Summary
Background and objectives
In autosomal dominant polycystic kidney disease, progressive renal enlargement secondary to expanding cysts is a hallmark. The total cyst load and range of cyst diameters are unknown. The purpose of this study was to quantify the total number and range of diameters of individual cysts in adults with preserved GFR.
Design, setting, participants, & measurements
A retrospective, morphometric analysis of renal cyst number and diameter using magnetic resonance images from eight adult autosomal dominant polycystic kidney disease patients was performed at baseline and after 6.9 years. Cyst number and diameter were measured in microscopic sections of nephrectomy specimens from five different adults.
Results
The diameters of 1010 cysts ranged from 0.9 to 77.1 mm in baseline T2 magnetic resonance images, and the mean total number of cysts increased from 682 to 1002 in 6.9 years. However, magnetic resonance imaging detects only cysts above the lower limit of detection. In 405 cysts measured in nephrectomy specimens, 70% had diameters <0.9 mm. Cyst counts by magnetic resonance in eight subjects compared with histology revealed approximately 62 times more cysts below the limit of magnetic resonance imaging detection than above it.
Conclusions
This study presents quantitative data indicating that renal cysts develop in a minority of renal tubules. Increased numbers detected by magnetic resonance imaging are caused primarily by cysts below detection at baseline enlarging to a detectable diameter over time. The broad range of diameters, with a heavy concentration of microscopic cysts, may be most appropriately explained by a formation process that operates continuously throughout life.
Introduction
In autosomal dominant polycystic kidney disease (ADPKD), total kidney volume seems to predict the risk of developing renal insufficiency (1). GFR is maintained nearly normal for several decades owing to compensatory glomerular hyperfiltration and tubule hypertrophy (2). Cysts disrupt the normal parenchyma and focally impair function by directly disturbing the adjacent microvasculature and displacing adjacent tubules and glomeruli in the cortex and by blocking the draining of large numbers of upstream nephrons when they form in or obstruct the flow of urine in medullary collecting ducts (3). This focal damage begins very early in the course of the disease in cysts that were either formed in utero or shortly after birth, and it becomes progressively worse as those cysts expand exponentially (4).
It is unknown whether new cysts form in childhood and throughout a patient’s lifespan. Although microscopic sections of noncystic parenchyma reveal cysts as small as glomeruli (5–7), a quantitative analysis of the number and the diameters of cysts in the noncystic parenchyma of children or adults with ADPKD has not been reported. The formation and expansion of new cysts within islands of preserved parenchyma may be especially important in older adults, because the loss of even a relatively few hyperfiltering glomeruli and hypertrophic renal tubules may transform a stable GFR into a precipitous decline (2).
The severity of ADPKD is highly associated with the number of cysts and their growth behavior after they are formed. In recent magnetic resonance (MR) imaging studies, the number of cysts in a single midcoronal slice of the left kidney increased in association with patient age, but this finding only shows that new cysts were possibly detected and did not address the formation of new cysts (8,9). To fully understand how renal cysts may provoke ESRD, we need to know more about how many cysts there are, how large they are, and if they continue to form throughout life.
In the current study, we have determined the total number of cysts that can be detected by state of the art MR imaging analysis and have supplemented these data with direct measurements of cyst number and diameter in histologic sections obtained from human nephrectomy specimens. The results illustrate that our best clinical imaging method overlooks substantial numbers of cysts that fall below the MR resolution limit.
Material and Methods
MR Imaging
T2-weighted MR images (Figure 1) were retrieved from baseline and ∼7-year follow-up visits of eight PKD1 subjects in the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) Institutional Review Board-approved study at Kansas University Medical Center (10). To reflect the broad range of kidney volumes observed in CRISP, we selected from the Kansas cohort four women and four men with total kidney volumes ranging from 401 to 2878 cm3 at baseline (Table 1). These subjects also exhibited relatively broad ranges of age and GFR. Total kidney volume (TKV) from MR images and GFR from iothalamate clearances were measured according to the CRISP study protocol (10,11). To measure the number of renal cysts, the retrieved MR images were displayed in a Digital Imaging and Communication in Medicine reader, and each serial coronal section (30–40) was analyzed with National Institutes of Health ImageJ software. A single analyst (C.J.G.) determined the total number of cysts in each kidney. For cysts <10 mm in diameter, we accepted circular and spheroid cysts with signal intensities that were equal to the intensities of spinal fluid. For cysts >10 mm, we accepted spheroid cysts with signal intensities that were higher than those intensities in liver or spleen. Calyx and renal pelvis were carefully identified and excluded. Septated cysts were counted as one. The mean coefficient of variation of five repeated counts of total cysts in single slices of combined kidneys in each of three subjects was 8.2% (range=6.2–9.7). To record diameter (in millimeters), we measured the mean length of two lines drawn at right angles through the center of the cyst and touching the outer wall of the cyst. The coefficients of variation of 10 repeated measures in four cysts with mean diameters of 3.4, 5.3, 18.0, and 23.5 mm were 26.4%, 12.7%, 4.3%, and 4.5%, respectively. We expect higher relative variability in the measurement of small cysts. To avoid counting large cysts more than one time in contiguous slices, the entire set of slices was assembled to represent the complete kidney volume, and the carryover cysts were identified in each section.
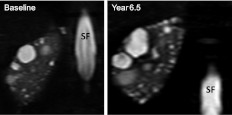
Figure 1.
T2 magnetic resonance image of right kidney in subject 5 at baseline and 6.5 years later. Baseline cysts are enlarged and new cysts are visible in the year 6.5 image. SF, spinal fluid.
Table 1.
Change in total kidney volume, cyst count, and GFR
| ID | Sex | Baseline | Change | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | GFR (ml/min per 1.73 m2) | TKV (cm3) | Cysts (n) | Age (yr) | GFR (ml/min per 1.73 m2) | TKV (cm3) | Cysts (n) | ||
| 1 | Female | 41 | 127 | 802 | 395 | 6.7 | −52 | 1 | 312 |
| 2 | Male | 19 | 95 | 1602 | 631 | 6.7 | −36 | 501 | 317 |
| 3 | Male | 34 | 72 | 2878 | 1064 | 7.5 | −54 | 2297 | 928 |
| 4 | Female | 34 | 113 | 401 | 632 | 7.0 | 17 | 28 | −36 |
| 5 | Female | 17 | 117 | 655 | 543 | 6.5 | −8 | 333 | 271 |
| 6 | Female | 45 | 98 | 1154 | 510 | 7.0 | −22 | 247 | 203 |
| 7 | Male | 36 | 76 | 1339 | 1144 | 6.8 | −8 | 889 | 457 |
| 8 | Male | 23 | 123 | 483 | 533 | 7.1 | −12 | 100 | 111 |
| Mean | 31 | 103 | 1164 | 682 | 6.9 | −22 | 550 | 320 | |
| Median | 34 | 106 | 978 | 587 | 6.9 | −17 | 290 | 292 | |
Histology
Histologic quantitation of cyst number and diameter was performed by S.M., C.J.G., and T.A.F. on renal sections of surgical specimens that had been fixed in formaldehyde and stained with para-aminosalicylic acid or hematoxylin and eosin. The deidentified specimens were obtained by the Institutional Review Board-approved PKD Biomaterials Core Program in the Kidney Institute from patients who had undergone nephrectomy for medical indications, including severe pain and uncontrolled hematuria. Kidney K001 from a local 34-year-old female patient (estimated GFR = 71 ml/min) with a ruptured cerebral aneurysm was donated for transplantation and not used after cysts were discovered. Sections were taken from residual parenchyma in portions of each kidney free of large cysts (K97: male, 40 years old, 42 ml/min; K106: male, 45 years old, 40 ml/min; K215: female, 39 years old, 97 ml/min; K236: male, 47 years old, 101 ml/min). GFR was estimated with the quadratic equation (12), because the weight and height were not routinely reported at outlying nephrectomy sites. The total area of kidney tissue in the slide mount was determined by tracing around the section using National Institutes of Health ImageJ analytic shareware. Although we counted and measured everything that looked like a cyst, we set a lower outer diameter limit of 200 µm (0.2 mm) on cysts to exclude empty glomerular capsules and sectioning artifacts. The measured cysts were lined by epithelium, and their cavities contained clear or proteinaceous liquid. Compound or complex cysts, most likely reflecting sectioning through a convoluted object, were counted as one. Glomeruli had circular or ellipsoidal configurations and contained an organized capillary network. We measured the outer diameters of 100 renal tubules and 769 glomeruli cut in cross-section.
Statistical Analyses
Mean and SD were used to express normally distributed data; range and median were used for highly skewed data. t test was used to determine significant differences in paired comparisons.
Results
MR Image Analysis
The baseline GFR values of the ADPKD patients ranged from 72 to 127 ml/min per 1.73 m2; GFR decreased to a variable extent in seven of eight subjects over the next 6.9 years (Table 1). Combined TKV ranged from 401 to 2878 cm3 at baseline and increased to a variable degree in all patients. The number of cysts in both kidneys combined at baseline ranged from 395 to 1144 (mean=681, median=587). In an average span of 6.9 years, the number of cysts increased in seven of eight patients (13 of 16 kidneys, 0–143 cysts/y), which was equivalent to an annual increase of 6.5%±4.2% (P<0.01).
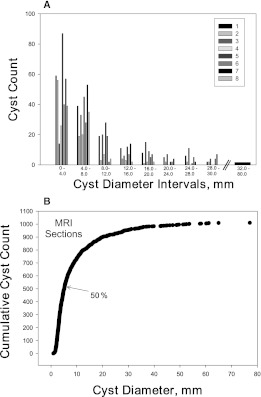
The diameters of 1010 individual cysts within three contiguous midcoronal MR slices of left and right kidneys (six hemisections of right and left kidneys combined) in eight subjects ranged from 0.9 to 77.1 mm (Table 2). Within individual patients, the average combined number of cysts per hemisection (mean of left and right midcoronal sections) ranged from 13.2 to 30.7. Minimum cyst diameters less than 2.0 mm were detected in each subject. The largest cysts ranged from 17.3 to 77.1 mm. The median diameters were much less than the means, reflecting a distribution skewed to the smaller cysts. Each of the kidneys exhibited a similar pattern of cyst distribution (Figure 2A); the majority of the cysts were <5.2 mm in diameter (Figure 2B).
Table 2.
Cyst counts and diameters determined in magnetic resonance and histology sections
| ID | Meana Slice Area (cm2/section) | Meana Count/Section (n) | Cysts/Areab (n/cm2) | Cyst Diameter (mm) | |||
|---|---|---|---|---|---|---|---|
| Mean | Median | Minimum | Maximum | ||||
| Magnetic resonance | |||||||
| 1 | 80 | 14.8 | 0.19 | 7.3 | 3.5 | 1.1 | 58.2 |
| 2 | 124 | 27.2 | 0.22 | 11.4 | 5.9 | 1.5 | 77.1 |
| 3 | 154 | 20.2 | 0.13 | 14.4 | 11.1 | 1.3 | 65.0 |
| 4 | 54 | 13.2 | 0.25 | 5.0 | 3.7 | 1.7 | 18.1 |
| 5 | 65 | 30.7 | 0.47 | 6.2 | 4.4 | 0.9 | 27.8 |
| 6 | 110 | 20.2 | 0.18 | 12.2 | 7.0 | 1.5 | 61.2 |
| 7 | 102 | 28.0 | 0.27 | 8.7 | 5.3 | 1.3 | 49.6 |
| 8 | 51 | 10.3 | 0.20 | 5.0 | 4.2 | 1.7 | 17.3 |
| Median | 91.0 | 20.2 | 0.21 | 8.0 | 4.9 | 1.4 | 53.9 |
| Histology | |||||||
| K001 | 14.8 | 204 | 13.8 | 1.12 | 0.91 | 0.20 | 3.58 |
| K215 | 6.6 | 44 | 6.6 | 0.80 | 0.40 | 0.21 | 3.50 |
| K236 | 0.5 | 23 | 46.0 | 0.46 | 0.39 | 0.25 | 1.14 |
| K97 | 7.4 | 69 | 9.3 | 1.15 | 0.91 | 0.21 | 3.64 |
| K106 | 5.0 | 65 | 13.0 | 0.95 | 0.69 | 0.21 | 3.80 |
| Median | 6.6 | 65.0 | 13.0 | 1.0 | 0.69 | 0.21 | 3.58 |
Mean of six coronal renal slices.
Ratio of means.
Figure 2.
Cyst distribution in MR scan sections. (A) Histogram from magnetic resonance imaging measurements of cyst count and diameter. Pattern of cyst distribution is similar among eight subjects. (B) Prevalence of cysts in midcoronal renal magnetic resonance sections of eight subjects. Arrow indicates that 50% of the diameter measurements were less than 5.2 mm.
Histologic Section Analysis
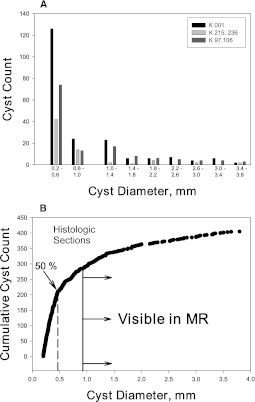
A total of 405 cysts were identified in 25 sections from five different kidneys with diameters ranging from 0.20 to 3.8 mm (Figure 3 and Table 2). The mean and median diameters ranged from 0.80 to 1.15 mm and from 0.39 to 0.91 mm, respectively; 70% (284) of the cysts were less than 0.9 mm in diameter. The skewed distribution of cyst diameters seen in the MR imaging analysis was also observed in the histologic section analysis (Figure 4A), where in 50%, the diameter was less than 0.454 mm (Figure 4B). Because the minimum diameter was set at 200 µm (0.2 mm), we excluded 196 spherical objects with diameters ranging from 50 to 198 µm that could have been cysts.
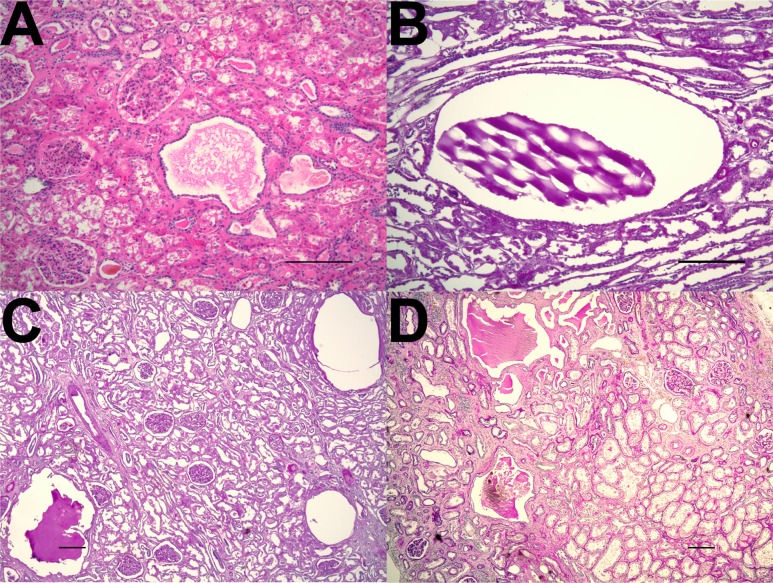
Figure 3.
Microcysts within early-stage autosomal dominant polycystic kidney disease. Representative images of kidney sections from patients with early-stage autosomal dominant polycystic kidney disease (A, K215; B and C, K001; D, K106). Sections stained by (A) hematoxylin and eosin and (B–D) periodic acid-Schiff methods showed multiple cysts measuring less than 1 mm in diameter within otherwise relatively preserved parenchyma. The small cysts were present within both (A, C, and D) the cortex and (B) the medulla. Scale bar, 200 µm.
Figure 4.
Cyst distribution in histologic sections. (A) Histogram from microscopic measurements of cyst count and diameter. Pattern of cyst distribution among individual kidneys was similar (not shown). Four kidneys with less total area for analysis and lower cyst numbers than K001were reduced to two groups. (B) Prevalence of microscopic cysts in parenchyma from five nephrectomy specimens. Arrows and dashed line indicate that 50% of the diameter measurements were less than 0.454 mm. The vertical line at 0.9 mm identifies the point at which cysts were visible by magnetic resonance imaging.
We measured the diameter of 100 tubules in each kidney to estimate the starting point of cyst formation in human kidneys. The mean and median diameters of tubules in the cortex were 35.8 and 34.8 µm, respectively, and the mean and median diameters in the medulla were 38.2 and 37.6 µm, respectively. The mean and median diameters of 769 glomeruli in three CKD stage 2 kidneys were 151 and 154 µm, respectively.
Comparing Histologic and MR Imaging-Derived Data
The relative contribution of microscopic cysts below the resolution of MR imaging detection to total kidney volume is difficult to assess, because the MR imaging and histologic analyses were highly variable in their coverage of renal tissue. To facilitate comparison of the two approaches, we counted the number of cysts per cross-sectional area in midcoronal 3- to 4-mm-thick slices of kidneys examined by MR imaging and 5-µm-thick histologic sections of nephrectomy specimens (Table 2). Cyst prevalence per unit of area ranged from 0.18 to 0.47 cysts/cm2 in the MR imaging study and from 6.6 to 46.0 cysts/cm2 in the microscopic examination. The median cyst prevalence in the microscopic examination (13.0 cysts/cm2) was 62 times greater than the median cyst prevalence in the MR imaging study (0.21 cysts/cm2). The extent of this difference in cyst prevalence is undoubtedly even greater, because we excluded 195 objects smaller than 200 µm (0.2 mm) in diameter, many of which were probably cysts.
Discussion
The current measurements of cyst count and diameter employed a standardization scale that was verified in previous studies involving three other institutions participating in CRISP. Rather than imposing a lower limit on cyst diameter, which we did in our previous MR imaging study (8), we recorded diameters of all circular objects that met the MR signal intensity criteria. We included cyst-like objects as small as 0.9 mm in diameter, lower than the commonly accepted MR imaging resolution limit of 2.0–3.0 mm. As noted previously, the coefficient of variation of cyst diameter measurements increased with declining cyst diameter. Consequently, the lower detection limit that we found should be considered an approximation. Nonetheless, the 0.9-mm cyst diameter minimum was still vastly insufficient to distinguish a large number of occult cysts of lesser size (Figure 4 and Table 2).
In light of finding so many occult cysts below MR imaging detection, we reexamined the possibility that a greater number of cystic renal tubules than previously appreciated might be a more serious threat to GFR. Consider in Table 1 that subject 3 had 1992 renal cysts detected by MR imaging in the combined kidneys after 7.5 years in the CRISP study. If, as illustrated in the histologic sections of polycystic kidneys (Table 2), there were ∼62 times more renal cysts than accounted for by MR imaging, the total number of cysts in the combined kidneys (1992 cysts×62=123,504 cysts) would still be relatively small compared with the 800,000–2,000,000 estimated nephrons in a single normal human kidney (13). Thus, the current study indicates, in more quantitative terms than previously described, that the progression to renal insufficiency in ADPKD probably depends on more than simply preventing cystic nephrons from excreting urine.
No study that we are aware of has used histology measurements to map the distribution of cyst diameters in the lowest range. Consequently, no quantitative data were available that could be used to judge if new cysts form in the course of ADPKD after renal development is completed. We determined here that the number of cysts detected by MR imaging increased over a span of 6.9 years, but this finding does not necessarily mean that new cysts were formed. Calculation reveals that baseline cysts as large as 675 µm in diameter growing at 17.3% per year, a mean cyst growth rate determined previously (4), could reach a diameter of 1 mm and be detected by MR imaging after 6.9 years. Figure 4 reveals an abundance of cysts between 675 and 900 µm that could rise into the range of MR imaging detection in a few years. Consequently, the most economical interpretation is that finding more cysts by MR imaging in the current study simply means that the occult cysts got large enough to detect, not that new cysts were formed.
Tissue processing for microscopy may cause shrinkage ranging up to 25% (14,15) and falsely elevate the number of cysts below 0.9 mm in diameter. To determine the extent to which fixation might reduce cyst diameter near the 0.9-mm MR limit, we computed cyst volume in hypothetical spherical cysts, and after shrinkage of 50%, we determined the new cyst diameter. We found that cysts initially 1.5 mm in diameter or greater would be unlikely to shrink below the 0.9-mm limit, whereas cysts with initial diameters between 0.9 and 1.5 mm could conceivably be reduced to less than 0.9 mm in diameter; however, as illustrated in Figure 4, these cysts would contribute a relatively small fraction of the cysts that were found in the tissue sections. More than likely, the shrinkage artifact is offset by the exclusion from the count of cysts below 200 µm in diameter. Consequently, innumerable small cysts in the histologic study undetected by MR imaging seem to be a true reflection of the in situ state, which is unaffected by shrinkage from tissue fixation.
The determination of mean tubule diameters in the current study indicates that cortical cysts probably originated in segments ranging from 30 to 40 µm in diameter. Assuming that new cysts enlarge at a rate of ∼17% per year, cysts 40, 100, or 200 µm in diameter would reach the range of MR imaging detection (1000 µm) in 57, 41, and 28 years, respectively. Figure 4B illustrates that the vast majority of the cysts measured in renal histologic sections fell within the MR imaging invisible range of diameters, with large numbers near the 200-µm cutoff. Moreover, Figure 4B does not include the 196 objects smaller than 200 µm that might have been tiny cysts. The pattern of cyst diameter distribution is consistent with a pathogenesis model in which cyst formation is relatively steady, and the progressive enlargement of newly formed cysts, growing collectively at the same average rate as established cysts, always lags behind the established cysts in the amounts of volume added year after year (16). Because of exponential growth, the larger cysts increase their absolute volumes and diameters to a greater extent than the newer cysts, leading to a skewed distribution of cyst diameters (Figure 4A). The skewed pattern of diameter distribution is carried forward to the MR imaging measurements, where the smallest cysts that can be detected are much larger to begin with than those cysts in the histologic study (Figure 2). In view of the smooth curvilinear patterns of prevalence illustrated in Figures 2 and 4, it seems reasonable to conclude that new cysts are added continuously in ADPKD. The rate of new cyst formation remains to be determined.
It is interesting to consider how much volume is contributed by the numerous cysts invisible to MR imaging. The median number of baseline cysts in the kidneys listed in Table 1 (587) times 62, the ratio of invisible to visible cysts, estimates that, overall, there were ∼36,394 cysts in both kidneys, and 35,807 of which were less than ∼1.0 mm in diameter. Based on the average median cyst diameter and equivalent volume of the five kidneys listed in Table 2 (0.66 mm=0.00015 cm3) times the median number of cysts (35,807×0.00015 cm3), we estimate that the collective volume of cysts below MR imaging detection is ∼5.4 cm3. This finding is quantitatively insignificant when viewed in relation to TKV values exceeding 400 cm3; however, the formation of new cysts may be especially important in patients in which older macroscopic cysts have led to the destruction of substantial amounts of functioning parenchyma.
In a recent analysis, we reviewed evidence indicating that cysts probably obstruct not only the tubules that they form in but adjacent tubules as well (3). This piecemeal obstruction apparently provokes secondary injury to adjacent parenchyma and upstream tubules that originally drained their urine into the cystic segment. In advanced disease in which compensatory glomerular hyperfiltration and tubule hypertrophy become inadequate to maintain GFR within normal limits, the kidneys cannot afford to lose additional functioning units. It is conceivable that the formation of new microcysts within hyperfunctioning nephrons could be an important factor that accelerates the downhill trajectory of GFR.
Recent studies report a computed tomography imaging method that seems to quantify the amount of residual functioning parenchyma in ADPKD (7,17). These investigators used morphometric methods to sort contrast-enhanced computed tomography scans into three regions: parenchyma, cysts, and intermediate volume. The work provides evidence to indicate that the hypoenhanced intermediate volume was comprised of fibrotic tissue, atrophic tubules, inflammatory cell infiltrates, vascular sclerosis, and dilated tubules (microcysts) (7). The estimated GFR declined as intermediate volume increased in ADPKD in patients with progressive disease. In light of the current findings, we think the intermediate volume probably represents the later stage of a process that begins within chunks of reasonably well preserved contrast-enhancing parenchyma that are littered with cysts too small to be detected.
The current study is limited by the small number of patients and nephrectomy specimens that were analyzed, and therefore, it is insufficient alone to determine the impact of total cyst number at baseline on the course of renal function. We also want to emphasize that the measurement of diameter in the smallest cysts was progressively less accurate as diameter approached the limit of MR imaging detection, which in this study, was ∼0.9 mm. We do not view this limit as an absolute limit of resolution; we think the limit of MR in our hands probably lies between 0.9 and 1.5 mm. MR imaging resolution will have to be substantially increased or alternative imaging strategies will need to be devised to detect the cyst load in the kidneys of young children in the earliest stages of the disease, where relatively few cysts are visible by ultrasound and MR imaging (9,18).
In summary, this study provides direct evidence from imaging and histologic measurements in support of the hypothesis that cysts form in a minority of renal tubules in ADPKD. We found that a vast number of microscopic cysts present in adult ADPKD kidneys remain undetected by clinically available imaging modalities. Consequently, finding additional cysts by MR, computed tomography, or ultrasound in sequential imaging studies is not proof of new cyst formation but rather, could be explained by the enlargement of previously undetected cysts to diameters above the threshold of detection. The broad range of cyst sizes, with a heavy concentration of microscopic cysts observed in the current study, may be most appropriately explained by a formation process that operates continuously throughout life. The extent to which small cysts that are invisible by imaging methods contribute to renal signs, symptoms, and decline of renal function in ADPKD remains to be determined. If, indeed, new cysts form within hyperfiltering nephrons, these occult, benign cysts may accelerate the end stage of the disease.
Disclosures
J.J.G. is a consultant to Otsuka Corp.
Acknowledgments
We thank our colleagues in the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease and the clinical coordinators at Kansas University Medical Center.
This work was supported in part by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK056943 and NCRR CTSA Grant RR025777.
A poster describing this work was presented at the 2011 Annual Meeting of the American Society of Nephrology, November 8–13, 2011, Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franz KA, Reubi FC: Rate of functional deterioration in polycystic kidney disease. Kidney Int 23: 526–529, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Grantham JJ, Mulamalla S, Swenson-Fields KI: Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7: 556–566, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Grantham JJ, Cook LT, Wetzel LH, Cadnapaphornchai MA, Bae KT: Evidence of extraordinary growth in the progressive enlargement of renal cysts. Clin J Am Soc Nephrol 5: 889–896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregoire JR, Torres VE, Holley KE, Farrow GM: Renal epithelial hyperplastic and neoplastic proliferation in autosomal dominant polycystic kidney disease. Am J Kidney Dis 9: 27–38, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Grantham JJ, Geiser JL, Evan AP: Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int 31: 1145–1152, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Caroli A, Antiga L, Conti S, Sonzogni A, Fasolini G, Ondei P, Perico N, Remuzzi G, Remuzzi A: Intermediate volume on computed tomography imaging defines a fibrotic compartment that predicts glomerular filtration rate decline in autosomal dominant polycystic kidney disease patients. Am J Pathol 179: 619–627, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP: Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Cadnapaphornchai MA, Masoumi A, Strain JD, McFann K, Schrier RW: Magnetic resonance imaging of kidney and cyst volume in children with ADPKD. Clin J Am Soc Nephrol 6: 369–376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF, Jr, Glockner JF, Wetzel LH, Brummer ME, O’Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort : Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int 64: 1035–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Bae KT, Tao C, Zhu F, Bost JE, Chapman AB, Grantham JJ, Torres VE, Guay-Woodford LM, Meyers CM, Bennett WM, Consortium for Radiologic Imaging Studies Polycystic Kidney Disease : MRI-based kidney volume measurements in ADPKD: Reliability and effect of gadolinium enhancement. Clin J Am Soc Nephrol 4: 719–725, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Brenner BM, Chertow GM: Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23: 171–175, 1994 [PubMed] [Google Scholar]
- 14.Stowell RE: Effect on tissue volume of various methods of fixation, dehydration and embedding. Stain Technol 16: 67–83, 1941 [Google Scholar]
- 15.Jonmarker S, Valdman A, Lindberg A, Hellström M, Egevad L: Tissue shrinkage after fixation with formalin injection of prostatectomy specimens. Virchows Arch 449: 297–301, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Grantham JJ, Cook LT, Torres VE, Bost JE, Chapman AB, Harris PC, Guay-Woodford LM, Bae KT: Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int 73: 108–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antiga L, Piccinelli M, Fasolini G, Ene-Iordache B, Ondei P, Bruno S, Remuzzi G, Remuzzi A: Computed tomography evaluation of autosomal dominant polycystic kidney disease progression: A progress report. Clin J Am Soc Nephrol 1: 754–760, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Reed B, Nobakht E, Dadgar S, Bekheirnia MR, Masoumi A, Belibi F, Yan XD, Cadnapaphornchai M, Schrier RW: Renal ultrasonographic evaluation in children at risk of autosomal dominant polycystic kidney disease. Am J Kidney Dis 56: 50–56, 2010 [DOI] [PubMed] [Google Scholar]