Summary
Background and objectives
This study examined the relationship between health-related quality of life and subsequent mortality among AKI survivors treated with renal replacement therapy.
Design, setting, participants, & measurements
Multivariable Cox regression models were used to assess the associations between Health Utilities Index Mark 3 (HUI3) and ambulation, emotion, cognition, and pain scores at 60 days and all-cause mortality at 1 year in 60-day AKI survivors (n=439 with evaluable HUI3 assessments) from a randomized multicenter study comparing less- with more-intensive renal replacement therapies.
Results
The median 60-day HUI3 index score was 0.32. Patients with evaluable HUI3 data who died between 60 days and 1 year (n=99) were more likely to have lower 60-day median HUI3 scores, higher comorbidity scores, and longer initial hospital stays, and they were more likely to be dialysis-dependent. A 0.1 higher HUI3 index score was associated with a 17% decrease (hazard ratio, 0.83; 95% confidence interval 0.77–0.89) in all-cause mortality after controlling for clinical risk factors. Similar associations were observed for HUI3 ambulation, emotion, cognition, and pain attribute scores.
Conclusions
Health-related quality of life measured by HUI3 is an independent predictor of mortality among survivors of AKI after adjusting for clinical risk variables. Poor ambulation and other health-related quality of life attributes are also associated with increased risk of death. Health-related quality of life may provide clinicians with additional information to help identify patients at high risk of mortality after AKI that required renal replacement therapy.
Introduction
AKI is a common complication of critical illness that develops in approximately 6% of patients admitted to intensive care units (1–3). Several studies have found associations between AKI and increased short- and long-term morbidity and mortality (1–10). Clinical predictors of long-term mortality after AKI can help identify high-risk patients, and they include such established biomedical risk factors as severity of acute illness, dialysis dependence, and number of comorbidities.
The literature has assessed health-related quality of life (HRQoL) in survivors of AKI using various tools, including the SF-36 and the Nottingham Health Profile, which measure psychometric health status, and the EQ-5D and Health Utilities Index Mark 3 (HUI3), which measure preference-based HRQoL (6,7,9,11–14). Although health status measures ask the respondent to measure the frequency and intensity of symptoms or feelings related to physical, psychological, and other types of functioning, preference-based HRQoL (or utilities) reflects the strength of a preference for a particular health state (15). For example, participants in the Veterans Affairs (VA) National Institute of Health Acute Renal Failure Trial Network (ATN) study recovering from AKI expressed a willingness to sacrifice 60% of their remaining life expectancy in current health for perfect health over a shorter time period (14).
Health status or preference-based HRQoL may provide unique information about the impact of AKI and its link to mortality beyond traditional clinical risk factors. Indeed, several studies have found that declines in HRQoL are significantly associated with increased mortality risk in nationally representative samples (16–19) and increased risk of death for patients with end stage kidney disease (20), advanced HIV infection (21), or cirrhosis (22) after controlling for various biomedical parameters. To date, however, no studies have evaluated HRQoL as a predictor of mortality after AKI.
Our objective was to determine whether HRQoL, as measured by the HUI3 index and select HUI3 attributes, was independently associated with mortality in patients who have survived an episode of AKI. We used data from a large randomized trial (the ATN study), which systematically collected preference-based HRQoL and assessed outcomes data for 1 year after the initiation of renal replacement therapy in patients with severe AKI. We hypothesized that lower HUI3 index and attribute scores 60 days after AKI onset would predict higher mortality at 12 months after controlling for clinical risk factors.
Materials and Methods
Study Design and Population
Study participants were enrolled in the ATN study (VA Cooperative Study 530, ClinicalTrials.gov Identifier NCT00076219), a randomized, multicenter, and parallel group trial that compared more- with less-intensive strategies for the management of renal replacement therapy. A detailed description of the study design and primary study outcomes has been previously published (10,23). Briefly, between November of 2003 and July of 2007, 1124 critically ill adults who had AKI, failure of one or more nonrenal organ systems or sepsis, and required renal replacement therapy were randomly assigned to receive either a less- (n=561) or more- intensive (n=563) renal replacement treatment strategy. Informed consent was obtained from patients or surrogates, and the study was approved by the Human Rights Committee at the West Haven VA Cooperative Studies Program Coordinating Center and the institutional review boards at each participating study site.
Data Collection and Measures
Data were collected at baseline, 60 days, and 1 year postrandomization. We selected the HUI3 as the HRQoL instrument to be used in the study, because it is a preference-based instrument that provides preference weights or utilities needed for planned cost-effectiveness analyses. The HUI3 includes 17 questions used to calculate eight attributes, including four prespecified attributes that we hypothesized would most likely be affected by AKI (ambulation, emotion, cognition, and pain). Each attribute contains five or six levels for a total of 972,000 possible health states (24). Preference weights were estimated with valuation data from a sample of Canadian adults and used in a multiplicative model to compute utility values ranging from −0.36 to 1.00.
Of the 533 patients alive at day 60, 299 (56%) patients completed the entire HUI3 assessment, whereas 159 (30%) patients partially completed the assessment; 75 (14%) patients did not complete the assessment. We followed the guidelines set in the work by Naeim et al. (25) and used inspection and logical deduction (n=38) or hot deck imputation (n=78) to impute 116 HUI3 index responses from the partially completed assessments. There were 24 additional assessments for which an HUI3 index score could not be generated but that did capture responses for at least one of four subscales of interest. Overall, our analyses include 439 patients who were alive at day 60 and had evaluable HUI3 index (n=415), ambulation (n=438), emotion (n=439), cognition (n=421), or pain (n=432) scores. Surrogates completed 138 (31%) of the assessments.
Candidate covariates included age, sex, and race/ethnicity as well as patients’ baseline health status using the Charlson Comorbidity Index (CCI) (26), whether a patient was admitted from home or a skilled nursing facility, and the Sequential Organ Failure Assessment score at baseline as a measure of severity of illness (27,28). We also adjusted for several treatment-related characteristics, including treatment group assignment (intensive versus less intensive), primary treating specialty (surgical versus medical), initial hospital length of stay, and whether the patient was dialysis-independent at day 60.
The outcome in this analysis was all-cause mortality, which was defined as death from any cause within 305 days of the first HUI3 assessment at day 60. We ascertained vital status at 1 year by patient/surrogate interview and use of the VA Beneficiary Identification and Records Locator System, the National Center for Health Statistics’ National Death Index database, or the Social Security Administration’s Death Master File.
Statistical Analyses
We tested for differences in characteristics and HRQoL between patients who were alive and patients who were deceased at the 1-year follow-up visit. We used chi-squared tests for categorical variables and independent t tests or Wilcoxon rank sum tests for normally and non-normally distributed continuous variables.
We examined differences in survival as a function of HRQoL by plotting Kaplan–Meier survival curves by tertiles of HUI3 index scores and select HUI3 attribute scores, including ambulation, emotion, cognition, and pain. We analyzed survival differences by using the log-rank test of equality.
We used Cox proportional hazards models (SAS PROC PHREG) to assess whether 60-day HRQoL predicted mortality 1 year after AKI while controlling for possible confounders. We ran collinearity diagnostics and found no collinearity issues among the potential confounders. However, because of moderate to high correlations between the HUI3 index score and the four selected HUI3 attribute scores (ranging from r=0.55 to r=0.84), we developed separate models for each HUI3 predictor. We examined Schoenfeld residuals to evaluate the proportionality assumption, which requires that the effect of any given predictor be constant over time, and we found that all predictors were in compliance with this assumption (29). We also compared the concordance of each model (Harrell’s C index) to assess model performance (30,31). As a sensitivity analysis, we reran each Cox proportional hazards model using only data obtained directly from patients (excluding surrogate responses) or original nonimputed data. We report relative hazards and 95% confidence intervals. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC) and STATA version 11.1 (StataCorp, College Station, TX).
Results
Of 1124 AKI patients enrolled at baseline, 533 (47%) patients were alive at day 60 (10). Table 1 summarizes the demographic, health status, 60-day HRQoL, and hospital stay characteristics of the 439 patients with AKI who were alive at day 60 and had evaluable HUI3 data. Overall, almost one-half (49%) of patients were 60 years of age or older, 70% of patients were male, and about three-quarters (76%) of patients were white. Compared with survivors, patients who died between days 60 and 365 (n=99) were more likely to have comorbidity scores of five or greater (24% versus 12%, P=0.005). Deceased patients were also more likely to have lower 60-day median HUI3 HRQoL scores (0.01 versus 0.46, P<0.001) and lower HUI3 subscale scores in several attributes, including ambulation (0.67 versus 0.81, P<0.001). Finally, patients who died had longer mean initial stays in the hospital (42 versus 31 days, P<0.001) and were more likely to remain dialysis-dependent at 60 days (30% versus 18%, P=0.008).
Table 1.
Characteristics of patients alive at day 60 with Health Utilities Index Mark 3 data in the Acute Renal Failure Trial Network study
| All (n=439) | By Mortality Status at 1 Year | P Value | ||
|---|---|---|---|---|
| Alive (n=340) | Deceased (n=99) | |||
| Demographics | ||||
| baseline age in years (%) | ||||
| <60 | 51 | 53 | 44 | 0.14 |
| 60–74 | 36 | 36 | 37 | 0.83 |
| ≥75 | 13 | 11 | 18 | 0.05 |
| male (%) | 70 | 70 | 70 | 0.99 |
| race/ethnicity (%) | ||||
| white | 76 | 75 | 79 | 0.47 |
| black | 15 | 15 | 15 | 0.91 |
| other | 9 | 10 | 6 | 0.23 |
| Baseline health status | ||||
| Charlson Comorbidity Index (%) | ||||
| 0 | 22 | 24 | 16 | 0.08 |
| 1–2 | 36 | 37 | 31 | 0.32 |
| 3–4 | 27 | 27 | 29 | 0.65 |
| ≥5 | 15 | 12 | 24 | 0.005 |
| admitted from home (%) | 91 | 90 | 92 | 0.62 |
| Sequential Organ Failure Assessment score mean (SD) | 12.4 (3.7) | 12.3 (3.7) | 13.1 (3.9) | 0.05 |
| 60-Day health-related quality of life: Health Utilities Index Mark 3 mean (SD) | ||||
| index score median (IQR) | 0.32 (−0.01, 0.78) | 0.46 (0.03, 0.84) | 0.01 (−0.19, 0.26) | <0.001 |
| vision | 0.96 (0.07) | 0.97 (0.06) | 0.93 (0.09) | <0.001 |
| hearing | 0.98 (0.07) | 0.98 (0.06) | 0.97 (0.08) | 0.04 |
| speech | 0.97 (0.08) | 0.98 (0.06) | 0.92 (0.12) | <0.001 |
| ambulation | 0.78 (0.18) | 0.81 (0.18) | 0.67 (0.14) | <0.001 |
| dexterity | 0.93 (0.15) | 0.95 (0.13) | 0.86 (0.20) | <0.001 |
| emotion | 0.92 (0.13) | 0.93 (0.12) | 0.87 (0.14) | <0.001 |
| cognition | 0.90 (0.16) | 0.92 (0.14) | 0.82 (0.20) | <0.001 |
| pain | 0.83 (0.18) | 0.85 (0.18) | 0.79 (0.20) | 0.005 |
| Treatment-related characteristics | ||||
| study treatment group (%) | ||||
| intensive strategy | 48 | 50 | 41 | 0.15 |
| less-intensive strategy | 52 | 50 | 59 | 0.15 |
| primary treating specialty (%) | ||||
| medical | 49 | 49 | 47 | 0.81 |
| surgical | 51 | 51 | 53 | 0.81 |
| mean initial hospital length of stay in days (SD) | 33.7 (17.7) | 31.3 (16.9) | 41.8 (17.8) | <0.001 |
| dialysis-independent at 60 days (%) | 79 | 82 | 70 | 0.008 |
Of 1124 patients enrolled at baseline, 533 (47%) patients were alive at day 60. This table reflects the characteristics of the 439 patients who were alive at day 60 and had evaluable Health Utilities Index Mark 3 (n=415), ambulation (n=438), emotion (n=439), cognition (n=421), or pain (n=432) data. IQR, interquartile range.
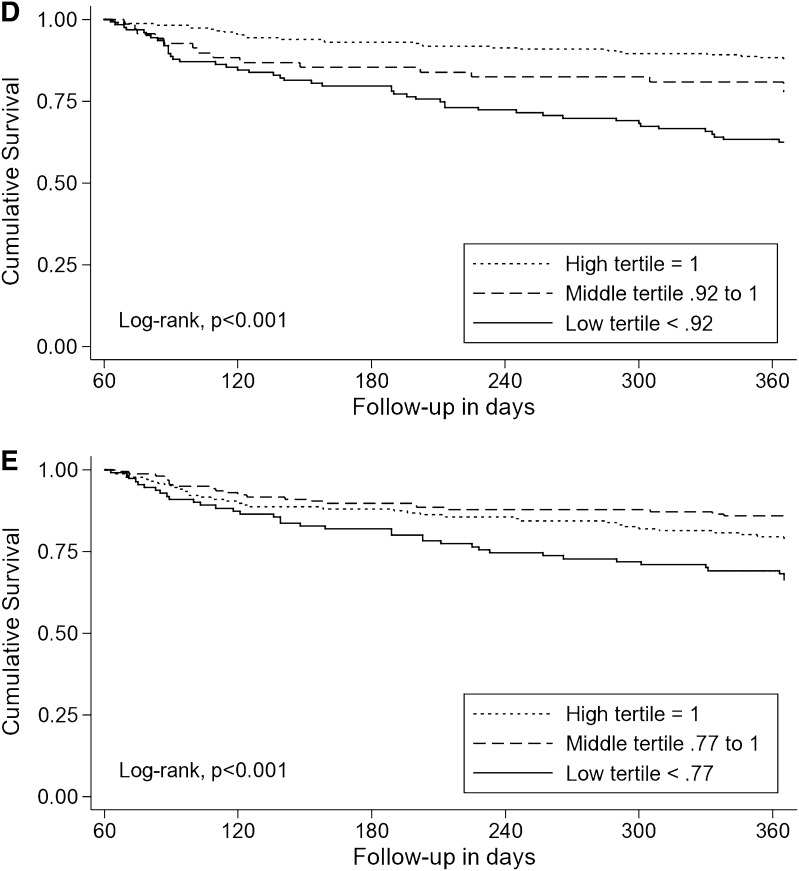
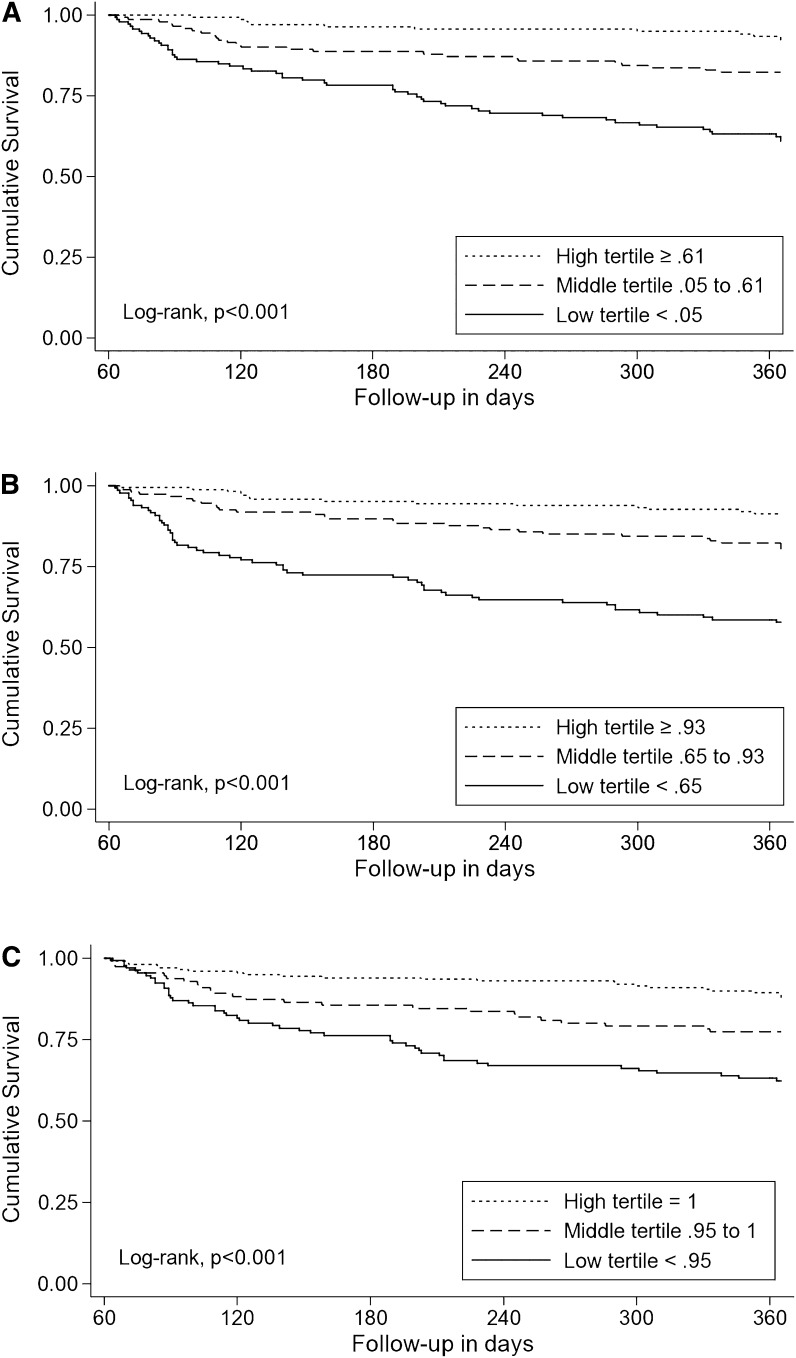
The Kaplan–Meier survival plots show that patients in the high tertile of HRQoL had significantly better survival than those patients in the middle and low tertiles (Figure 1). The 12-month survival rates for those patients in the high, middle, and low tertiles of HUI3 index scores were 92%, 81%, and 61% (log rank chi-squared, P<0.001) (Figure 1A); for those patients in the high, middle, and low tertiles of HUI3 ambulation, the survival rates were 92%, 79%, and 58% (log rank chi-squared, P<0.001) (Figure 1B).
Figure 1.
Kaplan–Meier survival plots showing that patients in the high Health Utilities Index (HUI3) score or subscale score tertile had significantly better survival than those in the middle and low tertiles. (A) Kaplan–Meier plot of overall survival by HUI3 index score. (B) Kaplan–Meier plot of overall survival by HUI3 ambulation subscale score. (C) Kaplan–Meier plot of overall survival by HUI3 emotion subscale score. (D) Kaplan–Meier plot of overall survival by HUI3 cognition subscale score. (E) Kaplan–Meier plot of overall survival by HUI3 pain subscale score.
In multivariable analyses, low HRQoL at day 60, adjusted for traditional clinical variables, was associated with higher mortality at 1 year (Table 2). A 0.1 increase in the 60-day HUI3 index score was associated with a 17% lower risk of death (hazard ratio [HR]=0.83, 95% confidence interval [CI]=0.77–0.89). Older age (≥75 years; HR=2.11, 95% CI=1.15–3.90), very high comorbidity scores (≥5; HR=2.75, 95% CI=1.28–5.93), and longer length of initial hospital stay (HR=1.02, 95% CI=1.00–1.03 per additional day) were also associated with increased hazard of death at 1 year. Although dialysis dependence at day 60 was associated with 1-year mortality in the univariate analysis, the association was not significant in the multivariable analysis (HR=0.73, 95% CI=0.44–1.19). Model performance was good, with the concordance index (C=0.77) meeting the 0.70 minimum for acceptable discrimination from the work by Hosmer and Lemeshow (30,31).
Table 2.
Adjusted proportional hazards models relating Health Utilities Index Mark 3 score to mortality
| HR | 95% CI | |
|---|---|---|
| Health Utilities Index Mark 3 (per 0.1 increment) | 0.83a | 0.77–0.89 |
| Baseline age in years (reference<60 years) | ||
| 60–74 | 1.09 | 0.67–1.77 |
| ≥75 | 2.11b | 1.15–3.90 |
| Male | 0.81 | 0.51–1.30 |
| Race/ethnicity (reference is white) | ||
| black | 0.78 | 0.42–1.46 |
| other | 0.48 | 0.19–1.20 |
| Charlson Comorbidity Index (reference=0) | ||
| 1–2 | 1.67 | 0.81–3.48 |
| 3–4 | 1.75 | 0.83–3.68 |
| ≥5 | 2.75c | 1.28–5.93 |
| Admitted from home | 1.06 | 0.48–2.32 |
| Sequential Organ Failure Assessment score | 1.03 | 0.97–1.10 |
| Study treatment group (reference is less-intensive strategy) | ||
| intensive strategy | 0.74 | 0.48–1.15 |
| Primary treating specialty (reference is surgical) | ||
| medical | 1.08 | 0.69–1.67 |
| Initial hospital length of stay (days) | 1.02b | 1.00–1.03 |
| Dialysis independent at 60 days | 0.73 | 0.44–1.19 |
Harrell’s C=0.77. HR, hazard ratio; CI, confidence interval.
P≤0.001 (two-tailed test).
P≤0.05.
P≤0.01.
Similarly, higher ambulation, emotion, cognition, and pain subscale scores were associated with a survival advantage (Table 3). A 0.1 increase in the 60-day HUI3 ambulation subscale score was associated with a 32% lower hazard of death (HR=0.68, 95% CI=0.57–0.81). Similar to the HUI3 model, older age, greater comorbidity, and longer initial hospital stays were consistently and significantly associated with higher mortality. Harrell’s C concordance indices of all subscale models indicated acceptable model performance, ranging from C=0.72 for the emotion and pain models to C=0.75 for the ambulation model.
Table 3.
Adjusted proportional hazards models relating select Health Utilities Index Mark 3 subscale scores to mortality
| Health Utilities Index Mark 3 (per 0.1 increment in subscale score) | HR | 95% CI |
|---|---|---|
| Ambulation | 0.68a | 0.57–0.81 |
| Emotion | 0.82b | 0.72–0.93 |
| Cognition | 0.82a | 0.74–0.91 |
| Pain | 0.89c | 0.79–1.00 |
Separate Cox models for each subscale were adjusted for the variables listed in Table 2 (excluding Health Utilities Index Mark 3 summary scores). Harrell’s C values by model are C=0.75 for ambulation, C=0.72 for emotion, C=0.74 for cognition, and C=0.72 for pain. HR, hazard ratio; CI, confidence interval.
P≤0.001 (two-tailed test).
P≤0.01.
P≤0.05.
On exclusion of surrogate responses, low HRQoL at day 60 remained significantly associated with higher 1-year mortality (HR=0.89, 95% CI=0.80–0.97, Harrell’s C=0.80). Older age (≥75 years; HR=4.57, 95% CI=1.78–11.73) and longer initial hospital length of stay (HR=1.04, 95% CI=1.02–1.06 per additional day) also remained significant; however, high comorbidity score (CCI≥5) was no longer associated with higher risk of death. Male sex and dialysis independence, however, were found to be associated with a 50% and 58% decreased risk of mortality, respectively (HR=0.50, 95% CI=0.26–0.96 and HR=0.42, 95% CI=0.22–0.79, respectively).
Estimates and levels of significance were similar regardless of whether imputed data were included in the analyses, except that older age (≥75 years) in the ambulation model and HUI3 pain subscale score in the pain model were no longer significant when using the dataset excluding imputed values.
Discussion
In this randomized, multicenter trial of critically ill patients with AKI, there was greater than 20% mortality between day 60 and 1 year. Those patients with poorer preference-based HRQoL 60 days after the onset of AKI had a higher risk of death at 1 year after controlling for established biomedical risk factors. Univariate analyses revealed significantly lower survival rates for patients with poor HRQoL scores, particularly those patients in the low HUI3 tertile. True to our hypothesis, the significant and inverse relationship between HUI3 scores and mortality remained after controlling for age, comorbidity, and length of initial hospital stay among other clinical risk factors. We also found that four prespecified HUI3 health status attributes—ambulation, emotion, cognition, and pain—were independent predictors of survival.
The relationship between HRQoL and mortality among AKI survivors has not been previously studied; however, the finding that HRQoL provides prognostic information above and beyond traditional clinical risk factors has been reported in other populations. The work by Mapes et al. (20) reported that lower Kidney Disease Quality of Life Short Form physical, mental, and kidney disease component scores were strongly associated with a higher risk of death for hemodialysis patients while controlling for possible demographic and biomedical risk factors. The work by Kanwal et al. (22) evaluated patients with cirrhosis and found that higher Short Form Liver Disease Quality of Life scores predicted lower mortality while controlling for Model for End Stage Liver Disease scores, CCI, and other variables. The work by Kaplan et al. (18) examined the association between HRQoL—specifically, the HUI3—and mortality in 12,375 Canadian men and women. They found a significant association between the HUI3 and subsequent mortality, even after controlling for numerous confounders like age, sex, chronic conditions, smoking frequency, and body mass index. However, the study by Plantinga et al. (32) evaluated incident hemodialysis patients and found that HRQoL, as measured using the time trade-off method, was not associated with mortality. The discrepancy between this study’s findings and the findings in the work by Plantinga et al. (32) could have several explanations, such as differences between the incident hemodialysis population in the work by Plantinga et al. (32) and the AKI population in this study or possibly, an indication that the time trade-off method is not as responsive to clinical events as the HUI3 (33).
Measuring health status or preference-based HRQoL may prove useful to clinicians who seek additional methods to identify patients at high risk for mortality after AKI. We found that HUI3 index and attribute scores help to explain the variance in mortality above and beyond traditional risk factors. The presence of comorbidities, for example, has been shown to predict death both at 6 months (34) and in longer-term follow-up between 5 and 88 months (7) in patients receiving renal replacement therapy for AKI in the intensive care unit; however, neither study adjusted for HRQoL. In contrast, this study’s prediction models included both a comorbidity index and an HRQoL instrument among other predictors. The mortality risks associated with lower HUI3 scores were not as great as the risks of age or CCI, but they show the importance of measuring several factors to capture the underlying disease severity and risk not fully explained by clinical factors alone. Furthermore, HRQoL subscale scores may also complement traditional clinical variables in predicting mortality. Ambulation, in particular, seems to draw on different aspects of health not fully assessed by clinical factors alone, a finding that is interesting in light of a recent pooled analysis measuring gait speed and survival in older adults (35). The work by Studenski et al. (35) determined that slower gait speed was associated with higher mortality and suggested that it reflected the “known and unrecognized disturbances in multiple organ systems” needed to walk and survive (35). Additional research is needed to more fully understand the mechanism behind the relationship between HRQoL—and its attributes—and mortality.
Our study has limitations. First, we included only 60-day survivors with HUI3 data. As noted in the work by Johansen et al. (14), those patients without HUI3 index data (n=118) were more likely to be nonwhite, discharged to skilled nursing or assisted living facilities, and dialysis-dependent at 60 days. Moreover, they were more likely to have longer initial hospital stays and longer intensive care unit stays. This finding may have introduced selection bias, because our sample is likely made up of lower-risk patients. There may also have been additional confounding by some unmeasured factor, such as socioeconomic status or baseline (pre-AKI) HRQoL. Also, we did not explore the relationship between changes in HRQoL over time and mortality, which might have the effect of tempering our results; in general, it may limit our ability to draw any conclusions about causality. Finally, a sizeable proportion (31%) of the HRQoL assessments was completed by surrogates, and they have been shown to give lower valuations to health states compared with patient self-response (36,37). We examined this concern by running the Cox regressions both with and without surrogate responses. The predictor of interest, overall HUI3 index score, remained statistically significant, although the HR dropped in magnitude. Other variables, such as dialysis independence, changed significance. We think that it is important to include the proxy responses, because survivors of AKI are a vulnerable and often critically ill patient population, and excluding surrogate responses effectively excludes those patients who are the most ill. Furthermore, we note a review of 23 patient-proxy HRQoL comparison studies in the work by Sneeuw et al. (37) that concluded that the judgments made by surrogates are reasonably accurate.
This study showed that the HUI3 index score, a preference-based measure of HRQoL, is an independent predictor of mortality after AKI. Ambulation, emotion, cognition, and pain attribute scores are also independently associated with mortality. Our results highlight the importance of measuring preference-based HRQoL in addition to traditional biomedical risk factors, and they suggest the usefulness of HRQoL in identifying patients at high risk for death after AKI. Studies are needed to determine whether interventions specifically targeted to improve HRQoL, such as rehabilitation efforts to address deficits in ambulation, improve mortality in these high-risk patients.
Disclosures
None.
Acknowledgments
This work was supported by the Cooperative Studies Program of the US Department of Veterans Affairs Office of Research and Development (Cooperative Studies Program #530) and the US National Institute of Diabetes and Digestive and Kidney Diseases (Interagency Agreement Y1-DK-3508-01). Trial registration is at http://clinicaltrials.gov (NCT00076219).
The Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Cooperative Studies Program, and the National Institute of Diabetes, Digestive and Kidney Diseases reviewed and approved the design and conduct of the trial but had no role in the writing of this report.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F: Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med 26: 915–921, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ, French Study Group on Acute Renal Failure : Acute renal failure in intensive care units—causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. Crit Care Med 24: 192–198, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Korkeila M, Ruokonen E, Takala J: Costs of care, long-term prognosis and quality of life in patients requiring renal replacement therapy during intensive care. Intensive Care Med 26: 1824–1831, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Morgera S, Kraft AK, Siebert G, Luft FC, Neumayer HH: Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis 40: 275–279, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Van Berendoncks AM, Elseviers MM, Lins RL, SHARF Study Group : Outcome of acute kidney injury with different treatment options: Long-term follow-up. Clin J Am Soc Nephrol 5: 1755–1762, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamel MB, Phillips RS, Davis RB, Desbiens N, Connors AF, Jr, Teno JM, Wenger N, Lynn J, Wu AW, Fulkerson W, Tsevat J, SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments : Outcomes and cost-effectiveness of initiating dialysis and continuing aggressive care in seriously ill hospitalized adults. Ann Intern Med 127: 195–202, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P, VA/NIH Acute Renal Failure Trial Network : Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maynard SE, Whittle J, Chelluri L, Arnold R: Quality of life and dialysis decisions in critically ill patients with acute renal failure. Intensive Care Med 29: 1589–1593, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Gopal I, Bhonagiri S, Ronco C, Bellomo R: Out of hospital outcome and quality of life in survivors of combined acute multiple organ and renal failure treated with continuous venovenous hemofiltration/hemodiafiltration. Intensive Care Med 23: 766–772, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Ahlström A, Tallgren M, Peltonen S, Räsänen P, Pettilä V: Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med 31: 1222–1228, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Johansen KL, Smith MW, Unruh ML, Siroka AM, O’Connor TZ, Palevsky PM, VA/NIH Acute Renal Failure Trial Network : Predictors of health utility among 60-day survivors of acute kidney injury in the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol 5: 1366–1372, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Revicki DA: Relationship between health utility and psychometric health status measures. Med Care 30[Suppl]: MS274–MS282, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Idler EL, Kasl S: Health perceptions and survival: Do global evaluations of health status really predict mortality? J Gerontol 46: S55–S65, 1991 [DOI] [PubMed] [Google Scholar]
- 17.Gold MR, Franks P, Erickson P: Assessing the health of the nation. The predictive validity of a preference-based measure and self-rated health. Med Care 34: 163–177, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Kaplan MS, Berthelot JM, Feeny D, McFarland BH, Khan S, Orpana H: The predictive validity of health-related quality of life measures: mortality in a longitudinal population-based study. Qual Life Res 16: 1539–1546, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Jerant A, Tancredi DJ, Franks P: Mortality prediction by quality-adjusted life year compatible health measures: Findings in a nationally representative US sample. Med Care 49: 443–450, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, Fukuhara S, Young EW, Kurokawa K, Saito A, Bommer J, Wolfe RA, Held PJ, Port FK: Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 64: 339–349, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Jacobson DL, Wu AW, Feinberg J, Outcomes Committee of the Adult AIDS Clinical Trials Group : Health-related quality of life predicts survival, cytomegalovirus disease, and study retention in clinical trial participants with advanced HIV disease. J Clin Epidemiol 56: 874–879, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Kanwal F, Gralnek IM, Hays RD, Zeringue A, Durazo F, Han SB, Saab S, Bolus R, Spiegel BM: Health-related quality of life predicts mortality in patients with advanced chronic liver disease. Clin Gastroenterol Hepatol 7: 793–799, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Palevsky PM, O’Connor T, Zhang JH, Star RA, Smith MW: Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: Intensive versus conventional renal support in acute renal failure. Clin Trials 2: 423–435, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feeny D, Furlong W, Torrance GW, Goldsmith CH, Zhu Z, DePauw S, Denton M, Boyle M: Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care 40: 113–128, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Naeim A, Keeler EB, Mangione CM: Options for handling missing data in the Health Utilities Index Mark 3. Med Decis Making 25: 186–198, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S: Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26: 1793–1800, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Singer JD, Willet JB: Applied Longitudinal Data Analysis, New York, Oxford University Press, 2003 [Google Scholar]
- 30.Harrell FE, Jr: Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis, New York, Springer-Verlag, 2001 [Google Scholar]
- 31.Hosmer DW, Lemeshow S: Applied Logistic Regression, New York, John Wiley & Sons, 2000 [Google Scholar]
- 32.Plantinga LC, Fink NE, Bass EB, Boulware LE, Meyer KB, Powe NR: Preferences for current health and their association with outcomes in patients with kidney disease. Med Care 45: 230–237, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Joyce VR, Barnett PG, Chow A, Bayoumi AM, Griffin SC, Sun H, Holodniy M, Brown ST, Kyriakides TC, Cameron DW, Youle M, Sculpher M, Anis AH, Owens DK: Effect of treatment interruption and intensification of antiretroviral therapy on health-related quality of life in patients with advanced HIV: A randomized, controlled trial. Med Decis Making 32: 70–82, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Delannoy B, Floccard B, Thiolliere F, Kaaki M, Badet M, Rosselli S, Ber CE, Saez A, Flandreau G, Guérin C: Six-month outcome in acute kidney injury requiring renal replacement therapy in the ICU: A multicentre prospective study. Intensive Care Med 35: 1907–1915, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J: Gait speed and survival in older adults. JAMA 305: 50–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Wit GA, Busschbach JJ, De Charro FT: Sensitivity and perspective in the valuation of health status: Whose values count? Health Econ 9: 109–126, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Sneeuw KC, Sprangers MA, Aaronson NK: The role of health care providers and significant others in evaluating the quality of life of patients with chronic disease. J Clin Epidemiol 55: 1130–1143, 2002 [DOI] [PubMed] [Google Scholar]