisms in reducing drug brain penetration in the in vitro epileptic BBB. The relative changes in CBZ levels depicted in Fig. 5A, C are also summarized in panel D.
Discussion
Our data support the hypothesis that the diseased BBB acts as a metabolic barrier, possibly confounding the pharmacokinetics of central nervous system (CNS) drugs. The results obtained from a relatively small cohort of patients suggest the presence of CYP enzymes at the diseased BBB, including endothelium derived from epileptics. We also discovered that hemodynamic conditions play an important role in regulating CYP expression and function. Cerebrovascular drug metabolisms could alter the pharmacokinetics of CNS drugs and possibly contribute to the multifaceted drug-resistant phenotype observed in refractory epilepsy.
CYPs at the human brain endothelium
Liver and gut cells ensure the metabolic transformation of xenobiotics and endogenous molecules (Martignoni et al., 2006). The data presented herein represent a significant departure from the concept of systemic drug metabolism, rather suggesting a local cerebrovascular drug transformation process. In the past, enzymes that are involved in hepatic drug metabolism have been mostly studied in the choroid plexus and leptomeninges (Ghersi-Egea et al., 1993). Our data reveal CYP expression in the diseased human brain endothelium (drug-resistant epileptic and aneurism-derived brain entothelial cells). Specifically, an abnormal pattern of CYP expression in the epileptic brain could influence the pharmacokinetics of AEDs. Recent evidence revealed the presence of CYP enzymes in a human brain–derived cell line (hCMEC/D3) and human isolated microvessels (Dauchy et al., 2008, 2009). Accordingly, our data obtained using freshly isolated endothelium showed the presence of CYP1A1, 1B1, 2B6, 2E1, and 2J2 genes (Dauchy et al., 2008). In accordance with previous findings (Dauchy et al., 2009), we generally measured higher CYP transcript levels in HUVEC compared to control brain endothelial cells (HBMEC).
We found levels of CYP3A4, CYPC9, CYP2A6, and CYP2J2 mRNA encoding for enzymes that are known to be responsible for the metabolism of AEDs (Figs. 1 and 3). We specifically confirmed the upregulation of CYP3A4 protein at the epileptic brain endothelium.
Effect of shear stress on CYP regulation and function
Physiologic shear stress affects brain endothelial cell differentiation, tight-junction formation, and regulates the cell cycle causing mitotic arrest (Arisaka et al., 1995; Desai et al., 2002). The DIV model used in our experiments generates shear stress resembling physiologic conditions (Stanness et al., 1996). Under exposure to flow, CYP and drug transporter mRNA ratios (shear/no-shear) were greater than 1 (Fig. 2), indicating the regulatory effect of shearing forces on transcription (Egnell et al., 2003). Previous findings have demonstrated that CYP1A1 and 1B1 mRNA levels are induced in control nonbrain endothelial cells under shear conditions (Eskin et al., 2004). The dissimilar effect provoked by shear stress in control and “epileptic” brain endothelial cells needs to be further clarified. Several variables such as cellular proliferation/differentiation and survival are involved when considering “diseased” endothelium (Desai et al., 2002; Han et al., 2008). We are also aware of the possible variations associated with the use of freshly isolated brain cells from patients. Nevertheless, human-based investigations provide valuable data to be compared with those obtained using animal models or cell lines.
In the epileptic brain, local brain hyperperfusion is a common ictal event. It is possible that hemodynamic changes could be involved in the expression of CYPs and drug transporter proteins at the epileptic BBB. It is important to understand under what circumstances the effect of flow becomes operant. It is not farfetched to suggest a dynamic model of brain drug resistance. The changes in cerebral blood perfusion in response to the ictal-interictal cycle acting in the diseased brain could shape the profile of endothelial expression of several proteins, including CYP enzymes and multidrug transporters.
We have previously demonstrated that the flow-based DIV-BBB recapitulates the physiologic permeability properties of the BBB in vivo and is also capable of mimicking a drug-resistant phenotype (Cucullo et al., 2007). We now propose that the DIV-BBB also reproduces the metabolic barrier properties of the human epileptic BBB (Fig. 5). Our results suggest that in vitro modeling of CNS pharmacodynamics requires appropriate models (such as those based on flow) and realistic cell types (as those taken from specific brain pathologies).
Our result also showed that preventive exposure to shear stress precludes any further induction of CYP expression (Fig. 3C–D). This highlights the importance of culturing conditions (e.g., those mimicking the physiologic parameters) when analyzing the pattern of gene and protein expression in brain-derived endothelial cells. The effect of shear stress exposure was specific for endothelial cells. Hepatocytes exposed to laminar flow did not significantly change their expression of CYP3A4 (Fig. S3). However, the relative contribution of variables associated with the epileptic condition (e.g., underlying pathology, hemodynamic changes, AED regimen, and seizure activity) in determining CYP/MDR overexpression needs to be further clarified. The possibility exists that EPI-EC deriving from patients treated with CBZ displays higher expression of CYP due to drug induction. However, within our cohort of patients with drug-resistant epilepsy, only three patients received CBZ (Table S1), whereas other AEDs were taken in combination.
Our HPLC data demonstrate that endothelial cells are capable of drug metabolism. In particular, EPI-EC displayed metabolic activity identical to that of cultured hepatocytes, which was significantly elevated compared to control endothelium (Fig. 5 and Table 1). Concomitant to CYP3A4 overexpression, exposure to shear stress increased the metabolic potency of endothelial cells (Table 1).
BBB CYP3A4 expression in the drug-resistant epileptic brain
Over the last decade, it has become evident that the epileptic brain has a tendency to overexpress a broad spectrum of multidrug transporter proteins (Dombrowski et al., 2001; Abbott et al., 2002; Aronica et al., 2004; Marchi et al., 2004; Loscher & Potschka, 2005; Aronica & Gorter, 2007; Janigro et al., 2007; Loscher, 2007). Immunohistochemical data revealed the presence of CYP3A4 in blood vessels, in particular in those brain areas characterized by reactive gliosis (Fig. 4). We are aware that one of the limitations of our study is the lack of normal brain tissue. Because of practical difficulties, control brain tissue was provided by histologically normal neocortex adjacent to the hippocampus with sclerosis. In such cases, control tissues are thus of the same age and have been exposed to the same environmental factors or drugs as has diseased tissue (Palmini et al., 2004; Sen et al., 2007). Further studies are required to confirm overexpression of CYP enzymes compared to “more adequate” control human brains. For instance, recent evidence has suggested an increased vascularization at the epileptic foci, underscoring the complexity of the pathophysiologic events characterizing the epileptic brain (Rigau et al., 2007).
P-Glycoprotein, multidrug resistance–associated proteins (MRPs), and CYP3A4 together constitute a highly efficient barrier for many drugs (Schuetz et al., 2000; Yasuda et al., 2002; Pal & Mitra, 2006). Our data have demonstrated that the epileptic BBB expresses both multi-drug transporter proteins and CYP enzymes. There is a striking overlap in CYP3A4 and P-glycoprotein inducers, substrates, and inhibitors (Yasuda et al., 2002; Pal & Mitra, 2006). Both MDR1 and CYP3A4 are under the control of the pregnane X receptor (PXR), a nuclear receptor family regulating a number of enzymes and transporters in mammals (Ma et al., 2008). One captivating hypothesis is that expression of CYP enzymes and drug transporters could be involved in cellular detoxification and promote a process whereby cells are allowed to survive in an otherwise hostile environment (Marroni et al., 2003; Marchi et al., 2004).
In conclusion, we demonstrated the expression and function of metabolic enzymes by the diseased endothelial cells at the BBB. Our results also underscore the effect of hemodynamic forces on cerebrovascular gene expression, with emphasis on BBB drug metabolism and transport. Further investigations are needed to elucidate the significance of these findings, specifically to refractory forms of epilepsy, and whether a “metabolic” vascular barrier influences AED brain access.
Supplementary Material
Figure S1. Distribution of genes within the cell types used and overall effect of shear stress. (A) Note that when comparing primary epileptic endothelial cells and control, several genes were outside of the confidence interval (green lines, 95%). Among these were CYP enzymes, but several other gene families were also regulated differently in epileptic endothelial cells. (B) Gene expression in cells grown under static (no-shear) or dynamic (DIV, 1 week, 3 dynes/cm2) conditions are compared by plotting the log of intensity values for each individual cDNA present in the filter.
Figure S2. CYP3A4 brain expression is associated with vascular abnormalities and neuronal heterotopias. (C–D) Cresyl violet staining revealed the presence of ectopic neurons and dysplastic vessels in drug-resistant epileptic tissue. (A–B) Note also the presence of relatively normal cortex within the samples analyzed.
Figure S3. Exposure to CBZ but not to shear stress significantly induced CYP3A4 protein in human hepatocytes. (A) Although shear stress positively regulated the levels of CYP transcripts and proteins in endothelial cells (Figs. 2 and 3), the effects on hepatocyte was modest and not significant. However, CBZ induced CYP3A4 expression (one-way ANOVA, *p < 0.05 hepato vs. hepato + CBZ and hepato-shear vs. hepato-shear + CBZ). (B) Exposure to shear stress led to a modest change in the amount of CBZ metabolized by hepatocytes after 72 h (one-way ANOVA, *p < 0.05 time zero vs. 72 h).
Table S1. Summary of the tissue donors used.
Table S2. Complete list of the genes analyzed (GenBank/Uni-Gene IDs).
Acknowledgments
This work was supported by NIH-RO1 NS43284, NIH-RO1 NS38195, and NIH-R21 HD057256 to DJ. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Drs Marchi, Cucullo and Janigro have served as consultants for Flocel, U.S.A.
References
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Khan EU, Rollinson CM, Reichel A, Janigro D, Dombrowski SM, Dobbie MS, Begley DJ. Drug resistance in epilepsy: the role of the blood-brain barrier. Novartis Found Symp. 2002;243:38–47. [PubMed] [Google Scholar]
- Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S, Yoshida Y. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann N Y Acad Sci. 1995;748:543–554. doi: 10.1111/j.1749-6632.1994.tb17359.x. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Ramkema M, Redeker S, Ozbas-Gerceker F, van Vliet EA, Scheffer GL, Scheper RJ, van der Valk P, Baayen JC, Troost D. Expression and cellular distribution of multidrug resistance-related proteins in the hippocampus of patients with mesial temporal lobe epilepsy. Epilepsia. 2004;45:441–451. doi: 10.1111/j.0013-9580.2004.57703.x. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA. Gene expression profile in temporal lobe epilepsy. Neuroscientist. 2007;13:100–108. doi: 10.1177/1073858406295832. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Hallene KL, Fazio V, Singhal SS, Cucullo L, Awasthi YC, Dini G, Janigro D. RLIP76, a non-ABC transporter, and drug resistance in epilepsy. BMC Neurosci. 2005;6:61. doi: 10.1186/1471-2202-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucullo L, Hossain M, Rapp E, Manders T, Marchi N, Janigro D. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia. 2007;48:505–516. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- Cucullo L, Couraud PO, Weksler B, Romero IA, Hossain M, Rapp E, Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab. 2008;28:312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, Scherrmann JM, De Waziers I, Decleves X. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem. 2008;107:1518–1528. doi: 10.1111/j.1471-4159.2008.05720.x. [DOI] [PubMed] [Google Scholar]
- Dauchy S, Miller F, Couraud PO, Weaver RJ, Weksler B, Romero IA, Scherrmann JM, De Waziers I, Decleves X. Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol. 2009;77:897–909. doi: 10.1016/j.bcp.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Desai SY, Marroni M, Cucullo L, Krizanac-Bengez L, Mayberg MR, Hossain MT, Grant GG, Janigro D. Mechanisms of endothelial survival under shear stress. Endothelium. 2002;9:89–102. doi: 10.1080/10623320212004. [DOI] [PubMed] [Google Scholar]
- Dombrowski SM, Desai SY, Marroni M, Cucullo L, Goodrich K, Bingaman W, Mayberg MR, Bengez L, Janigro D. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia. 2001;42:1501–1506. doi: 10.1046/j.1528-1157.2001.12301.x. [DOI] [PubMed] [Google Scholar]
- Egnell AC, Houston B, Boyer S. In vivo CYP3A4 heteroactivation is a possible mechanism for the drug interaction between felbamate and carbamazepine. J Pharmacol Exp Ther. 2003;305:1251–1262. doi: 10.1124/jpet.102.047530. [DOI] [PubMed] [Google Scholar]
- Eskin SG, Turner NA, McIntire LV. Endothelial cell cytochrome P450 1A1 and 1B1: up-regulation by shear stress. Endothelium. 2004;11:1–10. doi: 10.1080/10623320490432434. [DOI] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Walther B, Perrin R, Minn A, Siest G. Inducibility of rat brain drug-metabolizing enzymes. Eur J Drug Metab Pharmacokinet. 1987;12:263–265. doi: 10.1007/BF03189910. [DOI] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Perrin R, Leininger-Muller B, Grassiot MC, Jeandel C, Floquet J, Cuny G, Siest G, Minn A. Subcellular localization of cytochrome P450, and activities of several enzymes responsible for drug metabolism in the human brain. Biochem Pharmacol. 1993;45:647–658. doi: 10.1016/0006-2952(93)90139-n. [DOI] [PubMed] [Google Scholar]
- Han Z, Miwa Y, Obikane H, Mitsumata M, Takahashi-Yanaga F, Morimoto S, Sasaguri T. Aryl hydrocarbon receptor mediates laminar fluid shear stress-induced CYP1A1 activation and cell cycle arrest in vascular endothelial cells. Cardiovasc Res. 2008;77:809–818. doi: 10.1093/cvr/cvm095. [DOI] [PubMed] [Google Scholar]
- Janigro D, Rapp E, Hossein M, Batra A, Mayberg M, Marchi N, Manders T, Cucullo L. Development of a humanized in vitro blood-brain barrier model to study drug resistance. Stroke. 2007;38:585. doi: 10.1111/j.1528-1167.2006.00960.x. [DOI] [PubMed] [Google Scholar]
- Levy RH. Cytochrome P450 isozymes and antiepileptic drug interactions. Epilepsia. 1995;36(Suppl 5):S8–S13. doi: 10.1111/j.1528-1157.1995.tb06007.x. [DOI] [PubMed] [Google Scholar]
- Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2:86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W. Mechanisms of drug resistance in status epilepticus. Epilepsia. 2007;48(Suppl 8):74–77. doi: 10.1111/j.1528-1167.2007.01357.x. [DOI] [PubMed] [Google Scholar]
- Luo G, Cunningham M, Kim S, Burn T, Lin J, Sinz M, Hamilton G, Rizzo C, Jolley S, Gilbert D, Downey A, Mudra D, Graham R, Carroll K, Xie J, Madan A, Parkinson A, Christ D, Selling B, LeCluyse E, Gan LS. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab Dispos. 2002;30:795–804. doi: 10.1124/dmd.30.7.795. [DOI] [PubMed] [Google Scholar]
- Ma X, Idle JR, Gonzalez FJ. The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol. 2008;4:895–908. doi: 10.1517/17425255.4.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Hallene KL, Kight KM, Cucullo L, Moddel G, Bingaman W, Dini G, Vezzani A, Janigro D. Significance of MDR1 and multiple drug resistance in refractory human epileptic brain. BMC Med. 2004;2:37. doi: 10.1186/1741-7015-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Guiso G, Caccia S, Rizzi M, Gagliardi B, Noe F, Ravizza T, Bassanini S, Chimenti S, Battaglia G, Vezzani A. Determinants of drug brain uptake in a rat model of seizure-associated malformations of cortical development. Neurobiol Dis. 2006;24:429–442. doi: 10.1016/j.nbd.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Marroni M, Agrawal ML, Kight K, Hallene KL, Hossain M, Cucullo L, Signorelli K, Namura S, Bingaman W, Janigro D. Relationship between expression of multiple drug resistance proteins and p53 tumor suppressor gene proteins in human brain astrocytes. Neuroscience. 2003;121:605–617. doi: 10.1016/s0306-4522(03)00515-3. [DOI] [PubMed] [Google Scholar]
- Martignoni M, Groothuis G, de Kanter R. Comparison of mouse and rat cytochrome P450-mediated metabolism in liver and intestine. Drug Metab Dispos. 2006;34:1047–1054. doi: 10.1124/dmd.105.009035. [DOI] [PubMed] [Google Scholar]
- Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-drug interactions. J Neuroimmune Pharmacol. 2006;1:323–339. doi: 10.1007/s11481-006-9034-2. [DOI] [PubMed] [Google Scholar]
- Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, Jackson G, Luders HO, Prayson R, Spreafico R, Vinters HV. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–S8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- Patil KM, Bodhankar SL. Simultaneous determination of lamotrigine, phenobarbitone, carbamazepine and phenytoin in human serum by high-performance liquid chromatography. J Pharm Biomed Anal. 2005;39:181–186. doi: 10.1016/j.jpba.2005.02.045. [DOI] [PubMed] [Google Scholar]
- Pearce RE, Vakkalagadda GR, Leeder JS. Pathways of carbamazepine bioactivation in vitro I. Characterization of human cytochromes P450 responsible for the formation of 2- and 3-hydroxylated metabolites. Drug Metab Dispos. 2002;30:1170–1179. doi: 10.1124/dmd.30.11.1170. [DOI] [PubMed] [Google Scholar]
- Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P, Picot MC, Baldy-Moulinier M, Bockaert J, Crespel A, Lerner-Natoli M. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Umbenhauer DR, Yasuda K, Brimer C, Nguyen L, Relling MV, Schuetz JD, Schinkel AH. Altered expression of hepatic cytochromes P-450 in mice deficient in one or more mdr1 genes. Mol Pharmacol. 2000;57:188–197. [PubMed] [Google Scholar]
- Sen A, Martinian L, Nikolic M, Walker MC, Thom M, Sisodiya SM. Increased NKCC1 expression in refractory human epilepsy. Epilepsy Res. 2007;74:220–227. doi: 10.1016/j.eplepsyres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Stanness KA, Guatteo E, Janigro D. A dynamic model of the blood-brain barrier “in vitro”. Neurotoxicology. 1996;17:481–496. [PubMed] [Google Scholar]
- Volk B, Meyer RP, von Lintig F, Ibach B, Knoth R. Localization and characterization of cytochrome P450 in the brain. In vivo and in vitro investigations on phenytoin- and phenobarbital-inducible isoforms. Toxicol Lett. 1995;82–83:655–662. doi: 10.1016/0378-4274(95)03511-7. [DOI] [PubMed] [Google Scholar]
- Walther B, Ghersi-Egea JF, Minn A, Siest G. Subcellular distribution of cytochrome P-450 in the brain. Brain Res. 1986;375:338–344. doi: 10.1016/0006-8993(86)90754-7. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Lan LB, Sanglard D, Furuya K, Schuetz JD, Schuetz EG. Interaction of cytochrome P450 3A inhibitors with P-glycoprotein. J Pharmacol Exp Ther. 2002;303:323–332. doi: 10.1124/jpet.102.037549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of genes within the cell types used and overall effect of shear stress. (A) Note that when comparing primary epileptic endothelial cells and control, several genes were outside of the confidence interval (green lines, 95%). Among these were CYP enzymes, but several other gene families were also regulated differently in epileptic endothelial cells. (B) Gene expression in cells grown under static (no-shear) or dynamic (DIV, 1 week, 3 dynes/cm2) conditions are compared by plotting the log of intensity values for each individual cDNA present in the filter.
Figure S2. CYP3A4 brain expression is associated with vascular abnormalities and neuronal heterotopias. (C–D) Cresyl violet staining revealed the presence of ectopic neurons and dysplastic vessels in drug-resistant epileptic tissue. (A–B) Note also the presence of relatively normal cortex within the samples analyzed.
Figure S3. Exposure to CBZ but not to shear stress significantly induced CYP3A4 protein in human hepatocytes. (A) Although shear stress positively regulated the levels of CYP transcripts and proteins in endothelial cells (Figs. 2 and 3), the effects on hepatocyte was modest and not significant. However, CBZ induced CYP3A4 expression (one-way ANOVA, *p < 0.05 hepato vs. hepato + CBZ and hepato-shear vs. hepato-shear + CBZ). (B) Exposure to shear stress led to a modest change in the amount of CBZ metabolized by hepatocytes after 72 h (one-way ANOVA, *p < 0.05 time zero vs. 72 h).
Table S1. Summary of the tissue donors used.
Table S2. Complete list of the genes analyzed (GenBank/Uni-Gene IDs).