Synopsis
Despite being the most common sarcoma of the gastrointestinal tract, gastrointestinal stromal tumor (GIST) has only been widely recognized as a unique entity for just over a decade. The advent of tyrosine kinase inhibitors (TKIs) has revolutionized the diagnosis and treatment of GIST. While surgery remains the only chance for cure, multimodal treatment that includes molecular therapy continues to develop. Optimal management of GIST requires careful radiographic, pathologic, medical and surgical care, emphasizing the need for a multidisciplinary approach. In this review we highlight recent developments in the management of GIST.
Keywords: Gastrointestinal stromal tumor, KIT, tyrosine kinase, surgery
Epidemiology
GIST is the most frequently encountered mesenchymal tumor of the gastrointestinal tract. Although the annual incidence in the US is reported to be approximately 5000 cases per year (1), the true incidence is difficult to determine as it was only recently classified as a separate entity from leiyomyoma, leiyomyosarcoma, and leiomyoblastoma. Due to increased awareness and improved histopathological detection, the incidence of GIST seems to be increasing (2). GISTs affect men and women equally, except for pediatric GISTs that occur predominantly in girls (3, 4). While GISTs have been reported in all age groups including newborns, it is very uncommon in patients less than 30 years old. Most patients diagnosed with GIST are between 40–80 years old with a median age at diagnosis of 60 (5).
The most commonly encountered GIST is the sporadic form. Familial GISTs occur and result from a germline mutation in either the KIT or platelet-derived growth factor receptor alpha (PDGFRα) proto-oncogenes (6)((7). GIST can also occur in patients with neurofibromatosis type-1 (NF1) (8) and in young women as part of a syndrome that includes, paragangliomas, pulmonary chondromas, and gastric GISTs (i.e.,Carney’s triad) (9).
Clinical Presentation and Diagnosis
GISTs can cause a variety of symptoms ranging from vague abdominal pain to peritonitis as a result of tumor rupture and intraperitoneal bleeding. Other modes of presentation include abdominal fullness, early satiety, weakness, and fatigue secondary to anemia from occult gastrointestinal bleeding. Bowel obstruction is rare. Small GISTs (<3cm) are often detected incidentally on CT scans, endoscopy, or at the time of laparotomy for other indications (10). Lesions discovered incidentally and at autopsy have been shown to measure 2.7cm and 3.4cm respectively (11). Median tumor size at presentation in symptomatic patients is 5cm.
GISTs can occur anywhere in the gastrointestinal tract from the esophagus to the rectum. Stomach represents the most common site (60%), followed by the small bowel (30%), rectum (~5%), and esophagus (~5%) (5). The clinical course of GIST can range from benign to malignant. Up to 50% of patients will present with metastatic disease at the time of diagnosis, with the liver and peritoneum being the two most common sites of extraintestinal spread. Occasionally, patients will present with primary GISTs of the omentum, mesentery, or pancreas (12).
Due to the wide range of symptoms and its rarity, the diagnosis of GIST requires a high index of suspicion. The primary mode of diagnosis and assessment of extent of disease is by contrast enhanced CT scan of the abdomen and pelvis. Characteristic findings on CT scan include an enhancing, exophytic mass in close association with the stomach or bowel wall. Like other sarcomas, GISTs tend to displace rather than invade adjacent structures. Occasionally, larger GISTs (>10cm) can exhibit heterogeneity on CT that usually signifies hemorrhage or occasionally necrosis within the tumor. MRI can be useful in cases of rectal GIST. While PET is not used to diagnose GIST, it can be used to assess the response to tyrosine kinase therapy. PET can also be useful in patients with metastatic disease who are being considered for surgery or those on second line agents after failure of imatinib, in whom mixed responses may occur. On endoscopic evaluation, GIST appears as a submucosal mass. While endoscopic or percutaneous biopsy is recommended in cases in which neoadjuvant therapy or metastasis is suspected, the role of routine biopsy of isolated lesions is controversial. Endoscopic-guided fine needle aspiration has been shown to be ~80% sensitive in diagnosing GIST (13). Because GISTS tend to be soft and friable, biopsy carries the risk of tumor rupture, bleeding, and dissemination.
Pathologic Findings
There are three histologic sub-types of GIST. The spindle cell form is the most common (70%) and consists of uniform, intersecting fasicles with eosinophilic cytoplasm. The epitheliod (20%) and the rare mixed type (10%) forms show more rounded cells with nuclear atypia (14). Approximately 95% of GISTs stain positive for KIT (CD117) by immunohistochemistry (IHC). Epithelioid GISTs tend to have weaker KIT staining than the spindle cell type. Other commonly expressed markers include CD34 (70%), smooth muscle actin (30%) and desmin (<5%) (14). While immunophenotype is an important component in the diagnosis of GIST, it is not sufficient. Other malignancies that can stain positive for KIT include metastatic melanoma, angiosarcoma, small cell lung cancer and Ewing’s sarcoma (15). The diagnosis of GIST is based on concordance between the morphology and IHC. Nevertheless, mutation analysis is sometimes required.
GISTs are believed to arise from the interstitial cells of Cajal as a result of a gain of function mutation in the KIT proto-oncogene. KIT mutations can vary and occur in up to 85% of GISTs (16). The most common sites of KIT mutation include exon 11 (70%) and exon 9 (10%). Other described regions include exons 13, 14 and 17 (17, 18). Recently, ETV1 was shown to be a critical transcription factor in KIT oncogenesis and the development of GISTs (19). Approximately 10% of patients with GIST instead have a mutation in the PDGFRα proto-oncogene (20). Patients (~5–10%) who do not carry a mutation in either of the above-described proto-oncogenes are classed as having wild-type (WT) GISTs. A subset of these patients, have a BRAF mutation (21). DOG1 (a calcium-dependent chloride channel) is also expressed commonly in GIST and can be useful in establishing the diagnosis (22, 23).
Risk Stratification
Prognosis in GIST is highly variable. The critical determinants of GIST behavior include tumor size, mitotic rate, and location (24) (Table 1). Small tumors (<2cm) with low mitotic rates (<5 per 50 HPF) exhibit benign behavior, whereas larger tumors (>5cm) with high mitotic rates (>10 per 50 HPF) are associated with malignant behavior and display higher rates of recurrence after surgical resection. Tumors located in the stomach have favorable outcomes relative to small bowel tumors. Of the three aforementioned determinants of behavior, mitotic rate is considered the most significant (24). It is important to note that small tumors with low mitotic rates have been shown to display malignant behavior (25).
Table 1.
Rates of metastases in patients with GISTs of stomach, small bowel, and rectum grouped by tumor size and mitotic rate.
| Tumor parameters | Percentage of Patients with Progressive Disease During Long-term Follow-up and Characterization of Risk for Metastasis |
|||||
|---|---|---|---|---|---|---|
| Group | Size | Mitotic Rate | Gastric GSTS | Jojunal and Ileal GISTS |
Duodenal GISTS | Rectal GISTS |
| 1 | ≤ 2 cm | ≤ 5 per 50 HPF | 0, None | 0, None | 0, None | 0, None |
| 2 | >2 cm ≤ 5 cm | ≤5 per 50 HPFs | 1.9, Very low | 4.3, Low | 8.3, Low | 8.5, Low |
| 3a | >5 cm ≤ 10 cm | ≤ 5 per 50 HPF | 3.6, Low | 24, Moderate | ||
| 3b | > 10 cm | ≤ 5 per 50 HPF | 12, Moderate | 52, High | 34, Higha | 57, Higha |
| 4 | ≤ 2 cm | > 5 per 50 HPF | 0 | 50 | 54, High | |
| 5 | >2 cm ≤ 5 cm | > 5 per 50 HPF | 16, Moderate | 73, High | 50, High | 52, High |
| 6a | > 5 cm ≤ 10 cm | > 5 per 50 HPF | 55, High | 85, High | ||
| 6b | > 10 cm | > 5 per 50 HPF | 86, High | 90, High | 86, Higha | 71, Higha |
Groups 3a and 3b or 6a and 6b are combined in duodenal and rectal GISTs because of small number of cases. From Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagnostic Pathol 2006;23:70; with permission.
Gene locus as well as the type of mutation can also impact prognosis. Molecular analysis of the KIT proto-oncogene has revealed that tumors with exon 9 mutations or deletions in exon 11 are more aggressive when compared with those harboring either a point mutation or insertion in exon 11. Recurrence after surgery is more common in patients with a deletion mutation in exon 11 (24, 26, 27).
In patients with PDGFRα mutations, location is also important. Exon 18 D842V mutations are resistant to imatinib therapy whereas those in exon 12 are responsive to imatinib. Wild-type (WT) GISTs are associated with imatinib resistance and portend an unfavorable prognosis (28). Insulin-like growth factor receptor-1 (IGFR1) has been shown to be overexpressed in patients with WT GISTs. In vitro suppression of IGFR1 results in apoptosis of imatinib-sensitive and resistant WT GIST cells (29). Current trials to investigate the efficacy of IGFR1 inhibitors in patients with WT GISTs are underway. More recently, a germline mutation in the succinate dehydrogenase (SDH) gene was found in 12% of patients with WT GISTs. Defective cellular respiration as a result of SDH mutations in a subset of younger WT GIST patients is thought to contribute to GIST oncogenesis (30). Aneuploidy and telomerase expression have both been shown to correlate with worse outcome and the development of metastatic disease (31–33).
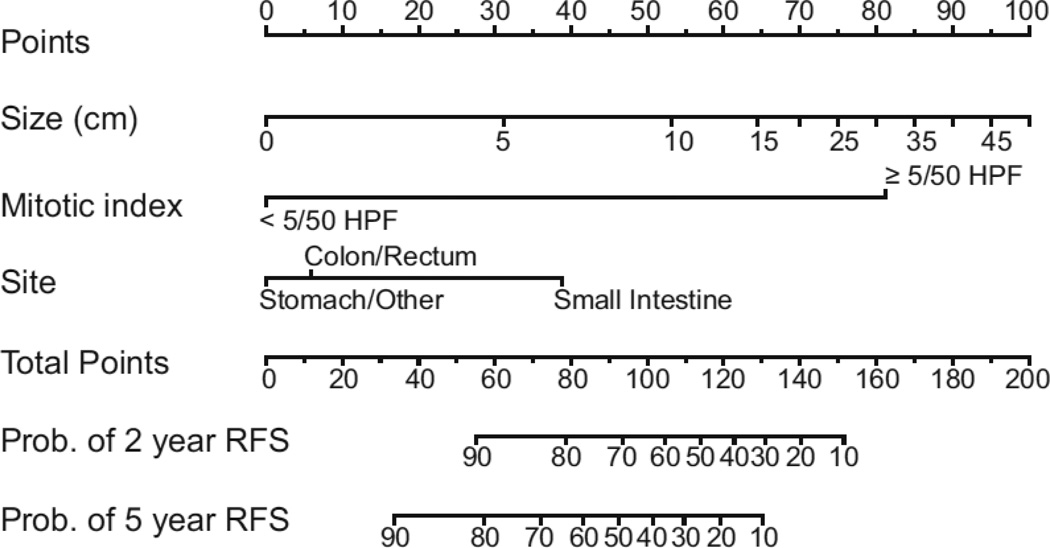
Tumor rupture before or during dissection portends a worse outcome manifested by higher rates of peritoneal recurrence. When examining a specimen, pathologists must consider a slew of prognostic factors that enable them to ultimately categorize GISTs as very low, low, intermediate, or high risk for malignancy (34). A prognostic nomogram developed at Memorial Sloan-Kettering Cancer Center (MSKCC) that takes into account tumor size, mitotic rate, and location can now be used to assess two and five year recurrence-free survival in patients undergoing potentially curative resection of localized primary GIST (35) (Figure 1). While the nomogram was developed using 127 patients at MSKCC, it has been validated using two patient cohorts from other institutions. The fact that inclusion of tyrosine kinase mutation status failed to improve discriminatory ability, may just reflect the number of patients in the study and the number of mutation subtypes.
Figure 1. Nomogram for predicting 2 and 5-year recurrence-free survival in patients with resected localized GIST.
An upward vertical line is drawn from the 2nd, 3rd, and 4th rows to the points line. The sum of points generated is marked on the total points line and a vertical line is drawn downward to determine the 2 and 5-year recurrence-free survival. From Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localized primary gastrointestinal stromal tumor: a retrospective analysis. Lancet Oncol 2009;10:1045–1052; with permission.
Treatment
Primary resectable disease
Surgery remains the only chance for cure in patients with localized, primary GIST. The goal is to achieve negative microscopic margins with an intact tumor pseudocapsule. Wide margins have not been shown to improve outcomes (5). Complete resection can usually be accomplished via wedge resection of the stomach or segmental resection of the bowel. Because GISTs spread hematogenously or by local invasion, lymphadenectomy is not routinely required unless adjacent nodes are obviously enlarged. En bloc resection is needed when adjacent organs appear to be involved.
While there is little disagreement that all tumors larger than 2cm should be resected, the management of incidentally discovered small GISTs less than 2cm is controversial. In the absence of high-risk features on endoscopic ultrasound (echogenic foci, ulceration, irregular margins), some have advocated following these lesions with serial imaging and/or endoscopy. A retrospective analysis looking at the rate of growth of smaller GISTs using endoscopic ultrasound (EUS) found that ~13% with low risk features on endoscopy progressed to a point where they were resected (36). The utility of EUS in the management of small GISTs remains unclear. The frequency of imaging is not well defined, and the need for potentially lifelong surveillance makes this option challenging for some patients and physicians. While endoscopic resection has been suggested by some, the risk of positive margins, perforation, and tumor spillage make this option generally less desirable. Current National Comprehensive Cancer Network (NCCN) guidelines for the management of gastric GISTs less than 2cm without high-risk features on EUS include surveillance endoscopy every 6–12 months (37).
Conventional adjuvant therapies such as chemotherapy and radiation have not been proven effective. Response rates of 5% (38) have been reported with chemotherapy, and radiation is seldom used due to the difficulty of sparing adjacent healthy tissue. Median survival for GIST patients treated with cytotoxic chemotherapy is approximately 12 months (39). Moreover, hepatic artery embolization and intraperitoneal chemotherapy have also resulted in discouraging outcomes (40).
With surgery alone, recurrence rates approached 50% irrespective of negative margins. The approval of imatinib mesylate for the treatment of GIST revolutionized the field. As a specific tyrosine kinase inhibitor (TKI), imatinib has shown efficacy in patients with both KIT and PDGFR α mutations (41). Imatinib is dosed orally once or twice a day and is generally well tolerated with rash, diarrhea and abdominal pain being the most commonly reported side effects (42).
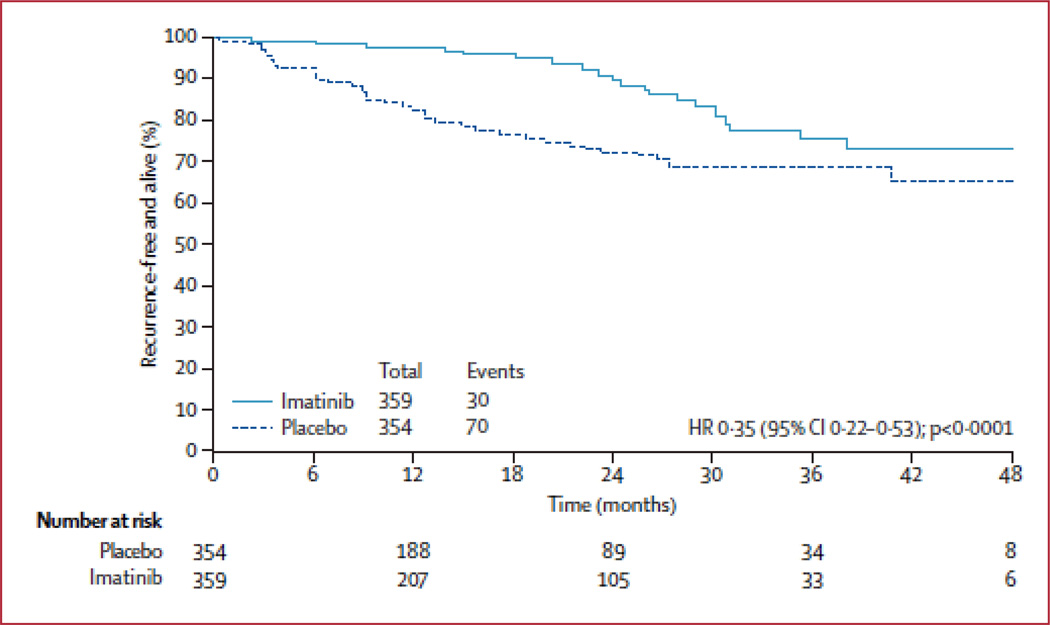
In a phase II trial led by the American College of Surgeons Oncology Group (ACOSOG), oral imatinib for twelve months following resection in patients with high risk GISTs was shown to improve recurrence-free survival and increase overall survival when compared with historical controls (43). High risk in this study was defined as a tumor greater than 10cm, spillage during resection, or more than 5 tumors per patient. In 2009, results from a randomized, placebo controlled, multicenter phase III trial were reported (44). Imatinib taken once a day for 1 year following surgery for localized, primary GIST (≥3cm) was compared with placebo in 713 patients. Recurrence-free survival was significantly higher in the imatinib arm (98%) when compared with the control group (83%) (Figure 2). Although overall survival was no different, longer follow up in this patient cohort will be needed to determine definitively whether adjuvant imatinib can improve overall survival. The cross-over study design may make overall survival similar between arms. In 2009, the FDA approved imatinib for use in the adjuvant setting. In order to define the most effective length of adjuvant imatinib therapy, the results of a recently completed randomized trial comparing one year to three years of adjuvant imatinib are being finalized. It appears that overall survival is longer with 3 years versus 1 year of adjuvant imatinib (45). The goal now will be to determine which subset of patients with resectable disease will truly benefit from adjuvant imatinib. The use of a prognostic nomogram (35) to assess risk of recurrence coupled with mutational analysis may shed some light on this important question.
Figure 2. Recurrence-free survival in patients treated for 1 year with adjuvant imatinib or placebo following resection of localized, primary GIST (≥3cm).
From DeMatteo RP, Ballman KV et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009; 373(9669): 1097–1104; with permission.
The role of laparoscopy in the management of patients with GIST continues to expand. The same principles of complete resection with careful intra-operative handling of tumors apply. Laparoscopic resection of localized gastric GISTs has been studied most extensively thus far. A recently published article from MKSCC studied patients with gastric tumors up to 8cm (46). Those undergoing laparoscopic resection had equivalent perioperative and oncologic outcomes when compared with case–matched controls undergoing open resection. There was no operative mortality, and 30-day morbidity was similar. Oncologic outcomes were also similar with no positive microscopic margins, and one recurrence in each group with a median follow-up of 34 months. Nishimura et al. reported similar results comparing laparoscopic resection with laparotomy in 67 patients with gastric GISTs ranging from 2 to 10cm (47).
Primary unresectable disease
The role of neoadjuvant imatinib in the setting of locally advanced disease has been investigated. The cytoreductive potential of imatinib in the pre-operative setting may enable surgeons to obtain R0 resections with less extensive resections and therefore lower morbidity. For example, pre-operative therapy for patients with rectal GISTs may increase rates of sphincter-preserving surgery. In addition, tumors located at the gastro-esophageal (GE)-junction may respond to imatinib such that esophageal resection is avoided. Both rectal and GE-junction GISTs have shown shrinkage with neoadjuvant imatinib (48).
Recent results from a phase II trial led by the Radiation Therapy Oncology Group (RTOG) revealed that imatinib is well tolerated in the neoadjuvant setting (49). The groups were divided into whether disease was locally advanced and >5cm (Group A) or recurrent/metastatic and >2cm (Group B). Imatinib administered at 600mg per day for 8 weeks pre-operatively was followed by surgery and an additional 2 years of imatinib. This regimen was associated with minimal toxicity and acceptable perioperative complications. Response rates after 8 weeks of pre-operative imatinib as determined by response evaluation criteria for solid tumors (RECIST) were similar between groups A and B (4–7% partial response, 83–90% stable disease, and 4–5% progressive disease). The 2-year progression-free survival rates were 83% and 77% in Groups A and B, respectively.
Another phase II trial from MD Anderson Cancer Center investigated either 3, 5, or 7 days of neoadjuvant imatinib in 19 patients (50). All patients received 2 years of post-operative imatinib as well. This regimen was tolerated well and response rates by FDG-PET were 69%. Median survival for patients treated in this manner was 47 months. Currently there are no published phase III studies investigating the role of neoadjuvant imatinib. The duration of neoadjuvant therapy and patient selection remain to be defined and are currently at the discretion of the surgeon and medical oncologist. Current NCCN guidelines suggest that in patients on neoadjuvant imatinib, once two successive CT scans fail to show any radiographic response, surgical resection should be considered. Incomplete resections in patients with advanced disease are generally only performed in the setting of palliation for bleeding, pain, or obstruction.
Recurrent and metastatic disease
In the pre-imatinib era, the recurrence rate following resection for primary localized GIST was greater than 50% and the median time to recurrence was 2 years (5, 32). Approximately two-thirds of patients with recurrence have liver metastases and about half have peritoneal disease. A true local recurrence at the site of prior resection is uncommon. Although patients with low metastatic burden were considered for surgery, re-resection alone was almost never curative.
In patients who develop recurrence, imatinib is the first line of therapy. Occasionally, patients with symptomatic primary tumors and limited synchronous metastases may be offered surgery before imatinib. The report of successfully treating a patient with metastatic GIST with imatinib (51) spurred a series of clinical trials. Up to 80% of patients with metastatic GIST attain a partial or complete response with imatinib (52). A recent meta-analysis of two, large, randomized studies (53, 54) comparing the efficacy of imatinib given either once (400mg) or twice daily, revealed that the higher dose confers a progression-free survival advantage among patients with exon 9 mutations (55). Overall survival however is unchanged with the higher dose. Since the toxicity of imatinib is dose dependent (56), current guidelines suggest initiating treatment at a dose of 400mg per day. Imatinib at 800mg per day should only be considered as a starting dose for patients with metastatic GIST and a confirmed mutation in exon 9. In patients on 400mg per day, dose escalation to 800mg is considered if progression has been documented and toxicity is acceptable. A summary of imatinib trials conducted in metastatic GIST is shown in Table 2.
Table 2.
Trials of imatinib in metastatic GIST
| Trial | Phase | Year | Dose (n) | Follow- up (months) |
PR | SD | PD | Notes |
|---|---|---|---|---|---|---|---|---|
| EORTC | I | 2001, 2002 |
400, 600, 800 or 1000mg/d (35) |
8–12 | 51% | 31% | 8% | MTD 800mg/d |
| US Multicenter |
II | 2002, 2004 |
400mg/d (73) 800mg/d (74) |
34 | 67% 66% |
16% 18% |
17% 8% |
No difference |
| EORTC | III | 2003 | 400mg/d (470) 800mg/d (472) |
48 | 50% 54% |
32% 32% |
13% 9% |
Improved PFS for 800mg/d |
| Intergroup | III | 2003 | 400mg/d (350) 800mg/d (352) |
12 | 49% 48% |
22% 22% |
No difference in PFS |
Abbreviations: PR, partial response; SD, stable disease; PD, progressive disease; TTR, time to recurrence; MTD, maximal tolerated dose; CR, complete response; PFS, progression-free survival.
In an effort to improve outcomes in patients with advanced disease, several investigators have looked at combining surgery with imatinib. The rationale for this is based on the fact that a complete pathologic response to imatinib occurs less than 5% of the time. Surgery in patients responding to medical therapy can therefore provide the only chance to render them completely free of disease (57, 58). With imatinib, the median survival following surgery for recurrent or metastatic disease has increased from 12–15 months to almost 5 years (53).
Timing of resection and patient selection based on pre-operative response to imatinib appear to be critical determinants of outcome. At MSKCC, patients are generally treated with imatinib for about 6 months, after which incremental shrinkage is uncommon. Then surgery is considered (59). Those who had lesions that were stable or responsive to imatinib had a 2-year progression-free survival of 61% and 2-year overall survival of 100% after surgical resection. In contrast, patients with focal resistance or multiple lesions that were resistant to imatinib did considerably worse with 2 survival of 36%. A similar study in 67 patients by Raut and colleagues confirmed that debulking surgery has little to offer patients with progressive metastatic disease, but may prolong survival in those who are either responsive to imatinib or have limited radiographic progression (60). Twelve-month progression-free survival was 80%, 33%, and 0% for patients with stable disease, limited progression, and widespread progression. A study by Gronchi et al., confirmed that surgery may be of value to a select subset of patients who develop responsive or stable disease while on pre-operative TKI therapy (61). The recommended time course of pre-operative therapy in patients who are responding to imatinib is not well established. Most experts would consider surgery after 6–12 months of medical therapy given the estimated time for development of secondary mutations is two years (62). ACOSOG and the EORTC have attempted unsuccessfully to assess the efficacy of surgery for locally advanced or metastatic GIST in combination with continued TKI therapy.
Other treatment options for patients with advanced disease include radiofrequency ablation (RFA), hepatic artery embolization, and liver transplantation. RFA is typically reserved for patients with unresectable liver disease. Select patients with multiple liver metastases can undergo combined resection with RFA. The use of hepatic artery embolization is reserved for patients with significant metastatic disease burden who have failed multiple TKIs (63, 64). There have been only a handful of case reports of patients undergoing liver transplantation for metastatic GIST (65). As such, the role of transplantation in the setting of metastatic disease remains uncertain.
Imatinib resistant disease
Primary resistance to imatinib is demonstrated by the development of radiographic progression during the first 6 months of treatment. It is important to note that size is not the sole criteria by which radiographic response is measured. GISTs can develop areas of necrosis while maintaining the same size and appearance on CT scan. In the absence of progressive disease, traditional RECIST criteria may be of limited utility in assessing response to TKI therapy (66). The best available option at this time may be to use modified RECIST criteria, where tumor density in addition to size is measured by CT scan (67). Determination of responsive disease may sometimes require functional assessment of tumors using PET.
The presence and location of mutations in KIT and PDGFRα can provide insight into the mechanism of resistance. WT GISTs, or those that contain mutations in exon 9 of KIT or a D842V mutation in PDGFRα, are likely to demonstrate primary resistance. Secondary resistance occurs later in the course of imatinib therapy (>6 months) most often as the result of a second mutation in the kinase domain of KIT or PDGFRα (68–70). Most GISTs that develop secondary resistance to imatinib have a primary mutation in KIT exon 11 and then develop an exon 13, 14 or 17 KIT mutation.
The second line agent for patients with imatinib-resistant disease is sunitinib (71). Sunitinib targets KIT and PDGFRα, as well as the vascular endothelial cell growth factor receptor (VEGFR), fms-like tyrosine kinase 3 (Flt3) receptor, and the RET receptor. In patients with advanced disease resistant to imatinib, sunitinib is a safe and effective second line agent (72). Patients randomized to the sunitinib arm had a median time to progression of 7 months compared with 1.5 months for patients in the placebo arm. Tolerability was acceptable with the most common side effects being fatigue, diarrhea, skin discoloration, and nausea. Raut et al. investigated the effect of surgery in patients with advanced disease resistant to imatinib (73). Fifty patients underwent surgery after a median time of 6.7 months on sunitinib therapy. Median progression-free survival after surgery was 6 months and overall survival was 16 months. Response to sunitinib at the time of surgery did not correlate with post-operative progression-free survival. Incomplete resections and complication rates were relatively high at 50%. The potential benefits of surgery for patients with advanced disease on 2nd line TKI therapy needs to be weighed carefully against the risks on an individual basis.
Options for patients with disease refractory to imatinib and sunitinib are limited. While several third line agents such as sorafenib, nilotinib, dasatinib, and most recently vatalanib (74) have been used in small numbers of patients, there is no clear optimal third line agent. Partial responses and stable disease in patients treated with sorafenib have been reported (75, 76). A phase III trial from the Cancer and Leukemia Group B is underway and compares sorafenib with nilotinib in patients with GIST resistant to imatinib and sunitinib.
Pediatric GIST
Pediatric GISTs are different from those occurring in the adult population. In contrast to adult GISTs, pediatric GISTs are more indolent, display higher rates of recurrence, and are more common in girls (37). Mutations in KIT and PDGFRα are uncommon in the pediatric population and most patients are WT for both proto-oncogenes (4). As a result, response rates to imatinib in this population are much lower when compared with adults. A recent study reported that SDH might play an important role in the oncogenesis of WT GISTs in younger patients (30). Surgery remains the only chance for cure in the children. A complete mutational analysis including SDH and referral to a specialty center and the NIH pediatric GIST clinic is recommended for pediatric patients diagnosed with GIST.
Familial GIST
Familial GISTs are characterized by germline mutations in either KIT or PDGFRα. Patients often present with associated abnormalities such as skin hyperpigmentation and a history of irritable bowel syndrome. Tumors tend to be multifocal, occur more commonly in the small bowel, and frequently have a low mitotic rate. Unlike sporadic GISTs, the type of mutation does not seem to impact the clinical course (77). Response to TKIs is uncertain.
Future strategies
Novel approaches aimed at enhancing response rates and reducing recurrence include combining TKI therapy with radiotherapy (78). Phase II trials are underway combining sunitinib with radiation in patients with progressive disease on imatinib. While investigational third line TKIs such as nilotinib, dasatinib, sorafenib, and vatalanib have shown some promise in patients with disease refractory to imatinib and sunitinib, additional targets of the oncogenic pathway are needed. Phase III trials are underway looking at the efficacy of other pathways such as mammalian target of rapamycin (mTOR) and heat shock proteins. With the recent discovery that BRAF mutations exist in a subset of patients with WT GISTs (21), the use of BRAF inhibitors are also being investigated.
Another innovative strategy that may show promise involves combining TKI therapy with immunomodulation. In a murine model, we recently found that part of imatinib’s effects on GIST are mediated by the immune system. The mechanism depended on imatinib lowering tumor production of indoleamine 2,3-dioxygenase (IDO), an immunosuppressive protein that blocks T cell function (79). The addition of ipilimumab (Yervoy) to TKI therapy may further enable tumor-specific T cells to kill GIST.
Summary
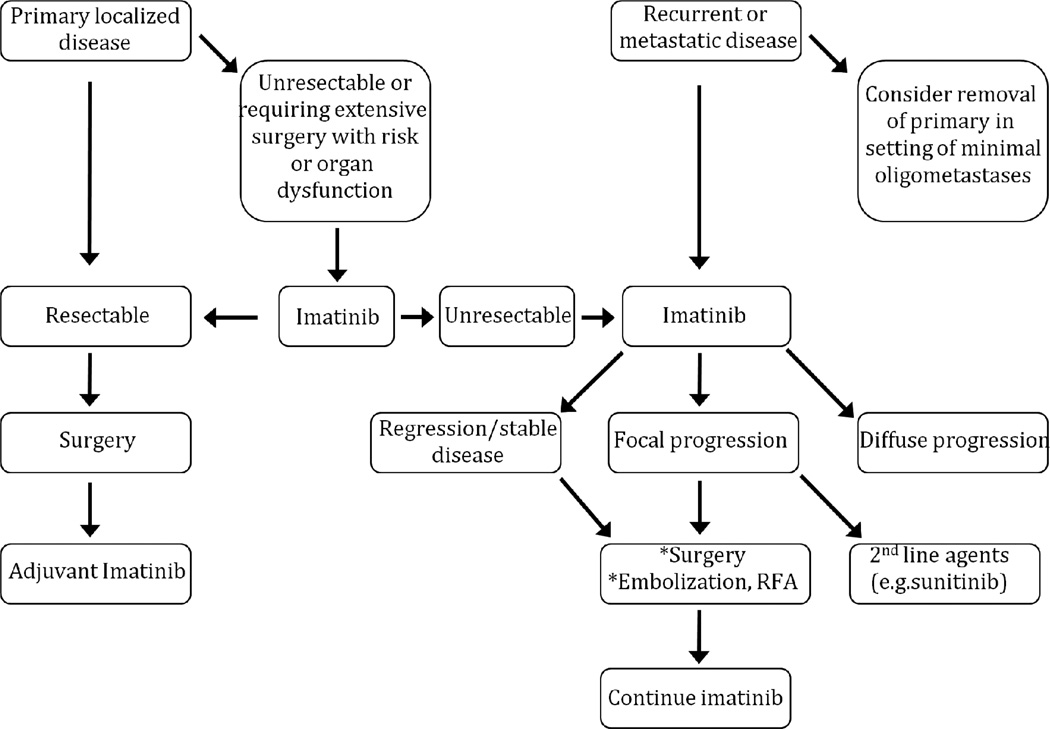
The goals in treating patients with GIST are to maximize the chance of cure, minimize recurrence, and limit the metastatic burden while maintaining a reasonable quality of life. As such, a multidisciplinary approach to patients with GISTs is necessary to optimize the timing of medical and surgical therapy. An evidence-based treatment algorithm is outlined in Figure 3.
Figure 3. Algorithm for the management of GIST.
RFA = Radiofrequency ablation. * If all gross disease or all imatinib-resistant disease is treatable.
Acknowledgments
Funding: This work was supported by NIH Grant CA102613.
Footnotes
Disclosures: Ronald P. DeMatteo is a consultant to and has received honoraria from Novartis.
References
- 1.Demetri GD, Baker LH, Benjamin RS, et al. Soft tissue sarcoma. J Natl Compr Canc Netw. 2007 Apr;5(4):364–399. doi: 10.6004/jnccn.2007.0034. [DOI] [PubMed] [Google Scholar]
- 2.Steigen SE, Eide TJ. Trends in incidence and survival of mesenchymal neoplasm of the digestive tract within a defined population of northern Norway. APMIS. 2006 Mar;114(3):192–200. doi: 10.1111/j.1600-0463.2006.apm_261.x. [DOI] [PubMed] [Google Scholar]
- 3.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005 Jan;100(1):162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 4.Prakash S, Sarran L, Socci N, et al. Gastrointestinal stromal tumors in children and young adults: a clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J Pediatr Hematol Oncol. 2005 Apr;27(4):179–187. doi: 10.1097/01.mph.0000157790.81329.47. [DOI] [PubMed] [Google Scholar]
- 5.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000 Jan;231(1):51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishida T, Hirota S, Taniguchi M, et al. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat Genet. 1998 Aug;19(4):323–324. doi: 10.1038/1209. [DOI] [PubMed] [Google Scholar]
- 7.Chompret A, Kannengiesser C, Barrois M, et al. PDGFRA germline mutation in a family with multiple cases of gastrointestinal stromal tumor. Gastroenterology. 2004 Jan;126(1):318–321. doi: 10.1053/j.gastro.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 8.Takazawa Y, Sakurai S, Sakuma Y, et al. Gastrointestinal stromal tumors of neurofibromatosis type I (von Recklinghausen's disease) Am J Surg Pathol. 2005 Jun;29(6):755–763. doi: 10.1097/01.pas.0000163359.32734.f9. [DOI] [PubMed] [Google Scholar]
- 9.Carney JA. The triad of gastric epithelioid leiomyosarcoma, functioning extra-adrenal paraganglioma, and pulmonary chondroma. Cancer. 1979 Jan;43(1):374–382. doi: 10.1002/1097-0142(197901)43:1<374::aid-cncr2820430152>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.van der Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor: 5 years later. Cancer. 2005 Nov 1;104(9):1781–1718. doi: 10.1002/cncr.21419. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005 Feb 15;103(4):821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 12.Graadt van Roggen JF, van Velthuysen ML, Hogendoorn PC. The histopathological differential diagnosis of gastrointestinal stromal tumours. J Clin Pathol. 2001 Feb;54(2):96–102. doi: 10.1136/jcp.54.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009 Aug;70(2):254–261. doi: 10.1016/j.gie.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002 May;33(5):459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005 Sep;13(3):205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 16.Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001 Nov 15;61(22):8118–8121. [PubMed] [Google Scholar]
- 17.Antonescu CR, Sommer G, Sarran L, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003 Aug 15;9(9):3329–3337. [PubMed] [Google Scholar]
- 18.Lux ML, Rubin BP, Biase TL, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol. 2000 Mar;156(3):791–795. doi: 10.1016/S0002-9440(10)64946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chi P, Chen Y, Zhang L, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumours. Nature. 2010 Oct 14;467(7317):849–853. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003 Jan 31;299(5607):708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 21.Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008 Oct;47(10):853–859. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Espinosa I, Lee CH, Kim MK, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008 Feb;32(2):210–218. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- 23.West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004 Jul;165(1):107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008 Feb 1;112(3):608–615. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 25.Franquemont DW. Differentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol. 1995 Jan;103(1):41–47. doi: 10.1093/ajcp/103.1.41. [DOI] [PubMed] [Google Scholar]
- 26.Martin J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557–558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS) J Clin Oncol. 2005 Sep;23(25):6190–6198. doi: 10.1200/JCO.2005.19.554. [DOI] [PubMed] [Google Scholar]
- 27.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006 May;42(8):1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Debiec-Rychter M, Wasag B, Stul M, et al. Gastrointestinal stromal tumours (GISTs) negative for KIT (CD117 antigen) immunoreactivity. J Pathol. 2004 Apr;202(4):430–438. doi: 10.1002/path.1546. [DOI] [PubMed] [Google Scholar]
- 29.Tarn C, Rink L, Merkel E, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U S A. 2008 Jun;105(24):8387–8392. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A. Jan;108(1):314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudolph P, Gloeckner K, Parwaresch R, Harms D, Schmidt D. Immunophenotype, proliferation, DNA ploidy, and biological behavior of gastrointestinal stromal tumors: a multivariate clinicopathologic study. Hum Pathol. 1998 Aug;29(8):791–800. doi: 10.1016/s0046-8177(98)90447-6. [DOI] [PubMed] [Google Scholar]
- 32.Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992 Jan;215(1):68–77. doi: 10.1097/00000658-199201000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunther T, Schneider-Stock R, Hackel C, et al. Telomerase activity and expression of hTRT and hTR in gastrointestinal stromal tumors in comparison with extragastrointestinal sarcomas. Clin Cancer Res. 2000 May;6(5):1811–1818. [PubMed] [Google Scholar]
- 34.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006 May;23(2):70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009 Nov;10(11):1045–1052. doi: 10.1016/S1470-2045(09)70242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lok KH, Lai L, Yiu HL, Szeto ML, Leung SK. Endosonographic surveillance of small gastrointestinal tumors originating from muscularis propria. J Gastrointestin Liver Dis. 2009 Jun;18(2):177–180. [PubMed] [Google Scholar]
- 37.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010 Apr;8(Suppl 2):S1–S41. doi: 10.6004/jnccn.2010.0116. quiz S2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002 May;33(5):466–477. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 39.Edmonson JH, Marks RS, Buckner JC, Mahoney MR. Contrast of response to dacarbazine, mitomycin, doxorubicin, and cisplatin (DMAP) plus GMCSF between patients with advanced malignant gastrointestinal stromal tumors and patients with other advanced leiomyosarcomas. Cancer Invest. 2002;20(5–6):605–612. doi: 10.1081/cnv-120002485. [DOI] [PubMed] [Google Scholar]
- 40.D'Amato G, Steinert DM, McAuliffe JC, Trent JC. Update on the biology and therapy of gastrointestinal stromal tumors. Cancer Control. 2005 Jan-Feb;12(1):44–56. doi: 10.1177/107327480501200106. [DOI] [PubMed] [Google Scholar]
- 41.Savage DG, Antman KH. Imatinib mesylate--a new oral targeted therapy. N Engl J Med. 2002 Feb;346(9):683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 42.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002 Aug;347(7):472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 43.Dematteo RPOC, Antonescu CR, et al. Efficacy of adjuvant imatinib mesylate following complete resection of localized, primary gastrointestinal stromal tumor (GIST) at high risk of recurrence: the US. Intergroup phase II trial ACOSOG Z9000. American Society of Clinical Oncology 2008; Gastrointestinal Cancers Symposium; Orlando (FL). January; 2008; 2008. p. A8. 2008. [Google Scholar]
- 44.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009 Mar;373(9669):1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joensuu H. Twelve versus 36 months of adjuvant imatinib (IM) as treatment of operable GIST with a high risk of recurrence: Final results of a randomized trial. J Clin Oncol. 2011;29 (suppl; abstract LBA1) [Google Scholar]
- 46.Karakousis GC, Singer S, Zheng J, et al. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol. Jun;18(6):1599–1605. doi: 10.1245/s10434-010-1517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura J, Nakajima K, Omori T, et al. Surgical strategy for gastric gastrointestinal stromal tumors: laparoscopic vs. open resection. Surg Endosc. 2007 Jun;21(6):875–878. doi: 10.1007/s00464-006-9065-z. [DOI] [PubMed] [Google Scholar]
- 48.Hohenberger POO, Licht T, et al. Neoadjuvant imatinib and organ preservation in locally advanced gastrointestinal stromal tumors (GIST) J Clin Oncol. 2009;27(Suppl 1) Abstract 10550. [Google Scholar]
- 49.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009 Jan;99(1):42–47. doi: 10.1002/jso.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol. 2009 Apr;16(4):910–919. doi: 10.1245/s10434-008-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001 Apr;344(14):1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 52.Katz SC, DeMatteo RP. Gastrointestinal stromal tumors and leiomyosarcomas. J Surg Oncol. 2008 Mar;97(4):350–359. doi: 10.1002/jso.20970. [DOI] [PubMed] [Google Scholar]
- 53.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004 Sep-Oct;364(9440):1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 54.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008 Feb;26(4):626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 55.(MetaGIST) GSTM-AG. Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. Mar;28(7):1247–1253. doi: 10.1200/JCO.2009.24.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Glabbeke M, Verweij J, Casali PG, et al. Predicting toxicities for patients with advanced gastrointestinal stromal tumours treated with imatinib: a study of the European Organisation for Research and Treatment of Cancer, the Italian Sarcoma Group, and the Australasian Gastro-Intestinal Trials Group (EORTC-ISG-AGITG) Eur J Cancer. 2006 Sep;42(14):2277–2285. doi: 10.1016/j.ejca.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 57.Bumming P, Andersson J, Meis-Kindblom JM, et al. Neoadjuvant, adjuvant and palliative treatment of gastrointestinal stromal tumours (GIST) with imatinib: a centre-based study of 17 patients. Br J Cancer. 2003 Aug 4;89(3):460–464. doi: 10.1038/sj.bjc.6600965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bauer S, Hartmann JT, de Wit M, et al. Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer. 2005 Nov;117(2):316–325. doi: 10.1002/ijc.21164. [DOI] [PubMed] [Google Scholar]
- 59.DeMatteo RP, Maki RG, Singer S, Gonen M, Brennan MF, Antonescu CR. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg. 2007 Mar;245(3):347–352. doi: 10.1097/01.sla.0000236630.93587.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006 May;24(15):2325–2331. doi: 10.1200/JCO.2005.05.3439. [DOI] [PubMed] [Google Scholar]
- 61.Gronchi A, Fiore M, Miselli F, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg. 2007 Mar;245(3):341–346. doi: 10.1097/01.sla.0000242710.36384.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008 Feb;26(4):620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 63.Kobayashi K, Szklaruk J, Trent JC, et al. Hepatic arterial embolization and chemoembolization for imatinib-resistant gastrointestinal stromal tumors. Am J Clin Oncol. 2009 Dec;32(6):574–581. doi: 10.1097/COC.0b013e31819cca35. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi K, Gupta S, Trent JC, et al. Hepatic artery chemoembolization for 110 gastrointestinal stromal tumors: response, survival, and prognostic factors. Cancer. 2006 Dec;107(12):2833–2841. doi: 10.1002/cncr.22336. [DOI] [PubMed] [Google Scholar]
- 65.Serralta AS, Sanjuan FR, Moya AH, et al. Combined liver transplantation plus imatinib for unresectable metastases of gastrointestinal stromal tumours. Eur J Gastroenterol Hepatol. 2004 Nov;16(11):1237–1239. doi: 10.1097/00042737-200411000-00025. [DOI] [PubMed] [Google Scholar]
- 66.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007 May;25(13):1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 67.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007 May;25(13):1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 68.Chen LL, Trent JC, Wu EF, et al. A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res. 2004 Sep;64(17):5913–5919. doi: 10.1158/0008-5472.CAN-04-0085. [DOI] [PubMed] [Google Scholar]
- 69.Debiec-Rychter M, Cools J, Dumez H, et al. Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology. 2005 Feb;128(2):270–279. doi: 10.1053/j.gastro.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 70.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005 Jun;11(11):4182–4190. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 71.Prenen H, Cools J, Mentens N, et al. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin Cancer Res. 2006 Apr;12(8):2622–2627. doi: 10.1158/1078-0432.CCR-05-2275. [DOI] [PubMed] [Google Scholar]
- 72.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006 Oct;368(9544):1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 73.Raut CP, Wang Q, Manola J, et al. Cytoreductive surgery in patients with metastatic gastrointestinal stromal tumor treated with sunitinib malate. Ann Surg Oncol. Feb;17(2):407–415. doi: 10.1245/s10434-009-0784-y. [DOI] [PubMed] [Google Scholar]
- 74.Joensuu H, De Braud F, Grignagni G, et al. Vatalanib for metastatic gastrointestinal stromal tumour (GIST) resistant to imatinib: final results of a phase II study. Br J Cancer. May;104(11):1686–1690. doi: 10.1038/bjc.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wiebe LKK, Maki RG, et al. Activity of sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): a phase II trial of the University of Chicago Phase II Consortium. J Clin Oncol. 2008;26(Suppl 1) Abstract 10502. [Google Scholar]
- 76.Reichardt PMM, Gelderblom H, et al. Sorafenib fourth-line treatment in imatinib-, sunitinib-, and nilotinib-resistant metastatic GIST: a retrospective analysis. J Clin Oncol. 2009;27(Suppl 1) Abstract 10564. [Google Scholar]
- 77.Antonescu CR. Gastrointestinal stromal tumor (GIST) pathogenesis, familial GIST, and animal models. Semin Diagn Pathol. 2006 May;23(2):63–69. doi: 10.1053/j.semdp.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Kao J, Packer S, Vu HL, et al. Phase 1 study of concurrent sunitinib and image-guided radiotherapy followed by maintenance sunitinib for patients with oligometastases: acute toxicity and preliminary response. Cancer. 2009 Aug;115(15):3571–3580. doi: 10.1002/cncr.24412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balachandran VP, Cavnar M, Zeng S, et al. Imatinib mesylate potentiates anti-tumor T cell responses in gastrointestinal stromal tumor through the inhibition of indoleamine 2,3-dioxygenase. Nat Med. 2011 doi: 10.1038/nm.2438. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]