Summary
Background and objectives
AKI leads to increased morbidity and mortality and progression to chronic kidney injury is a frequent consequence of AKI. Surgical treatment of mesothelioma is associated with increased risk for kidney injury. However, sustained kidney injury may limit therapeutic options for treating residual cancer. This study hypothesized that patients with significant serum creatinine (sCr) elevation within 48 hours of surgery would be at risk for sustained kidney injury. The goal was to determine the best acute sCr measure predictive of sustained kidney injury defined as a 50% increase in sCr from baseline measured 2–4 weeks after surgery.
Design, setting, participants, & measurements
In a prospective, observational cohort of surgical patients with mesothelioma, receiver operator characteristic curves were generated for the 24- and 48-hour absolute difference and relative sCr change over baseline in the derivation cohort (n=279). The prediction was tested in a validation cohort (n=207). The ability of various other AKI definitions to predict sustained kidney injury was evaluated.
Results
Sustained kidney injury occurred in 9.8% of patients in the derivation cohort. A ≥59% increase in sCr 48 hours after surgery was most predictive of sustained kidney injury (c statistic=0.78). Among other AKI definitions, a sCr increase of 0.3 mg/dl in 24 hours or 0.5 mg/dl increase in 48 hours (Waikar and Bonventre criteria) also reliably predicted sustained kidney injury.
Conclusions
Development of clinically significant sustained kidney injury can be predicted by acute postoperative sCr elevation in patients treated for mesothelioma.
Introduction
Perioperative AKI is a complex disorder characterized by rapid decline in GFR, electrolyte and acid-base imbalance, accumulation of nitrogenous waste products, and increased morbidity and mortality (1–5). Patients who survive AKI are at high risk for developing long-term adverse outcomes such as CKD, ESRD, and death, even though their serum creatinine (sCr) values may have normalized (6–8). Although studies support a causal link between AKI and CKD, few clinical studies have addressed methods of predicting subchronic, mid-term kidney injury (sustained kidney injury) in which progression to CKD or ESRD can potentially be prevented or halted (1).
The incidence of severe AKI (sCr elevation >3 times upper limit of normal) is nearly 10% in patients undergoing surgical resection of malignant pleural mesothelioma (9–11), a procedure in which intraoperative cisplatin chemotherapy is commonly administered. Because of the high risk of AKI and the devastating consequences of CKD and ESRD in this cancer cohort, we studied the ability of early postoperative changes in sCr to predict longer-term changes in renal function. We hypothesized that acute sCr elevation, within 24–48 hours after surgery, can predict sustained, clinically significant decline in kidney function defined as doubling of sCr from baseline, 2–4 weeks after surgery (12).
Materials and Methods
Data Sources, Data Collection, and Patient Characteristics
We studied a cohort of patients undergoing surgical resection (extrapleural pneumonectomy) for the treatment of malignant pleural mesothelioma at the Brigham and Women’s Hospital between 1998 and 2009. Patient data (demographics, clinical details of the operative procedure and postoperative care, and laboratory values) were obtained from our electronic medical records. Clinical criteria for surgical therapy included the following: age ≥18 years, Karnofsky performance status >70, normal liver function (aspartate aminotransferase <80 IU/L, total bilirubin <1.9 mg/dl, prothrombin time <15 seconds), sCr <1.5 mg/dl, estimated GFR (eGFR) >45 ml/min, room air arterial PaCO2 <45 mmHg, room air PaO2 >65 mmHg, predicted postoperative FEV1 >0.8 L by pulmonary function tests and quantitative ventilation-perfusion scans, and grossly normal cardiac function by electrocardiography and echocardiography (left ventricular ejection fraction >45%) (13,14).
Patients scheduled to receive optional administration of intraoperative intracavitary heated cisplatin chemotherapy were admitted to the hospital the night before surgery. They were hydrated with 150 ml/h of normal saline, were given sodium thiosulfate intraoperatively to promote binding of the absorbed cisplatin (4 g/m2 followed by a 12 g/m2 infusion), and were given liberal fluid replacement intraoperatively to maintain intravascular volume expansion.
All patients had a thoracic epidural catheter placed for pain control and the surgical procedure was performed under general anesthesia (15). The procedure consists of an en bloc resection of the lung, pleura, ipsilateral diaphragm, and pericardium, followed by reconstruction of the diaphragm and pericardium with a prosthetic patch made of Gore-Tex (W.L. Gore and Associates, Flagstaff, AZ) (16). After resection of the entire specimen, some patients received intraoperative intracavitary lavage with a solution of 225 mg/m2 cisplatin in dialysate (Baxter, Deerfield, IL) maintained at 42°C, as previously described (10). This was given because pharmacokinetic studies showed that patients could benefit from the high local concentration of cisplatin while minimizing systemic toxicity (only 3% of intracavitary cisplatin is absorbed systemically) (17).
Serum creatinine was measured at our central clinical laboratory using a kinetic colorimetric assay based on the Jaffe method. The isotope dilution mass spectrometry traceable calibrator was introduced in December 2009 (Roche/Hitachi Cobas c Systems) (18). The coefficient of variance for both methods of measuring sCr was 1%–3%. All patients had blood drawn for sCr during their first 4–5 postoperative days. Baseline sCr was defined as the most recent sCr available before surgery and within 2 weeks of surgery. Preoperative GFR was estimated by the isotope dilution mass spectrometry–traceable Modification of Diet in Renal Disease study equation as recommended by the National Kidney Foundation’s Kidney Disease Outcomes Initiative clinical practice guidelines (19).
Determining Sustained Kidney Injury as an Outcome Measure
We defined sustained kidney injury as doubling of sCr (relative to preoperative baseline) during the 2- to 4-week period after surgery or the latest sCr value before discharge. This specific time was chosen because we considered it to reflect a subacute, ongoing injury, which might be predictive of long-term outcome or the potential for intervention that may mitigate kidney disease progression.
The maximum sCr value during the 2- to 4-week postoperative period in patients who had >1 measurement was used. The assumption that the latest sCr value before discharge represented the patients’ stable kidney function was confirmed by comparing their last sCr value available before discharge (used for our analysis) with sCr values available beyond the fourth postoperative week (obtained during follow-up visits). Of 173 patients, 93 had sCr values beyond the fourth postoperative week. The mean difference between the discharge sCr and that obtained later than 4 weeks after surgery was 0.17 (±0.18).
Statistical Analyses
All patients undergoing extrapleural pneumonectomy were included, except two patients who died within 48 hours of surgery. We divided the cohort by year of surgery into a derivation cohort (1998–2004; n=279) and a validation cohort (2005–2008; n=207).
Receiver operator characteristic (ROC) curves were generated to examine the diagnostic ability of 24- and 48-hour changes in sCr over baseline to identify sustained kidney injury. The ROC curve represents a graphical relationship between sensitivity (true positive) and 1 − specificity (false positive). The optimal sCr cutoffs were identified by the point on the ROC curve where the distance from the ideal point (sensitivity=1 and specificity=1) was the shortest. The areas under the ROC curves were compared by a nonparametric method. The discriminative ability of the optimal cutoff for each measure was tested in the validation cohort. The c statistics and their 95% confidence intervals were determined.
To characterize the time course of sustained kidney injury, we followed the trend for mean sCr values plotted over time for all patients who had 2- to 4-week postoperative sCr values (n=313). We plotted the changes of mean sCr from baseline measured at various postoperative times up to 100 days. We then used an independent two-sample t test to compare the mean sCr values between patients who developed sustained kidney injury and those who did not. In addition, we examined the association of median length of both the intensive care unit (ICU) and hospital stay with sustained kidney injury and AKI. We compared median length of stay between the two groups using the Wilcoxon two-sample test.
We then compared the performance of the criteria we derived to various other criteria used to characterize AKI to predict the risk of sustained kidney injury, such as the Acute Kidney Injury Network (AKIN); risk, injury, failure, loss, ESRD (RIFLE); and Waikar and Bonventre criteria (20–22). We used a >1.5-fold increase in sCr within 48 hours (RIFLE risk criteria) as our reference and compared the c statistics. We then computed the net reclassification index (NRI) and the integrated discrimination improvement (IDI) to compare the ability of the various criteria to predict sustained kidney injury (23,24). Specifically, we used various formerly tested AKI criteria and our optimal sCr cutoff to ascertain whether any of these criteria are equivalent or better in assigning patients to be at risk for developing sustained kidney injury.
Statistical analysis was performed using SAS version 9.2 and JMP version 8.0 (SAS Institute, Cary, NC). Summaries of continuous variables are presented as mean (SD) and categorical variables are reported as absolute numbers (percentages). The level of statistical significance used was 0.05.
Results
Incidence of Sustained Kidney Injury and Clinical Characteristics of the Patient Cohorts
Sustained kidney injury, defined as doubling of sCr measured 2–4 weeks after surgery, occurred in 8.9% (n=25) of patients in the derivation cohort and 10.1% (n=21) in the validation cohort. There were no differences in baseline characteristics (including comorbid factors) between the derivation and validation cohorts except for age and baseline sCr; in addition, more patients in the validation cohort received intraoperative cisplatin (P<0.001) (Table 1). All patients except six had a preoperative baseline sCr <1.5 mg/dl. One patient in the derivation cohort and five patients in the validation cohort had a preoperative baseline sCr >1.5 mg/dl (range, 0.3–1.98 mg/dl) and none of these patients developed sustained kidney injury. Baseline preoperative characteristics of patients in the derivation cohort who developed sustained kidney injury and those who did not were similar except for age, in which those who developed the outcome were older by 4.3 years (Table 2). Patients who developed sustained kidney injury in the derivation cohort had a higher incidence of receiving intracavitary cisplatin (P=0.01) and requiring renal replacement therapy (RRT) (P<0.001), and had higher sCr values at 2–4 weeks after surgery (mean 3.09 mg/dl; P<0.001) and lower eGFR values (mean 29.3 ml/h per 1.73 m2; P<0.001) (Table 2).
Table 1.
Perioperative characteristics of the derivation and validation cohorts
| Characteristic | Derivation Cohort (n=279) | Validation Cohort (n=207) | P Value |
|---|---|---|---|
| Age (yr), mean (SD) | 58.8 (9.5) | 61.5 (9.2) | 0.002 |
| Sex (male), n (%) | 219 (78.5) | 168 (81.2) | 0.47 |
| Height (in), mean (SD) | 68.7 (3.7) | 68.8 (3.6) | 0.93 |
| Weight (kg), mean (SD) | 80.5 (16.1) | 81.0 (16.3) | 0.74 |
| Body mass index, mean (SD) | 26.4 (4.4) | 26.4 (4.2) | 0.94 |
| Diabetes requiring medication, n (%) | 7 (2.5) | 4 (1.9) | 0.77 |
| Diabetes not requiring medication, n (%) | 2 (0.7) | 6 (2.9) | 0.08 |
| Hypertension, n (%) | 73 (26.2) | 63 (30.1) | 0.33 |
| Received intracavitary cisplatin, n (%) | 113 (40.5) | 165 (79.0) | <0.001 |
| Preoperative sCr, mean (SD) | 0.87 (0.21) | 0.90 (0.23) | 0.04 |
| Preoperative eGFR, mean (SD)a | 91.49 (27.7) | 86.6 (22.8) | 0.02 |
| Need for renal replacement therapy, n (%) | 3 (1.1) | 9 (4.4) | 0.02 |
| Doubling of sCr at 2–4 wk, n (%) | 25 (8.9) | 21 (10.1) | 0.66 |
| 30-d mortality, n (%) | 9 (3.2) | 4 (1.9) | 0.38 |
sCr, serum creatinine; eGFR, estimated GFR.
Calculated from sCr by the following Modification of Diet in Renal Disease formula: eGFR=175 × (sCr)−1.54 × (age) −0.203 × 0.742 (if female) × 1.212 (if African American), expressed as milliliters per hour per 1.73 m2.
Table 2.
Perioperative characteristics of patients who did and did not develop SKI in the derivation cohort (n=279)
| Characteristic | SKI (n=25) | No SKI (n=254) | P Value |
|---|---|---|---|
| Age (yr), mean (SD) | 62.8 (8.7) | 58.5 (9.5) | 0.02 |
| Sex (male), n (%) | 19 (6.0) | 200 (78.7) | 0.75 |
| Height (in), mean (SD) | 69.1 (2.8) | 68.7 (3.7) | 0.52 |
| Weight (kg), mean (SD) | 81.6 (16.9) | 80.4 (16.0) | 0.73 |
| Body mass index, mean (SD) | 26.5 (4.9) | 26.4 (4.4) | 0.89 |
| Diabetes requiring medication, n (%) | 1 (4.0) | 6 (2.4) | 0.49 |
| Diabetes not requiring medication, n (%) | 0 (0.0) | 1 (0.8) | 1.00 |
| Hypertension, n (%) | 7 (28.0) | 66 (26.0) | 0.83 |
| Received intracavitary cisplatin, n (%) | 16 (64.0) | 97 (38.2) | 0.01 |
| Preoperative sCr, mean (SD)a | 0.84 (0.26) | 0.87 (0.20) | 0.55 |
| Preoperative eGFR, mean (SD) | 96.3 (34.1) | 91.0 (27.0) | 0.46 |
| 2- to 4-wk sCr, mean (SD) | 3.09 (2.46) | 0.92 (0.28) | <0.001 |
| 2- to 4-wk eGFR, mean (SD) | 29.3 (16.8) | 87.0 (27.3) | <0.001 |
| Need for renal replacement therapy, n (%) | 3 (12.0) | 0 (0.0) | <0.001 |
| 30-d mortality, n (%) | 2 (8.0) | 7 (2.8) | 0.19 |
SKI, sustained kidney injury; sCr, serum creatinine; eGFR, estimated GFR.
Calculated from sCr by the following Modification of Diet in Renal Disease formula: eGFR=175 × (sCr)−1.54 × (age) −0.203 × 0.742 (if female) × 1.212 (if African American), expressed as milliliters per hour per 1.73 m2.
The overall incidence of RRT was 2.5% (12 of 486) (Table 1). There were three patients in the derivation cohort and nine in the validation cohort. The 30-day mortality rate was 3.1% (n=15 of 488). Two patients died within 48 hours of surgery and an additional six patients died within 2 weeks. All patients who died within 2 weeks of surgery had a preoperative baseline sCr <0.9 mg/dl except one, who had a sCr of 1.6 mg/dl; three patients had no increase in their sCr. Of the remaining three patients with increased sCr, only one patient required RRT and died on postoperative day 12.
Acute Elevation of sCr Predicts Sustained Kidney Injury
Using ROC curve analyses in our derivation cohort, we found that a 59% increase sCr (1.59-fold) at 48 hours had the highest area under the ROC curve (AUCROC=0.798; 68% sensitivity and 87% specificity) (Table 3). A 0.30-mg/dl increase in sCr at 48 hours was similar (AUCROC=0.779; 84% sensitivity and 63% specificity).
Table 3.
sCr increases predicting the development of SKI in the derivation cohort
| sCr-Based Optimal Diagnostic Thresholds | Optimal Cutoff | Inclusive (n=279) | ||
|---|---|---|---|---|
| Sensitivity | Specificity | AUCROC (95% CI) | ||
| 24-h sCr differencea (mg/dl increase) | 0.30 | 0.56 | 0.80 | 0.70 (0.58–0.81) |
| 24-h sCr ratiob (-fold increase) | 1.44 | 0.52 | 0.87 | 0.71 (0.60–0.83) |
| 48-h sCr differencec (mg/dl increase) | 0.30 | 0.84 | 0.63 | 0.78 (0.68–0.88) |
| 48-h sCr ratiod (-fold increase) | 1.59 | 0.68 | 0.87 | 0.80 (0.70–0.90) |
Unadjusted estimates are reported with 95% CIs. sCr, serum creatinine; SKI, sustained kidney injury; AUCROC, area under the receiver operating characteristic curve; 95% CI, 95% confidence interval.
24-h sCr – preoperative baseline sCr.
24-h sCr/preoperative baseline sCr.
48-h sCr – preoperative baseline sCr.
48-h sCr/preoperative baseline sCr.
We confirmed our findings in the validation cohort, in which the 59% increase in sCr performed best for identifying patients at risk for sustained kidney injury (Table 4). The 59% increase in sCr had a higher c statistic (0.798 versus 0.759) compared with the 0.30 mg/dl increase at 48 hours. It correctly predicted 15 of 21 patients who had sustained kidney injury and 164 of 186 patients who did not (71% sensitivity and 88% specificity). The positive predictive value was 41% and the negative predictive value was 96%.
Table 4.
Performance of our model in the validation cohort (n=207) using sCr thresholds obtained from the derivation study to predict SKI
| sCr Diagnostic Markersa | Patients Predicted to Have SKI (n=21) | Patients Predicted with No SKI (n=186) | Sensitivity | Specificity | PPV | NPV | c Statistic (95% CI) |
|---|---|---|---|---|---|---|---|
| 24-h sCr differenceb of 0.30 mg/dl | 14 | 152 | 0.67 | 0.82 | 0.29 | 0.96 | 0.74 (0.64–0.85) |
| 24-h sCr ratioc of 1.44-fold increase | 10 | 169 | 0.48 | 0.91 | 0.37 | 0.94 | 0.69 (0.58–0.80) |
| 48-h sCr differenced 0.30 mg/dl | 19 | 114 | 0.90 | 0.61 | 0.21 | 0.98 | 0.76 (0.69–0.83) |
| 48-h sCr ratioe of 1.59-fold increase | 15 | 164 | 0.71 | 0.88 | 0.41 | 0.96 | 0.80 (0.70–0.90) |
sCr, serum creatinine; SKI, sustained kidney injury; 95% CI, 95% confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Cutoffs derived from receiver operating characteristic analysis of the derivation cohort.
24-h sCr – preoperative baseline sCr.
24-h sCr/preoperative baseline sCr.
48-h sCr – preoperative baseline sCr.
48-h sCr/preoperative baseline sCr.
Serial Creatinine Measurements Identify Distinct Cohorts with and without Sustained Kidney Injury
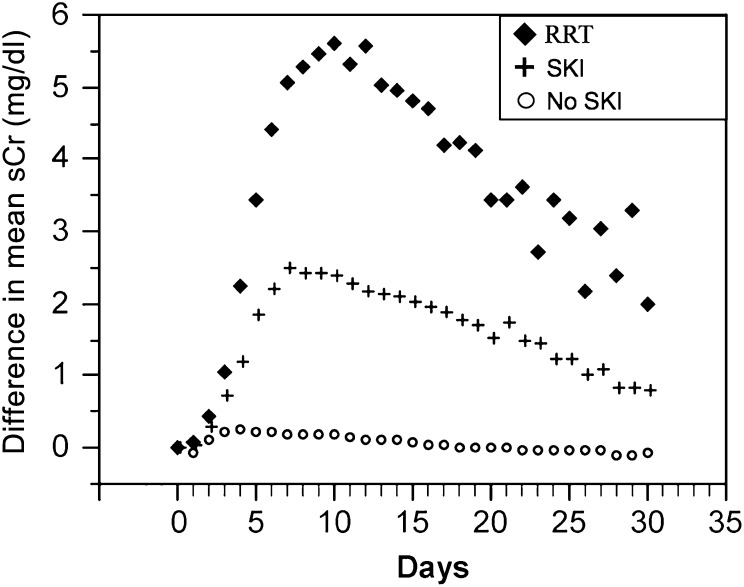
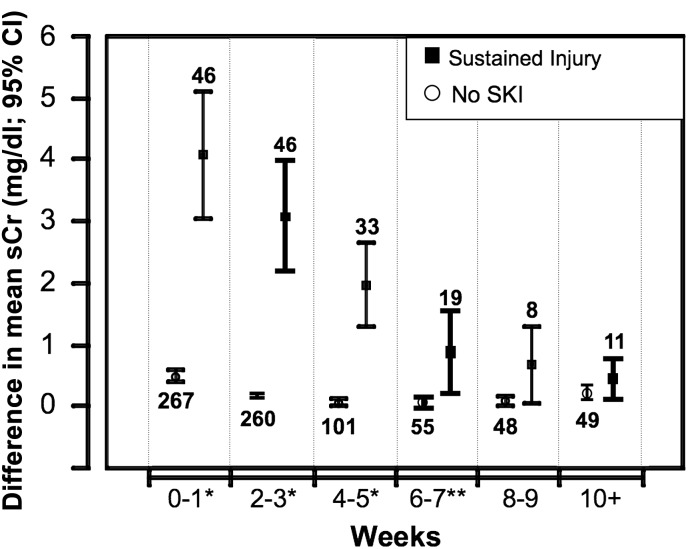
To characterize the time course of sustained kidney injury, we followed the trend for mean sCr values plotted over time (n=313). We plotted the changes of mean postoperative sCr to 30 days (Figure 1) and up to 100 days (Figure 2) and we identified three distinct cohorts. The nonsustained injury group showed a slight rise in sCr that normalized within a short period of time, whereas the sustained kidney injury group showed an immediate and larger magnitude of sCr increase that peaked on day 8 (mean sCr 3.3 mg/dl). We also plotted the sCr changes for 11 patients who required RRT. This curve had an even steeper initial slope that peaked on postoperative day 8 (mean sCr 5.6 mg/dl) (Figure 1). Furthermore, among patients who survived 100 days, the separation between the cohorts with and without sustained kidney injury persisted (Figure 2). For weeks 0–7, there was a significant difference in the mean sCr values between the two cohorts (P<0.001 for weeks 0–5; P<0.02 for weeks 6–7). However, beyond week 8, we were unable to demonstrate a significant difference although the number of patients was small.
Figure 1.
Short-term changes of mean sCr over time for patients undergoing extrapleural pneumonectomy (n=313). sCr changes that occurred over the first 30 postoperative days were plotted. For every time point, the increase in sCr was plotted (after subtracting the mean baseline sCr value from the mean sCr value of that particular time point) for both the group that showed SKI (+) and the group that did not develop SKI (○). The sCr trend for 11 patients who required RRT is plotted (◆). The x axis denotes the postoperative day. sCr, serum creatinine; SKI, sustained kidney injury; RRT, renal replacement therapy.
Figure 2.
Long-term sCr trends of patients undergoing extrapleural pneumonectomies. sCr changes that occurred over the first 100 postoperative days are plotted. As the number of patients with data points declined with time, we plotted the mean sCr values between 2-week periods after subtracting the mean preoperative baseline sCr. The x axis represents the time since preoperative baseline divided into 2-week segments as follows: 0–1 week, n=313; 2–3 weeks, n=306; 4–5 weeks, n=134; 6–7 weeks, n=74; 8–9 weeks, n=59; and ≥10 weeks (10 weeks–100 days), n=60. Numbers above and below the bars indicate the number of patients during that time period. *Denotes that patients during this time period had a P<0.001 between those who developed SKI and those who did not. **During the 6- to 7-week time frame, the difference between the two groups was still significant (P<0.02). sCr, serum creatinine; SKI, sustained kidney injury; RRT, renal replacement therapy.
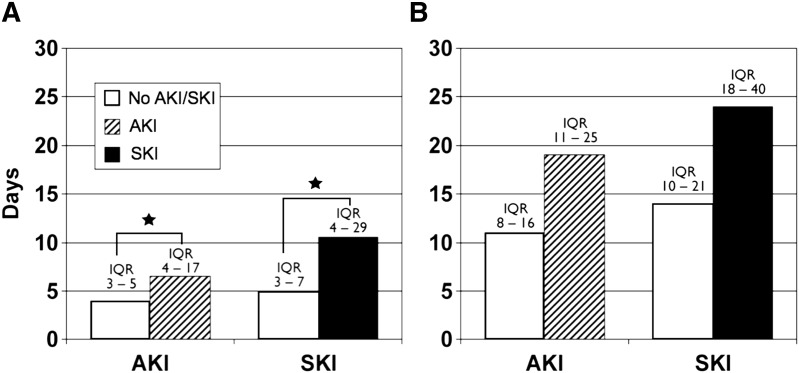
Effect of Sustained Kidney Injury on Length of Hospital Stay and ICU Stay
Patients who developed initial AKI and continued to show signs of sustained kidney injury, defined by our criteria of >1.59 increase in sCr from baseline at 48 hours postoperatively, had a significantly longer ICU stay. However, the length of hospital stay was not significantly different (Figure 3).
Figure 3.
Effect of AKI and SKI on hospital and ICU length of stay. (A) Median ICU length of stay (days) for patients with AKI and SKI. The IQRs for each cohort are noted above the bars. Compared with those with no AKI or SKI, the patients who developed AKI or SKI had significantly longer length of ICU stays (P<0.001). (B) Median length of hospital stay (days) for the same cohorts of patients with AKI and SKI. The IQRs for each cohort are noted above the bars. *P<0.001. Although the hospital length of stay was prolonged for patients with both AKI and SKI, this difference did not reach statistical significance. SKI, sustained kidney injury; ICU, intensive care unit; IQR, interquartile range.
Sustained Kidney Injury Is Better Predicted by Derived Criteria Compared with Other Criteria of Kidney Injury
We compared various criteria (RIFLE, AKIN, and Waikar and Bonventre) to our prediction model (1.59-fold increase of sCr from baseline at 48 hours). We used the RIFLE risk criteria (i.e., 1.5-fold increase) as our reference. We found that our prediction model had the highest c statistic (0.78); compared with the RIFLE risk (c statistic=0.73), the difference was statistically significant (P<0.001) (Table 5).
Table 5.
Comparison of various sCr-based criteria to the RIFLE risk criteria (48-h ratio of 1.5-fold increase)
| Criterion | Sensitivity | Specificity | c Statistic (95% CI) | P Valuea | IDI (SEM) | P Value | NRI (SEM) | P Value |
|---|---|---|---|---|---|---|---|---|
| 48-h sCr ratio of 1.50-fold increaseb | 0.68 | 0.79 | 0.73 (0.64–0.83) | Reference | ||||
| 24-h sCr differenced of 0.3 mg/dl | 0.56 | 0.80 | 0.68 (0.58–0.78) | 0.13 | 0.01 (0.007) | 0.07 | −0.03 (0.07) | 0.64 |
| 24-h sCr ratioe of 1.44-fold increase | 0.52 | 0.87 | 0.70 (0.60–0.80) | 0.34 | 0.02 (0.01) | 0.11 | −0.07 (0.08) | 0.43 |
| 48-h sCr differencef 0.3 mg/dlg | 0.84 | 0.63 | 0.73 (0.65–0.81) | 0.99 | 0.01 (0.01) | 0.007 | 0.01 (0.007) | 0.08 |
| 48-h sCr ratioc of 1.59-fold increase | 0.68 | 0.87 | 0.78 (0.68–0.87) | <0.001 | 0.08 (0.08) | <0.001 | 0.08 (0.02) | <0.001 |
| 24-h sCr ratioe of 1.50-fold increase | 0.48 | 0.89 | 0.68 (0.58–0.79) | 0.23 | 0.01 (0.01) | 0.23 | −0.10 (0.09) | 0.27 |
| 24-h sCr differenced of 0.3 mg/dl or 48-h sCr differencef of 0.5 mg/dlh | 0.68 | 0.75 | 0.72 (0.62–0.81) | 0.12 | 0.02 (0.02) | <0.001 | 0.01 (0.01) | <0.001 |
| 24-h sCr difference of 0.3 mg/dl or 48-h sCr difference of 0.3 mg/dl | 0.63 | 0.84 | 0.73 (0.65–0.81) | 0.99 | 0.01 (0.01) | 0.007 | 0.01 (0.007) | 0.08 |
sCr, serum creatinine; RIFLE, risk, injury, failure, loss, ESRD; 95% CI, 95% confidence interval; IDI, integrated discrimination improvement; NRI, net reclassification index.
P values were obtained by comparing c statistics with that of the RIFLE criteria (48-h sCr ratio of 1.50-fold increase).
RIFLE risk criteria was used as the reference.
48-h sCr/preoperative baseline sCr.
24-h sCr – preoperative baseline sCr.
24-h sCr/preoperative baseline sCr.
48-h sCr – preoperative baseline sCr.
AKIN criteria.
Waikar and Bonventre criteria.
Subsequently, the net NRI and the IDI were calculated to further characterize the discriminatory ability of our criteria over various other definitions (Table 5). We found that our criteria and the Waikar and Bonventre criteria for AKI, in regard to NRI and IDI, performed equally well (P values of <0.001 and <0.001 for NRI and IDI, respectively, for both criteria) (22).
Discussion
In this study, we identified a cohort that developed sustained kidney injury (defined as doubling of sCr present at 2–4 weeks after surgery). We investigated the performance of absolute and relative increases in sCr at 24 and 48 hours in a unique cancer population, using ROC curve analyses, rather than using known definitions of AKI (i.e., RIFLE, AKIN, and Waikar and Bonventre criteria), to determine the specific degree of sCr elevation that best predicts clinically significant sustained kidney injury defined as doubling of sCr at 2–4 weeks after surgery. We found that a 59% (1.59-fold) sCr elevation at 48 hours was the best predictor of sustained kidney injury (41% positive predictive value, and 96% negative predictive value) (Table 4). It had the highest, statistically significant c statistic (0.78; P<0.001). We also found that the Waikar and Bonventre criteria, which defines AKI as a sCr increase of 0.3 mg/dl within the first 24 hours or a sCr increase of 0.5 mg/dl within the first 48 hours, performed well according to the NRI and IDI (Table 5) (22).
Although the concept of sustained kidney injury is well accepted, its definition and role in the development of CKD and renal failure is still unclear. Thus, we chose to define sustained kidney injury as doubling of sCr because it represents significant (50% or potentially >50%) loss of renal function at 2–4 weeks after surgery. Early identification of patients at risk of developing sustained kidney injury (within 48 hours after surgery) will motivate close follow-up of their renal status after discharge, allowing early implementation of interventions to attempt to mitigate kidney disease progression. When kidney injury is severe enough to lead to a sustained increase in creatinine over 2–4 weeks, it is likely that histologic evidence of injury will continue to be present even if the sCr then returns close to baseline (1).
Because this study was a retrospective, database-driven cohort study, we could not determine which perioperative variable was most responsible for sustained kidney injury, nor were we able to determine the effect of additional postoperative insults occurring at 1–2 weeks after surgery on our outcome. Thus, unadjusted crude estimates are presented here and future prospective studies designed to adjust for confounders such as fluid shift, hemodynamic instability, and intraoperative cisplatin are necessary. It is likely that some patients had reduced renal reserve not reflected in their baseline sCr value (98% of our cohort had sCr <1.5 mg/dl) secondary to preoperative chemotherapy or other comorbid conditions in this population. In addition, because we defined sustained kidney injury using sCr values 2–4 weeks after surgery, our rule may not reliably predict sustained kidney injury at other time points. Finally, we did not address the applicability of our prediction rule in patients with pre-existing kidney disease. Hence, for patients with pre-existing severe renal dysfunction, a similar but independent calibration strategy assessing the value of sCr as a diagnostic marker for sustained kidney injury should be performed (22).
Our results are specific to our unique cohort and thus are not generalizable to other cohorts and may not be predictive of other types of AKI such as septic AKI. However, our methodology may be applicable to other unique populations undergoing specific procedures in which no general risk scoring system will perform ideally. A customized risk prediction rule as created here by using ROC curve analysis can help refine and predict risks more accurately. However, the Waikar and Bonventre criteria may be useful in practices in which constructing a customized prediction rule may be impractical because of limited resources or sample size. We suggest deriving ROC curves specific to a population whenever possible; however, when this is not feasible, the Waikar and Bonventre criteria could be useful as an early predictive tool for sustained kidney injury (Table 5).
Disclosures
None.
Acknowledgments
We thank Ian Shempp for coordinating our efforts with the development of our database and James Bell for help with the graphics.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Sear JW: Kidney dysfunction in the postoperative period. Br J Anaesth 95: 20–32, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Thadhani R, Pascual M, Bonventre JV: Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Brady HR, Singer GG: Acute renal failure. Lancet 346: 1533–1540, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Esson ML, Schrier RW: Diagnosis and treatment of acute tubular necrosis. Ann Intern Med 137: 744–752, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Coca SG: Long-term outcomes of acute kidney injury. Curr Opin Nephrol Hypertens 19: 266–272, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG, University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zellos L, Richards WG, Capalbo L, Jaklitsch MT, Chirieac LR, Johnson BE, Bueno R, Sugarbaker DJ: A phase I study of extrapleural pneumonectomy and intracavitary intraoperative hyperthermic cisplatin with amifostine cytoprotection for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 137: 453–458, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Sugarbaker DJ, Jaklitsch MT, Bueno R, Richards W, Lukanich J, Mentzer SJ, Colson Y, Linden P, Chang M, Capalbo L, Oldread E, Neragi-Miandoab S, Swanson SJ, Zellos LS: Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 128: 138–146, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Tilleman TR, Richards WG, Zellos L, Johnson BE, Jaklitsch MT, Mueller J, Yeap BY, Mujoomdar AA, Ducko CT, Bueno R, Sugarbaker DJ: Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: A phase II prospective study. J Thorac Cardiovasc Surg 138: 405–411, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Lewis J, Greene T, Appel L, Contreras G, Douglas J, Lash J, Toto R, Van Lente F, Wang X, Wright JT, Jr, AASK Study Group : A comparison of iothalamate-GFR and serum creatinine-based outcomes: Acceleration in the rate of GFR decline in the African American Study of Kidney Disease and Hypertension. J Am Soc Nephrol 15: 3175–3183, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Karnofsky DA, Burchenal JH: The Clinical Evaluation of Chemotherapeutic Agents, New York, : Columbia University Press, 1949 [Google Scholar]
- 14.Chang MY, Sugarbaker DJ: Extrapleural pneumonectomy for diffuse malignant pleural mesothelioma: Techniques and complications. Thorac Surg Clin 14: 523–530, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Ng JM, Hartigan PM: Anesthetic management of patients undergoing extrapleural pneumonectomy for mesothelioma. Curr Opin Anaesthesiol 21: 21–27, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Wolf AS, Daniel J, Sugarbaker DJ: Surgical techniques for multimodality treatment of malignant pleural mesothelioma: Extrapleural pneumonectomy and pleurectomy/decortication. Semin Thorac Cardiovasc Surg 21: 132–148, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Rusch VW, Niedzwiecki D, Tao Y, Menendez-Botet C, Dnistrian A, Kelsen D, Saltz L, Markman M: Intrapleural cisplatin and mitomycin for malignant mesothelioma following pleurectomy: Pharmacokinetic studies. J Clin Oncol 10: 1001–1006, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Stöckl D, Reinauer H: Candidate reference methods for determining target values for cholesterol, creatinine, uric acid, and glucose in external quality assessment and internal accuracy control. I. Method setup. Clin Chem 39: 993–1000, 1993 [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup : Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waikar SS, Bonventre JV: Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20: 672–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, discussion 207–212, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Sundström J, Byberg L, Gedeborg R, Michaëlsson K, Berglund L: Useful tests of usefulness of new risk factors: Tools for assessing reclassification and discrimination. Scand J Public Health 39: 439–441, 2011 [DOI] [PubMed] [Google Scholar]