Abstract
The pandemic of HIV-1 has continued for decades, yet there remains no licensed vaccine. Previous research has demonstrated the effectiveness of a multi-envelope, multi-vectored HIV-1 vaccine in a macaque-SHIV model, illustrating a potential means of combating HIV-1. Specifically, recombinant DNA, vaccinia virus (VV) and purified protein (DVP) delivery systems were used to vaccinate animals with dozens of antigenically-distinct HIV-1 envelopes for induction of immune breadth. The vaccinated animals controlled disease following challenge with a heterologous SHIV. This demonstration suggested that the antigenic cocktail vaccine strategy, which has succeeded in several other vaccine fields (e.g. pneumococcus), might also succeed against HIV-1. The strategy remains untested in an advanced clinical study, in part due to safety concerns associated with the use of replication-competent VV. To address this concern, we designed a macaque study in which psoralen/ultraviolet light-inactivated VV (UV VV) was substituted for replication-competent VV in the multi-envelope DVP protocol. Control animals received a vaccine encompassing no VV, or no vaccine. All VV vaccinated animals generated an immune response toward VV, and all vaccinated animals generated an immune response toward HIV-1 envelope. After challenge with heterologous SHIV 89.6P, animals that received replication-competent VV or UV VV experienced similar outcomes. They exhibited reduced peak viral loads, maintenance of CD4+ T cell counts and improved survival compared to control animals that received no VV or no vaccine; there were 0/15 deaths among all animals that received VV and 5/9 deaths among controls. Results define a practical means of improving VV safety, and encourage advancement of a promising multi-envelope DVP HIV-1 vaccine candidate.
Keywords: HIV-1 vaccine, pathogenic SHIV, non-human primate, envelope cocktail, ultra violet-inactived vaccinia virus
INTRODUCTION
Despite important advances in the fields of immunology, virology and infectious diseases, no vaccine has yet matched the capacity of vaccinia virus (VV) to induce durable immunity and eradicate a human disease [1]. However, VV usage in humans has been associated with rare adverse events. The vaccine is not well controlled in immunodeficient patients or patients with excema, who may be exposed either by direct vaccination or by inadvertent transmission [2;3], and has been associated with myopericarditis [4]. Precautions can be taken to prevent adverse events, such as the use of subcutaneous (SQ) rather than percutaneous routes of vaccination [5], and the implementation of stringent training.
As one additional means of reducing risk, investigators have tested attenuated VV such as modified vaccinia virus Ankara (MVA)[6;7] [8], although some problems may be encountered during at the manufacturing stage due to slow growth. Another means of improving VV safety involves VV inactivation with psoralen and UV light [9]. This strategy can exploit (i) the robust growth characteristics of non-attenuated VV during manufacturing, and (ii) an inactivation methodology that has already been approved for clinical use [10–12].
The current study was designed to test a UV-inactivated VV product in the context of a multi-envelope, multi-vectored (DVP) HIV-1 vaccine. This vaccine was previously shown to control viral load and disease in macaques following a heterologous SHIV 89.6P challenge, even though no 89.6P envelope or any SIV component was represented by sequence in the vaccine [13–19]. A multi-envelope DVP vaccine was also tested in a brief clinical study in which it was both immunogenic and well-tolerated [16;20]. Cocktail vaccines have been successfully designed in other fields to combine mutually-exclusive membrane antigens and thereby recruit different populations of lymphocytes to function in unison to provide immune breadth. The study described here tested the UV-inactivation strategy in the context of DVP [14–16;19;21–23] as a means to improve safety and advance an attractive HIV-1 multi-envelope vaccine approach.
MATERIALS AND METHODS
Psoralen and UV inactivation of VV
VV were amplified on TK-143B cells and purified by sucrose gradient sedimentation. Psoralen (UVADEX® methoxsalen, 10 μg/ml final concentration, Therakos, Inc., Eaton, PA) was added to viruses with preset titers of 2×107 – 2×108 pfu/ml. Human serum albumin was added to a final concentration of 0.1% and samples were incubated at room temperature for 10 minutes. Virus/psoralen mixtures were transferred to a polystyrene 6-well culture plate, 1 ml/well. Plates were placed in a Stratalinker 1800 (Stratagene, LaJolla, CA) and irradiated under 365 nm long-wave UV light in 3 minute intervals, with swirling between intervals, for a total of 12 minutes. Virus titers were determined by plaque assays on TK-143B cells. Cells were stained with 10% Formalin/1% Methylene blue after 3 days of incubation for plaque enumeration. Plaques were only identified prior to UV treatments.
Macaque immunizations
Vaccine vector production has been described previously [24–27]. Delivery systems included recombinant DNA (D), recombinant VV (V) and recombinant protein (P). The DNA vaccine was a combination of 52 different constructs, each expressing a different envelope protein (including envelopes from clades A,B,C,D and E) for a final total dose of 150 μg administered in a volume of 1.5 ml per animal by intramuscular (IM) injection. DNA was formulated either in PBS or in alum (500 micrograms per dose, Rehydrophos aluminum phosphate, Reheis, Inc.). The VV vaccine was a combination of 23 different constructs. VV was either replication competent (Live VV or Late VV) or UV inactivated (UV VV). Viruses were represented in equal quantities and the total dose per animal was 6.5 × 107 pfu in 1 ml delivered subcutaneously (SQ) between shoulder blades. The protein vaccine included four different purified envelope proteins from virus HIV-11007 (clade B gp140, isolated in Memphis TN, purified by affinity chromatography from recombinant CHO cell supernatants), HIV-1UG92005 (clade D gp140, purified by affinity chromatography from recombinant CHO cell supernatants), HIV-1MN (clade B gp120, Protein Sciences Corp, Meriden, CT) and HIV-1CM (clade E gp120, Protein Sciences Corp). The proteins were represented equally by weight, totaling 100 micrograms in 500 micrograms alum (Rehydrogel HPA, Reheis) in a 1 ml dose per animal, delivered IM. The majority of the proteins in the composite DVP vaccines were gp140 and a fraction of sequences were shared between the delivery vehicles. The SHIV 89.6P stock challenge virus was kindly provided by Drs. N. Letvin and K. Reimann [28]. Stock was diluted 1:1000 and delivered IV with 1 ml per animal (approximately 10 MID50).
HIV-1 antibody assays
The HIV-1/2 Abbott ELISA (Abbott Laboratories, Abbott Park, Ill) was used. Antibody titers were calculated with curve-fitting software, defining the reciprocal serum dilution associated with an O.D. 492 nm reading of 0.1 (GraphPad Prism, San Diego, CA). Neutralization assays were performed as described previously [29]. To avoid false positives, serum immunoglobulin was purified on protein G columns and brought to its original sample volume prior to the preparation of serial dilutions. Percent neutralization was defined for test monkey samples by comparisons with a ‘no serum’ control.
Macaque SHIV virus loads and CD4 counts
To measure virus loads, branched DNA (bDNA) assays were performed by Bayer Reference Testing Laboratory (Berkeley, CA) on plasma samples. The CD4+ T cells were enumerated using Trucount kits (Becton Dickinson). Statistics were evaluated with GraphPad Prism Software using the Fisher’s Exact Test or Unpaired T Test as described in the text.
RESULTS
Inactivation of recombinant VV with psoralen and UV light
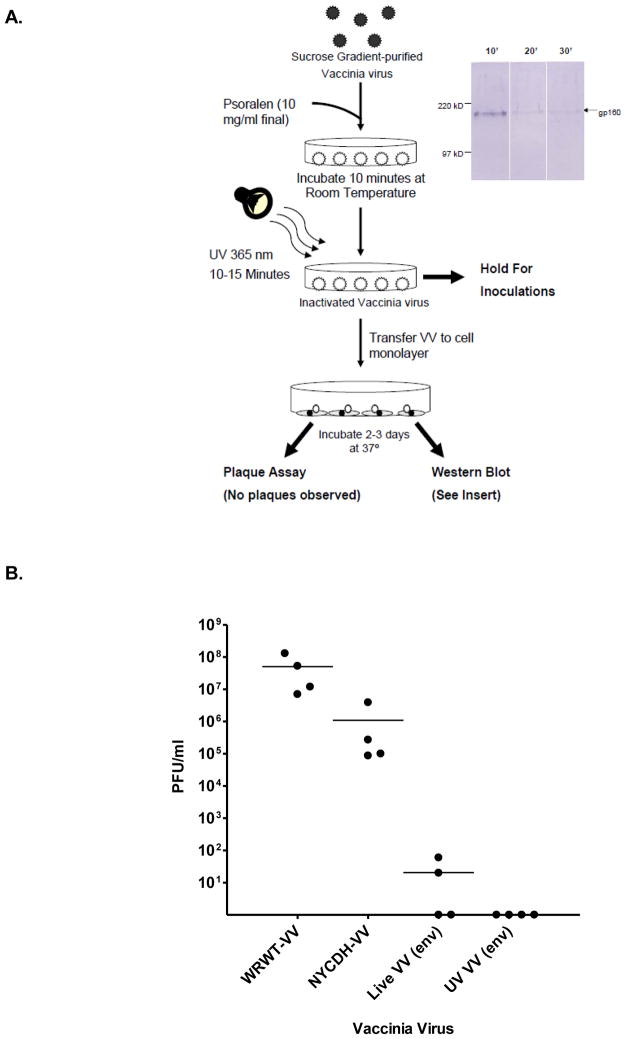
A first goal in the current study was to demonstrate that our recombinant VV could be inactivated with psoralen and UV light (Figure 1A). Following treatment, virus was incapable of producing plaques, but maintained capacity to express recombinant protein in tissue culture, confirmed by Western blot analyses. To ensure the replication incompetence of UV-inactivated VV, we injected product IP into SCID mice (Figure 1B). Ovaries were isolated 7 days later to test for plaque formation in vitro. Controls included replication competent viruses: (i) WRWT VV, (ii) non-recombinant NYCDH VV, and (iii) untreated HIV-1 envelope recombinant VV. The WRWT VV was used at a dose of 107 pfu (due to its high virulence), while the remaining viruses were used at a higher dose of 108 pfu. We found that the WRWT strain of VV was amplified to a much higher titer in ovaries compared to the NYCDH VV strain even though the original dose was ten-fold lower. The recombinant VV was further attenuated, and the UV-treated recombinant VV did not generate any plaques. When wildtype C57BL/6 mice were immunized twice with a DNA vaccine that expressed the clade D HIV-1UG92005 envelope protein, followed by one VV injection (either untreated or UV-inactivated VV expressing HIV-1UG92005 envelope), anti-envelope antibody activity was boosted in all test mice (data not shown).
Figure 1. Inactivation of VV.
Panel A. A flowchart of VV inactivation is shown. Virus was purified by sucrose gradient and then treated with psoralen and UV light. UV-treated virus was measured by plaque assay and Western blot to ensure inactivation and to ensure capacity for protein expression.
Panel B. SCID mice were administered live VV of the WRWT strain (WRWT-VV, 107 pfu), live VV of the NYCDH strain (NYCDH-VV, 108 pfu), live recombinant NYCDH VV (Live VV (env),108 pfu) and inactivated live recombinant NYCDH VV (UV VV (env), originally 108 pfu). After 7 days, ovaries were assessed for plaque formation on mammalian cell monolayers. Results from individual mice and means are shown.
Recombinant UV-inactivated VV is immunogenic in macaques
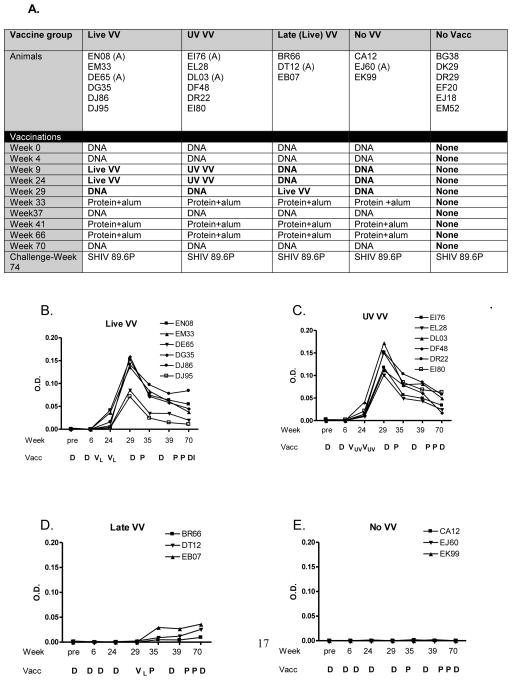
The UV-inactivated VV was next tested in macaques. Animals were assigned to 5 groups (Figure 2A). The first four groups were immunized with multi-envelope vaccines designed to represent HIV-1 diversity as described previously [16;30;31], whereas the fifth group was not immunized. Vaccine delivery vehicles included recombinant DNA (D), recombinant VV (in some cases termed ‘V’) and recombinant protein (P), each formulated as cocktails to represent multiple HIV-1 envelope proteins [15;32]. Differences between groups are bolded in Figure 2A. The ‘Live VV’ Group received a DVP vaccine including two doses of replication-competent VV. The ‘UV VV’ Group received the same vaccine, but with UV/psoralen-inactivated VV. The ‘Late VV’ Group was the same as ‘Live VV’, except that there was only one dose of virus which was given relatively late in the regimen. This group was set up to test responses to replication-competent VV after multiple doses of DNA. The ‘No VV’ group received only DNA and protein. The ‘No Vacc’ group received no immunizations. One additional variable was that a portion of animals received DNA formulated in alum rather than PBS (indicated by ‘(A)’ in animal names) to determine if alum would enhance DNA-based immune activities as described in previous literature [33].
Figure 2. DVP elicits VV-specific antibody activities in macaques.
Panel A. The schedule of monkey immunizations is shown. DNA (150 μg, 52 envelopes) was delivered IM in 1.5 ml PBS. An (A) designates an animal that received all DNA vaccines formulated in 500 μg alum rather than PBS. VV was delivered SQ (6.5×107 original pfu; 23 envelopes) and protein was delivered IM (100 μg in 500 μg alum, 4 envelopes). Bolded entries highlight differences between the monkey groups
Panels B-E. Macaques received a DVP vaccine (panels A, B, C) or a control vaccine including no VV (panel D). Vaccinations are shown below each graph. Groups received two doses of live VV (VL, panel A), two doses of inactivated VV (VUV, panel B), one dose of live VV (panel C), or no VV (panel D). ELISAs were conducted to monitor VV-specific antibody titers throughout the immunization scheme.
Of note, all vaccines were well tolerated. All VV doses were administered by the SQ route and there were no cutaneous skin lesions in ‘Live VV’, ‘UV VV’ or ‘Late VV’ vaccinated animals [34]. To determine how virus inactivation affected the VV-specific antibody response, VV ELISAs were performed. VV-specific antibodies were detected in all animals in ‘Live VV’, ‘UV VV’ and ‘Late VV’ groups, but not in the ‘No VV’ group (Figure 2B). The first two groups exhibited similar antibody profiles suggesting that the UV-inactivation treatment did not significantly alter VV immunogenicity. Antibodies peaked after the second VV dose and then waned, but persisted throughout a 6 month period of evaluation. For ‘Late VV’ animals, the peak VV-specific antibody response was significantly reduced, perhaps because there was only one VV injection and because the anti-envelope responses induced by four previous DNA immunizations reduced the number and/or persistence of VV-infected cells [35].
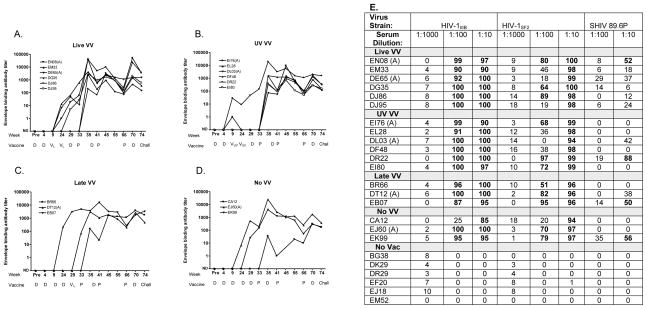
Blood samples were also tested for HIV-1 envelope binding antibodies throughout the course of immunizations. As shown in Figure 3A-D, all vaccinated animals generated anti-envelope antibody responses and there were not significant differences between ‘Live VV’ and ‘UV VV’ animals, or between animals that received DNA in PBS or DNA in alum. Neutralizing assays were conducted with week 70 sera, taken one month prior to the SHIV challenge. Serially diluted samples were tested on target viruses including HIV-1IIIB, HIV-1SF2 and the SHIV 89.6P. Neutralization scores of 50% or greater were considered positive. As shown in Figure 3E, all vaccinated animals neutralized HIV-1 IIIB and HIV-1SF2, and there were sporadic responses toward SHIV 89.6P. Again, there were not significant differences between ‘Live VV’ and ‘UV VV’ animals.
Figure 3. DVP elicits HIV-specific antibody activities in macaques.
Macaques received Live VV (panel A), UV VV (panel B), Late VV (panel C) or No VV (panel D). The Abbott ELISA was conducted with serially diluted serum samples to monitor envelope-specific antibody titers throughout the immunization scheme. Curve fitting software defined reciprocal serum dilutions associated with an O.D. reading of 0.1, graphed on a log scale. ND-No detected antibody. E. Neutralization was tested pre-challenge (week 70) against HIV-1IIIB and HIV-1SF2 and SHIV 89.6P. Entries exceeding 50% were considered positive and were highlighted in bold.
Vaccine-induced protection against SHIV: reduced viral load
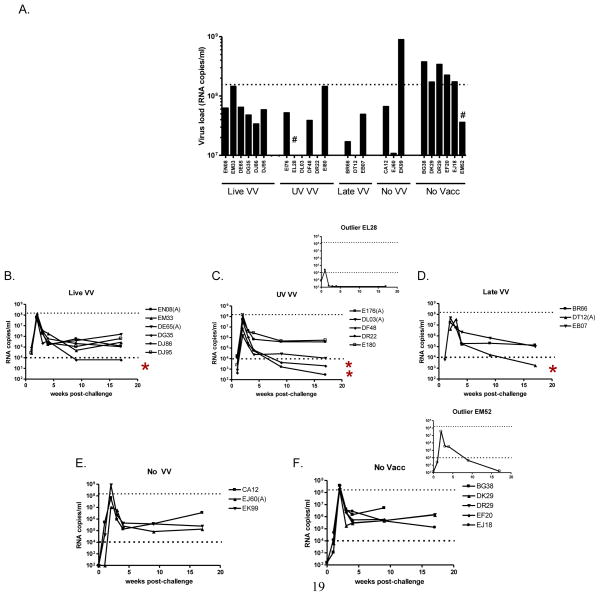
To examine the protective capacity of DVP in the context of ‘Live VV’, ‘UV VV’, ‘Late VV’ or ‘No VV’, animals were challenged intravenously with SHIV 89.6P. Viral loads were then monitored by bDNA analyses for several months. Peak viral loads were apparent two weeks after challenge as shown in Figure 4, panel A. The full time course of viral loads is shown in panels 4B-F. There were two outlier monkeys, EL28 in the ‘UV VV’ group and EM52 in the ‘No Vacc’ group that exhibited complete virus control. These animals are indicated by ‘#’ in panel 4A and by inserts in panels 4C and 4F. The EL28 outlier result was explained by a known technical difficulty experienced during the challenge procedure. EL28 results were therefore excluded from statistical analyses. For animal EM52, there was no known technical difficulty during the challenge procedure and the results were therefore included in statistical analyses. As shown in panel 4A, the highest peak viral loads were in the control ‘No VV’ or ‘No Vacc’ (no vaccine) groups. Several of these animals (6/9) exhibited peak viral loads that exceeded 1.5 × 108 copies per ml, while none of the animals that received ‘Live VV’, ‘UV VV’ or ‘Late VV’ experienced such high virus levels. The ‘Live VV’, ‘UV VV’, and ‘Late VV’ groups were not different, but each differed significantly from the ‘No Vacc’ group, despite exclusion of EL28 data (Fishers Exact test; p<.05). As shown in panels 4B-F, virus loads at later time points also trended lower for animals that received ‘Live VV’, ‘UV VV’ or ‘late VV’ compared to ‘No VV’ and ‘No Vacc’ controls, although statistically significant differences were not noted. Four animals that received VV (as well as the two outliers) achieved virus loads below 104 copies per ml as indicated by asterisks.
Figure 4. DVP protects macaques from SHIV challenge by reducing peak virus loads Peak virus loads are shown in panel A (RNA copies per ml, Bayer bDNA assay; samples taken 2 weeks after challenge).
‘#’ identifies outlier animals described in the text. A time course of virus loads is also shown following SHIV challenge for the five animal groups: Live VV (panel B), UV VV (panel C), Late VV (Panel D), No VV (Panel E) and No Vacc (Panel F). Inserts include data for outlier animals described in the text. Dotted lines highlight virus levels of 1.5×108 (panel A) and 1 × 104 (panels B-F) copies/ml to assist animal comparisons. Asterisks identify animals that were not outliers with levels lower than 104.
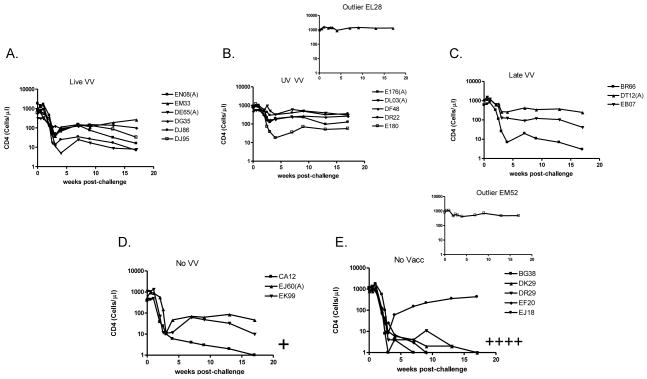
Vaccine-induced protection against SHIV disease: maintenance of CD4+ T cell counts
An additional analysis was of CD4+ T cell counts following challenge. As shown in Figure 5, animals that received VV (‘Live VV’, ’UV VV’, or ’Late VV’) maintained higher CD4+ T cell counts than the ‘No VV’ and ‘No Vacc’ control animals. The majority of ‘No VV’ and ‘No Vacc’ animals (5/9) showed CD4+ T cell counts that dropped below 1 cell/μl. All of these animals ultimately died as indicated by ‘+’ marks in Figure 5. All animals that received a VV vaccine component maintained levels of CD4+ T cells above 1 cell/μl and survived. There were not significant differences between ‘Live VV’ and ‘UV VV’ groups. When groups were combined for analyses, those that received a VV component survived at a significantly higher rate than did control animals (‘No VV’ or ‘No Vacc’; Fishers Exact Test p<.05). When examined independently, the ‘Live VV’ and ‘UV VV’ groups were also significantly better than controls in terms of CD4+ T cell maintenance and animal survival (Fishers Exact Test, p<.05). When the ‘UV VV’ group was compared directly to the ‘No VV’ group, CD4+ T cell numbers were yet again superior (week 17 values, unpaired T Test, p<.05).
Figure 5. DVP protects macaques from SHIV challenge by controlling CD4+ T cell counts.
CD4+ T cell counts/μl are shown throughout the course of immunizations for the five animal groups. Animals received Live VV (panel A), UV VV (panel B), Late VV (panel C), No VV (panel D) or No Vacc (panel E). Inserts include data for outlier animals described in the text. ‘+’ indicates animals with CD4+ T cell counts <1 cell/μl; these animals subsequently died.
DISCUSSION
The experiments described here were designed to address concerns related to the use of replication-competent VV in the context of a multi-envelope DVP vaccine. To improve safety, a clinically approved psoralen/UV light-inactivation technology was tested [36]. The treatment of VV by UV and psoralen may provide an advantage over virus attenuation, because attenuated virus growth poses difficulties at the manufacturing stage. In contrast, the strategy of VV inactivation occurs only after facile preparation of high-titered virus products.
Results in this report showed that ‘UV VV’ was comparable to ‘Live VV’ in terms of immunogenicity and protection in the context of DVP. In macaques that were vaccinated with multi-envelope DVP vaccines and challenged with SHIV 89.6P, the animals that received VV vaccines (Live VV, UV VV or Late VV) were better protected than controls (No VV or No Vacc) with regard to lower peak viral loads, CD4+ T cell maintenance, and animal survival.
All vaccines were well tolerated in the macaque study. VV vaccines were administered by the SQ route resulting in no cutaneous lesions even in the context of Live VV. The psoralen/UV light inactivation step provided an additional safety feature to support the continued use of VV in the clinical arena.
The success of the HIV-1 multi-envelope DVP vaccine was statistically significant, but full prevention of virus infection was not achieved. The reason for this limitation may relate, at least in part, to the origin of pathogenic SHIVs. When chimeric viruses were first produced to express HIV-1 envelope sequences with the SIV backbone, resultant viruses were not highly pathogenic. It was only after multiple monkey passages that SHIVs were isolated with greater monkey-tropism and pathogenicity [37], but this associated with mutations in the HIV-1 envelope. Immune responses elicited by natural HIV-1 envelopes may not be sufficiently cross-reactive with the mutated SHIV envelopes to prevent infection. Despite the limitations of the monkey model, our results demonstrated that a heterologous, multi-envelope vaccine devoid of SIV components could prevent disease and death following SHIV challenge.
For animals that initially control virus infection in the SHIV challenge model, why do some progress toward severe disease and others do not? This perhaps relates to the acute damage caused when peak viral titers are reached. Of the six animals in the current study that reached peak viral loads above 1.5×108 copies per ml, most (66%) ultimately suffered severe CD4+ T cell loss and death. Among the 18 animals that experienced peak viral loads below 1.5×108, there was only 1 death (<10%). Such associations between peak viral load and animal survival illustrate the attributes of vaccination, even in a monkey model in which full protection is difficult to achieve. The precise correlates of protection conferred by the DVP vaccine described in this report were not fully discerned. There are numerous mechanisms of antibody-mediated protection against virus that deserve further analyses, as well as the robust CD4+ and CD8+ T cell responses known to be induced by the DVP vaccine [38–44].
How can the full capacity of the immune system be harnessed? We propose that a HIV-1 vaccine should be used to pre-activate not just one type of lymphocyte [45;46], but a variety of lymphocytes with diverse receptor specificities, capable of recognizing different HIV-1 envelope structures. This strategy has been highly successful in other fields. The licensed Pneumovax encompasses 23 different components and vaccines of lesser complexity often associate with an increase in escaped bacterial serotypes [47;48]. The Pneumovax experience demonstrates the need to achieve a fine balance between vaccine simplicity and antigenic coverage when targeting a diverse pathogen. In the HIV-1 field, this balance may ultimately be achieved by advancing a multi-envelope vaccine approach.
HIGHLIGHTS.
UV-inactivated vaccinia virus is a successful HIV-1 vaccine vehicle
A multi-envelope HIV-1 vaccine protects against heterologous SHIV in macaques
DNA-vaccinia virus-protein prime-boost vaccine protects against heterologous SHIV
Acknowledgments
We thank Harold P. Stamey and the Tennessee Blood Services, Inc. for providing blood donor samples to the study, and N. Letvin and K. Reimann for the challenge virus stock and helpful discussions. We thank the AIDS Research and Reference Reagent Program, NIAID and the World Health Organization/UNAIDS for providing certain virus and antibody samples (Specific appreciation goes to J. Bradac, F. Gao, B. Hahn, K. Nelson and the WHO for the UG92005 and CMU06 viruses and the p92BR025.9 and p92RW020.5 clones from which some of the envelope sequences were derived, to R. V. Srinivas, R Gallo, and J Levy for HIV-1IIIB and HIV-1SF2 viruses for neutralization assays, to B. Chesebro and H. Chen for the p24 hybridoma 183-H12-5C, to V KewalRamani and D Littman for Ghost cells). We thank J. Mullins and H. Robinson for the pJW4303 vector used in the process of recombinant Chinese hamster ovary cell preparation. We thank S. D. Rencher, T.D. Lockey, D. Dawson, Q. Rodgers, B. Brown, A. Zirkel, K. W. Ryan, R.J. Owens and K.S. Slobod for assistance with vaccine production and valuable discussions. We thank the Tulane National Primate Research Center veterinary and clinical staff for animal care. This work was supported in part by grants from the NIH NCI Cancer Center Support Core Grant P30-CA21765, NIH-NIAID: P01 AI45142, R21-AI56974 and R01 AI078819, NIH NCRR base grant P51-RR00164 to the Tulane National Primate Research Center, the Aboussie Fund, the Federated Department Stores, the Mitchell Fund, the Carl C. Anderson Sr. and Marie Joe Anderson Charitable Foundation, the James B. Pendleton Charitable Trust, the Pioneer Fund and the American Lebanese Syrian-Associated Charities (ALSAC).
Footnotes
Disclosure Statement: The multi-envelope HIV-1 vaccine concept has been patented. A vector that may facilitate multi-envelope HIV-1 vaccine production has also been patented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003 Nov 15;171(10):4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 2.Garde V, Harper D, Fairchok MP. Tertiary contact vaccinia in a breastfeeding infant. JAMA. 2004 Feb 11;291(6):725–7. doi: 10.1001/jama.291.6.725. [DOI] [PubMed] [Google Scholar]
- 3.Cono J, Casey CG, Bell DM. Smallpox vaccination and adverse reactions. Guidance for clinicians. MMWR Recomm Rep. 2003 Feb 21;52(RR–4):1–28. [PubMed] [Google Scholar]
- 4.Arness MK, Eckart RE, Love SS, et al. Myopericarditis following smallpox vaccination. Am J Epidemiol. 2004 Oct 1;160(7):642–51. doi: 10.1093/aje/kwh269. [DOI] [PubMed] [Google Scholar]
- 5.Slobod KS, Lockey TD, Howlett N, et al. Subcutaneous administration of a recombinant vaccinia virus vaccine expressing multiple envelopes of HIV-1. Eur J Clin Microbiol Infect Dis. 2004 Feb;23(2):106–10. doi: 10.1007/s10096-003-1075-3. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez JC, Gherardi MM, Esteban M. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J Virol. 2000 Jan;74(2):923–33. doi: 10.1128/jvi.74.2.923-933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen TM, Vogel TU, Fuller DH, et al. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J Immunol JID - 2985117R. 2000 May 1;164(9):4968–78. doi: 10.4049/jimmunol.164.9.4968. [DOI] [PubMed] [Google Scholar]
- 8.Earl PL, Americo JL, Wyatt LS, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004 Mar 11;428(6979):182–5. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 9.Lin L, Hanson CV, Alter HJ, et al. Inactivation of viruses in platelet concentrates by photochemical treatment with amotosalen and long-wavelength ultraviolet light. Transfusion. 2005 Apr;45(4):580–90. doi: 10.1111/j.0041-1132.2005.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin L, Hanson CV, Alter HJ, et al. Inactivation of viruses in platelet concentrates by photochemical treatment with amotosalen and long-wavelength ultraviolet light. Transfusion. 2005 Apr;45(4):580–90. doi: 10.1111/j.0041-1132.2005.04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pamphilon D. Viral inactivation of fresh frozen plasma. Br J Haematol. 2000 Jun;109(4):680–93. doi: 10.1046/j.1365-2141.2000.02019.x. [DOI] [PubMed] [Google Scholar]
- 12.Subklewe M, Chahroudi A, Schmaljohn A, Kurilla MG, Bhardwaj N, Steinman RM. Induction of Epstein-Barr virus-specific cytotoxic T-lymphocyte responses using dendritic cells pulsed with EBNA-3A peptides or UV-inactivated, recombinant EBNA-3A vaccinia virus. Blood. 1999 Aug 15;94(4):1372–81. [PubMed] [Google Scholar]
- 13.Brown SA, Surman SL, Sealy R, et al. Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre-Clinical and Clinical Trials. Viruses. 2010 Feb 1;2(2):435–67. doi: 10.3390/v2020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richmond JFL, Mustafa F, Lu S, et al. Screening of HIV-1 Env glycoproteins for the ability to raise neutralizing antibody using DNA immunization and recombinant vaccinia virus boosting. Virology. 1997;230:265–74. doi: 10.1006/viro.1997.8478. [DOI] [PubMed] [Google Scholar]
- 15.Caver TE, Lockey TD, Srinivas RV, Webster RG, Hurwitz JL. A novel vaccine regimen utilizing DNA, vaccinia virus and protein immunizations for HIV-1 envelope presentation. Vaccine. 1999;17:1567–72. doi: 10.1016/s0264-410x(98)00355-7. [DOI] [PubMed] [Google Scholar]
- 16.Sealy R, Slobod KS, Flynn P, et al. Preclinical and clinical development of a multi-envelope, DNA-virus- protein (D-V-P) HIV-1 vaccine. Int Rev Immunol. 2009;28(1):49–68. doi: 10.1080/08830180802495605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhan X, Martin LN, Slobod KS, et al. Multi-envelope HIV-1 vaccine devoid of SIV components controls disease in macaques challenged with heterologous pathogenic SHIV. Vaccine. 2005 Nov 16;23(46–47):5306–20. doi: 10.1016/j.vaccine.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Stambas J, Brown SA, Gutierrez A, et al. Long lived multi-isotype anti-HIV antibody responses following a prime-double boost immunization strategy. Vaccine. 2005 Mar 31;23(19):2454–64. doi: 10.1016/j.vaccine.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Hurwitz JL, Zhan X, Brown SA, et al. HIV-1 vaccine development: Tackling virus diversity with a multi- envelope cocktail. Frontiers Bioscience. 2008;13:609–20. doi: 10.2741/2706. [DOI] [PubMed] [Google Scholar]
- 20.Brown SA, Surman SL, Sealy R, et al. Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre- Clinical and Clinical Trials. Viruses. 2010 Feb 1;2(2):435–67. doi: 10.3390/v2020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown SA, Surman SL, Sealy R, et al. Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre- Clinical and Clinical Trials. Viruses. 2010 Feb 1;2(2):435–67. doi: 10.3390/v2020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan X, Martin LN, Slobod KS, et al. Multi-envelope HIV-1 vaccine devoid of SIV components controls disease in macaques challenged with heterologous pathogenic SHIV. Vaccine. 2005 Nov 16;23(46–47):5306–20. doi: 10.1016/j.vaccine.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Stambas J, Brown SA, Gutierrez A, et al. Long lived multi-isotype anti-HIV antibody responses following a prime-double boost immunization strategy. Vaccine. 2005 Mar 31;23(19):2454–64. doi: 10.1016/j.vaccine.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 24.Stambas J, Brown SA, Gutierrez A, et al. Long lived multi-isotype anti-HIV antibody responses following a prime-double boost immunization strategy. Vaccine. 2005 Mar 31;23(19):2454–64. doi: 10.1016/j.vaccine.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Ryan KW, Owens RJ, Hurwitz JL. Preparation and use of vaccinia virus vectors for HIV protein expression and immunization. In: Lefkovits I, editor. Immunology methods manual. 1. London, England: Academic Press; 1997. pp. 1995–2015. [Google Scholar]
- 26.Smith GL, Murphy BR, Moss B. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7155–9. doi: 10.1073/pnas.80.23.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith GL, Mackett M, Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–5. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- 28.Reimann KA, Li JT, Veazey R, et al. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996 Oct;70(10):6922–8. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhan X, Martin LN, Slobod KS, et al. Multi-envelope HIV-1 vaccine devoid of SIV components controls disease in macaques challenged with heterologous pathogenic SHIV. Vaccine. 2005 Nov 16;23(46–47):5306–20. doi: 10.1016/j.vaccine.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Zhan X, Martin LN, Slobod KS, et al. Multi-envelope HIV-1 vaccine devoid of SIV components controls disease in macaques challenged with heterologous pathogenic SHIV. Vaccine. 2005 Nov 16;23(46–47):5306–20. doi: 10.1016/j.vaccine.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Brown SA, Surman SL, Sealy R, et al. Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre- Clinical and Clinical Trials. Viruses. 2010 Feb 1;2(2):435–67. doi: 10.3390/v2020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stambas J, Brown SA, Gutierrez A, et al. Long lived multi-isotype anti-HIV antibody responses following a prime-double boost immunization strategy. Vaccine. 2005 Mar 31;23(19):2454–64. doi: 10.1016/j.vaccine.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Ulmer JB, DeWitt CM, Chastain M, et al. Enhancement of DNA vaccine potency using conventional aluminum adjuvants. Vaccine. 1999 Aug 20;18(1–2):18–28. doi: 10.1016/s0264-410x(99)00151-6. [DOI] [PubMed] [Google Scholar]
- 34.Slobod KS, Lockey TD, Howlett N, et al. Subcutaneous administration of a recombinant vaccinia virus vaccine expressing multiple envelopes of HIV-1. Eur J Clin Microbiol Infect Dis. 2004 Feb;23(2):106–10. doi: 10.1007/s10096-003-1075-3. [DOI] [PubMed] [Google Scholar]
- 35.Brown SA, Surman SL, Sealy R, et al. Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre-Clinical and Clinical Trials. Viruses. 2010 Feb 1;2(2):435–67. doi: 10.3390/v2020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin L, Cook DN, Wiesehahn GP, et al. Photochemical inactivation of viruses and bacteria in platelet concentrates by use of a novel psoralen and long-wavelength ultraviolet light. Transfusion. 1997 Apr;37(4):423–35. doi: 10.1046/j.1537-2995.1997.37497265344.x. [DOI] [PubMed] [Google Scholar]
- 37.Cayabyab M, Karlsson GB, Etemad-Moghadam BA, et al. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2) J Virol. 1999 Feb;73(2):976–84. doi: 10.1128/jvi.73.2.976-984.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):19985–90. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhan X, Slobod KS, Surman S, Brown SA, Coleclough C, Hurwitz JL. Minor components of a multi-envelope HIV vaccine are recognized by type-specific T-helper cells. Vaccine. 2004;22:1206–13. doi: 10.1016/j.vaccine.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Zhan X, Slobod KS, Surman S, et al. Limited breadth of a T-helper cell response to a human immunodeficiency virus envelope protein. J Virol. 2003 Apr;77(7):4231–6. doi: 10.1128/JVI.77.7.4231-4236.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown SA, Slobod KS, Surman S, Zirkel A, Zhan X, Hurwitz JL. Individual HIV type 1 envelope-specific T cell responses and epitopes do not segregate by virus subtype. Aids Res Hum Retroviruses. 2006 Feb;22(2):188–94. doi: 10.1089/aid.2006.22.188. [DOI] [PubMed] [Google Scholar]
- 42.Surman S, Lockey TD, Slobod KS, et al. Localization of CD4+ T cell epitope hotspots to exposed strands of HIV envelope glycoprotein suggests structural influences on antigen processing. Proc Natl Acad Sci (USA) 2001;98:4587–92. doi: 10.1073/pnas.071063898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown SA, Hurwitz JL, Zirkel A, et al. A recombinant Sendai virus is controlled by CD4+ effector T cells responding to a secreted human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 2007 Nov;81(22):12535–42. doi: 10.1128/JVI.00197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surman SL, Brown SA, Jones BG, Woodland DL, Hurwitz JL. Clearance of HIV Type 1 Envelope Recombinant Sendai Virus Depends on CD4(+) T Cells and Interferon-gamma But Not B Cells, CD8(+) T Cells, or Perforin. Aids Res Hum Retroviruses. 2010 Jul 12; doi: 10.1089/aid.2009.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haynes BF, Fleming J, St Clair EW, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005 Jun 24;308(5730):1906–8. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 46.Sealy R, Chaka W, Surman S, Brown SA, Cresswell P, Hurwitz JL. Target peptide sequence within infectious human immunodeficiency virus type 1 does not ensure envelope-specific T-helper cell reactivation: influences of cysteine protease and gamma interferon-induced thiol reductase activities. Clin Vaccine Immunol. 2008 Apr;15(4):713–9. doi: 10.1128/CVI.00412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paradiso PR. Advances in pneumococcal disease prevention: 13-valent pneumococcal conjugate vaccine for infants and children. Clin Infect Dis. 2011 May;52(10):1241–7. doi: 10.1093/cid/cir142. [DOI] [PubMed] [Google Scholar]
- 48.Hurwitz JL, Slobod KS, Lockey TD, Wang S, Chou TH, Lu S. Application of the Polyvalent Approach to HIV-1 Vaccine Development. Curr Drug Targets Infect Disord. 2005 Jul;5(2):143–56. doi: 10.2174/1568005054201517. [DOI] [PubMed] [Google Scholar]