Summary
Background and objectives
Clinical heart failure (HF) is associated with CKD and faster rates of kidney function decline. Whether subclinical abnormalities of cardiac structure are associated with faster kidney function decline is not known. The association between cardiac concentricity and kidney function decline was evaluated.
Design, setting, participants, & measurements
This is a longitudinal study of 3866 individuals from the Multi-Ethnic Study of Atherosclerosis (2000–2007) who were free of clinical cardiovascular disease, with an estimated GFR (eGFR) ≥60 ml/min per 1.73 m2 at baseline and 5 years of follow-up. Concentricity, a measurement of abnormal cardiac size, was assessed by magnetic resonance imaging and evaluated as a continuous measurement and in quartiles. GFR was estimated by creatinine (eGFRcr) and cystatin C (eGFRcys). The association of concentricity with annual eGFR decline, incident CKD, and rapid kidney function decline (>5% per year) was investigated using linear mixed models as well as Poisson and logistic regression, respectively. Analyses adjusted for demographics, BP, diabetes, and inflammatory markers.
Results
Median decline was −0.8 (interquartile range, −3.1, −0.5) by eGFRcr. Compared with the lowest quartile of concentricity, persons in the highest quartile had an additional 21% (9%–32%) decline in mean eGFRcr in fully adjusted models. Concentricity was also associated with incident CKD and with rapid kidney function decline after adjustment.
Conclusions
Subclinical abnormalities in cardiac structure are associated with longitudinal kidney function decline independent of diabetes and hypertension. Future studies should examine mechanisms to explain these associations.
Introduction
CKD, defined by an elevated creatinine or an estimated GFR (eGFR) <60 ml/min per 1.73 m2, has been identified as an independent risk factor for heart failure (HF) (1,2). Clinical HF has also been independently associated with kidney function decline and development of kidney disease (3). The mechanisms to explain these observations remain unclear, and the complex interdependence of heart and kidney function is referred to as the cardiorenal syndrome (4). Most studies examining these associations have evaluated persons with established CKD, clinically symptomatic HF, or both.
Less is known about the associations of kidney and heart disease at subclinical stages. In cross-sectional studies, subclinical cardiac structural abnormalities such as left ventricular hypertrophy (LVH) are detectable in early CKD (5). Decreased kidney function in preclinical stages (defined by eGFR measured by cystatin C <75 ml/min per 1.73 m2) is associated with higher odds of LVH (6). LVH in turn is an independent risk factor for myocardial infarction, HF, and death (7–9) and was recently found to be associated with progression to dialysis (10). The pattern of ventricular remodeling may also confer additional cardiovascular risk (11).
The use of cardiac magnetic resonance imaging (MRI) over echocardiography may allow insight into the pathophysiology of myocardial remodeling at subclinical disease stages (12). Higher left ventricular (LV) mass and higher concentricity detected by cardiac MRI have been associated with increased cardiovascular events (12), whereas clinical cardiovascular disease (CVD) has been associated with the development of kidney disease in longitudinal studies (13). Whether subclinical cardiac abnormalities are associated with longitudinal decline in kidney function and development of CKD is not known. Concentricity, the ratio of LV mass to LV end-diastolic volume, is a sensitive measure of subclinical cardiac remodeling. Establishing associations between subclinical heart and kidney disease would help to elucidate the pathophysiology of the early cardiorenal relationship. We designed this study to evaluate the associations between subclinical cardiac abnormalities as detected by cardiac MRI and kidney function decline and incident CKD among adults without clinical heart disease or decreased eGFR. We hypothesized that early abnormalities in heart structure manifest by higher concentricity would be associated with kidney function decline and incident CKD.
Materials and Methods
Participants
We included participants from the Multi-Ethnic Study of Atherosclerosis (MESA), which is a large cohort established to understand predictors of CVD. MESA recruited 6814 men and women, aged 45–84 years, who were free of CVD at the time of the baseline examination and who self-identified as white, African American, Hispanic, or Chinese American. Details of recruitment and examinations have been described previously (14). The baseline visit took place between July 2000 and September 2002, and participants returned for three visits at years 2002–2004 (examination 2), years 2004–2005 (examination 3), and years 2005–2007 (examination 4). Repeat measures of kidney function were done at examinations 3 and 4. Individuals were excluded if they had a physician-diagnosed heart attack, angina, HF, stroke or transient ischemic attack, or atrial fibrillation; had undergone coronary artery bypass grafting, angioplasty, valve replacement, or pacemaker placement; or weighed >136.4 kg. At baseline, 990 participants (15%) had CKD, defined as an eGFR <60 ml/min per 1.73 m2 (by either creatinine or cystatin C). Additional details on the MESA rationale and design are available at http://www.mesa-nhlbi.org.
In this study, we included all MESA participants with an eGFRcr ≥60 who had cardiac MRI and kidney function measures at baseline. We excluded persons with no measures of cardiac MRI (n=1810) and persons who did not have any follow-up measures of creatinine or cystatin C (n=423), for a total sample size of 3866 persons. The primary reason for missing cardiac MRI measurements was patients’ declining of the procedure.
Primary Predictors
Cardiac MRI is a well established method for assessment of three-dimensional LV mass and geometry. Advantages over alternative methods for the assessment of cardiac structure and function include less reliance on geometric assumptions required in cardiac echocardiography (15). The MESA MRI protocol has been described in detail previously (14,16). Briefly, LV mass, LV end-diastolic (LVEDV) and LV end-systolic volumes (LVESV), and LV ejection fraction (LVEF) were determined by 1.5-T MRI scanners. Measurements were performed at the six MESA field centers (Wake Forest University, Columbia University, Johns Hopkins University, University of Minnesota, Northwestern University, and University of California at Los Angeles), and all images were read at the central MESA cardiac MRI review center at Johns Hopkins Hospital (Baltimore, MD).
Our predictor of interest was cardiac concentricity measured by MRI. We specifically chose concentricity as our primary predictor because it has been shown to predict incident non-HF cardiovascular events more consistently than LV mass in the MESA cohort (12). Concentricity was estimated as the ratio of LV mass to LVEDV (12). Conceptually, concentricity refers to LV mass in excess of the quantity expected for a given level of LV end-diastolic volume. LV mass was determined by the sum of the myocardial area multiplied by slice thickness plus image gap in the end-diastolic phase multiplied by the specific gravity of myocardium (1.05 g/ml). LV end-diastolic volume and LV end-systolic volume were calculated using Simpson’s rule (12). LV stroke volume was calculated as the difference between LVEDV and LVESV. LVEF was calculated as LV stroke volume divided by LVEDV multiplied by 100. We also examined the variables LV mass, LVEDV, and LVEF as secondary predictors to determine whether each cardiac parameter had independent associations with the outcomes.
Adjusted Variables
Age, sex, race/ethnicity, education level, and smoking status were ascertained by standardized questionnaires (14). Height and weight were measured using calibrated scales with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. BP measurements were obtained using the Dinamap automated BP device (Dinamap Monitor Pro 100). Three sequential measures were obtained and the average of the second and third measurements was recorded. Hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or current use of antihypertensive medication. After a 12-hour fast, participants underwent phlebotomy to measure total cholesterol, HDL cholesterol, triglycerides, and glucose. Fasting blood was collected and stored at −70°F until needed for the appropriate assays. HDL cholesterol was measured using the cholesterol oxidase cholesterol method (Roche Diagnostics). The Friedewald equation was used to calculate LDL cholesterol (17). Impaired glucose tolerance was defined by a fasting glucose level of 100–125 mg/dl without diabetes. Diabetes was defined as either a fasting glucose ≥126 mg/dl or use of oral hypoglycemic medication or insulin (18). Urine albumin and creatinine were measured in a single morning urine sample by nephelometry and the rate Jaffe reaction, respectively, and expressed as urine albumin/creatinine ratio (ACR) in milligrams to grams. High-sensitivity C-reactive protein (CRP) was measured by using the BN II nephelometer at the Laboratory for Clinical Biochemistry Research (Dade Behring Inc). IL-6 was measured by ultra-sensitive ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN). Study personnel recorded the use of antihypertensive medication (i.e., β-blockers, calcium channel blockers, angiotensin converting enzyme inhibitors/angiotensin receptor blockers, diuretics, phosphodiesterase inhibitors, or nitrates) and lipid-lowering medications.
Outcomes
Kidney function was measured by serum creatinine and cystatin C. All assays were performed in frozen serum specimens that were stored at –70°C. Serum creatinine was measured by rate reflectance spectrophotometry using thin film adaptation of the creatine amidinohydrolase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics Inc, Rochester, NY) at the Collaborative Studies Clinical Laboratory at Fairview University Medical Center (Minneapolis, MN) and calibrated to the Cleveland Clinic (Cleveland, OH). Cystatin C was measured by means of a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring) with a nephelometer (BNII; Dade Behring) and calibrated for assay drift. We estimated the GFR with the use of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation (19) to determine eGFRcr (141× min(Scr/κ,1)α × max(Scr/κ, 1)–1.209 × 0.993Age × 1.018 [if female] × 1.159 [if black]) and the CKD-EPI cystatin C equation to determine eGFRcys (76.7 × cys C−1.19). These formulae were developed from the pooling of several cohorts with GFR measured from iothalamate (20).
Our primary outcomes were kidney function decline and incident CKD. Kidney function decline was assessed using repeated measures of eGFR available at examinations 3 and 4 and expressed as milliliters per minute per 1.73 m2 per year. Incident CKD was defined as an eGFR <60 ml/min per 1.73 m2 with a concomitant eGFR decline ≥1 ml/min per year at any follow-up examination. We chose this definition in order to reduce misclassification due to changes close to the eGFR threshold of 60 ml/min per 1.73 m2. We determined the outcome of incident CKD using eGFRcr and eGFRcys separately and also analyzed the outcome among those who met both criteria simultaneously.
A secondary outcome was rapid kidney function decline, assessed by using repeated measures of eGFR, defined as a change of >5% per year, which approximates the highest quartile of kidney function decline. This cutoff was established based on the association between the highest quintile of kidney function decline assessed by eGFRcys and CVD outcomes in ambulatory elderly individuals (21). All analyses were performed using both the eGFRcr and the eGFRcys.
Statistical Analyses
We first described the baseline characteristics of the study participants categorized by quartiles of concentricity. We then evaluated the association between concentricity and kidney function decline by eGFRcr and eGFRcys separately using linear mixed models with random intercepts and slopes to estimate and compare linear trends in mean eGFR. Linear mixed models were used to account for the correlation of observations by subject in this longitudinal study with repeated measurements (22). We used concentricity as a continuous variable (per SD) and also categorized as sex-specific quartiles (<0.95, 0.95–1.06, 1.07–1.2, and ≥1.2 g/ml for women; <1.06, 1.06–1.17, 1.18–1.35, and ≥1.35 g/ml for men).
Given the discrete time period of our study, we used Poisson regression to calculate adjusted relative risks to evaluate the association of concentricity and incident CKD (a relatively rare outcome). The association of concentricity with the secondary outcome of rapid kidney function decline as a dichotomous outcome (absolute change >5% annually versus <5%) was assessed by multivariable logistic regression. Secondary predictors LV mass, LVEDV, and LVEF were evaluated separately.
For all regression models, candidate covariates (chosen a priori) included age, race, education, smoking, BMI, total cholesterol, HDL, LDL, triglycerides, lipid-lowering medications, systolic BP, diastolic BP, hypertension, hypertension medications, diabetes, IL-6, CRP, and urine ACR. After applying a stepwise selection procedure with P values of 0.20 as the threshold for entry into the model and 0.10 as criterion for retention, model 1 included demographic variables including age, race, education, and baseline eGFR. Model 2 included model 1 plus BMI, systolic BP, antihypertensive medications, diabetes, HDL, IL-6, and CRP. In addition, we performed a sensitivity analysis excluding persons with an ACR ≥30 mg/g.
Results
Participant Characteristics
Among 3866 persons without CKD at baseline, the mean age was 60 (SD 10) years, 52% were female, and 39% self-identified as white, 13% as Chinese, 25% as black, and 23% as Hispanic. Overall, 11% of participants had diabetes, 38% had hypertension, and 7% had a urine ACR ≥30 mg/g. Mean baseline eGFRcr was 82 (SD ±13) ml/min per 1.73 m2 and eGFRcys was 97 (SD ±16). Mean LV concentricity was 1.15 (SD 0.24) g/ml. Persons in the highest quartile of concentricity had higher BMI, higher systolic BP, and higher prevalence of hypertension and diabetes (Table 1). Persons in the highest quartile of concentricity had the lowest baseline eGFRcr and eGFRcys.
Table 1.
Characteristics of MESA participants with baseline eGFRcr ≥60 ml/min per 1.73 m2 by concentricity quartiles
| Characteristic | Concentricity Quartiles (g/ml) | |||
|---|---|---|---|---|
| ≤1.00 | 1.01–1.13 | 1.14–1.29 | ≥1.30 | |
| Number of participants | 1016 | 994 | 957 | 899 |
| Age (yr) | 58 (9) | 59 (9) | 60 (10) | 63 (9) |
| Men | 302 (30) | 439 (44) | 519 (54) | 606 (67) |
| Race | ||||
| white | 453 (45) | 414 (42) | 341 (36) | 286 (32) |
| Chinese | 163 (16) | 140 (14) | 128 (13) | 85 (10) |
| black | 169 (17) | 219 (22) | 253 (26) | 339 (38) |
| Hispanic | 231 (23) | 221 (22) | 235 (25) | 189 (21) |
| Body mass index (kg/m2) | 26.7 (5.0) | 27.5 (4.8) | 28.0 (4.9) | 28.9 (4.7) |
| Weight (kg) | 73 (16) | 76 (16) | 78 (16) | 82 (15) |
| Smoking | ||||
| never | 581 (57) | 532 (54) | 476 (50) | 410 (46) |
| former | 340 (34) | 338 (34) | 345 (36) | 346 (39) |
| current | 93 (9) | 123 (12) | 134 (14) | 141 (16) |
| Hypertension | 234 (23) | 347 (35) | 411 (43) | 488 (54) |
| Diabetes mellitus | 59 (6) | 98 (10) | 95 (10) | 157 (18) |
| Hypertension medication | 211 (21) | 288 (29) | 325 (34) | 388 (43) |
| Lipid-lowering medication | 115 (11) | 122 (12) | 146 (15) | 174 (19) |
| Systolic BP | 116 (19) | 122 (19) | 126 (20) | 132 (21) |
| Diastolic BP | 68 (10) | 71 (10) | 73 (10) | 76 (10) |
| Total cholesterol | 196 (36) | 194 (34) | 194 (34) | 193 (35) |
| HDL (mg/dl) | 55 (16) | 51 (15) | 49 (15) | 48 (13) |
| LDL (mg/dl) | 117 (31) | 117 (31) | 118 (31) | 118 (31) |
| Triglycerides (mg/dl) | 99 (70, 145) | 112 (79, 160) | 113 (78, 162) | 116 (82, 175) |
| Urine albumin/creatinine ratio (mg/g) | 4.4 (3.0, 6.8) | 4.6 (3.1, 8.3) | 5.1 (3.3, 10.1) | 6.4 (3.8, 13.6) |
| IL-6 (pg/ml) | 1.01 (0.66, 1.58) | 1.02 (0.69, 1.59) | 1.12 (0.69, 1.76) | 1.21 (0.83, 1.84) |
| C-reactive protein (mg/L) | 1.54 (0.70, 3.94) | 1.52 (0.70, 3.87) | 1.70 (0.77, 3.94) | 1.98 (0.87, 4.11) |
| eGFRcr at baseline (ml/min per 1.73 m2) | 83 (13) | 82 (13) | 82 (13) | 82 (13) |
| eGFRcys at baseline (ml/min per 1.73 m2) | 101 (15) | 98 (15) | 96 (15) | 92 (16) |
Data are presented as n (%) or median (interquartile range). MESA, Multi-Ethnic Study of Atherosclerosis; eGFRcr, estimated GFR by creatinine; eGFRcys, estimated GFR by cystatin C.
We compared the characteristics between individuals in our cohort and those who were excluded due to missing MRI measurements; there were no major differences between groups. For example, among those missing MRI, the mean age was 62 years (SD ±10) and 54% were female. Baseline eGFRcr was 82 (SD ±13) ml/min per 1.73 m2 and eGFRcys was 93 (±17). Urinary ACR was 5.5 (3.4–12.1) mg/g in those missing MRI measurements versus 5.0 (3.2–9.3) mg/g in our cohort. Participants in both groups were equally distributed among all sites.
Concentricity and Kidney Function Decline
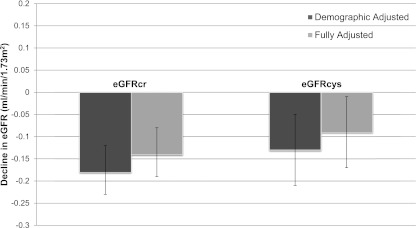
Over a median follow-up time of 4.8 years, median decline by eGFRcr was −0.8 (interquartile range [IQR], −3.1, −0.5) ml/min per 1.73 m2 per year and −0.8 (IQR, −3.4, 1.2) ml/min per 1.73 m2 per year by eGFRcys. Each SD higher concentricity (0.24 g/ml) was associated with a 9% (95% confidence interval [95% CI], 5%–13%) and 8% (95% CI, 0%–16%) faster rate of kidney function decline by eGFRcr and eGFRcys, respectively, in fully adjusted models (Figure 1). Compared with the lowest quartile of concentricity, participants in the highest quartile had the fastest rate of kidney function decline in demographic adjusted models (Table 2). Full adjustment for confounders including hypertension attenuated these associations somewhat but still demonstrated a −0.3 (−0.5, −0.2) ml/min per year faster decline for eGFRcr (−0.2 [−0.4, 0.1] for eGFRcys) (Table 2).
Figure 1.
Decline in estimated GFR per SD increment concentricity among MESA participants with a baseline GFR estimated by creatinine ≥60 ml/min per 1.73 m2. Error bars represent 95% confidence intervals. MESA, Multi-Ethnic Study of Atherosclerosis; eGFRcr, estimated GFR by creatinine; eGFRcys, estimated GFR by cystatin C.
Table 2.
Association of concentricity with decline in eGFRcr and eGFRcys among MESA participants with baseline eGFRcr ≥60 ml/min per 1.73 m2 by linear mixed models
| Predictors | n | Demographic Adjusted Change in eGFRa | Fully Adjusted Change in eGFRb |
|---|---|---|---|
| eGFRcr | |||
| concentricity | |||
| continuous (per SD=0.24) | 3866 | −0.18 (−0.24, −0.12) | −0.14 (−0.20, −0.08) |
| quartile (g/ml) | |||
| 1 (<0.95 for women, <1.06 for men) | 966 | Reference | Reference |
| 2 (0.95–1.06 for women, 1.06–1.17 for men) | 967 | −0.15 (−0.30, 0.01) | −0.11 (−0.26, 0.04) |
| 3 (1.07–1.20 for women, 1.18–1.35 for men) | 967 | −0.12 (−0.27, 0.03) | −0.08 (−0.23, 0.08) |
| 4 (≥1.20 for women, ≥1.35 for men) | 966 | −0.44 (−0.60, −0.28) | −0.32 (−0.49, −0.15) |
| eGFRcys | |||
| concentricity | |||
| continuous (per SD=0.24) | 3866 | −0.13 (−0.21, −0.05) | −0.09 (−0.18, −0.003) |
| quartile (g/ml) | |||
| 1 (<0.95 for women, <1.06 for men) | 966 | Reference | Reference |
| 2 (0.95–1.06 for women, 1.06–1.17 for men) | 967 | −0.14 (−0.35, 0.07) | −0.08 (−0.30, 0.14) |
| 3 (1.07–1.20 for women, 1.18–1.35 for men) | 967 | −0.03 (−0.25, 0.18) | 0.03 (−0.19, 0.25) |
| 4 (≥1.20 for women, ≥1.35 for men) | 966 | −0.28 (−0.51, −0.05) | −0.16 (−0.41, 0.08) |
Values in parentheses are 95% confidence intervals. eGFRcr, estimated GFR by creatinine; eGFRcys, estimated GFR by cystatin C; MESA, Multi-Ethnic Study of Atherosclerosis; eGFR, estimated GFR.
Demographic adjusted: adjusted for age, race, and education level.
Fully adjusted for above variables plus body mass index, systolic BP, hypertension medications, diabetes, HDL, IL-6, and C-reactive protein.
We also studied the associations of LV mass and LV end-diastolic volume with kidney function decline separately. Using eGFRcr, the association of LV mass with kidney function decline was significant; every SD higher LV mass was associated with a faster rate of kidney function decline per year (−0.1 [−0.2, 0] ml/min per year). LVEDV was not significantly associated with kidney function. By eGFRcys, there was no significant association between LV mass or LVEDV and kidney function decline in fully adjusted models.
We conducted a sensitivity analysis excluding participants with ACR ≥30 mg/g. Overall, only 7% had an ACR ≥30. Among these persons, the association of concentricity and KF decline was not materially different (−0.1 [−0.2, −0.1] ml/min per year by eGFRcr; −0.1 [−0.2, 0] by eGFRcys) in fully adjusted models.
Concentricity and Incident CKD
Each SD higher concentricity (0.24 g/ml) was associated with a 17% (9%–26%) and 19% (4%–35%) higher risk of incident CKD in demographic adjusted models by eGFRcr and eGFRcys, respectively (Table 3). Adjustment for BMI, SBP, hypertensive medications, diabetes, HDL, IL-6, and CRP attenuated this association, but it remained statistically significant (8% [0%–18%] for eGFRcr; 16% [0–35]) for eGFRcys). Persons in the highest quartile of concentricity had a 43% (12%–82%) and 72% (4%–282%) increased risk of incident CKD by eGFRcr and eGFRcys respectively, adjusted for baseline eGFR (Table 3). This association was attenuated by adjustment for BMI, SBP, hypertensive medications, diabetes, HDL, IL6, and CRP (21% [−6%, 56%] for eGFRcr; 63% [8%, 288%] for eGFRcys). In an analysis using eGFR <60 by both creatinine and cystatin to define incident CKD, the highest quartile of concentricity had a nearly 3-fold by eGFRcr (incident rate ratio 2.73 [1.45–5.14]) and more than two-fold by eGFRcys (incident rate ratio 2.23 [1.09–4.54]) increased rate of incident CKD after full adjustment (Table 3).
Table 3.
Association of cardiac concentricity with incident CKDa among MESA participants with baseline eGFRcr ≥60 ml/min per 1.73 m2 by Poisson regression
| Predictor | n | Demographic Adjusted IRRb | Fully Adjusted IRRc |
|---|---|---|---|
| Incident CKD defined by eGFRcr | 457 | ||
| concentricity | |||
| continuous (per SD=0.24) | 3866 | 1.17 (1.09, 1.26) | 1.08 (1.00, 1.18) |
| quartile (g/ml) | |||
| 1 (<0.95 for women, <1.06 for men) | 966 | Reference | Reference |
| 2 (0.95–1.06 for women, 1.06–1.17 for men) | 967 | 1.03 (0.79, 1.35) | 0.99 (0.76, 1.30) |
| 3 (1.07–1.20 for women, 1.18–1.35 for men) | 967 | 1.12 (0.86, 1.44) | 1.07 (0.83, 1.40) |
| 4 (≥1.20 for women, ≥1.35 for men) | 966 | 1.43 (1.12, 1.82) | 1.21 (0.94, 1.56) |
| Incident CKD defined by eGFRcys | 158 | ||
| concentricity | |||
| continuous (per SD=0.24) | 3866 | 1.19 (1.04, 1.35) | 1.16 (1.00, 1.35) |
| quartile (g/ml) | |||
| 1 (<0.95 for women, <1.06 for men) | 966 | Reference | Reference |
| 2 (0.95–1.06 for women, 1.06–1.17 for men) | 967 | 1.17 (0.67, 2.05) | 1.18 (0.64, 2.18) |
| 3 (1.07–1.20 for women, 1.18–1.35 for men) | 967 | 1.44 (0.85, 2.43) | 1.57 (0.88, 2.78) |
| 4 (≥1.20 for women, ≥1.35 for men) | 966 | 1.72 (1.04, 2.82) | 1.63 (0.92, 2.88) |
| Incident CKD defined by both eGFRcr and eGFRcys | 116 | ||
| concentricity | |||
| continuous (per SD=0.24) | 3866 | 1.21 (1.05, 1.40) | 1.20 (1.01, 1.41) |
| quartile (g/ml) | |||
| 1 (<0.95 for women, <1.06 for men) | 966 | Reference | Reference |
| 2 (0.95–1.06 for women, 1.06–1.17 for men) | 967 | 1.38 (0.68, 2.81) | 1.37 (0.64, 2.92) |
| 3 (1.07–1.20 for women, 1.18–1.35 for men) | 967 | 1.98 (1.02, 3.86) | 1.94 (0.94, 4.03) |
| 4 (≥1.20 for women, ≥1.35 for men) | 966 | 2.73 (1.45, 5.14) | 2.23 (1.09, 4.54) |
Values in parentheses are 95% confidence intervals. MESA, Multi-Ethnic Study of Atherosclerosis; eGFRcr, estimated GFR by creatinine; eGFRcys, estimated GFR by cystatin C; IRR, incident rate ratio.
Incident CKD defined as eGFR <60 ml/min per 1.73 m2 and a decline in eGFR of >1 ml/min per year.
Demographic adjusted for age, race, education level, baseline eGFR (creatinine for creatinine models; cystatin C for cystatin C models; both for third model).
Fully adjusted for the above variables plus body mass index, systolic BP, hypertension medications, diabetes, HDL, IL-6, and C-reactive protein.
Concentricity and Rapid Kidney Function Decline
The absolute annual change in eGFR corresponding to a >5% annual decline (rapid kidney function decline) was 2.1 ml/min per 1.73 m2 using eGFRcr and 1.9 ml/min per 1.73m2 using eGFRcys. Each SD higher concentricity was associated with 13% (4%–22%) higher odds of rapid decline with eGFRcr and 8% (−2, 17%) higher odds of rapid decline with eGFRcys after full adjustment (Table 4).
Table 4.
Association of cardiac concentricity with rapid declinea in eGFRcr and eGFRcys among MESA participants with baseline eGFRcr ≥60 ml/min per 1.73 m2 by logistic regression
| Predictors | n | Participants With Rapid Decline (n) | Demographic Adjusted ORb | Fully Adjusted ORc |
|---|---|---|---|---|
| eGFRcr | ||||
| concentricity | ||||
| continuous (per SD=0.24) | 3866 | 1037 | 1.17 (1.08, 1.26) | 1.13 (1.04, 1.22) |
| quartile (g/ml) | ||||
| 1 (<0.95 for women, <1.06 for men) | 966 | 208 | 1.00 (Reference) | 1.00 (Reference) |
| 2 (0.95–1.06 for women, 1.06–1.17 for men) | 967 | 242 | 1.15 (0.92, 1.43) | 1.09 (0.87, 1.36) |
| 3 (1.07–1.20 for women, 1.18–1.35 for men) | 967 | 273 | 1.25 (1.01, 1.56) | 1.18 (0.95, 1.48) |
| 4 (≥1.20 for women, ≥1.35 for men) | 966 | 314 | 1.43 (1.15, 1.79) | 1.27 (1.01, 1.60) |
| eGFRcys | ||||
| concentricity | ||||
| continuous (per SD=0.24) | 3866 | 867 | 1.16 (1.06, 1.26) | 1.08 (0.98, 1.17) |
| quartile (g/ml) | ||||
| 1 (<0.95 for women, <1.06 for men) | 966 | 174 | 1.00 (Reference) | 1.00 (Reference) |
| 2 (0.95–1.06 for women, 1.06–1.17 for men) | 967 | 214 | 1.34 (1.06, 1.69) | 1.27 (1.00, 1.61) |
| 3 (1.07–1.20 for women, 1.18–1.35 for men) | 967 | 219 | 1.27 (1.01, 1.61) | 1.13 (0.89, 1.44) |
| 4 (≥1.20 for women, ≥1.35 for men) | 966 | 260 | 1.48 (1.16, 1.87) | 1.22 (0.96, 1.56) |
Values in parentheses are 95% confidence intervals. eGFRcr, estimated GFR by creatinine; eGFRcys, estimated GFR by cystatin C; MESA, Multi-Ethnic Study of Atherosclerosis; OR, odds ratio.
Rapid decline defined as >5% per year.
Demographic adjusted for age, race, education, baseline estimated GFR (creatinine for creatinine models; cystatin C for cystatin C models).
Fully adjusted for the above variables plus body mass index, systolic BP, hypertension medications, diabetes, HDL, IL-6, and C-reactive protein.
Discussion
In this cohort of individuals free of established CKD and CVD, we found that subclinical heart abnormalities (measured as concentricity by MRI) were associated with faster rates of decline in kidney function, independent of hypertension, diabetes, or other established CVD risk factors. Higher concentricity was associated with higher incidence of CKD and with rapid kidney function decline, although these associations were attenuated by adjustment. Our findings suggest that the relationship between cardiac concentricity and kidney function decline may be present at very early stages of disease. Early cardiac structural changes such as higher concentricity may contribute to kidney disease progression.
Our findings expand on previous studies that have identified clinical CVD as an independent risk factor for subsequent CKD (3,13). Investigations of early stages of disease are less well established. For example, in the Cardiovascular Health Study, no association was found between increased LV mass by echocardiography and kidney function decline. This may have been due to a different spectrum of disease in that elderly cohort, or perhaps because concentricity was not evaluated as a predictor. Extrapolating on the established associations of CVD and HF with CKD, we hypothesize that disease progression in both organs occurs by parallel processes. In other words, the commonly accepted direction of disease progression from kidney disease to CVD may not be the only pathway for disease development in this population, especially in early stages. This may be the result of subclinical endothelial injury and vessel stiffness preceding the development of both diseases (23) or be in part mediated by early effects of the renin-angiotensin-aldosterone axis, which has been shown to induce proteinuria and fibrosis (24).
Our finding on the association between concentricity and incident CKD raises interesting questions about the pathway of early kidney disease progression. Although increased LV mass and CKD are often both attributed to antecedent hypertension, our multivariable models suggest that higher BP does not fully account for the observed associations between concentricity and kidney function decline. Of note, increased LV mass has previously been shown to precede the development of hypertension, suggesting that cardiac remodeling may be part of the abnormal physiologic response accompanying vascular stiffness observed in incident hypertension (25,26). A recent longitudinal study in the MESA cohort demonstrated that concentricity is associated with incident hypertension (27). The mechanisms driving incident hypertension in this population may also contribute to kidney disease progression, such as subclinical impaired salt regulation and extracellular fluid volume expansion preceding clinical hypertension (28). Thus, early relationships between these subclinical diseases are intriguing and their independent effects, while subtle, may be of long-term importance.
Our study has several strengths. This large, ethnically diverse cohort free of CVD and CKD is the ideal population for studying the effects of subclinical cardiac disease. The longitudinal design with a 5-year follow-up period allowed the assessment of temporal causality in the early cardiorenal relationship, which has not been previously explored. In addition, we included two measures of kidney function, creatinine and cystatin C. These have different non-GFR determinants and thus improved our ability to detect associations. Our study also has some limitations. We do not have directly measured GFR, but this is cumbersome and not available in clinical practice or in population-based studies. In addition, access to cardiac MRI is not readily generalizable to most patient populations. However, cardiac MRI is recognized as a sensitive tool for evaluating cardiac structure and function, and it is a powerful research tool that may become more applicable to clinical settings. Although we may have been limited in power due to a relatively low prevalence of MRI abnormalities and incident CKD in this cohort, we were still able to detect significant associations. As some of our results may be driven by findings in the highest quartile of concentricity, future studies with repeat MRI measurements and longer follow-up are warranted to fully characterize the observed associations between cardiac morphology and CKD risk. However, the strong association of concentricity with incident CKD as determined by the strictest definition is striking, suggesting that detection of CKD is also an important factor in our understanding of this relationship. As in all epidemiologic studies, residual confounding may remain, although we attempted to adjust for the most relevant comorbidities and confounders. Follow-up measurements of BP would also have strengthened our analyses, but these are unavailable in MESA. In addition, we have adjusted for variables that may act as mediators rather than confounders in the association of concentricity and kidney function decline. Nevertheless, we were missing some variables such as hemoglobin, which was not measured in MESA, because this was a relatively healthy cohort at baseline. Finally, although our design allows for longitudinal examination of kidney function decline, information about trajectory of kidney disease before inclusion is lacking and further studies are needed to elucidate the likely parallel pathways of decline in early cardiac and kidney disease.
In conclusion, our study demonstrates an independent association between subclinical heart dysfunction (measured as higher cardiac concentricity) and kidney function decline at early stages of disease. Our findings suggest that the complex interplay of subclinical cardiac remodeling and kidney dysfunction may happen very early in both disease states. Future studies should be designed to evaluate the pathophysiology of these relationships.
Disclosures
None.
Acknowledgments
This project is supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (Grant R01 DK066488) and the American Heart Association (Grant AHA 0640012N) (M.G.S., principal investigator). M.P. was supported by an American Heart Association Western Affiliates Fellowship Grant and is currently supported by the National Institutes of Health (Grant NRSA F32 DK093231).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB: Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 41: 1364–1372, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Siscovick D, Sarnak MJ: Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis 204: 298–303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R: Cardiorenal syndrome. J Am Coll Cardiol 52: 1527–1539, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O: Left ventricular mass index increase in early renal disease:Impact of decline in hemoglobin. Am J Kidney Dis 34: 125–134, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Moran A, Katz R, Jenny NS, Astor B, Bluemke DA, Lima JA, Siscovick D, Bertoni AG, Shlipak MG: Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: The multi-ethnic study of atherosclerosis (MESA). Am J Kidney Dis 52: 839–848, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS: Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: The Cardiovascular Health Study. J Am Coll Cardiol 43: 2207–2215, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC: Predictors of congestive heart failure in the elderly: The Cardiovascular Health Study. J Am Coll Cardiol 35: 1628–1637, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KKL: The progression from hypertension to congestive heart failure. JAMA 275: 1557–1562, 1996 [PubMed] [Google Scholar]
- 10.Chen SC, Su HM, Hung CC, Chang JM, Liu WC, Tsai JC, Lin MY, Hwang SJ, Chen HC: Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol 6: 2750–2758, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krumholz HM, Larson M, Levy D: Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol 25: 879–884, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Bluemke DA, Kronmal RA, Lima JAC, Liu K, Olson J, Burke GL, Folsom AR: The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 52: 2148–2155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, Sarnak MJ, Weiner DE: Cardiovascular disease and subsequent kidney disease. Arch Intern Med 167: 1130–1136, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP: Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol 156: 871–881, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Lyne JC, Pennell DJ: Cardiovascular magnetic resonance in the quantitative assessment of left ventricular mass, volumes and contractile function. Coron Artery Dis 16: 337–343, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA: Cardiovascular function in multi-ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. AJR Am J Roentgenol 186[Suppl 2]: S357–S365, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 18.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Expert Committee on the Diagnosis and Classification of Diabetes Mellitus : Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 26[Suppl 1]: S5–S20, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, Rifkin D, Sarnak MJ: Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 20: 2625–2630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laird NM, Ware JH: Random-effects models for longitudinal data. Biometrics 38: 963–974, 1982 [PubMed] [Google Scholar]
- 23.Peralta CA, Katz R, Madero M, Sarnak M, Kramer H, Criqui MH, Shlipak MG: The differential association of kidney dysfunction with small and large arterial elasticity: The multiethnic study of atherosclerosis. Am J Epidemiol 169: 740–748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiyomoto H, Rafiq K, Mostofa M, Nishiyama A: Possible underlying mechanisms responsible for aldosterone and mineralocorticoid receptor-dependent renal injury. J Pharmacol Sci 108: 399–405, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Peralta CA, Adeney KL, Shlipak MG, Jacobs D, Jr, Duprez D, Bluemke D, Polak J, Psaty B, Kestenbaum BR: Structural and functional vascular alterations and incident hypertension in normotensive adults: The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 171: 63–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iso H, Kiyama M, Doi M, Nakanishi N, Kitamura A, Naito Y, Sato S, Iida M, Konishi M, Shimamoto T, Komachi Y: Left ventricular mass and subsequent blood pressure changes among middle-aged men in rural and urban Japanese populations. Circulation 89: 1717–1724, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Shimbo D, Muntner P, Mann D, Barr RG, Tang W, Post W, Lima J, Burke G, Bluemke D, Shea S: Association of left ventricular hypertrophy with incident hypertension: The multi-ethnic study of atherosclerosis. Am J Epidemiol 173: 898–905, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasavada N, Agarwal R: Role of excess volume in the pathophysiology of hypertension in chronic kidney disease. Kidney Int 64: 1772–1779, 2003 [DOI] [PubMed] [Google Scholar]