Summary
Background and objectives
Expanded-criteria donor (ECD) kidneys are used to expand the number of deceased-donor kidney transplants, often for elderly recipients. This study sought to determine whether older recipients had significantly worse outcomes from receiving ECD kidneys and whether outcomes of ECD versus standard-criteria donor (SCD) kidneys differed in younger recipients.
Design, setting, participants, & measurements
This is a single-center, retrospective review of all primary deceased-donor kidney transplantations performed between 2000 and 2005. Group 1 consisted of patients ≥60 years of age (n=189) who received an ECD (n=96) or an SCD (n=93) kidney. Group 2 consisted of patients 40–59 years of age (n=370) who received an ECD (n=105) or an SCD (n=265) kidney.
Results
Older recipients (group 1) who received ECD kidneys demonstrated significantly shortened 5-year actuarial patient and graft survival rates compared with older recipients of SCD allografts. Group 1 ECD recipients also had significantly worse outcomes than younger (group 2) ECD recipients. In multivariate analysis, ECD kidneys remained an independent predictor of poorer outcome in group 1.
Conclusions
Morbidity and mortality were increased in elderly recipients of ECD kidneys. These findings may have implications in kidney allocation policy developments that encourage placement of ECD kidneys for older recipients.
Introduction
Kidney transplantation improves quality of life and saves lives compared with other alternatives for renal replacement therapy in the uremic patient (1–3). Each year more patients are being listed for transplantation, with only a moderate increase in the number of deceased donors. In an effort to address this excess demand, centers have sought to use expanded-criteria donors (ECDs) who otherwise may not have previously been considered for transplantation (4).
In 2002, the term ECD was defined as deceased donors ≥60 years of age or donors 50–59 years of age with two of the three following characteristics: terminal serum creatinine level >1.5 mg/dl, hypertension, or death from a cerebrovascular event (United Network for Organ Sharing [UNOS] policy 3.5.1). A recent analysis of the UNOS database listed the 5-year and projected 10-year graft survival rates for standard-criteria donor (SCD) kidneys (patients analyzed, 33,118) at 68.8% and 50.9% and for ECD kidneys (patients analyzed, 5943) at 51.8% and 32.9%, respectively (5). Because of this discrepancy in graft survival, UNOS allocation policy has required that patients who enter the waiting list for transplantation consent for consideration of ECD kidneys.
A landmark study examined the mortality of patients in two groups: (1) recipients of ECD kidneys and (2) recipients of SCD kidneys and candidates remaining on dialysis while awaiting SCD kidneys. That study assessed whether a patient would enjoy a survival benefit from an ECD kidney versus waiting on dialysis for an SCD kidney. In organ procurement organizations with short waiting times (<1350 days), there was no survival benefit from ECD transplantation over waiting for an SCD organ (6).
That study did not directly compare differences in morbidity or outcomes for recipients ≥60 versus those 40–59 years of age. We sought to determine whether older recipients had significantly worse outcomes from receiving ECD kidneys rather than SCD kidneys and whether outcomes of ECD versus SCD kidneys differed in younger recipients. We hypothesized that elderly patients may be the population most likely to experience significantly inferior outcomes from transplantation with ECD rather than SCD kidney allografts.
Materials and Methods
This is a single-center, retrospective study of all deceased-donor ECD and SCD kidney transplantations performed between 2000 and 2005. Recipients were ≥40 years of age because few ECD transplantations are performed in younger patients nationwide. The resulting study population of 559 patients consisted of 201 recipients of ECD kidneys and 358 recipients of SCD kidneys. Recipients were divided into two cohorts according to age: Group 1 consisted of patients ≥60 years of age, and group 2 consisted of patients 40–59 years of age. All recipients were assessed for patient survival, graft survival, acute rejection, need for acute dialysis, and complications. We also directly compared recipients of ECD kidneys 40–59 years of age (from group 2) with those ≥60 years of age (from group 1).
Immunosuppression was typically triple-drug maintenance therapy (steroids, mycophenolate mofetil, and cyclosporine or tacrolimus), and a majority of patients received induction with basiliximab, rabbit antithymocyte globulin, or alemtuzumab. Although choice of induction was strongly related to era of the transplant, in general antithymocyte globulin was given to patients deemed to be at higher immunologic risk. An effort was made to use machine perfusion for all deceased-donor kidneys. All ECD and donation-after-cardiac-death (DCD) kidneys underwent biopsy before implantation. No dual transplants are included in this study.
Differences between groups were tested with a Wilcoxon rank-sum test, a Kruskal-Wallis test (continuous variables), or a Fisher exact test (categorical variables). The first test was used if two groups were being compared, and the second test if there were three or more groups. For time-to-event data, Kaplan-Meier actuarial estimates were used to estimate survival, and differences in survival between groups were tested with the log-rank test. All P values were two tailed, and P<0.05 was used as the criterion for statistical significance. All computations and figures were done in R for Windows, version 2.5.1 patched (2007–07–10 r42164) (R Development Core Team, 2005).
For multivariate analysis, multivariate Cox proportional hazards models were fitted to both graft and patient survival in the two age groups (≥60 and the 40–59 years) as follows: First, univariate Kaplan-Meier (for categorical data) and Cox (for continuous data) models were fitted to the set of risk factors. If P<0.15, then that risk factor was included in a multivariate Cox model. These models were estimated with Proc TPHREG in SAS software, version 9.1.3 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
Demographic information and baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics
| Characteristic | Group 1: Recipients Age ≥60 yr | Group 2: Recipients Age 40–59 yr | ||
|---|---|---|---|---|
| SCD | ECD | SCD | ECD | |
| Patient age (yr) | 66 | 67 | 50a | 53a |
| Donor age (yr) | 41b | 64b | 37a | 61a |
| Waiting time (yr) | 1.70b | 1.72b | 1.70a | 1.95a |
| Preemptive transplantc | 9 | 8 | 16 | 12 |
| Diabetes | 29 | 33 | 31 | 30 |
| Hypertension | 96 | 97 | 98 | 96 |
| Coronary artery disease | 42 | 43 | 28 | 21 |
| Cerebrovascular accident | 1 | 0 | 0 | 0 |
| Preservation time (h) | 21.31 | 20.86 | 21.36 | 21.92 |
| Female recipients | 39 | 45 | 38 | 45 |
| Female donors | 39 | 51 | 37 | 53a |
| Induction | ||||
| alemtuzumab | 48 | 51 | 51 | 50 |
| basiliximab | 46 | 45 | 43 | 37 |
| antithymocyte globulin | 6 | 4 | 7 | 12 |
| sirolimus | 8b | 19b | 12 | 20 |
Values are percentage of patients unless otherwise noted. In the induction category, 10% of the patients in group 2 did not have data. Also in group 2, there was a significant difference in induction between the 3 groups when compared as a whole by Fisher exact test. SCD, standard-criteria donor; ECD, extended-criteria donor.
Differences between group 2 values were significant (P<0.05).
Differences between group 1 values were significant (P<0.05).
Patients underwent transplantation before dialysis.
Patient Survival
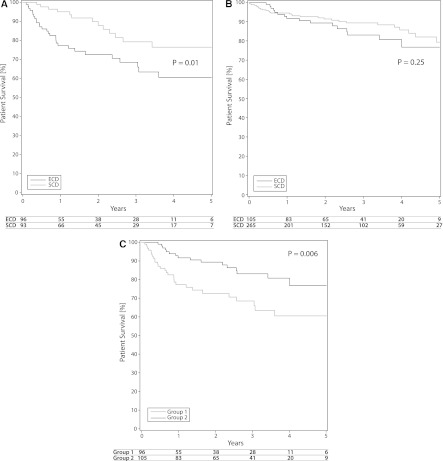
In older recipients (group 1), the 1-, 3-, and 5-year patient survival rates were 95%, 79%, and 76%, respectively, for SCD recipients (n=93) and 77%, 69%, and 61% for ECD recipients (n=96). The survival curves are shown in Figure 1A, and the P values indicate statistical significance (Figure 1A).
Figure 1.
Five-year patient survival in groups 1 and 2. (A) Group 1 (age ≥60 years) recipients of an expanded-criteria donor (ECD) or standard-criteria donor (SCD) kidney. (B) Group 2 (age 40–59 years) recipients of an ECD or SCD kidney. (C) Group 1 versus group 2 recipients of ECD kidneys.
In the younger recipients (group 2), survival curves and outcomes were less distinct. The younger recipients of SCD kidneys (n=265) had 95%, 89%, and 79% patient survival at 1, 3, and 5 years compared with 92%, 83%, and 77%, respectively, in younger recipients of ECD kidneys (n=105). The survival curves for SCD and ECD recipients were not statistically different (Figure 1B).
When the survival of ECD recipients was compared between groups according to age, the 5-year survival rates were 77% for patients age 40–59 years and 61% for those age ≥60 years. The survival curves showed a statistically significant difference (Figure 1C).
Graft Survival
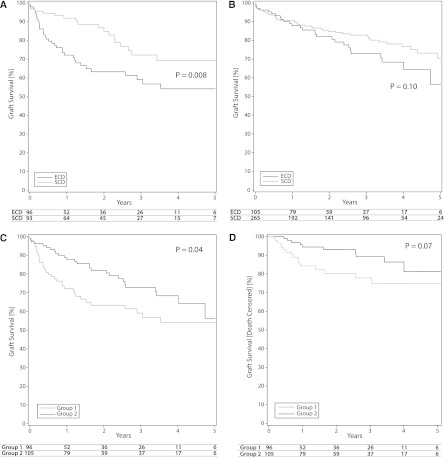
Older recipients (group 1) of SCD kidneys had 1-, 3-, and 5-year graft survival rates of 92%, 72%, and 69%, respectively. Rates in recipients of ECD kidneys were 72%, 59%, and 54%, respectively. The graft survival curves for this group are shown in Figure 2A. These differences in graft survival were statistically significant, as represented by the P values in Figure 2.
Figure 2.
Five-year graft survival in groups 1 and 2. (A) Group 1 (age ≥60 years) recipients of an expanded-criteria donor (ECD) or standard-criteria donor (SCD) kidney. (B) Group 2 (age 40–59 years) recipients of an ECD or SCD kidney. (C) Group 1 versus group 2 recipients of ECD kidneys (age ≥60 years versus <60 years). (D) Group 1 and group 2 recipients of ECD kidneys (death-censored graft survival).
In younger recipients (group 2) who received SCD kidneys, the 1-, 3-, and 5-year graft survival rates were 91%, 83%, and 70%, respectively. In the ECD group, these rates were 88%, 73%, and 56%. There was no statistically significant difference in graft survival between the SCD and ECD recipients (Figure 2B).
When recipients of ECD kidneys were compared between groups, those ≥60 years age had 1-, 3-, and 5-year survival rates of 72%, 59%, and 54%, respectively. These rates were significantly lower than those in younger recipients (group 2)—88%, 73%, and 56% (Figure 2C). Although the preceding data show that patient and graft survival of ECD kidneys are worse in older recipients, it is well known that death with a functioning graft is common in the older age group. To further investigate whether ECD kidneys performed worse in older recipients than younger recipients, even when this cause of graft loss was not considered, we analyzed death-censored graft survival in group 1 versus group 2. As can be seen in Figure 2D, even with censoring for death, the 1-, 3-, and 5-year survival rates were 84%, 78%, and 74% in group 1 and 94%, 89%, and 81% in group 2. The association was marginally significant (P=0.07).
Delayed Graft Function
Rates of delayed graft function (DGF), defined in our analysis as need for dialysis in the first 7 days after transplantation, were as follows: group 1, 23.7% with SCD kidneys and 34.4% with ECD kidneys (P=0.11); group 2, 22.3% in SCD recipients and 33.3% in ECD recipients (P=0.03). DGF rates for ECD kidneys did not differ between the two recipient groups.
Although DGF rates were similarly high in all recipients of ECD kidneys, patient and graft survival were dramatically different depending on the age of recipients and the type of kidney received. Group 1 patients receiving ECD kidneys who developed DGF had 1- and 3-year patient survival rates of 69% and 56%, respectively. Their graft survival rates at 1 and 3 years were 63% and 45%. When DGF was not seen, patient survival at 1 and 3 years was 82% and 76% (P=0.04 compared with DGF), and graft survival was 77% and 67% (P=0.09). For group 2 patients receiving ECD kidneys with DGF, 1- and 3-year patient survival rates of 90% and 73% and graft survival rates of 85% and 60%. Patients in this group without DGF had patient survival rates of 92% and 88% at 1 and 3 years (P=0.06 compared with DGF) and graft survival rates of 89% and 79%, respectively (P=0.06).
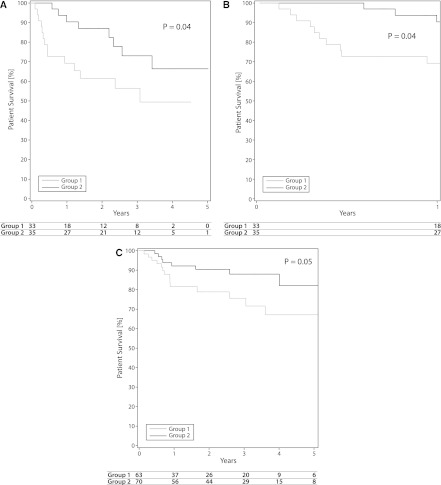
The difference in survival between ECD recipients with DGF is shown in Figure 3A. The survival for group 1 patients was significantly worse, with a P value of 0.04 when analyzed over a 5-year period. Figure 3B highlights that the majority of this difference was seen in the first year, where group 1 recipients experienced a very high death rate in the setting of DGF. After the first year, the two curves appear to have a similar slope of decline. Figure 3C compares patient survival between group 1 and group 2 recipients, with ECD donors censored for DGF. Although patient survival was significantly better for group 1 patients without DGF than those with DGF, as mentioned earlier, patient survival was still significantly worse compared with group 2 recipients. When group 1 patients who received ECD kidneys with DGF were compared with group 1 patients who received SCD kidneys with DGF, the long-term effects on patient survival were the same, although the 1-year survival rate was different. ECD donors with DGF had 1- and 3-year survival rates of 69% and 56%, compared with 89% and 63% for SCD donors with DGF (P=0.26).
Figure 3.
Patient survival with expanded-criteria donor (ECD) kidneys with delayed graft function (DGF). (A) Five-year patient survival in group 1 and group 2 recipients of ECD kidneys with DGF. (B) One-year patient survival in group 1 and group 2 recipients of ECD kidneys with DGF. (C) Five-year patient survival in group 1 and group 2 recipients of ECD kidneys without DGF (survival censored for DGF).
Complications
Perioperative complications were examined (Table 2). In group 1, there were significantly more acute rejection episodes, cases of pneumonia, readmissions, and opportunistic infections among recipients of ECD kidneys than among recipients of SCD kidneys. Additionally, ureteral complications were also significantly increased in the ECD recipients in this group (10%) compared with the SCD (3%) recipients.
Table 2.
Complications after transplantation
| Complication | Group 1 (Age ≥60 yr) | Group 2 (Age 40–59 yr) | Patients Age <60 yr | Patients Age ≥60 yr | ||
|---|---|---|---|---|---|---|
| SCD | ECD | SCD | ECD | |||
| Acute rejection | 17a | 35a | 27a | 43a | 43 | 35 |
| Myocardial infarction | 9 | 15 | 5 | 9 | 9 | 15 |
| Pulmonary embolism | 1 | 3 | 1 | 2 | 2 | 3 |
| Lymphocele | 4 | 4 | 5 | 7 | 7 | 4 |
| Ureteral injury | 3a | 10a | 6 | 4 | 4a | 10a |
| Vascular injury | 10 | 12 | 8 | 12 | 12 | 12 |
| Wound | 8 | 12 | 13 | 10 | 10 | 12 |
| Abscess | 1 | 2 | 1 | 2 | 2 | 2 |
| Hematoma | 5 | 10 | 5 | 7 | 7 | 10 |
| Pneumonia | 22a | 35a | 14 | 21 | 21a | 35a |
| Readmission | 66a | 80a | 62 | 72 | 72a | 80a |
| Return to operating roomb | 17 | 25 | 19 | 13 | 13a | 25a |
| Nonopportunistic infection | 65 | 74 | 50 | 61 | 61a | 74a |
| Opportunistic infection | 27a | 60a | 31a | 52a | 52 | 60 |
Data expressed as percentage of patients experiencing complication. SCD, standard-criteria donor; ECD, expanded-criteria donor.
Statistically significant versus other members of group (P<0.05).
Within 90 days of transplantation.
Group 2 showed less disparity between subgroups. Only acute rejection episodes and opportunistic infections were increased in ECD over SCD recipients.
Recipients of ECD kidneys who were ≥60 years of age were more likely to have ureteral strictures, develop pneumonia, be readmitted to the hospital, return to the operating room, and suffer infection than recipients 40–59 years of age.
Multivariate Analysis
To assess independent predictors of outcome in this retrospective study, initially a univariate model was fitted for the following risk factors: donor group (SCD versus ECD), DCD, African-American recipients, class IA matching, class IB matching, class II DR matching, DGF, recipient diabetes, induction (basiliximab versus antithymocyte globulin versus alemtuzumab), body mass index > 30 kg/m2, cold ischemic time, and panel of reactive antibodies. As described in the Materials and Methods section, all factors with P<0.15 were then included in a multivariate Cox model. The statistically significant results of this analysis are included in Table 3. The independent predictors for outcome in group 1 included ECD donor, DGF, and recipient diabetes. For group 2, ECD kidneys were not an independent predictor for graft or patient survival, although DGF remained significant. Additionally, induction with basiliximab and alemtuzumab seemed to effect better graft and patient survival in group 2 than antithymocyte globulin induction. This could be a consequence of selection bias because antithymocyte globulin was chosen for high-risk patients and retransplants during this time period. No other factor analyzed was significant in multivariate analysis.
Table 3.
Multivariate analysis of graft and patient survival
| Variable | ECD HRa (P Value) (95% CI) | DGF HRb (P Value) (95% CI) | Recipientc DM HR (P Value) (95% CI) | Induction HRd (P Value) (95% CI) |
|---|---|---|---|---|
| Group 1: graft survival | 1.90 (0.03) | 2.17 (0.01) | 1.48 (0.17) | Univariate: antithymocyte globulin (0.85) |
| (1.07–3.38) | (1.24–3.77) | (0.85–2.58) | Alemtuzumab (0.52) | |
| Group 1: patient survival | 1.97 (0.05) | 1.96 (0.04) | 2.05 (0.03) | Univariate: antithymocyte globulin (0.98) |
| (0.99–3.90) | (1.02–3.76) | (1.08–3.89) | Alemtuzumab (0.50) | |
| Group 2: graft survival | 1.23 (0.41) | 1.93 (0.01) | 1.27 (0.35) | Antithymocyte globulin 2.42 (0.01) |
| (0.75–2.01) | (1.18–3.17) | (0.77–2.08) | (1.22–4.78) | |
| Group 2: patient survival | Univariate (0.25) | 2.49 (0.004) | 1.51 (0.19) | Antithymocyte globulin 3.18 (0.001) |
| (1.34–4.60) | (0.81–2.80) | (1.59–6.37) |
Univariate P values indicate that the differences were nonsignificant and were not included in the multivariate analysis. ECD, expanded-criteria donor; HR, hazard ratio; CI, confidence interval; DGF, delayed graft function; DM, diabetes mellitus.
Compared with standard-criteria donor.
Compared with no DGF.
Compared with no DM.
Compared with basiliximab.
Paired Analysis of ECD Recipients
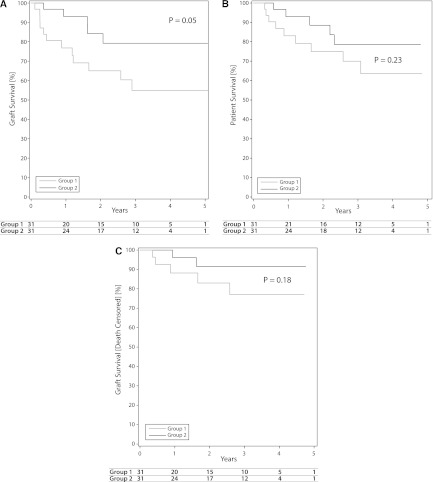
To further highlight differences between outcomes of similar kidneys in recipients of different ages, we examined our cohort for ECD donors wherein one kidney was used in a recipient from group 1 and the other in a recipient from group 2. We identified 31 donors that met these criteria, with 62 kidneys transplanted. When we looked at graft survival, group 1 recipients showed 1- and 3-year survival rates of 77% and 55%, whereas group 2 recipients revealed survival rates of 93% and 79% (P=0.05) (Figure 4A). Patient survival in this small group was less disparate; survival rates at 1 and 3 years were 83% and 70% in group 1 and 93% and 79% in group 2 (P=0.23) (Figure 4B).
Figure 4.
Paired analysis of recipients of expanded-criteria donor kidneys. For each panel, 31 donors yielded 62 kidneys where 1 was used for group 1 and 1 for group 2. (A) Comparison of graft survival. (B) Comparison of patient survival. (C) Comparison of death-censored graft survival.
Discussion
Our data demonstrate that patients ≥60 years who received ECD kidneys had significantly worse patient survival and graft survival, higher rates of acute rejection, and more complications in the perioperative period than similarly aged recipients receiving SCD kidneys. Further, upon comparing younger (age 40–59 years) ECD recipients with those ≥60 years of age, patient and graft survival rates and perioperative complications were significantly higher in the older age group. In a smaller analysis, ECD kidneys from the same donors exhibited longer graft survival when transplanted into younger recipients.
ECD donor remained an independent predictor of graft and patient survival in recipients ≥60 years according to multivariate analysis, but not in younger ECD recipients. DGF also remained an independent predictor of poorer outcome in all recipients. It is clear from the data that DGF is a serious complication for all groups, but the striking and rapid effect this had on 1-year survival of group 1 recipients of ECD kidneys is worth reiterating. Patients in this group who received an ECD kidney with DGF had a 31% chance of dying by 1 year.
The preceding data illustrate worse outcomes than those reported in some previous analyses from both single-center and registry studies (7,8). We believe this relates to an era when our center was using a high percentage of ECD kidneys, without being selective with donors, recipients, or donor-recipient matching. We have since instituted criteria for selection of donors as candidates for single or dual transplantation based on biopsy characteristics (9); reduced immunosuppression in elderly recipients of ECD kidneys; and, perhaps most important, focused on avoiding DGF in elderly recipients of these kidneys. This has included shortening cold ischemic time; minimizing warm ischemic time; using machine perfusion in all ECD kidneys; minimizing recipient obesity, diabetes, and high plasma renin activity; placing small kidneys into large recipients; and avoiding the combination of ECD and DCD, all factors known to increase rates of DGF (10). We have also strongly encouraged living-donor kidneys for elderly recipients. By applying these changes, we have improved recent outcomes in elderly recipients of ECD kidneys, a finding consistent with data from other centers (11). At the same time, however, our discard rate and use of dual kidneys has increased, hence decreasing the number of recipients receiving transplants.
This study had some limitations. It is a retrospective, nonrandomized study from a single center, and although large for one center’s experience, it does not have the numbers and regional variability of a multicenter study. Thus, it may reflect biases present at this single transplant center and in our patients. Nevertheless, it has allowed us to better understand how best to use the kidneys available to our patients.
This topic has become particularly relevant as consideration for changes to the current allocation system of deceased donor kidneys has come to the forefront of Organ Procurement and Transplantation Network policies. In response to the aging population of patients with ESRD nationally, the proportion of older patients on the transplant waiting list has increased, leading to more kidneys being allocated to elderly recipients. Because of this, many “ideal” kidneys are going to older recipients, and for that reason, “death with a functioning graft” has become a more common cause of graft loss (12). The Organ Procurement and Transplantation Network issued proposed changes to the system for public comment in 2008, with a central goal for kidney allocation to maximize estimated life-years from transplant, directing higher-quality kidneys to healthier recipients to maximize life-years from transplant (www.optn.org). This proposal was met with mixed reviews (13,14) and was ultimately rejected. In 2011, an updated policy was released, proposing that the top 20% of deceased donor kidneys would be allocated to candidates with the highest estimated post-transplant survival, and the remaining 80% of kidneys would be allocated to recipients with the highest priority on the list with an age within 15 years of the donor. The goal remains to maximize life-years from transplant, but with a more incremental change to the current system than previously proposed (15).
We are concerned that the combination of “more marginal” kidneys into “more marginal” patients will also maximize complications and bad outcomes in elderly recipients, to an extent that is not fully captured by current data and analysis. Some of the concepts in the proposal are reasonable, as it may not be appropriate to allocate very young donor kidneys to older donors purely on the basis of time on the list (16). One of the stated aims, however, is to decrease discard rates, and it is not clear this will be accomplished in the new system. When older kidneys are used with elderly recipients, it will be crucial to evaluate the donors carefully, consider dual transplantation, and have a low threshold for alternate patient selection or discard if DGF is likely, which may not be supported in the proposed system. Additionally, it is not clear how informed consent will be managed, given our knowledge of inferior outcomes with older donors. Older recipients will need to understand that they may experience increased complications or worse outcomes unless they can identify a living donor, without any access for younger deceased donors.
This study sheds light on the importance of appropriately matching organs with recipients, particularly for ECD organs. This can be difficult in regions where multiple transplant centers are represented by a single organ procurement organization. Modifying allocation rules for ECD kidneys should be considered in an effort to match the appropriate kidney to the appropriate recipient, and still minimize the amount of transportation time for the kidney. Perhaps combining some of the proposed allocation changes with more flexibility in matching recipients for an accepted ECD kidney, as opposed to automatically attaching it to the first patient on the list on the basis of time, would be allow for more donor-recipient matching at the local level.
Disclosures
None.
Acknowledgments
The project described was supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources Grant 1UL1RR025011 and now by National Center for Advancing Translational Sciences Grant 9U54TR000021. J.D.M. and D.P.F. are the recipients of this CTSA award and are also supported by ICTR at the University of Wisconsin. J.D.M. is a John Merrill Scholar sponsored by the American Society of Nephrology.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Sureshkumar KK, Patel BM, Markatos A, Nghiem DD, Marcus RJ: Quality of life after organ transplantation in type 1 diabetics with end-stage renal disease. Clin Transplant 20: 19–25, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Ogutmen B, Yildirim A, Sever MS, Bozfakioglu S, Ataman R, Erek E, Cetin O, Emel A: Health-related quality of life after kidney transplantation in comparison intermittent hemodialysis, peritoneal dialysis, and normal controls. Transplant Proc 38: 419–421, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Sung RS, Guidinger MK, Lake CD, McBride MA, Greenstein SM, Delmonico FL, Port FK, Merion RM, Leichtman AB: Impact of the expanded criteria donor allocation system on the use of expanded criteria donor kidneys. Transplantation 79: 1257–1261, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Cecka JM: The OPTN/UNOS renal transplant registry. Clin Transpl 1–16, 2004 [PubMed] [Google Scholar]
- 6.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Schold JD, Meier-Kriesche HU: Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol 1: 532–538, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Bentas W, Jones J, Karaoguz A, Tilp U, Probst M, Scheuermann E, Hauser IA, Jonas D, Gossmann J: Renal transplantation in the elderly: Surgical complications and outcome with special emphasis on the Eurotransplant Senior Programme. Nephrol Dial Transplant 23: 2043–2051, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Remuzzi G, Cravedi P, Perna A, Dimitrov BD, Turturro M, Locatelli G, Rigotti P, Baldan N, Beatini M, Valente U, Scalamogna M, Ruggenenti P, Dual Kidney Transplant Group : Long-term outcome of renal transplantation from older donors. N Engl J Med 354: 343–352, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Siedlecki A, Irish W, Brennan DC: Delayed graft function in the kidney transplant. Am J Transplant 11: 2279–2296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stratta RJ, Rohr MS, Sundberg AK, Farney AC, Hartmann EL, Moore PS, Rogers J, Iskandar SS, Gautreaux MD, Kiger DF, Doares W, Anderson TK, Hairston G, Adams PL.Intermediate-term outcomes with expanded criteria deceased donors in kidney transplantation: A spectrum or specter of quality? Ann Surg 243594–6012006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK: Long-term survival in renal transplant recipients with graft function. Kidney Int 57: 307–313, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Freeman RB, Matas AT, Henry M, Segev DL, Kaufman DB, Roberts JP: Moving kidney allocation forward: The ASTS perspective. Am J Transplant 9: 1501–1506, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Hippen BE, Thistlethwaite JR, Jr, Ross LF: Risk, prognosis, and unintended consequences in kidney allocation. N Engl J Med 364: 1285–1287, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Leichtman AB, McCullough KP, Wolfe RA: Improving the allocation system for deceased-donor kidneys. N Engl J Med 364: 1287–1289, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Blumberg JM: A piece of my mind. The perfect match. JAMA 306: 2197–2198, 2011 [DOI] [PubMed] [Google Scholar]