Abstract
The idea of using platelet rich plasma (PRP) in medicine has been around since the 1970s. It is only more recently that its use has been employed in the area of musculoskeletal science. Platelet rich plasma in this area has received much media attention being used by many celebrity sports athletes for musculoskeletal injuries. Therefore it is important for the musculoskeletal practitioner to be aware of the concepts surrounding its use and application. In this article we cover what platelet rich plasma is, how it is prepared and administered, its potential clinical application, and what the current literature discusses in the various areas of musculoskeletal science.
Introduction
Platelet rich plasma (PRP) is an autologous blood-derived product that has an increased concentration of platelets that are rich in growth factors, and has the potential to enhance the healing of tissue at the cellular level via the recruitment, proliferation, and differentiation of cells involved in tissue regeneration. There have been a number of studies on the effect of PRP at the preclinical and clinical level in musculoskeletal medicine, some of which we will highlight in this article.
Methods
The basic science of PRP
Platelets are key components in haemostasis, and stimulate the construction of new connective tissue, and revascularization. Platelets are small, regularly shaped, clear cells measuring between 2–3 µm in diameter. They are derived from the fragmentation of precursor megakaryocytes and have a lifespan of 5–9 days. The physiological range for platelets in humans is between 150 and 400 × 109 per litre. They normally circulate in the blood and are involved in the formation of the haemostatic plug.1
A sample of blood will normally contain 93% red blood cells, 6% platelets, and 1% white blood cells.2 In PRP the ratio of red blood cell to platelets is reversed, thereby increasing factors that would be more useful in healing. The exact ratio of red and white blood cells to platelets in PRP is variable depending on the way in which the PRP is prepared.
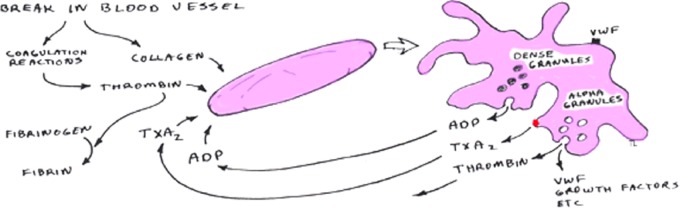
There are three stages of platelet involvement in the formation of the blood clot: activation, secretion, and aggegration. Platelets in the body are activated when they come in to contact with von Willebrand factor, collagen (exposed endothelium), or by the action of thrombin. Once activated, they secrete the contents of their alpha and dense granules (Figure 1 and Table 1). The alpha granules facilitate platelet adherence to the exposed endothelium and the dense granules lead to activation of the intrinsic coagulation pathway. The growth factors released from these granules facilitate the three stages of healing: inflammation, proliferation, and remodelling by: (1) initiating the clotting cascade (2) releasing histamine and serotonin that increase capillary permeability of the area allowing inflammatory cells greater access to the site and (3) encouraging the migration of white blood cells. This leads to cell proliferation while fibroblasts begin to lay the ground substance.5 It is thought by creating a concentrated formulation of the above factors in PRP, an optimal environment could be created to accelerate healing.
Figure 1.
Activation and secretion of platelets Source: Getgood, A. Articular Cartilage Tissue Engineering. 2009. Cambridge University Library
Table 1.
| Growth Factor | Effect |
|---|---|
| PDGF | Macrophage activation and angiogenesis Fibroblast chemotaxis and proliferative activity Enhances collagen synthesis Enhances the proliferation of bone cells |
| TGF-Beta | Enhances the proliferative activity of fibroblasts Stimulates biosynthesis of type I collagen and fibronectin Induces osteoclast formation and bone resorption |
| IGF-I | Chemotactic for fibroblasts and stimulates protein synthesis Enhances bone formation by proliferation and differentiation of osteoblasts |
| PDEGF | Promotes wound healing by stimulating the proliferation of keratinocyes and dermal fibroblasts |
| PDAF | Induces vascularization by stimulating vascular endothelial cells |
| PF-4 | Stimulates the initial reflux of neutrophils into wounds A chemoattractant for fibroblasts A potent antiheparin agent |
| EGF | Cellular proliferation Differentation of epithelial cells |
| VEGF | Angiogenesis Migration and mitosis of endothelial cells Creation of blood vessel lumen Creates fenestrations Chemotactic for macrophages and granulocytes Vasodilation (indirectly by release of nitrous oxide) |
PDGF = Platelet-Derived Growth Factor; TGF = Transforming Growth Factor; IGF = Insulin Growth Factor; PDEGF = Platet-derived endothelial growth factor; PDAF = Platelet-derived angiogenesis factor; PF-4 = Platelet Factor 4; EGF = Endothelial Growth Factor; VEGF = Vascular Endothelial Growth Factor;
Formulation of PRP
PRP is prepared from blood which is treated to concentrate the maximum amount of platelets.Citrate is added to PRP to inhibit the coagulation process, as the clot will contain the platelets. The blood sample is then placed into a centrifuge that will separate out the platelet rich plasma. The next step is to release the associated growth factors from the platelet. This can be done by adding either: (1) Bovine thrombin to the platelet rich plasma. This releases 70% of stored growth factors within 10 minutes, and nearly 100% within one hour.5 A small amount of growth factors will be released throughout the lifespan of the platelet. (2) Calcium chloride to convert autologous thrombin to prothrombin resulting in platelets being trapped in a fibrin matrix. As a small amount of thrombin is formed, release of growth factors is gradual over 7 days. (3) Use type I collagen to activate platelet rich plasma. Figure 2 is one example of a commercial process to produce platelet rich plasma.
Figure 2.
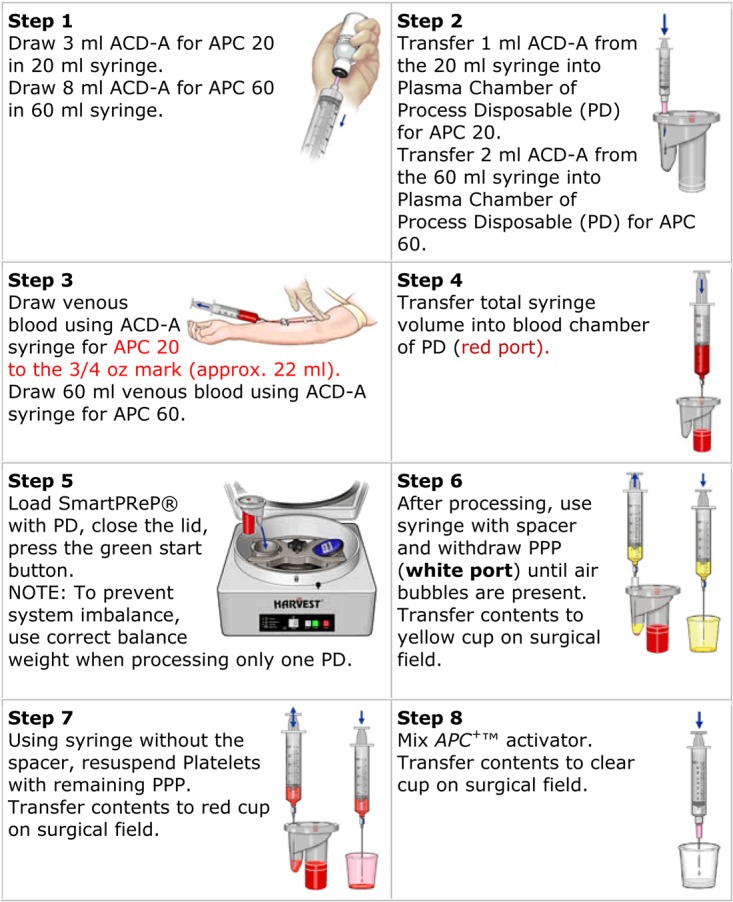
SmartPrep PRP set up
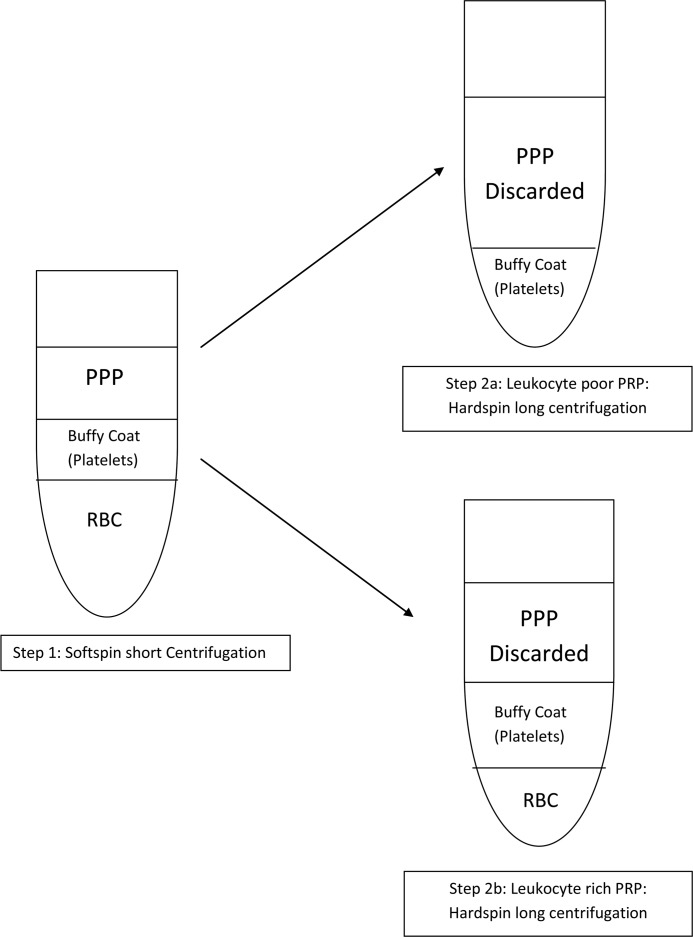
The various commercial preparation methods for PRP do not produce the same product (Table 2 and Figure 3). Some are leukocyte rich, others are leukocyte poor. The ideal preparation has yet to be determined – there are papers on this which suggest optimum values for PRP. There are a variety of methods by which PRP could be applied including: (1) By direct application, (2) By single or multiple injections, (3) As a gel and (4) In a collagen sponge. The most effective method for each proposed use, has yet to be determined.
Table 2.
A sample of various commercially available
| System, company | Whole Blood Volume (ml) | Activator | Centrifuge Time (min) | Volume of PRP (ml) | Leukocytes |
|---|---|---|---|---|---|
| Cascade, PRFM | 18 | Calcium Chloride | 6 | 7.5 | No |
| Harvest, SmartPrep 2 | 20–120 | Bovine thrombin | 14 | 3–20 | Yes |
| Biomet, GPS III | 27–110 | Autologous thrombin with calcium chloride | 15 | 3–12 | Yes |
Figure 3.
Two-step centrifugation procedure – Production of leukocyte rich/poor PRP (Taken from Dohan Ehrenfest, Rasmusson et al. 2009)
Current Clinical Applications
Bone
The standard approach to promoting bone repair is to use bone grafting, a procedure that can enable healing of both critical-sized defects and refractory lesions. There are several areas where there is a need for improvement; to reduce the need for autograft harvest with its associated morbidity, to improve the outcome seen when using allogeneic bone, to reduce healing time thereby reduce time to recovery and ideally to provide off-the-shelf products with reproducible outcomes that would replace the requirement for grafting. Treatments that could enhance bone grafting or be used to augment bone graft substitutes are attractive and PRP offers this potential as it contains a number of growth factors that can stimulate the proliferation and differentiation of cells of the osteogenic lineage.
The first use of PRP for bone repair was described by Marx in 1998.6 He reported a series of 88 cases of mandibular continuity defects treated with autograft bone of which half had added PRP. Radiographs, produced at 2, 4 and 6 months, showed a significant increase in maturity and consolidation of the graft in patients treated with PRP. These results were confirmed by bone biopsy at 6 months which showed a significant increase in trabecular bone density within the PRP augmented graft. Subsequently, several pre-clinical studies were reported investigating the ability of PRP to improve healing with bone graft7 or with collagen and other graft substitute materials8 for various repair requirements. The results of these studies have been equivocal with some studies reporting improved healing with PRP and others showing no effect. One of the reasons for the discrepancy between studies has been ascribed to differences in PRP activation protocols.9 Interestingly, a comparison of the augmentation of autologous graft with either PRP or bone morphogenetic protein (BMP) for the repair of mandibular defects in rats, showed improved healing with BMP but not PRP.10
Alongside this work there have been a number of reports of clinical periodontal and maxillofacial bone repair augmentations following on from the work of Marx. Whilst a small number of these showed a beneficial effect of PRP, the majority showed no effect.11 Using a split-mouth technique to control for PRP augmentation, Hanna et al. showed improved clinical periodontal response whilst Raghoebar et al. showed no effect.12 One meta analysis of the use of PRP in sinus bone grafting indicated a small but significant increase in the odds ratio for increased bone formation but no effect on subsequent implant survival,13 whilst another meta analysis of sinus lifting procedures concluded that there was no significant effect of PRP augmentation.14 There have been several reports of clinical studies using human PRP in a variety of orthopaedic procedures. A beneficial effect of PRP was described in distraction osteogenesis, although this study may have been biased by treatment self-selection.15 In a randomized trial of augmented grafting in wedge osteotomy of the proximal tibia, Dallari et al. reported improved osseointegration at follow-up times up to 52 weeks and a significant increase in bone formation in a biopsy taken at 6 weeks.16 PRP has also been reported to promote healing in a series of fracture non-unions.17 However, a randomised clinical trial of non-union healing showed only 68.3 % healing with PRP compared to 86.7 % healing with BMP718 and a prospective randomized trial of the use of PRP in knee arthroplasty found no significant clinical effect.19 A systematic review of the use of PRP to promote bone healing concluded that although PRP treatment was safe, there was as yet no clinical evidence of benefit.20
The lack of unequivocal evidence for the effectiveness of PRP augmentation of bone repair may be due to a number of factors. The varied conditions being treated and their site within the skeleton are probably, in part, responsible for the variation in efficacy. Reported studies have used a variety of different methods for the production of PRP and this will have an effect on the composition of the PRP produced. One key factor is the number of platelets in the concentrate and evidence has been put forward for an optimal concentration. Other factors are the activation method used and the interaction of the platelets and released growth factors with the graft material used. The timing of analysis may also be important; in a study of PRP augmentation of the repair of frontal skull defects, changes were seen in the production of bone matrix proteins and bone regeneration at early time points but no long-term effect on bone formation.21 Further prospective randomized clinical trials, with sufficient patients to provide statistical power, are required for recommendations regarding the use of PRP to augment bone repair to be given and these have been proposed for fracture repair.22
Tendon and ligaments
Tendon and ligaments heal more slowly than most tissues due to poor vascular supply.23 This can result in new tendon tissue that does not have the same structural and functional properties as the original tissue when healed and leads to scar tissue. One possible explanation is that the poor blood supply results in a lack of adequate growth factors being delivered to the site of injury.24 Given, then, that there are problems with tendon and ligament healing, it is possible that PRP, as a good source of growth factors, may enhance healing.
Certain rotator cuff tears (including massive tears and chronic injuries) have a high failure rate of repair. There have been several randomized controlled trials investigating the effects of PRP in rotator cuff repair, and the results have been disappointing. For example, one randomized controlled trial25 that examined the effect of PRP on the efficacy of arthroscopic rotator cuff repair, found no statistically significant difference in clinical or imaging outcomes compared to the placebo group.
PRP has also been explored in anterior cruciate ligament reconstruction. Two randomized controlled trials26,27 showed no statistically significant difference in bone filling of the bone tunnels in ACL reconstructions as visualized on MRI, though one of these found an improvement in the clinical score. Another randomized controlled trial28 of 108 patients, showed at 6 months that PRP had an enhancing effect on the maturation of the graft as visualized by MRI.
The effect of PRP on Achilles tendinopathy treatment has also been investigated. In this condition it appears that the outcome may depend on whether the injury is acute or chronic. A recent randomized controlled trial by de Vos et al. showed at 24 weeks,29 and 1-year follow-up,30 that PRP had no statistically significant benefit in clinical scoring outcome or ultrasound findings in chronic Achilles tendinopathy. However, in PRP treatment of acute Achilles tendon damage, studies have shown a quicker recovery time31 and a better Achilles tendon rupture score.32
Other areas explored include lateral epicondylitis, where it has been shown at two years that injecting PRP improved healing as compared to injecting corticosteroids at two33 years post-treatment in a 100 patient trial. The use of PRP n patellar tendinopathy appears to be favourable.34
However, in general, very few randomized controlled trials have been conducted on small cohorts of patients in each specific area of clinical tendinopathy. Further studies must be conducted before a specific conclusion can be made.
Muscle
As with the other tissues already discussed, it can be hypothesized that the use of PRP will accelerate muscle healing. At the current time, little evidence exists to support this hypothesis. Indeed, some researchers have suggested that PRP may actually lead to unwanted fibrotic healing in muscle.35
In surgically created tibialis anterior muscle defects in rats, PRP injections led to a quicker time to recovery than in platelet poor plasma or sham treated animals.36 Cugat et al. conducted a cohort study of 14 professional athletes with acute muscle injuries who were treated with ultrasound-guidance injections of PRP. The patients showed a quick return to play and enhanced healing in tears assessed ultrasonographically. These findings agree with Sanchez et al.37 who reported a similar study in 20 athletes who found that patients recovered in half of the expected time. These results are not from randomized, well controlled trials involving large numbers of patients but are, nevertheless, supportive of the idea that PRP could play a key role in muscle healing. A number of prospective randomized trials are currently being conducted in this area and will hopefully help to formulate future treatment recommendations.38
Cartilage
Articular cartilage lacks the vascularization and inflammatory processes that enable effective repair. Endogenous chondrocytes can synthesize fibrous repair tissue but not sufficiently to fill even small defects with a cartilage-like matrix.39
PRP has been promoted as a potential agent for therapeutic repair of cartilage40 due to the range of beneficial growth factors released on activation.41 Of these it is likely that PDGF, TGF and FGF exert the greatest beneficial effects on cartilage and chondrocytes. Some anti-inflammatory properties of PRP have also been demonstrated,42 but the variable nature of PRP has led to conflicting data making an overall assessment of its worth in tissue repair difficult. Donor variability, PRP preparation method, scaffold type, cell origin and culture conditions have all been shown to alter the levels of growth factor release or cell responses.
Many authors agree that PRP increases chondrocytes proliferation, but there is still considerable debate as to the influence of PRP on chondrogenic differentiation and cartilage matrix accumulation. Akeda et al.43 demonstrated that porcine chondrocytes entrapped in alginate, produced the highest proteoglycan and collagen synthesis, and the majority of the collagen expressed was type II collagen when treated with PRP compared to chondrocytes cultured with PPP or FCS. Spreafico et al.44 cultured human chondrocytes in a PRP/fibrin gel and demonstrated reduced de-differentiation and increased matrix synthesis compared to cells treated with FCS or PPP. However, conflicting data was reported by Kaps et al.45 (bovine chondrocytes), Gaissmair et al.46 (human chondrocytes) and Drengk et al.47 (sheep chondrocytes). These studies suggested that PRP enhanced de-differentiation of chondrocytes and did not contribute to cartilage matrix synthesis in in vitro 3D culture. However, it should be emphasized that most of these studies use different sources and preparation methods for the cells, different expansion conditions and supplements for expanding the cells, and different methods of PRP preparation. One key factor affecting PRP potency is the type of activation that it undergoes during preparation or when incorporated into the experiment. Thrombin activation can eliminate the chondrogenic and osteoinductive potential of PRP.48 Two other studies49,50 achieved chondrogenic induction of BMSCs by using freeze–thaw cycles to activate PRP, whilst Getgood et al.51 demonstrated enhanced activation of PRP when it was combined with a collagen/glycosaminoglycan scaffold for osteochondral repair.
PRP also has potential for articular cartilage repair by direct application into the damaged joint; either as a liquid, gel or entrapped in a delivery device. Sampson et al52 carried out a single-centre, uncontrolled, prospective preliminary study on 14 patients with primary and secondary knee osteoarthritis. The patients received three PRP injections in the affected knee at 4-wk intervals. Although designed as a preliminary safety study for PRP, the results demonstrated significant and almost linear improvements in knee injury and osteoarthritis outcome scores, including pain and symptom relief.
Kon et al.53 carried out a study on 100 patients (115 knees) treated with PRP which was evaluated by IKDC- and EQ-VAS scores. The study demonstrated that PRP treatment is safe, reduces pain and improves knee function, especially in younger patients. However, at the 24-month follow-up, the outcome (IKDC score) worsened from 67% to 59% of normal or nearly normal knees between the 12- and 24-month evaluations.54 A study by the same group using an ovine model concluded that PRP had a negative effect on osteochondral repair when compared to scaffold alone.55 However, Milano et al.56 demonstrated positive effects of PRP in an ovine micro fracture model with PRP gel being more effective than a PRP injection. The beneficial effects of PRP on cartilage repair have also been reported in a rabbit model of osteoarthritis57 and in a rabbit osteochondral defect model.58
In conclusion, PRP is showing promise in the field of cartilage repair but the adoption of standardized preparation and usage protocols will be required for future studies to enable meaningful statistical data to be generated.
Meniscus
There are few studies specifically targeting menisci with PRP. However, isolated meniscal cells grown in vitro have been demonstrated to respond to individual growth factors present in PRP including TGF, IGF and PDGF.59 Ishida and co-workers60 demonstrated an in vitro reaction to PRP using cultured meniscal fibroblasts and observed improved tissue infilling in gelatine/PRP inserts 12 weeks postoperatively in a rabbit meniscal defect. However, Zellner and co-workers,61 using a double centrifugation PRP preparation method, found no benefit to adding their PRP to a hyaluronan/collagen plug in a rabbit meniscal defect by comparison to the following groups: empty defects, plugs with bone marrow aspirate, MSC with and without prechondrogenic in vitro treatment. Fibrin clots, produced by agitating blood with glass beads until a clot formed, were used by Arzonscky and co-workers.62 The clots were rinsed of excess red blood cells using sterile saline and used to pack 2 mm meniscal defects in dogs. Opposite knees were operated in the same way but with extensive saline rinsing before finishing. The fibrin packed defects filled with fibrocartilagenous-like tissue at six months, although the tissue was histologically distinct to the mature tissue.
Although menisci are not essential for joint function, meniscal resection leads to long-term destabilization and degradation of the articular surfaces.63 Autologous blood products such as PRP, Fibrin and blood provide an increase of factors demonstrated to influence meniscal cells and may be one initial, simple, route to improve healing. However, the studies presented here are limited in the PRP types used, and the numbers of patient studies specifically investigating PRP on the menisci remains relatively low at the current time.
Discussion
It is difficult to make a general conclusion on the use of platelet-rich plasma in musculoskeletal medicine. Commercial preparations of platelet-rich plasma vary in terms of growth factors, activation, and platelet concentration. This is compounded by the fact that clinical investigations of platelet-rich plasma differ in application methods, timing, and volume of platelet-rich plasma used. Many of the published studies are not randomized controlled trials, and may not be at the appropriate statistical power to identify whether the treatment works or not. We are reluctant to dismiss PRP, as its clinical benefits could be varied by a number of factors in its production and application. PRP also offers many benefits such as being safe, easy to extract, relatively simple and short time to process, offering multiple growth factors at a relatively inexpensive cost, as compared to obtaining individual growth factors or even stem cells. It is for these reasons, and because we believe that the PRP potential has yet to be fully explored, that we would consider further investigation of PRP be warranted. We suggest as others have done that scientific studies need to be conducted to determine the optimal: (1) process method (2) volume (3) delivery (4) timing (5) indication (6) single vs series of injections (7) and rehabilitation protocol – for PRP.
Interventions in musculoskeletal medicine aim to achieve predictable, rapid tissue repair, and enhance tissue repair for the quickest recovery time. PRP may have the potential to bring this about, however a number of further high-level investigations need to be conducted before a conclusion can be made on PRP.
DECLARATIONS
Competing interests
None declared
Funding
None
Ethical approval
Not applicable
Guarantor
ZA
Contributorship
All author contributed equally
Acknowledgements
The authors would like to gratefully acknowledge the support of the National Institute for Health Research and the Engineering and Physical Sciences Research Council.
References
- 1.Campbell NAB Biology. International , 8th ed. / Campbell Neil A. … [et al.]. ed. 2008, San Francisco; London: Pearson/Benjamin Cummings; 1 v. (various pagings) [Google Scholar]
- 2.Marx RE, Garg AKDMD Dental and craniofacial applications of platelet-rich plasma. 2005; Chicago, IL; London: Quintessence Pub. Co. ix, 154 p [Google Scholar]
- 3.Alsousou J, Thompson M, Hulley P, Noble A, Willett K The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone and Joint Surg Br 2009;91:987–96 [DOI] [PubMed] [Google Scholar]
- 4.Molloy T, Wang Y, Murrell G The roles of growth factors in tendon and ligament healing. Sports Med 2003;33:381–94 [DOI] [PubMed] [Google Scholar]
- 5.Bennett NT, Schultz GS Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg 1993;165:728–37 [DOI] [PubMed] [Google Scholar]
- 6.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR Platelet-rich plasma - Growth factor enhancement for bone grafts. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics 1998;85:638–646 [DOI] [PubMed] [Google Scholar]
- 7.Jensen TB, Rahbek O, Overgaard S, Soballe K No effect of platelet-rich plasma with frozen or processed bone allograft around noncemented implants. International Orthopaedics 2005;29:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkar MR, Augat P, Shefelbine SJ, et al. Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials 2006;27:1817–1823 [DOI] [PubMed] [Google Scholar]
- 9.Intini G The use of platelet-rich plasma in bone reconstruction therapy. Biomaterials 2009;30:4956–4966 [DOI] [PubMed] [Google Scholar]
- 10.Roldan JC, Jepsen S, Miller J, et al. Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone 2004;34:80–90 [DOI] [PubMed] [Google Scholar]
- 11.Raghoebar GM, Schortinghuis J, Liem RSB, Ruben JL, van der Wal JE, Vissink A Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clinical Oral Implants Research 2005;16:349–356 [DOI] [PubMed] [Google Scholar]
- 12.Weibrich G, Kleis WK, Hafner G Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants 2002;17:184–90 [PubMed] [Google Scholar]
- 13.Bae JH, Kim YK, Myung SK Effects of Platelet-Rich Plasma on Sinus Bone Graft: Meta-Analysis. Journal of Periodontology 2011;82:660–667 [DOI] [PubMed] [Google Scholar]
- 14.Esposito M, Grusovin MG, Rees J, et al. Effectiveness of sinus lift procedures for dental implant rehabilitation: a Cochrane systematic review. European Journal of Oral Implantology 2010;3:7–26 [PubMed] [Google Scholar]
- 15.Kitoh H, Kitakoji T, Tsuchlya H, Katoh M, Ishiguro N Transplantation of culture expanded bone marrow cells and platelet rich plasma in distraction osteogenesis of the long bones. Bone 2007;40:522–528 [DOI] [PubMed] [Google Scholar]
- 16.Dallari D, Fini M, Stagni C, et al. In vivo study on the healing of bone defects treated with bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone allografts, alone and in combination. Journal of Orthopaedic Research 2006;24:877–888 [DOI] [PubMed] [Google Scholar]
- 17.Sanchez M, Anitua E, Cugat R, et al. Nonunions treated with autologous preparation rich in growth factors. J Orthop Trauma 2009;23:52–9 [DOI] [PubMed] [Google Scholar]
- 18.Calori GM, Tagliabue L, Gala L, d'Imporzano M, Peretti G, Albisetti W Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions A prospective randomised clinical study on 120 patients. Injury-International Journal of the Care of the Injured 2008;39:1391–1402 [DOI] [PubMed] [Google Scholar]
- 19.Horstmann WG, Slappendel R, van Hellemondt GG, Wymenga AW, Jack N, Everts PA Autologous platelet gel in total knee arthroplasty: a prospective randomized study. Knee Surg Sports Traumatol Arthrosc 2011;19:115–21 [DOI] [PubMed] [Google Scholar]
- 20.Griffin XL, Smith CM, Costa ML The clinical use of platelet-rich plasma in the promotion of bone healing: A systematic review. Injury-International Journal of the Care of the Injured 2009;40:158–162 [DOI] [PubMed] [Google Scholar]
- 21.Thorwarth M, Wehrhan F, Schultze-Mosgau S, Wiltfang J, Schlegel KA PRP modulates expression of bone matrix proteins in vivo without long-term effects on bone formation. Bone 2006;38:30–40 [DOI] [PubMed] [Google Scholar]
- 22.Griffin XL, Parsons N, Achten J, Costa ML Warwick Hip Trauma Study: a randomised clinical trial comparing interventions to improve outcomes in internally fixed intracapsular fractures of the proximal femur. Protocol for The WHiT Study. Bmc Musculoskeletal Disorders 2010;11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson S, Gerhardt M, Mandelbaum B Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med 2008;1(3–4):165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramachandran M Basic orthopaedic sciences : the Stanmore guide. 2007, LondonNew York: Hodder Arnold; Distributed in the U.S. by Oxford University Press. x, 304 p [Google Scholar]
- 25.Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med 2011;39:258–65 [DOI] [PubMed] [Google Scholar]
- 26.Cervellin M, de Girolamo L, Bait C, Denti M, Volpi P Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: a randomized, controlled clinical study. Knee Surg Sports Traumatol Arthrosc 2011 [DOI] [PubMed] [Google Scholar]
- 27.Nin JR, Gasque GM, Azcarate AV, Beola JD, Gonzalez MH Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy 2009;25:1206–13 [DOI] [PubMed] [Google Scholar]
- 28.Orrego M, Larrain C, Rosales J, et al. Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy 2008;24:1373–80 [DOI] [PubMed] [Google Scholar]
- 29.de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA 2010;303:144–9 [DOI] [PubMed] [Google Scholar]
- 30.de Jonge S, de Vos RJ, Weir A, et al. One-year Follow-up of Platelet-Rich Plasma Treatment in Chronic Achilles Tendinopathy: A Double-Blind Randomized Placebo-Controlled Trial. Am J Sports Med 2011 [DOI] [PubMed] [Google Scholar]
- 31.Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med 2007;35:245–51 [DOI] [PubMed] [Google Scholar]
- 32.Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med 2011;39:38–47 [DOI] [PubMed] [Google Scholar]
- 33.Gosens T, Peerbooms JC, van Laar W, den Oudsten BL Ongoing Positive Effect of Platelet-Rich Plasma Versus Corticosteroid Injection in Lateral Epicondylitis: A Double-Blind Randomized Controlled Trial With 2-year Follow-up. Am J Sports Med 2011;39:1200–8 [DOI] [PubMed] [Google Scholar]
- 34.Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop 2010;34:909–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan YS, Li Y, Foster W, Fu FH, Huard J The use of suramin, an antifibrotic agent, to improve muscle recovery after strain injury. Am J Sports Med 2005;33:43–51 [DOI] [PubMed] [Google Scholar]
- 36.Cugat et al. 2005. The International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine, Florida.
- 37.Sanchez M, Anitua E, Orive G, Mujika I, Andia I Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med 2009;39:345–54 [DOI] [PubMed] [Google Scholar]
- 38.Mishra A, Woodall J Jr., Vieira A Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med 2009;28:113–25 [DOI] [PubMed] [Google Scholar]
- 39.Mankin HJ The response of articular cartilage to mechanical injury. J Bone Joint Surg Am 1982;64:460–6 [PubMed] [Google Scholar]
- 40.Hildner F, Albrecht C, Gabriel C, Redl H, van Griensven M State of the art and future perspectives of articular cartilage regeneration: a focus on adipose-derived stem cells and platelet-derived products. J Tissue Eng Regen Med 2011 [DOI] [PubMed] [Google Scholar]
- 41.Weibrich G, Kleis WK, Hafner G, Hitzler WE Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg 2002;30:97–102 [DOI] [PubMed] [Google Scholar]
- 42.Bendinelli P, Matteucci E, Dogliotti G, et al. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-kappaB inhibition via HGF. J Cell Physiol 2010;225:757–66 [DOI] [PubMed] [Google Scholar]
- 43.Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage 2006;14:1272–80 [DOI] [PubMed] [Google Scholar]
- 44.Spreafico A, Chellini F, Frediani B, et al. Biochemical investigation of the effects of human platelet releasates on human articular chondrocytes. J Cell Biochem 2009;108:1153–65 [DOI] [PubMed] [Google Scholar]
- 45.Kaps C, Loch A, Haisch A, et al. Human platelet supernatant promotes proliferation but not differentiation of articular chondrocytes. Med Biol Eng Comput 2002;40:485–90 [DOI] [PubMed] [Google Scholar]
- 46.Gaissmaier C, Fritz J, Krackhardt T, Flesch I, Aicher WK, Ashammakhi N Effect of human platelet supernatant on proliferation and matrix synthesis of human articular chondrocytes in monolayer and three-dimensional alginate cultures. Biomaterials 2005;26:1953–60 [DOI] [PubMed] [Google Scholar]
- 47.Drengk A, Zapf A, Sturmer EK, Sturmer KM, Frosch KH Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs 2009;189:317–26 [DOI] [PubMed] [Google Scholar]
- 48.Han B, Woodell-May J, Ponticiello M, Yang Z, Nimni M The effect of thrombin activation of platelet-rich plasma on demineralized bone matrix osteoinductivity. J Bone Joint Surg Am 2009;91:1459–70 [DOI] [PubMed] [Google Scholar]
- 49.Doucet C, Ernou I, Zhang Y, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol 2005;205:228–36 [DOI] [PubMed] [Google Scholar]
- 50.Zaky SH, Ottonello A, Strada P, Cancedda R, Mastrogiacomo M Platelet lysate favours in vitro expansion of human bone marrow stromal cells for bone and cartilage engineering. J Tissue Eng Regen Med 2008;2:472–81 [DOI] [PubMed] [Google Scholar]
- 51.Getgood A, Henson F, Brooks R, Fortier LA, Rushton N Platelet-rich plasma activation in combination with biphasic osteochondral scaffolds-conditions for maximal growth factor production. Knee Surg Sports Traumatol Arthrosc 2011 [DOI] [PubMed] [Google Scholar]
- 52.Sampson S, Reed M, Silvers H, Meng M, Mandelbaum B Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study. Am J Phys Med Rehabil 2010;89:961–9 [DOI] [PubMed] [Google Scholar]
- 53.Kon E, Buda R, Filardo G, et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 2010;18:472–9 [DOI] [PubMed] [Google Scholar]
- 54.Filardo G, Kon E, Buda R, et al. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2011;19:528–35 [DOI] [PubMed] [Google Scholar]
- 55.Kon E, Filardo G, Delcogliano M, et al. Platelet autologous growth factors decrease the osteochondral regeneration capability of a collagen-hydroxyapatite scaffold in a sheep model. BMC Musculoskelet Disord 2010;11:p. 220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milano G, Sanna Passino E, Deriu L, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage 2010;18:971–80 [DOI] [PubMed] [Google Scholar]
- 57.Saito M, Takahashi KA, Arai Y, et al. Intraarticular administration of platelet-rich plasma with biodegradable gelatin hydrogel microspheres prevents osteoarthritis progression in the rabbit knee. Clin Exp Rheumatol 2009;27:201–7 [PubMed] [Google Scholar]
- 58.Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop 2010;34:589–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stewart K, Pabbruwe M, Dickinson S, Sims T, Hollander AP, Chaudhuri JB The effect of growth factor treatment on meniscal chondrocyte proliferation and differentiation on polyglycolic acid scaffolds. Tissue Eng 2007;13:271–80 [DOI] [PubMed] [Google Scholar]
- 60.Ishida K, Kuroda R, Miwa M, et al. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng 2007;13:1103–12 [DOI] [PubMed] [Google Scholar]
- 61.Zellner J, Mueller M, Berner A, et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J Biomed Mater Res A 2010;94:1150–61 [DOI] [PubMed] [Google Scholar]
- 62.Arnoczky SP, Warren RF, Spivak JM Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J Bone Joint Surg Am 1988;70:1209–17 [PubMed] [Google Scholar]
- 63.Englund M, Roos EM, Roos HP, Lohmander LS Patient-relevant outcomes fourteen years after meniscectomy: influence of type of meniscal tear and size of resection. Rheumatology (Oxford) 2001;40:631–9 [DOI] [PubMed] [Google Scholar]