Summary
Background and objectives
Micropolitan and rural patients face challenges when initiating dialysis, including healthcare access. Previous studies have shown little association of nonurban residence with dialysis outcomes but have not examined the association of dialysis modality with residence location.
Design, setting, participants, & measurements
This retrospective cohort study used data from the U.S. Renal Data System. Adults who initiated maintenance dialysis between January 1, 2006, and December 31, 2007, were classified as rural, micropolitan, or urban. Early and long-term mortality and kidney transplantation were examined with Cox regression stratified by dialysis modality.
Results
Of 204,463 patients, 80% were urban; 10.2%, micropolitan; and 9.8%, rural. Micropolitan and rural patients were older, were less racially diverse, had more comorbid conditions, and were more likely to start peritoneal dialysis (PD). Median follow-up was 2.0 years. Early mortality or long-term hemodialysis (HD) mortality did not significantly differ by geographic residence. After adjustment, micropolitan and rural PD patients had higher risk for long-term mortality (hazard ratio [HR], 1.21 [95% confidence interval (CI), 1.09–1.35] and 1.12 [95% CI, 1.01–1.24], respectively) than urban PD patients. After adjustment, kidney transplantation was more likely in micropolitan and rural HD patients (HR, 1.19 [95% CI, 1.11–1.28] and 1.30 [CI, 1.21–1.40]) than urban HD patients, and micropolitan PD patients (HR, 1.31 [95%, CI 1.13–1.51]) than urban PD patients.
Conclusions
Micropolitan and rural residence is associated with higher mortality in PD patients and similar or higher likelihood of kidney transplantation among HD and PD patients. Studies examining the underlying mechanisms of these associations are warranted.
Introduction
Approximately 20% of the United States population lives in micropolitan or rural areas (1), an important underserved population. On the basis of disparities research, Healthy People 2020 identifies rural living as an important social determinant of health (2). Micropolitan and rural residence, defined as a population of 10,000–50,000 individuals or <10,000 individuals, respectively, have been associated with increased morbidity and mortality in chronic diseases that require longitudinal care, including chronic obstructive pulmonary disease and heart disease (3). The association of micropolitan and rural living on outcomes among patients with CKD and those who develop ESRD is less clear.
Rural and micropolitan patients initiating dialysis face unique challenges that could ultimately affect outcomes. Patients living in nonurban areas have fewer options for maintenance dialysis and are more likely to start with peritoneal dialysis (PD) (4). Those who initiate hemodialysis (HD) face long travel times, which has been associated with worse mortality (5). Despite the importance of PD as an alternative modality in this population, PD units are increasingly consolidating to urban areas (6), rural units are less likely to offer home modalities (7), and rural units with PD programs have worse overall outcomes (8). Previous studies that examined the association of rural and micropolitan living on mortality and likelihood of kidney transplantation have had conflicting findings (9–12); most show little or no difference in outcomes compared with urban populations. However, none of these studies directly examined how permanent dialysis modality may differentially affect outcomes among patients in micropolitan and rural communities.
This study examined the association of micropolitan and rural living on mortality and kidney transplantation after initiation of dialysis while stratifying for dialysis treatment modality. We hypothesized that rural and micropolitan patients would have higher mortality and lower likelihood of kidney transplantation than their urban counterparts.
Materials and Methods
This retrospective cohort study used patient-level data obtained from the U.S. Renal Data System (USRDS). This study was approved by the institutional review board at Vanderbilt University Medical Center, Nashville, Tennessee, and by the USRDS.
Data Source
The USRDS (www.usrds.org) is a central dialysis registry that includes epidemiologic data on all ESRD patients who initiate dialysis or who receive a kidney transplant in the United States. The USRDS data include patient-level variables describing demographic characteristics, residence location, and treatment history.
Patient Selection
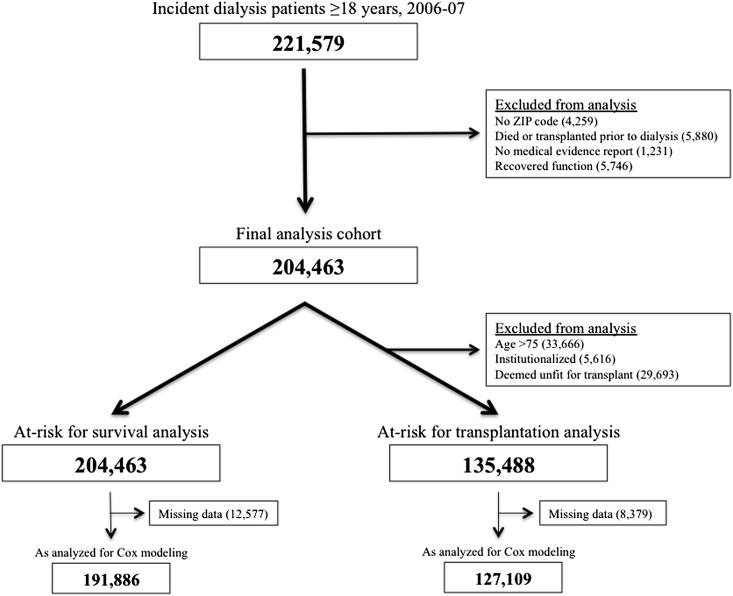
Patients were included if they initiated dialysis for the first time between January 1, 2006, and December 31, 2007, and were 18 years of age or older at initiation. Patients were required to have their residence ZIP code and ESRD medical evidence form available within USRDS. Individuals were excluded if they received a kidney transplant or died before initiation of maintenance dialysis or if they recovered kidney function and thus discontinued dialysis (a surrogate for recovering acute kidney injury) at any time after initiation. Figure 1 depicts the patient flow, which included 204,463 patients for analysis.
Figure 1.
Study flow.
Identification of Residence Location
Rural and micropolitan residence was determined by the use of rural-urban commuting area (RUCA) codes (13). This method combines measures of urbanization, population density, and urban commuting to categorize locations by assigning a numeric code from 1 to 10, with higher values corresponding to higher degrees of rural living. ZIP code–specific RUCA codes were used because they represent the smallest feasible geographic area to study (14). The primary residence was defined as the ZIP code at the time of dialysis initiation, as documented in the file RESIDENC. Patients were further grouped as urban, micropolitan, or rural on the basis of RUCA code aggregates (see Supplemental Table S1) (15). The nonurban population was studied in two distinct categories because we hypothesized that barriers to dialysis access may be more severe in more remote populations. Patient movement after dialysis initiation was infrequent, and group changes were rare (8.9% of patients moved within the first year; 3.2% of nonurban patients moved to urban locales). The RUCA method was chosen because it is a robust and flexible method for characterizing rurality and is commonly used in rural epidemiology research (16).
Outcomes of Interest
All-cause mortality and kidney transplantation were the primary outcomes of interest. The date of death and date of kidney transplantation as documented in USRDS were used to calculate time-to-death and time-to-kidney transplantation from the documented first service date. As shown in Figure 1, patients who were older than age 75 years at dialysis initiation, were deemed unfit for transplant on their medical evidence report, or were institutionalized at initiation were excluded from transplant analysis because these patients were unlikely to undergo transplantation and therefore were not “at risk” for transplantation analysis. The transplantation rate among this excluded subpopulation was low (0.3%). Censoring was determined at the date of last follow-up or October 1, 2009, whichever occurred earlier. Patients who received a kidney transplant but later died during follow-up (3.7%) were censored at the time of their transplant for survival analysis.
Adjustment Covariates
Demographic information was obtained from the PATIENTS file and included age at initiation, sex, and race. Because USRDS does not collect individual measures of socioeconomic status, ecologic surrogates from the United States Census were used, including ZIP code–median household income and ZIP code–proportion older than age 25 with a high school diploma (17). Other measures of socioeconomic status, such as insurance coverage and employment status, were included as documented in the medical evidence report. Characteristics of kidney disease were obtained from treatment history and medical evidence report and included presumed underlying cause of ESRD, estimated GFR at initiation, and dialysis modality at initiation and at 90 days after initiation. For patients who survived less than 90 days, modality was assumed to be the same as when they initiated dialysis. Covariates related to underlying chronic medical comorbid conditions included heart failure; coronary artery disease; diabetes mellitus; hypertension; chronic obstructive pulmonary disease; cancer; history of stroke; and use of tobacco products, alcohol, or illicit drugs. Documented history of institutionalization (yes/no) and impairment of activities of daily living (yes/no) was included. The distance between a patient’s residence and dialysis unit was calculated by measuring the direct vector distance between the geo-coded coordinates for each ZIP code, which were obtained from the Zip Code Database Project (http://zips.sourceforge.net).
Statistical Analyses
Baseline characteristics were calculated for all covariates and summarized as percentages, medians with interquartile ranges, or means ± SDs, as appropriate on the basis of the variable type and frequency distribution. Comparisons across all groups were made using the Pearson chi-squared test for categorical data and the Kruskal-Wallis test for continuous data. Statistical significance was defined at a P value less than 0.05, and all tests were two tailed.
The association of nonurban residence and likelihood of PD as an initial modality and at 90 days was assessed using Poisson regression with robust variances, which provides relative risk estimates and correct confidence intervals (CIs) (18). The unadjusted association of urban, micropolitan, and rural residence with longitudinal outcomes was assessed using the Kaplan-Meier method for time-to-death and time-to-transplantation and the log-rank test for hypothesis testing. Because of a higher rate of death within the first 90 days of dialysis therapy, the time-to-death analysis was separated into early dialysis mortality (<90 days) and long-term mortality (≥90 days). The latter was assessed by excluding patients who died before 90 days. A strong interaction was noted between dialysis modality and geographic residence on long-term time-to-death (P for interaction < 0.001) and time-to-kidney transplantation (P for interaction = 0.003). Kaplan-Meier curves were generated by geographic residence with stratification by modality.
Because of the observed interaction, adjusted analyses were performed using Cox regression models with stratification by modality, yielding two models per outcome for each modality strata. This study was designed to make within-strata comparisons (i.e., rural PD versus urban PD) and not between-strata comparisons. All regression models were adjusted for demographic characteristics (age, sex, race), body mass index, United States region (by ESRD network: Northeast, 1–5; Midwest, 9–12; South, 6–8 and 13–14; and West, 15–18), socioeconomic status (insurance, employment, ZIP code–median household income), kidney disease–related factors (presumed primary cause of ESRD and estimated GFR at initiation) and comorbid conditions (as listed in “adjustment covariates”). In an effort to limit selection bias from stratification by dialysis modality, each comorbid disease was adjusted individually. All Cox models were tested for the proportional hazard assumption through use of log-log survival plots and were found to satisfy the assumption. Multicollinearity was assessed using variance inflation factors and resulted in exclusion of the education covariate in the final models because it was highly correlated with income. In addition, we performed a sensitivity analysis to evaluate the potential of informative censoring and did not find any effect on the study findings. Missing data were rare but highest among the surrogates for socioeconomic status (approximately 3% missing) and were handled through use of list-wise deletion for the adjusted regression models, with the analysis including only patients with complete data on file. Data management and statistical analysis were performed using Stata software, version 11.2 (College Station, TX).
Results
Patient Characteristics
Baseline characteristics of the cohort by geographic residence are summarized in Table 1. Rural and micropolitan patients were modestly older than urban patients, with mean ages of 64 ± 15 years and 64 ± 15 years, respectively, compared with 63 ± 15 years for urban patients. Blacks accounted for only 21.9% of micropolitan and 20.8% of rural patients compared with 31.6% of urban patients. PD was the treatment modality for 6.8% of micropolitan and 8.4% of rural patients at 90 days after initiation compared with 5.9% of urban patients (P<0.001), but HD was still the dominant treatment modality for all patients. Micropolitan and rural patients lived farther from their dialysis units, with greater distances noted in the PD subgroup. Micropolitan and rural patients had higher prevalence of comorbid conditions, including heart failure, heart disease, stroke, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease. Institutionalization or inability to perform activities of daily living was more common among micropolitan and rural patients. Micropolitan and rural communities were less wealthy and had fewer residents with a high school diploma.
Table 1.
Baseline characteristics
| Characteristic | Urban (n=163,592) | Micropolitan (n=20,811) | Rural (n=20,060) | P Value |
|---|---|---|---|---|
| Demographic | ||||
| age (yr) | 63.0±15.4 | 63.7±14.9 | 63.9±14.6 | <0.001 |
| men (%) | 55.9 | 55 | 55.4 | 0.03 |
| body mass index (kg/m2) | 27.0 (23.1–32.3) | 27.8 (23.7–33.3) | 27.9 (23.9–33.4) | <0.001 |
| race (%) | <0.001 | |||
| white | 62.9 | 74.7 | 73.9 | |
| black | 31.6 | 21.9 | 20.8 | |
| other | 5.6 | 3.5 | 5.4 | |
| any insurance coverage (%) | 92.1 | 93.3 | 93.0 | <0.001 |
| employment (%) | <0.001 | |||
| unemployed | 44.5 | 44.9 | 45.8 | |
| employed | 15.4 | 13.1 | 12.5 | |
| retired | 40.1 | 42.0 | 41.7 | |
| distance to dialysis unit (miles) | ||||
| hemodialysis | 4.2 (<2.0–7.7) | 4.7 (<2.0–14) | 18.4 (10.1–29.0) | <0.001 |
| peritoneal dialysis | 7.3 (3.8–13.1) | 22.1 (8.3–37.1) | 36.0 (23.8–54.0) | <0.001 |
| Kidney disease | ||||
| primary disease causing ESRD (%) | <0.001 | |||
| diabetes mellitus | 45.4 | 47.6 | 48.0 | |
| hypertension | 28.8 | 27.7 | 27.2 | |
| GN | 6.8 | 6.3 | 5.9 | |
| cystic disease | 2.1 | 2.2 | 2.1 | |
| other | 16.9 | 16.2 | 16.8 | |
| modality as peritoneal dialysis (%) | ||||
| at initiation | 5.7 | 6.3 | 7.9 | <0.001 |
| at 90 d | 5.9 | 6.8 | 8.4 | <0.001 |
| estimated GFR (ml/min per 1.73 m2) | 9.9 (7.2–13.2) | 10.2 (7.5–13.5) | 10.1 (7.4–13.3) | <0.001 |
| Medical comorbid conditions (%) | ||||
| congestive heart failure | 33.2 | 36.1 | 35.2 | <0.001 |
| heart disease (includes valvular) | 32.2 | 37.9 | 37.9 | <0.001 |
| stroke | 9.5 | 10.6 | 11.1 | <0.001 |
| hypertension | 84.2 | 85.4 | 85.2 | <0.001 |
| diabetes mellitus | 52.5 | 56.5 | 56.6 | <0.001 |
| chronic obstructive pulmonary disease | 8.4 | 11.9 | 12.0 | <0.001 |
| cancer | 7.2 | 7.8 | 8.3 | <0.001 |
| assistance with activities of daily living | 13.0 | 15.2 | 15.0 | <0.001 |
| institutionalized (nursing home) | 6.8 | 7.8 | 7.7 | <0.001 |
| tobacco use | 5.9 | 7.9 | 8.5 | <0.001 |
| alcohol use | 1.5 | 1.6 | 1.5 | 0.45 |
| illicit drug use | 1.5 | 1.0 | 0.8 | <0.001 |
| ZIP code–based measures of community socioeconomic status | ||||
| household income ($ thousands) | 40.2 (31.8–51.4) | 33.0 (29.3–37.9) | 30.6 (26.8–35.3) | <0.001 |
| high school diploma (% of residents) | 77.3±13.3 | 75.4±9.9 | 72.5±10.5 | <0.001 |
Statistical testing performed by Pearson chi-squared test for categorical variables and Kruskal-Wallis test for continuous variables. Values expressed with a plus/minus sign are means ± SDs. Values expressed with a range are medians (25th–75th percentile interquartile ranges). Percentages may not add to 100% because of rounding. Estimates of ZIP code income and education are based on calculations from the U.S. Census, 2000.
Dialysis Modality
As demonstrated in Table 2, micropolitan and rural residence were associated with PD as the initial and long-term treatment modality, an observation that was strengthened through adjustment for potential confounders (relative risk, 1.25 [95% CI, 1.18–1.32] and 1.57 [95% CI, 1.49–1.66], respectively, at 90 days).
Table 2.
Likelihood of peritoneal dialysis as the permanent modality by geographic residence
| Variable | Relative Risk (95% CI)a | ||
|---|---|---|---|
| Urban (n=153,611) | Micropolitan (n=19,153) | Rural (n=19,140) | |
| Likelihood of PD at dialysis initiation | |||
| unadjusted | 1.00 (reference) | 1.10 (1.04–1.17) | 1.37 (1.30–1.45) |
| adjustedb | 1.00 (reference) | 1.20 (1.13–1.27) | 1.52 (1.44–1.61) |
| Likelihood of PD at 90 d after dialysis initiation | |||
| unadjusted | 1.00 (reference) | 1.15 (1.09–1.21) | 1.42 (1.35–1.49) |
| adjustedb | 1.00 (reference) | 1.25 (1.18–1.32) | 1.57 (1.49–1.66) |
CI, confidence interval; PD, peritoneal dialysis.
By Poisson regression.
Models adjusted for demographic characteristics (age, sex, race, body mass index, United States region), socioeconomic status (insurance status, employment status, community median income), kidney disease–related features (presumed cause of ESRD, estimated GFR at initiation), and medical comorbid conditions.
Dialysis Mortality
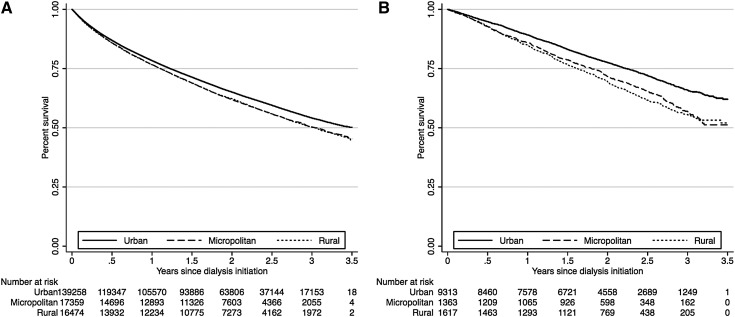
Of 204,463 patients, 17,730 (8.7%) died within the first 90 days of dialysis therapy, with an unadjusted mortality rate of 36.4 deaths per 100 person-years. Median follow-up was 2.0 years. Early mortality risk did not significantly differ by degree of rurality, regardless of modality (Supplemental Table S2). Among the 185,434 patients who survived or were not censored during the first 90 days, 71,814 (38.7%) died during the remainder of follow-up, with an unadjusted mortality rate of 21.4 deaths per 100 person-years. Kaplan-Meier survival curves for all-cause mortality after 90 days by geographic residence and stratified by modality are depicted in Figure 2. These unadjusted curves demonstrate significantly increased mortality in micropolitan and rural populations compared with urban in both HD and PD strata (log-rank P<0.001 for both strata). As summarized in Table 3 (full estimates in Supplemental Table S3), micropolitan and rural residence was associated with increased mortality within the PD strata (adjusted hazard ratio [HR], 1.21 [95% CI, 1.09–1.35], P≤0.001, and 1.12 [95% CI, 1.01–1.24], P=0.025, respectively). A sensitivity analysis examined the influence of permanent modality changes after 90 days by censoring patients at the time of their modality change. Micropolitan and rural residence remained associated with long-term mortality with PD despite this assumption (adjusted HR, 1.16 [95% CI, 1.03–1.31] and 1.14 [95% CI, 1.02–1.27], respectively). As summarized in Table 4, a subanalysis examined the relationship of distance from the dialysis unit and long-term mortality risk, which showed increasing mortality risk the farther patients lived from their dialysis unit. Mortality risk was worst among PD patients living >50 miles away (adjusted HR, 1.30 [95% CI, 1.12–1.50]).
Figure 2.
Long-term dialysis mortality by Kaplan-Meier method, stratified by treatment modality. (A) Hemodialysis. (B) Peritoneal dialysis.
Table 3.
Long-term mortality risk (>90 days) by geographic residence, stratified by modality at 90 days
| Variable | Urban | Micropolitan | Rural |
|---|---|---|---|
| Hemodialysis | |||
| patients (n) | 131,076 | 15,975 | 15,733 |
| unadjusted HR | 1.00 (reference) | 1.11 (1.08–1.14) | 1.12 (1.09–1.14) |
| HR adjusted for age, sex, race, BMI, region, SES | 1.00 (reference) | 1.02 (0.99–1.04) | 1.02 (0.99–1.05) |
| fully adjusted HRa | 1.00 (reference) | 1.00 (0.98–1.03) | 1.00 (0.98–1.03) |
| Peritoneal dialysis | |||
| patients (n) | 8885 | 1299 | 1566 |
| unadjusted HR | 1.00 (reference) | 1.34 (1.21–1.48) | 1.43 (1.31–1.57) |
| HR adjusted for age, sex, race, BMI, region, SES | 1.00 (reference) | 1.26 (1.13–1.41) | 1.18 (1.07–1.31) |
| fully adjusted HRa | 1.00 (reference) | 1.21 (1.09–1.36) | 1.12 (1.01–1.24) |
Hazard ratios by Cox regression. Numbers in parentheses are 95% confidence intervals. HR, hazard ratio; BMI, body mass index; SES, socioeconomic status.
Models adjusted for demographic characteristics (age, sex, race, BMI, United States region), SES (insurance status, employment status, community median income), kidney disease–related features (presumed cause of ESRD, estimated GFR), and medical comorbid conditions.
Table 4.
Long-term mortality risk (>90 days) by distance from dialysis unit, stratified by modality at 90 days
| Variable | <5 Miles | 5–10 Miles | 11–20 Miles | 21–50 Miles | >50 Miles |
|---|---|---|---|---|---|
| Hemodialysis | |||||
| patients (n) | 82,464 | 34,044 | 18,770 | 11,018 | 2959 |
| unadjusted HR | 1.00 (reference) | 1.04 (1.02–1.06) | 1.10 (1.08–1.13) | 1.18 (1.14–1.21) | 1.25 (1.18–1.31) |
| fully adjusted HRa | 1.00 (reference) | 1.03 (1.01–1.05) | 1.05 (1.02–1.08) | 1.08 (1.05–1.11) | 1.07 (1.01–1.13) |
| Peritoneal dialysis | |||||
| patients (n) | 3185 | 2584 | 2117 | 1948 | 734 |
| unadjusted HR | 1.00 (reference) | 1.06 (0.96–1.18) | 1.17 (1.05–1.30) | 1.33 (1.20–1.48) | 1.48 (1.28–1.71) |
| fully adjusted HRa | 1.00 (reference) | 1.04 (0.94–1.16) | 1.11 (0.99–1.24) | 1.14 (1.02–1.27) | 1.30 (1.12–1.50) |
Hazard ratios by Cox regression. Numbers in parentheses are 95% confidence intervals. HR, hazard ratio.
Models adjusted for demographic characteristics (age, sex, race, body mass index, United States region), socioeconomic status (insurance status, employment status, community median income), kidney disease–related features (presumed cause of ESRD, estimated GFR), and medical comorbid conditions.
Kidney Transplantation
A total of 68,975 (33.7%) patients were excluded from transplantation analysis because they were older than age 75 years, were deemed unfit for transplantation, or were institutionalized. The unadjusted transplantation rate in the remaining cohort was 4.5 transplants per 100 person-years. Unadjusted and adjusted HRs for time-to-transplantation are shown in Table 5 (full estimates in Supplement Table S4). Micropolitan PD patients were more likely to receive a kidney transplant after adjustment compared with urban PD patients (adjusted HR, 1.31 [95% CI, 1.13–1.51]). Micropolitan and rural HD patients were modestly more likely to undergo transplantation during follow-up compared with urban HD patients (adjusted HR, 1.19 [95% CI, 1.11–1.28] and 1.30 [95% CI, 1.21–1.40], respectively).
Table 5.
Likelihood of kidney transplantation after initiation of dialysis, stratified by modality at 90 days
| Variable | Urban | Micropolitan | Rural |
|---|---|---|---|
| Hemodialysis | |||
| patients (n) | 94,378 | 11,386 | 11,140 |
| unadjusted HR | 1.00 (reference) | 0.99 (0.93–1.06) | 1.01 (0.94–1.08) |
| HR adjusted for age, sex, race, BMI, region, SES | 1.00 (reference) | 1.18 (1.10–1.27) | 1.29 (1.20–1.38) |
| fully adjusted HRa | 1.00 (reference) | 1.19 (1.11–1.28) | 1.30 (1.21–1.40) |
| Peritoneal dialysis | |||
| patients (n) | 7761 | 1140 | 1304 |
| unadjusted HR | 1.00 (reference) | 1.04 (0.91–1.19) | 0.80 (0.69–0.92) |
| HR adjusted for age, sex, race, BMI, region, SES | 1.00 (reference) | 1.26 (1.09–1.45) | 1.01 (0.87–1.18) |
| fully adjusted HRa | 1.00 (reference) | 1.31 (1.13–1.51) | 1.06 (0.91–1.24) |
Hazard ratios by Cox regression. Numbers in parentheses are 95% confidence intervals. HR, hazard ratio; BMI, body mass index; SES, socioeconomic status.
Models adjusted for demographic characteristics (age, sex, race, BMI, United States region), SES (insurance status, employment status, community median income), kidney disease–related features (presumed cause of ESRD, estimated GFR), and medical comorbid conditions.
Discussion
We examined the association of geographic residence on longitudinal outcomes after initiation of maintenance dialysis. Our analyses suggest that micropolitan and rural patients undergoing PD have higher overall mortality risk than urban PD patients, an important finding considering that these patients are more likely to receive PD. This association was not observed in HD patients. Both modalities are associated with higher mortality risk the farther patients live from their dialysis unit, with worse outcomes among PD patients living >50 miles away. Micropolitan and rural patients were as likely or more likely to receive a kidney transplant compared with urban patients, regardless of modality.
To our knowledge, our study is among the first to describe increased mortality among United States micropolitan and rural PD patients based on residential location, although similar observations have been suggested previously. Tonelli and colleagues described an association between remoteness and increased mortality among Canadian PD patients, with a 15% increased risk for death among patients living >300 km away (4). Although this is an important finding, it has limited generalizability to the United States population because of differences in healthcare delivery between the United States and Canada. Mehrotra and colleagues (8) used private dialysis-unit data and described an association between rural PD unit care and increased mortality; however, this study lacked data on patient residential location and did not examine rural PD patients who received care from more urban units. Our findings along with these previous studies create questions as to the underlying mechanisms for these associations.
Rural and micropolitan patients may choose PD because of a lack of alternative options. Although HD is the dominant strategy nationally, micropolitan and rural patients are more likely to be treated with PD compared with urban patients (6,9), a finding that was corroborated in this study. The underlying reasons are probably diverse, although limited availability of in-center HD units in remote areas and difficulty with transportation are likely reasons (19). This mismatch between modality and patient, a form of selection bias, could lead to further imbalances in comorbid conditions in addition to exposing patients to a modality they may not be comfortable with. This could potentially increase the risk for dialysis-related complications.
Our study demonstrated that PD patients residing in micropolitan and rural areas live farther from their dialysis unit and that farther distance is associated with higher mortality risk. Increased distance could lead to deficiencies in PD training, dietary education, and response time for complications. Home visits and PD training reinforcement, which have reduced peritonitis episodes in several observational studies (20,21), are difficult to provide for patients living farther away. In addition, local healthcare factors could greatly affect micropolitan and rural PD patients. Rural hospitals are poorly equipped to handle patients with acute cardiovascular events or sepsis, resulting in higher 30-day mortality compared with urban hospitals (22). Rural areas have reduced access to medical subspecialists (23) with clustering of providers in urban centers (24). This study was not designed to examine these factors, and further research is necessary to understand the potential barriers that could contribute to the higher mortality risk observed in micropolitan and rural PD patients.
Although it is important to recognize that micropolitan and rural PD patients may have higher long-term mortality risk, it does not mean PD is an inappropriate modality. PD allows considerable flexibility, allowing individualized care while reducing the number of dialysis unit visits, which could be burdensome for rural patients on hemodialysis. Until additional research can be performed to elucidate the underlying mechanisms of this mortality risk, dialysis units should focus on providing optimum support to rural patients, ensuring delivery of high-quality PD through nursing communication, home visits, and training reinforcement. This is especially relevant given recent changes in Medicare reimbursement due to bundling, which are expected to increase PD use nationally as a result of projected cost savings (25).
The lack of differences in outcomes among urban and nonurban HD patients is consistent with previous work (9). O’Hare and colleagues examined incident dialysis patients from 1995 to 2002 and found no substantial difference in mortality (9). This observation may reflect the more equitable access HD patients have to their dialysis unit, which is an important limitation in home dialysis. In addition, it is important to acknowledge the impact of vascular access on HD mortality, as patients with central venous catheters have higher dialysis mortality than patients with an arteriovenous fistula or graft (26,27). There was no meaningful difference in the prevalence of catheter use at dialysis initiation between urban and nonurban populations in our cohort; thus, this factor was unlikely to influence our results.
Kidney transplantation should share similar barriers to access, as discussed previously. One study using data from the Organ Procurement and Transplantation Network supported these concerns because rural patients appeared to receive solid organ transplants (including kidney) at modestly lower rates compared with an urban population (10). These findings came into question when a subsequent study using data from USRDS and the United Network for Organ Sharing concluded the contrary: that rural patients who ultimately receive dialysis therapy receive kidney transplants at similar rates or perhaps higher rates than urban patients (11). Our cohort study, which used more recent USRDS data, appears to support the latter conclusion. This observation may seem counterintuitive but could be related to differences in rural case-mix because those who are eligible for transplant may be healthier. Racial segregation within urban areas, which is associated with decreased kidney transplantation, could influence the comparison group (28). Finally, there is always the potential for residual confounding.
This study had important limitations. The classification of rural and micropolitan status was defined at the time of initiation and is a potential source for misclassification. The data source was retrospective and limited by the data measurement techniques of USRDS. Dialysis modality was assessed only at two time points; thus, persistent modality changes after 90 days could lead to additional misclassification. Most late changes in dialysis modality are from PD to HD, and more often occur because of changes in medical condition. This may create the potential for selection bias and residual confounding (29). In addition, the use of community-level surrogates for socioeconomic status could introduce information bias. The source of data on medical comorbid conditions was the medical evidence report, which can have inconsistencies and errors, especially when completed by nonphysicians (30). Although every effort was made to reduce the effect of confounding, unmeasured confounders may limit the interpretation of the results. Finally, the results reported are associations and cannot imply causality.
In conclusion, micropolitan and rural residence is associated with higher mortality in patients receiving PD, which may be related to reduced access to healthcare providers and dialysis programs, barriers to high-quality longitudinal PD care, and lack of local resources for dialysis support and acute issues. These data emphasize the need for more research related to appropriate allocation of CKD patients to dialysis modality options. Nonurban patients receive kidney transplants at rates similar to or higher than those in urban patients, suggesting remote living is not a significant barrier in those eligible to receiving kidney transplant for ESRD.
Disclosures
None.
Acknowledgments
S.M. is supported by an institutional training grant from the National Institutes of Health (T32 DK007569). K.L.C. and T.A.I. are supported by grants from the National Institutes of Health (K23 DK080952 and K24 DK062849, respectively). Funding for patient-level data, provided by the USRDS, was supported by Vanderbilt CTSA Grant UL1 RR024975-01 from the National Center for Research Resources, National Institutes of Health.
This paper does not represent the opinion of the USRDS or the National Institutes of Health. S.M. and K.L.C. assume full responsibility for data management and statistical analysis.
The findings of this study were presented as a poster at the American Society of Nephrology Annual Meeting, November 10, 2011, Philadelphia, Pennsylvania.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10831011/-/DCSupplemental.
See related editorial, “Dialysis and Mortality: Does It Matter Where You Live?,” on pages 1055–1057.
References
- 1.US Census Bureau. Census 2000 Summary File (SF-1) 100-Percent Data. Available at: http://www.census.gov/census2000/sumfile1.html Accessed March 8, 2011 [Google Scholar]
- 2.Healthy People 2020: Foundation Health Measures US Department of Health and Human Services, 2010. Available at: http://www.healthypeople.gov/2020/about/DisparitiesAbout.aspx Accessed August 10, 2011 [Google Scholar]
- 3.Eberhardt MS, Pamuk ER: The importance of place of residence: Examining health in rural and nonrural areas. Am J Public Health 94: 1682–1686, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonelli M, Hemmelgarn B, Culleton B, Klarenbach S, Gill JS, Wiebe N, Manns B, Alberta Kidney Disease Network : Mortality of Canadians treated by peritoneal dialysis in remote locations. Kidney Int 72: 1023–1028, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Moist LM, Bragg-Gresham JL, Pisoni RL, Saran R, Akiba T, Jacobson SH, Fukuhara S, Mapes DL, Rayner HC, Saito A, Port FK: Travel time to dialysis as a predictor of health-related quality of life, adherence, and mortality: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 51: 641–650, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Wang V, Lee SY, Patel UD, Weiner BJ, Ricketts TC, Weinberger M: Geographic and temporal trends in peritoneal dialysis services in the United States between 1995 and 2003. Am J Kidney Dis 55: 1079–1087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker DR, Inglese GW, Sloand JA, Just PM: Dialysis facility and patient characteristics associated with utilization of home dialysis. Clin J Am Soc Nephrol 5: 1649–1654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehrotra R, Story K, Guest S, Fedunyszyn M: Neighborhood location, rurality, geography, and outcomes of peritoneal dialysis patients in the United States [published online ahead of print December 1, 2011]. Perit Dial Int doi: 10.3747/pdi.2011.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Hare AM, Johansen KL, Rodriguez RA: Dialysis and kidney transplantation among patients living in rural areas of the United States. Kidney Int 69: 343–349, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Axelrod DA, Guidinger MK, Finlayson S, Schaubel DE, Goodman DC, Chobanian M, Merion RM: Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA 299: 202–207, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M, Klarenbach S, Rose C, Wiebe N, Gill J: Access to kidney transplantation among remote- and rural-dwelling patients with kidney failure in the United States. JAMA 301: 1681–1690, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM: Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol 20: 1333–1340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Measuring rurality: rural-urban commuting area codes. Economic Research Service, United States Department of Agriculture. Available at: http://www.ers.usda.gov/briefing/Rurality/RuralUrbanCommutingAreas Accessed: March 8, 2011
- 14.ZIP code RUCA approximation methodology. Washington Wyoming Alaska Montana Idaho Rural Health Research Center. Available at: http://depts.washington.edu/uwruca/ruca-data.php Accessed: March 8, 2011.
- 15.Using RUCA data. Washington Wyoming Alaska Montana Idaho Rural Health Research Center. http://depts.washington.edu/uwruca/ruca-uses.php Accessed: June 7, 2011
- 16.Hart LG, Larson EH, Lishner DM: Rural definitions for health policy and research. Am J Public Health 95: 1149–1155, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Census Bureau: Census 2000 Summary File (SF-3) Sample Data: Median Household Income (P053) and Education Attainment for Population 25 Years or Older (P037). Available at: http://www.census.gov/census2000/sumfile3.html Accessed March 8, 2011
- 18.Barros AJ, Hirakata VN: Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 3: 21, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennette CE, Vupputuri S, Hogan SL, Shoham DA, Falk RJ, Harward DH: Community perspectives on kidney disease and health promotion from at-risk populations in rural North Carolina, USA. Rural Remote Health 10: 1388, 2010 [PubMed] [Google Scholar]
- 20.Bordin G, Casati M, Sicolo N, Zuccherato N, Eduati V: Patient education in peritoneal dialysis: An observational study in Italy. J Ren Care 33: 165–171, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Castro MJ, Celadilla O, Muñoz I, Martínez V, Mínguez M, Auxiliadora Bajo M, del Peso G: Home training experience in peritoneal dialysis patients. EDTNA ERCA J 28: 36–39, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Joynt KE, Harris Y, Orav EJ, Jha AK: Quality of care and patient outcomes in critical access rural hospitals. JAMA 306: 45–52, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook NL, Hicks LS, O’Malley AJ, Keegan T, Guadagnoli E, Landon BE: Access to specialty care and medical services in community health centers. Health Aff (Millwood) 26: 1459–1468, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Rucker D, Hemmelgarn BR, Lin M, Manns BJ, Klarenbach SW, Ayyalasomayajula B, James MT, Bello A, Gordon D, Jindal KK, Tonelli M: Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney Int 79: 210–217, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Golper TA, Guest S, Glickman JD, Turk J, Pulliam JP: Home dialysis in the new USA bundled payment plan: Implications and impact. Perit Dial Int 31: 12–16, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Lacson E, Jr, Lazarus JM, Himmelfarb J, Ikizler TA, Hakim RM: Balancing Fistula First with Catheters Last. Am J Kidney Dis 50: 379–395, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Pastan S, Soucie JM, McClellan WM: Vascular access and increased risk of death among hemodialysis patients. Kidney Int 62: 620–626, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O’Hare AM: Geography matters: Relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med 146: 493–501, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Jaar BG, Plantinga LC, Crews DC, Fink NE, Hebah N, Coresh J, Kliger AS, Powe NR: Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: A prospective study. BMC Nephrol 10: 3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Layton JB, Hogan SL, Jennette CE, Kenderes B, Krisher J, Jennette JC, McClellan WM: Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol 5: 2046–2052, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]