Abstract
AI-2–mediated quorum sensing has been identified in various bacteria, including both Gram-negative and Gram-positive species, and numerous phenotypes have been reported to be regulated by this mechanism, using the luxS-mutant strain. But the AI-2 production process confused this regulatory function; some considered this regulation as the result of a metabolic change, which refers to an important metabolic cycle named activated methyl cycle (AMC), caused by luxS-mutant simultaneously with the defect of AI-2. Herein we hypothesized that the quorum sensing system—not the metabolic aspect—is responsible for such a regulatory function. In this study, we constructed plasmids infused with sahH and induced protein expression in the luxS-mutant strain to make the quorum-sensing system and metabolic system independent. The biofilm-related genes were investigated by real-time polymerase chain reaction (PCR), and the results demonstrated that the quorum-sensing completed strain restored the gene expression of the defective strain, but the metabolically completed one did not. This evidence supported our hypothesis that the autoinducer-2-mediated, quorum-sensing system, not the AMC, was responsible for luxS mutant regulation.
Keywords: quorum sensing, AI-2, luxS, sahH
1. Introduction
Quorum sensing (QS) is an intercellular signal mechanism that bacteria use to control their gene expression for adapting to changes in their surroundings. This process involves the production, secretion, and recognition of signal molecules and regulation of gene expression [1]. Among these signal molecules, autoinducer-2 (AI-2), which was first identified as a regulator of bioluminescence in Vibrio harveyi [2], supposedly participates in interspecies cell-to-cell communication because of its generating gene luxS that conservatively exists in a broad range of species [3].
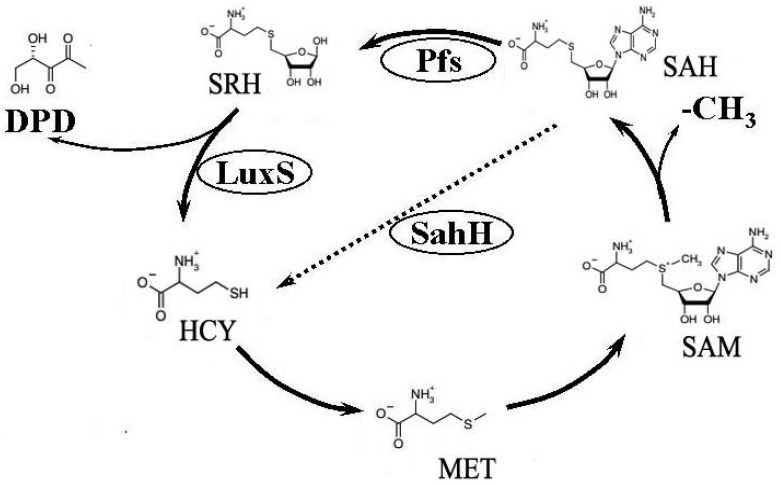
Previous studies have used the luxS mutant strain to demonstrate that AI-2–mediated QS plays an important regulative role in many biological behaviours of bacteria such as cell division, DNA processing, virulence, and motility [4–8]. Furthermore, as an important etiological factor of infectious diseases [9–11], biofilm formation also differs between luxS mutant and its wild-type strain [12–15]. There is much controversy over the origin of these regulating behaviors. AI-2 is a byproduct of the activated methyl cycle (AMC) [16] (Figure 1). This important metabolic cycle begins with homocysteine (HCY), then involves the formation of methione (MET), S-adenosylmethionine (SAM), and S-adenosylhomocysteine (SAH). During the cycle, SAM provides an activated methyl group (CH3), which is used for the methylation of RNA, DNA, certain metabolites, and proteins [17,18]. To complete the cycle, HCY is generated from SAH through two independent pathways. Some bacteria, like Escherichia coli, travel one pathway that needs Pfs enzyme to first produce S-ribosylhomocysteine (SRH), then LuxS catalyzes the conversion from SRH to HCY, simultaneously generating the precursor of AI-2, 4,5-dihydroxy-2,3-pentanedione (DPD). The other route involves a one-step conversion catalyzed by SAH hydrolase (SahH), which catalyzes the conversion from SAH to adenosine and HCY but without generating DPDs [19–21], bacteria such as P. aeruginosa which do not contain luxS passing through this way. Since most previous studies on mutated luxS considered it a QS-defective strain and that AMC was also disrupted at the LuxS level, a series change would occur in the bacteria's metabolism [20]. Thus arose the controversy as to whether the change in gene expression is caused by QS, as previously described, or by AMC metabolic pathway disruption [21,22].
Figure 1.
Process of activated methyl cycle (AMC; adapted from Vendeville et al. [18]). E. coli uses a two-step mechanism involving the Pfs and LuxS enzymes to produce the AI-2 precursor and homocysteine (HCY), while P. aeruginosa synthesizes HCY from S-adenosylhomocysteine (SAH) in a one-step reaction involving the SahH enzyme.
Although the luxS mutant leads to AMC obstruction, it is not believed to arouse so much regulated gene expression belonging to such diverse biological behaviors as gene production, virulence, and motility [7,23]. Such a wide influence is likely to be due to a monolithic mechanism, such as AI-2-induced QS. Furthermore, in previous studies, mutated luxS was not fatal to the strain, so we speculated that the influential range of the AMC obstacle was limited. To this end, we hypothesized that the QS role, instead of an AMC obstacle, is responsible for luxS mutant regulation. To verify our hypothesis, we intended to express SahH in an E. coli luxS mutant strain, aiming to repair the AMC of this strain but retain the defective QS system, then detect whether the genes reported to be regulated by AI-2–mediated QS would be restored.
2. Materials and Methods
2.1. Bacterial Strains, Plasmid Construction, and Culture Condition
The strains and plasmids used in this study are listed in Table 1, the primers in Table 2. The sahH gene was amplified from genomic DNA of the P. aeruginosa strain PAO1 using primers SF and SR, and the luxS gene was amplified from genomic DNA of E. coli W3110 with primers LF and LR. The restriction enzymes chosen in this part were BamH I and EcoR I for sahH and vector, EcoR I and Bgl II for luxS. And Bgl II is the isocaudamer of BamH I. To construct the plasmids pluxS and psahH, amplified products were inserted into the expression vector pGEX4T-1, using the standard method of preparing clones [24]. Generally, DNA amplification products were digested by the corresponding restriction enzymes and ligated with the vector. After sequencing, pluxS, psahH, and pGEX4T-1 were transformed into strain MDAI2 (luxS mutant strain) to construct strains M-L, M-S, and M-P, respectively.
Table 1.
Plasmids and strains used in this study.
| Strain or plasmid Plasmids | Relevant genotype | Reference or source |
|---|---|---|
| pGEX4T-1 | expression vector, Apr | Shanghai key laboratory of stomatology |
| pluxS | luxS from E. coli W3110 cloned in pGEX4T-1 | this study |
| psahH | sahH from P. aeruginosa PAO1 cloned in pGEX4T-1 | this study |
| Strains | ||
| E.coli | ||
| W3110 | K-12 strain, wild type | [25] |
| MDAI2 | W3110 luxS∷TcrW3110-derived luxS mutant strain | [25] |
| M-L | MDAI2 pluxS | this study |
| M-S | MDAI2 psahH | this study |
| M-P | MDAI2 pGEX4T-1 | this study |
| P. aeruginosa | ||
| PAO1 | wild type | Shanghai key laboratory of stomatology |
Table 2.
Primers used in this study.
| Primer | Sequence (5′-3′) |
|---|---|
| SF | GGCGGATCCATGAGCGCTGTCATGACGC * |
| SR | GGCGAATTCTTAGTAGCGATAGGTGTCCGG |
| LF | GGCAGATCTATGCCGTTGTTAGATAGCTTCAC |
| LR | GGCGAATTCCTAGATGTGCAGTTCCTGCAACT |
| fliAF | CCGCAACGCCACGGAAACTGA |
| fliAR | GCTCTTCGCGCCACTCATCGTA |
| fliCF | ATTGCTAACCGTTTCACCTCTAA |
| fliCR | CGCTGTAAGTTGTTGTTGATTTCG |
| motAF | CGTCGCTCCAAATACACCAA |
| motAR | CAGCGAAAACATCCCCATCT |
| motBF | GCCAGCGGTGAGAAAGGA |
| motBR | CAACCCTCCGACCATCAGTT |
| rpoAF | GCGCTCATCTTCTTCCGAAT |
| rpoAR | CGCGGTCGTGGTTATGTG |
Underline sequence is reference the restriction enzymes.
All strains were precultured in a 2 × YT medium at 37 °C overnight with shaking at 220 rpm, and the concentration of ampicillin for selection was 100 μg/mL. These overnight cultures were inoculated into a fresh medium and cultivated at 37 °C with 250-rpm shaking. When the strains had reached the exponential phase, a final concentration of 0.1 mmol/L isopropyl-β-D-thiogalactopyranoside (IPTG) was added, and cultivation was continued for about 6 h.
2.2. Western Blotting
After cultivation, the OD600 of five strains was tested and total proteins were extracted. Briefly, about 2 × 108 E. coli were collected and a corresponding volume of double-distilled water (ddH2O) and a 5× sample of buffer (20% sodium dodecyl sulfate [SDS], 20% glycerol, 200 mM Tris base, pH 6.8, 0.001% bromophenol blue) was used to subsequently suspend the pellets. The samples were heated at 100 °C for 10 min and centrifuged for 1 min. Equal amounts of total proteins were electrophoresed in SDS–10% polyacrylamide gel electrophoresis. Western blotting was performed as previously described [24]. The antibodies used in this study were monoclonal anti-glutathione-S-transferase (anti-GST) and rabbit antimouse antibody with a green fluorescent group.
2.3. RNA Extraction and Reverse Transcription
After IPTG induction and cultivation for 6 h, the total RNA of these strains was extracted. Culture volumes equivalent to 10 mL with an OD600 of 1.0 were harvested by centrifugation. The pellet was suspended with TRIzol (Invitrogen, Carlsbad, CA, USA). The reagent and RNA extraction process followed the manufacturer's specifications. The resulting RNA was dissolved in diethyl pyrocarbonate-treated water and stored at −80 °C. The PrimerScript gDNA eraser RT reagent kit (Takara, Otsu, Shiga, Japan) was used to generate cDNA; about 1 μg of total RNA was used for each strain. To detect the target exogenous gene, cDNA of each strain was used as a template for polymerase chain reaction (PCR). Other compositions were forward/reverse primer (SF, SR, LF, LR), PrimeStar HS DNA Polymerase (Takara) and buffer, dNTP mixture, and ddH2O. PCR conditions included an initial denaturation at 98 °C for 5 min, followed by 30 cycles amplification consisting of 98 °C for 15 s, 55 °C for 15 s, and 72 °C for 90 s, then final extension at 72 °C for 5 min. The amplified products were electrophoresed in 1% agarose gel.
2.4. Real-Time PCR
To determinae whether the AMC-completed strain would restore the biofilm formation-related genes affected by luxS mutant, the expression of these genes was compared with the real-time PCR. The target genes and their primers are shown in Table 2. The amplification efficiency and template specificity of each primer pair were verified, then the amplification was performed with the following 15-μL reaction mixture: 7.5 μL 2× Thunderbird SYBRqPCR Mix (Toyobo, Osaka, Japan), 5 μL cDNA template, 1 μL PCR primers mix (10 μM), and 1.5 μL ddH2O. PCR conditions included an initial denaturation at 98 °C for 5 min, followed by 40 cycles amplification of 98 °C for 15 s, 55 °C for 15 s, and 72 °C for 30 s. To check DNA contamination, the production of RT-PCR step 1 (without reverse transcriptase) served as a negative control. The rpoA gene was used in this study as the normalising gene for all reactions [26]. Applied Biosystems (Carlsbad, CA, USA) 7900HT Fast Real Time PCR System was used for this test, and fold changes of the expression levels were calculated by SDS Software v2.3 with RQ Study 1.2 (Applied Biosystems). All the assays were conducted with each sample in triplicate. Here, the Kruskal-Wallis test was used to analyse the gene expression difference among the five strains. A P-value less than 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Identification of Exogenous Gene Expression
To test and verify our hypothesis, the QS system and AMC must be independent. Previous investigators have collected supernatant that had cultivated a wild-type strain for some time, believing it to contain AI-2 [27,28]. They then used it as a conditioning medium in which to cultivate the luxS mutant strain. In a sense, this method could make the QS system and AMC independent, but it allowed the other substance in the supernatant—secreted by the bacteria—to influence the results. This study, aiming to restore the obstructed AMC and simultaneously keep the defective QS mechanism, transformed the sahH into an E. coli luxS mutant strain. Consequently, we could now check whether the AMC or the QS system took responsibility for changes caused by the luxS mutant.
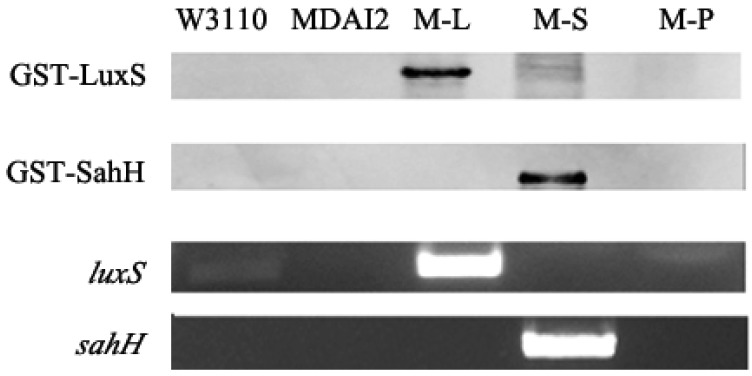
The RT-PCR and Western blot were performed to prove the expression of target genes in these five strains. As shown in Figure 2, the bands detectable by anti-GST represented the fusion proteins GST-LuxS and GST-SahH, whose molecular weights were about 44.9 kDa and 77.7 kDa, respectively. For luxS RT-PCR, the primer pairs LF and LR could spot both W3110 and M-L, whereas sahH was detected only in M-S. Moreover, the band amplified from M-L is more remarkable than W3110, which amplification should be attributed to the overexpression of pluxS.
Figure 2.
GST-tagged LuxS and SahH were detected in strains M-L and M-S, respectively, by Western blotting. RT-PCR showed that luxS existed in both W3110 and M-L, while M-L is more likely caused by gene overexpression. sahH was detectable only in M-S.
3.2. Gene Expression Differences Caused by luxS Mutant are Regulated by the QS System, not by AMC
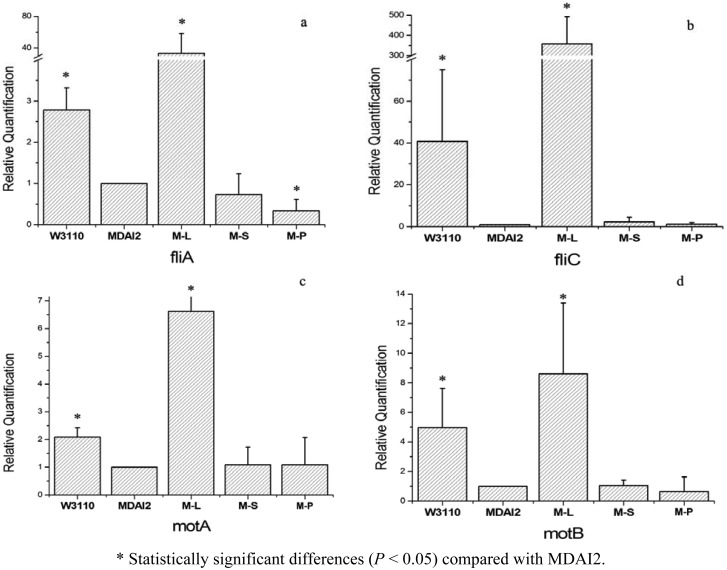
To determine whether AMC regulates gene expression of the luxS mutant strain, a real-time PCR was conducted. Biofilm formation is one of the main causative factors in infectious diseases. And as described before, biofilm formation was reported to be regulated by quorum sensing system. Genes such as fliA, fliC, motA, and motB, which were responsible for flagellar biosynthesis and rotation, also played an important role in the initial stage of the biofilm formation process [29–31]. They were also reported to have different expression levels between the wild-type and luxS mutant strain [14,15], so here these four genes were tested [32–34].
The results of real-time PCR are shown in Figure 3. In general, M-S, relative to M-L, could not restore the gene expression of the luxS mutant strain. In practical terms, all the detected genes showed the trend that the wild-type strain expressed more than the luxS defective strain. They increased about 2.8-fold, 40.8-fold, 2.1-fold, and 5.0-fold for fliA, fliC, motA, and motB, respectively. But M-S, whose AMC disorder may be partly repaired, meanwhile the AI-2 producing process obstructed, had an expression level similar to the luxS mutant strain, MDAI2, and their relative expression folds were 0.73 for fliA, 2.3 for fliC, and 1.1 for motA and motB. That means SahH failed to restore the changes caused by luxS mutant. Under the same experimental conditions, the expression of target genes in the M-L strain was recovered or even up-regulated compared with the wild-type strain. These results showed that there was no significant restoration by expression of SahH which was intended to complete the AMC, thus the luxS-mediated QS system impacted the biofilm-related gene expression.
Figure 3.
Real-time PCR was used to demonstrate that motility-related genes are restored in the luxS-mutant strain by the expression of luxS, although it was not restored in the mutant strain with the plasmid-expressing sahH. The results represent the means and standard deviations (SDs) of relative quantification.
This study also showed that, the genes, downregulated by the luxS mutant, were even strongly upregulated when LuxS was overexpressed. This demonstrated that the mutant strain with LuxS expression may complete the QS system and cause AI-2 secretion to recover the expression of the target genes. And theoretically, overexpressed LuxS synthetized more active AI-2, so these target genes were upregultaed much more than the wild-type strain. But under the same experimental conditions, the mutant strain with SahH expression, which might recover the AMC system without AI-2 secretion, kept the expression of target genes at a low level (Figure 3), which means that, without integrated QS mediating, the phenotype could not be recovered, although its role in metabolism may be intact.
4. Conclusions
The genetic test results confirm our hypothesis that the QS role—rather than metabolism—is the primary regulator of luxS mutant physiological changes. We intend, in a future study, to further verify our hypothesis by means of an E.coli function test.
Acknowledgments
We thank William E. Bentley of the University of Maryland Biotechnology Institute for the W3110 and MDAI2 strains used in this work. This study was supported by a grant from the National Natural Science Foundation of China (NSFC) No. 81070826/30872886, and partly supported by a university grant of YG2011MS67.
References
- 1.Xavier K.B., Bassler B.L. Regulation of Uptake and Processing of the Quorum-Sensing Autoinducer AI-2 in Escherichia coli. J. Bacteriol. 2005;187:238–248. doi: 10.1128/JB.187.1.238-248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassler B.L., Wright M., Showalter R.E., Silverman M.R. Intercellular Signalling in Vibrio Harveyi: Sequence and Function of Genesregulating Expression of Luminescence. Mol. Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 3.Xavier K.B., Bassler B.L. luxS Quorum Sensing: More than just a Numbers Game. Curr. Opin. Microbiol. 2003;6:191–197. doi: 10.1016/s1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 4.Walters M., Sircili M.P., Sperandio V. AI-3 Synthesis is not Dependent on luxS in Escherichia coli. J. Bacteriol. 2006;188:5668–5681. doi: 10.1128/JB.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J., Attila C., Wang L., Wood T.K., Valdes J.J., Bentley W.E. Quorum Sensing in Escherichia coli is Signaled by AI-2/LsrR Effects on Small RNA and Biofilm Architecture. J. Bacteriol. 2007;189:6011–6020. doi: 10.1128/JB.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling H., Kang A., Tan M.H., Qi X., Chang M.W. The Absence of the luxS Gene Increases Swimming Motility and flagella Synthesis in Escherichia coli K12. Biochem. Biophys. Res. Commun. 2010;401:521–526. doi: 10.1016/j.bbrc.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 7.Delisa M.P., Wu C.P., Wang L., Valdes J.J., Bentley W.E. DNA Microarray-Based Identification of Genes Controlled by Autoinducer 2-Stimulated Quorum Sensing in Escherichia coli. J. Bacteriol. 2001;183:5239–5247. doi: 10.1128/JB.183.18.5239-5247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLisa M.P., Valdes J.J., Bentley W.E. Mapping Stress-Induced Changes in Autoinducer AI-2 Production in Chemostat-Cultivated Escherichia coli K-12. J. Bacteriol. 2001;183:2918–2928. doi: 10.1128/JB.183.9.2918-2928.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Z.W., Meric G., Liu Z., Ma R., Tang Z.S., Lejeune P. luxS-Based Quorum-Sensing Signaling Affects Biofilm Formation in Streptococcus mutans. J. Mol. Microbiol. Biotechnol. 2009;17:12–19. doi: 10.1159/000159193. [DOI] [PubMed] [Google Scholar]
- 10.Ma R., Liu J., Jiang Y.T., Liu Z., Tang Z.S., Ye D.X., Zeng J., Huang Z.W. Modeling of Diffusion Transport through Oral Biofilms with the Inverse Problem Method. Int. J. Oral Sci. 2010;2:190–197. doi: 10.4248/IJOS10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z.W., Jiang Y.T., Liang J.P. Pathogenesis could be One of the Anti-Cheating Mechanisms for Pseudomonas aeruginosa Society. Med. Hypoth. 2011;76:166–168. doi: 10.1016/j.mehy.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Balestrino D., Haagensen J.A.J., Chantal R., Forestier C. Characterization of Type 2 Quorum Sensing in Klebsiella pneumoniae and Relationship with Biofilm Formation. J. Bacteriol. 2005;187:2870–2880. doi: 10.1128/JB.187.8.2870-2880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J., Maeda T., Hong S.T., Wood T.K. Reconfiguring the Quorum-Sensing Regulator SdiA of Escherichia coli to Control Biofilm Formation via Indole and N-Acylhomoserine Lactones. Appl. Microbiol. Biotechnol. 2009;75:1703–1716. doi: 10.1128/AEM.02081-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren D., Bedzyk L., Ye R.W., Thomas S., Wood T.K. Differential Gene Expression Shows Natural Brominated Furanones Interfere with the Autoinducer-2 Bacterial Signaling System of Escherichia coli. Biotechnol. Bioeng. 2004;88:630–642. doi: 10.1002/bit.20259. [DOI] [PubMed] [Google Scholar]
- 15.Sperandio V., Torres A.G., Giron J.A., Kaper J.B. Quorum Sensing is a Global Regulatory Mechanism in Enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 2001;183:5187–5197. doi: 10.1128/JB.183.17.5187-5197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winzer K., Hardie K.R., Burgess N., Doherty N., Kirke D., Holden M.T.G., Linforth R., Cornell K.A., Taylor A.J., Hill P.J., Williams P. luxS: Its Role in Central Metabolism and the in vitro Synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology. 2002;148:909–922. doi: 10.1099/00221287-148-4-909. [DOI] [PubMed] [Google Scholar]
- 17.Winzer K., Hardie K.R., Williams P. luxS and Autoinducer-2: Their Contribution to Quorum Sensing and Metabolism in Bacteria. Adv. Appl. Microbiol. 2003;53:291–396. doi: 10.1016/s0065-2164(03)53009-x. [DOI] [PubMed] [Google Scholar]
- 18.Vendeville A., Winzer K., Heurlier K., Tang C.M., Hardie K.R. Making ‘Sense’ of Metabolism: Autoinducer 2, luxS and Pathogenic Bacteria. Nat. Rev. Microbiol. 2005;3:383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 19.Nikhat P., Kenneth A.C. Methylthioadenosine/S-Adenosylhomocysteine Nucleosidase, a Critical Enzyme for Bacterial Metabolism. Mol Microbiol. 2011;79:7–20. doi: 10.1111/j.1365-2958.2010.07455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliday N.M., Hardie K.R., Williams P., Winzer K., Barrett D.A. Quantitative Liquid Chromatography-Tandem Mass Spectrometry Profiling of Activated Methyl Cycle Metabolites Involved in luxS-Dependent Quorum Sensing in Escherichia coli. Anal. Biochem. 2010;403:20–29. doi: 10.1016/j.ab.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Sun J.B., Daniel R., Wagner-Döbler I., Zeng A.-P. Is Autoinducer-2 a Universal Signal for Interspecies Communication: A Comparative Genomic and Phylogenetic Analysis of the Synthesis and Signal Transduction Pathways. BMC Evol. Biol. 2004;4 doi: 10.1186/1471-2148-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winzer K., Hardie K.R., Williams P. Bacterial Cell-to-Cell Communication: Sorry, can't Talk Now—Gone to Lunch! Curr. Opin. Microbiol. 2002;5:216–222. doi: 10.1016/s1369-5274(02)00304-1. [DOI] [PubMed] [Google Scholar]
- 23.Wang L., Li J., March J.C., Valdes J.J., Bentley W.E. luxS-Dependent Gene Regulation in Escherichia coli K-12 Reveale by Genomic Expression Profiling. J. Bacteriol. 2005;187:8350–8360. doi: 10.1128/JB.187.24.8350-8360.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperandio V., Li C.C., Kaper J.B. Quorum-Sensing Escherichia coli Regulator A: A Regulator of the LysR Family Involved in the Regulation of the Locus of Enterocyte Effacement Pathogenicity Island in Enterohemorrhagic E. coli. Infect. Immun. 2002;70:3085–3093. doi: 10.1128/IAI.70.6.3085-3093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsao C.Y., Wang L., Hashimoto Y., Yi H., March J.C., DeLisa M.P., Wood T.K., Valdes J.J., Bentley W.E. luxS Coexpression Enhances Yields of Recombinant Proteins in Escherichia coli in Part through Posttranscriptional Control of GroEL. Appl. Environ. Microbiol. 2011;77:2141–2152. doi: 10.1128/AEM.02347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melissa M.K., Rasko D.A., Sperandio V. Global Effects of the Cell-to-Cell Signaling Molecules Autoinducer-2, Autoinducer-3, and Epinephrine in a luxS Mutant of Enterohemorrhagic Escherichia coli. Infect. Immun. 2007;75:4875–4884. doi: 10.1128/IAI.00550-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperandio V., Torres A.G., Kaper J.B. Quorum Sensing Escherichia coli Regulators B and C (QseBC): A Novel Two-Component Regulatory System Involved in the Regulation of fllagella and Motility by Quorum Sensing in E. coli. Mol. Microbiol. 2002;43:809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoon Y., Sofos J.N. Absence of Association of Autoinducer-2-Based Quorum Sensing with Heat and Acid Resistance of Salmonella. J. Food Sci. 2010;75:M444–M448. doi: 10.1111/j.1750-3841.2010.01744.x. [DOI] [PubMed] [Google Scholar]
- 29.Pratt L.A., Kolter R. Genetic Analysis of Escherichia coli Biofilm Formation: Roles of fllagella, Motility, Chemotaxis and Type I pili. Mol. Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 30.Habdas B.J., Smart J., Kaper J.B., Sperandio V. The LysR-Type Transcriptional Regulator QseD Alters Type Three Secretion in Enterohemorrhagic Escherichia coli and Motility in K-12 Escherichia coli. J. Bacteriol. 2010;192:3699–3712. doi: 10.1128/JB.00382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrios A.G., Zuo R., Hashimoto Y., Yang L., Bentley W.E., Wood T.K. Autoinducer 2 Controls Biofilm Formation in Escherichia coli through a Novel Motility Quorum-Sensing Regulator (MqsR, B3022) J. Bacteriol. 2006;188:305–316. doi: 10.1128/JB.188.1.305-316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domka J., Lee J., Bansal T., Wood T.K. Temporal Gene-Expression in Escherichia coli K-12 Biofilms. Environ. Microbiol. 2007;9:332–346. doi: 10.1111/j.1462-2920.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- 33.Blattner F.R., Plunkett G., Bloch C.A., Perna N.T., Burland V., Riley M., Collado-Vides J., Glasner J.D., Rode C.K., Mayhew G.F., Gregor J., Davis N.W., Kirkpatrick H.A., Goeden M.A., Rose D.J., Mau B., Shao Y. The Complete Genome Sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 34.Ren D., Bedzyk L., Thomas S., Ye R.W., Wood T.K. Gene Expression in Escherichia coli Biofilms. Appl. Microbiol. Biotechnol. 2004;64:515–524. doi: 10.1007/s00253-003-1517-y. [DOI] [PubMed] [Google Scholar]