Abstract
A CD4 T-lymphocyte count determines eligibility for antiretroviral therapy (ART) with patients recently diagnosed with HIV and also monitors the efficacy of ART treatment thereafter. ART slows the progression of HIV to AIDS. In the developing world, CD4 tests are often performed in centralized laboratories, typically in urban areas. The expansion of ART programs into rural areas has created a need for rapid CD4 counting as logistical barriers can delay the timely dissemination of test results and affect patient care through delay in intervention or loss of follow-up care. CD4 measurement at the point-of-care (POC) in rural areas could help facilitating ART and monitoring of treatment. This review highlights recent technology developments with applications towards determining CD4 counts at the POC.
The need for Point of Care technologies
A study by the Joint United Nations Program on HIV/AIDS (UNAIDS) estimated that, in 2009, there were 33.3 million global cases of infection with HIV— 68% in sub-Saharan Africa [1]. The HIV virus infects cells of the immune system, primarily CD4+ T lymphocytes (CD4+ cells). Chronic HIV infection depletes CD4 levels, weakening the immune system and progressing to AIDS and death from cancer or opportunistic infections [2]. Anti-retroviral therapy, ART, especially when CD4+ cells are not yet depleted, can reduce viremia and slow its progression to AIDS [3]. Furthermore, if CD4 counts are established, antiretroviral prophylaxis can reduce the risk of transmission in pregnant women to prevent mother to child transmission of HIV during pregnancy, childbirth and breastfeeding [4]. Therefore it is critical to count CD4+ cells upon diagnosis of HIV and before initiating ART. The World Health Organization currently recommends ART initiation if absolute CD4 counts are below 350/cells mm3 [4]; the median CD4+ cell count from a non HIV infected adult cohort was 828 cells mm3 [5]. CD4+ cells levels can be expressed as an absolute count for a set volume, as a percentage of the total lymphocyte population or as ratio with another specific lymphocyte subset. While absolute CD4 counts are acceptable for assessing the status of adult patients, populations of lymphocytes including CD4+ are greater in children and so it is more informative to measure the CD4 % in the total lymphocyte population or ratios of CD4/CD8, lymphocytes. Other cell types expressing CD3 (all lymphocytes), CD14 and CD45 (both on monocytes) are often used in CD4 counting methods to discriminate CD4+ T lymphocytes from the total lymphocyte population or from monocytes in whole blood. Monocytes express lower level of the CD4 surface antigen and so discriminatory methods must be employed to get an accurate count of the CD4+ cells from whole blood [6]. Point of Care (POC) testing either at or n ear the patient, could improve enrollment into ART and consequently its management in rural areas[7,8]. Rapid, reliable, and affordable POC CD4 tests are not yet widely available [9,10]. In this review, we identify emerging technologies that have the potential for CD4 testing and will also discuss some of the commercial CD4 tests that are currently marketed or in development for POC use.
Flow Cytometry
Most methods described to count CD4+ cells use antibodies to human CD4 (anti-hCD4) as part of specific cell labeling, cell capture strategies or both for subsequent detection. Alternatives to direct CD4+ cell counting are discussed in Box 1. Fluorescence-activated cell sorting (FACS) analysis by flow cytometry (a fluidic technique combined with optics for counting cells and other microscopic particles) is the established gold standard for CD4 counting via fluorescently labeled antibody conjugates, using equipment, such as the FACSCalibur™ (Becton Dickinson, USA) and EPICS XL/MCL™ (Beckman Coulter, USA) [11]. Dual flow cytometry utilizes a hematological analyser in addition to a flow cytometer to determine the absolute CD4 count: the fraction of CD4 in a small percentage of the leukocyte population derived from FACS multiplied by the total leukocyte population established with a hematological analyser [8]. Single flow platforms use only a flow cytometer and either count the absolute CD4 count in a fixed volume (Guava EasyCD4™ Merck; CyFlow CD4™, Partec, and the PointCare Now™) or calculate the absolute number of CD4 cells from a ratio of known concentration of beads to CD4+ cells (FACScan™ and FACSCount™, Becton Dickinson). Single platform technology is less expensive and easier to perform than dual platform and is now used outside of large reference laboratories [7–9]. The Guava instrument uses a microcapillary for cell focusing rather than a fluid sheath and is subsequently termed sheathless; an advantage, because minimizing the volume of sheath fluid required for operation reduces the need for highly pure water and produces much less liquid waste during continual operation. Simplified FACS protocols that require minimal pre-analytical manipulation have also been proposed and validated in a multi center study [12]. Flow cytometry is limited to laboratory use so that specimens acquired in rural areas are sent to urban laboratories making dissemination of test results and follow-up care difficult [9].
Box 1: Alternatives to direct CD4+ cell counting.
In addition to direct cell counting, assays that are based on molecules extracted from whole blood have also been developed. For example, the Burnet Institute is developing a commercial immunochromatographic strip (ICS) to detect the CD4 antigen that is described in the next section. In addition, fully mature T cell-specific genomic DNA, such as the rearranged T cell receptor-β (rTCR-β) gene has been evaluated as a predictor of the mature total T lymphocyte counts (TLC) [62]. Quantitative real-time PCR using cellular DNA extracted from dried blood spots confirms that rTCR-β counts can determine antiretroviral initiation with an accuracy that is comparable to TLC. The potential to combine TLC with viral load tests on the same platform makes it an attractive tool in terms of expanding the utility of existing equipment.
Manual CD4 Counting Methods
Manual CD4 counting requires refrigeration of reagents, a microscope with a 40X objective, a hemocytometer, calibrated pipettes, test tubes, and a manual counter. Two kits are commercially available (Table 1) and have been evaluated in low-resource settings (LRS) with good correlation with flow cytometry data [13–19]. However, sample preparation and manual counting are laborious, especially when reading CD4 counts above 500 cells/mm3. To improve on throughput using the manual T4 Quant method (Invitrogen, USA), the Sysmex pocH-100i hematological analyzer can be used to count stained nuclei and showed 93% concordance with FACS counting [20].
Table 1.
Specifications of existing POC CD4 test technologies on the market or in development. e
| Instrument | Sample Volume (µL) |
Stabilized reagents |
CD4 /CD 4% |
Time to Result (min) |
Instrument required |
Automati on/Throu ghput Hr−1 |
Data storage |
Battery Run time per charge (Hrs) |
Marketed | QC | Regulatory Status |
Dimensionsa L × W × H (cm) |
Weight (Kg) |
Manufacturer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PointCare NOW™ | 40 | Y | Y/Y | 8 | Y | Y/NA | Y | Y/NA | Y | Y | FDA | 33.7 × 25.0 × 35.4 | <12 | www.pointcare.net/ |
| CyFlow® miniPOC | 20 | Y | Y/Yb | 1 | Y | Y/30–40 | Y | Y/4–5 | Y | Y | CE | 18.6 × 26.8 × 24.3 | <5 | www.partec.com/ |
| Pima™ | 25 | Y | Y/Yb | 20 | Y | Y/3 | Y | Y/8 | Y | Y | CE | 22 × 13 × 16 9.7 × 3.2 × 0.6 |
2.54 0.11 |
www.alere.com/ |
| Coulter® CD4 Count Kit | 100 | N | Y/N | 30 | Y | N/2c | N | N | Y | Y | CE | NA | NA | www.beckmancoulter.com/ |
| Dynal T4 Quant Kit | 125 | N | Y/N | 30 | Y | N/6c | N | N | Y | NA | CE | NA | NA | www.invitrogen.com/ |
| Daktari | 10 | Y | Y/N | 8 | Y | Y/7 | Y | Y/NA | N | NA | NA | 22.9 × 12.7 × 17.8 0.80 × 5.65 × 1.08 |
2.5 0.03 |
www.daktaridx.com/ |
| mBio | 10 | Y | Y/N | 20 | Y | SA/8–10 | Y | Y/NA | N | NA | NA | 38 × 19 × 15 0.88 × 0.30 × 0.13 |
3 0.016 |
www.mbiodx.com/ |
| Burnet | 30 | Y | Y/N | 40 | N | SA/10 | Yd/N | N | N | NA | NA | 12 × 7.7 × 8.5 9 × 2 × 0.5 |
0.37 0.03 |
www.axxin.com/ www.burnet.edu.au/ |
| Zyomyx | 100 | Y | Y/N | 6 | N | N/10 | N | N | N | NA | NA | 10 × 1.4 | 0.06 | www.zyomyx.com/ |
Instrument dimensions precede cassette dimensions where listed.
This is the CD4% versus total lymphocyte reading.
To minimize reader error, a limit of 8 specimens per day should be read.
The ICT CD4 device can be read manually or with the Axxin CubicTM instrument platform to automatically score the test
The cost per test and in terms of capital equipment and training was not addressed due to limited information from some manufacturers and the variety of settings where testing may be performed.
Acronyms: Y=Yes, N=No, S /A=Semi automated, QC=quality control, NA=Not available, FDA=Food and Drug Administration (USA), CE= Conformité Européene, L=length, W=width and H=height.
Several groups have created microdevices to separate T lymphocytes. An enclosed microfluidic device simplifies the multiple processing steps needed in bead count assays [21]. In one example, an array of micropillars is coated with a layer of heparin to promote surface hydrophilicity and to automate blood filling by capillary force [22,23]. The micropillars are then further functionalized with anti-hCD4 antibodies [21]. Blood flows through the microchannel and is removed by wicking buffer and absorbent pads. The CD4+ cells are captured on the pillars and following nuclear staining, are counted under a fluorescence microscope. The performance of the device has not been demonstrated with CD4 counts under 500 cells/µl; for high CD4 counts above 600 cells/µl, the lymphocyte capture yield is 79% [21]. While direct cell counting via microscopy has clear application for CD4 counting at the POC, its effectiveness is limited by the need for training programs, quality control measures and the quality and maintenance of microscopes [24]. By combining simple and low cost CD4 capture techniques with automated optical measurement systems as discussed next, throughput and data capture are greatly improved at relatively low cost [25].
Automated imaging cytometry in cell capture devices
The development of microfluidic technology to create cell capture and counting devices has been extensively explored. As the devices are microfluidic, only small volumes of whole blood are needed for analysis (Table 1) eliminating the need for a venous blood draw permitting use by less skilled users; the amount of critical reagents and liquid waste is greatly reduced to permit housing in a single disposable cartridge. Simple ‘plug and play’ formats permit the user to add the specimen to a cartridge and then place it into a reader to perform specimen preparation, analysis and data interpretation. In this section we describe several microfluidic cell capture technologies and various detection formats that have been described for performing CD4+ counting.
Imaging cytometry in cell capture devices is a single-platform approach, i.e. the absolute CD4 count in a fixed volume is obtained without a separate hematology analyzer. Typically, an optical detector images a surface on which fluorescently stained CD4+ cells are selectively captured. Blood is first mixed with anti-hCD3 conjugated to magnetic beads and anti-hCD4 and anti-hCD8 each labeled with a different fluorescent tag [24]. The mixture is then injected into a microchannel device, in which magnetic bead captured CD3+ cells are attracted to the roof of the flow cell by a magnetic field. Fluorescently labeled CD4+ and CD8+ cells are then excited by light-emitting diode (LED) illumination and imaged with a charge-coupled device camera that is controlled with a single-board computer on battery power. The associated image analysis software automatically provides both absolute CD4 counts and the CD4/CD8% ratios from these images [25,26]. A recent study showed similar performance to flow cytometry in an HIV infected cohort [27]. Furthermore, a separate study showed that T cells can be distinguished from monocytes based on their different intensity after staining with fluorescent anti-hCD4 [28]. The CD4 counts from this microchannel method correlate closely with results of flow cytometry in 100 specimens, but blood dilution and staining are yet to be automated. Another study uses anti-hCD3 and anti-hCD4, each conjugated with a different quantum dot to double label CD4+ cells and improve the signal to background ratio in imaging [29]. Apart from the cell imaging systems described above, other generic, inexpensive, and portable fluorescence technologies have been proposed for CD4 counting from a fixed volume of whole blood [30–32].
To overcome the complex optics requirement in fluorescence imaging, chemiluminsence has been used to determine absolute CD4 counts from cells captured on a microfabricated solid matrix [33]. In this approach, CD4+ cells are first captured inside an immunoaffinity microchannel after which they are labeled with an anti-hCD3 antibody that is conjugated with an enzyme to catalyze a chemiluminescent reaction detectable with a photodetector. The sandwich structure procedure eliminates nonspecific signals arising from captured monocytes, and the photo signal has been found to be proportional to the number of captured cells [33]. An alternative to fluorescence or luminescence detection uses surface bound antibody to immobilize CD4+ cells and a wide-field lens-free imager to detect and count the shadows the cells cast on an optoelectronic sensor array [34]. As a further development, CD4 cells immuno-captured on the interior surface of a capillary tube are detected by a ring resonator. The near-surface refractive index changes associated with cell binding are detected through the shift of the resonant wavelength inside the capillary wall, which is proportional to the total number of captured cells [35].
Methods that report CD4 percentages or ratios require simultaneous detection of two or more types of cell. Typical two-parameter detection uses two dyes with different emission wavelengths to tag two separate antibodies, thus requiring two detectors. A method that uses anti-hCD45 tagged with Fluoroscein isothiocyanate (FITC) to label all leukocytes and anti-hCD4 conjugated to FITC-doped silica nanoparticles to label CD4+ cells reduces the complexity associated with dual detection [35]. The nanoparticles have a 100 times higher intensity than FITC alone, so that absolute CD4 counts and CD4/CD45 ratios can be determined with a single detector [36]. However, it is unclear if this design would allow differentiation of CD4+ cells from monocytes.
Imaging cytometry has a few distinct advantages over FACS in POC applications. In addition to being single platform and potentially portable, imaging cytometry detects a large number of cells simultaneously, while FACS measures one cell at a time. Of the several imaging systems described in this section above, detection of the sandwich structures has a higher accuracy by eliminating monocyte interference [25,26,29,33], while label-free imaging of all cells captured by a single antibody [28,34–37] offers greater operation simplicity and faster turn-around time. The field performances of these systems are yet to be established to determine if the strength of each system outweighs its shortcomings.
Microflow cytometry
Several research groups have turned to microfabrication to create a low-cost flow cytometer for mobile CD4 counting. The development of microcytometers has concentrated on creating microchips to align and position the cells for detection in a manner analogous to flow cytometry but without requiring the liters/day of sheath fluid used in conventional FACS [36]. Various methods to produce a single and focused stream of cells without sheath fluid have been described including mechanical structures [36,38], dielectrophoresis (DEP) [39], optical forces [40], hydrodynamic forces [41], electrokinetic transport [42] and ultrasound effect [43]. Moreover, prototypes of automated micro-flow cytometers have been presented by several groups. For example, a pneumatically driven microchip in which erythrocyte depleted blood is first mixed and incubated with fluorescent CD3, CD4 and CD8 antibodies and then automatically transported to a detection channel has been developed. The labeled CD3+, CD4+ and CD8+ cells are separately detected through a home built optical sensor containing 3 sets of fluorescence detectors. The resulting absolute CD4 counts and CD4/CD8 ratios are in close agreement with a benchtop flow cytometer using healthy donor blood [44].
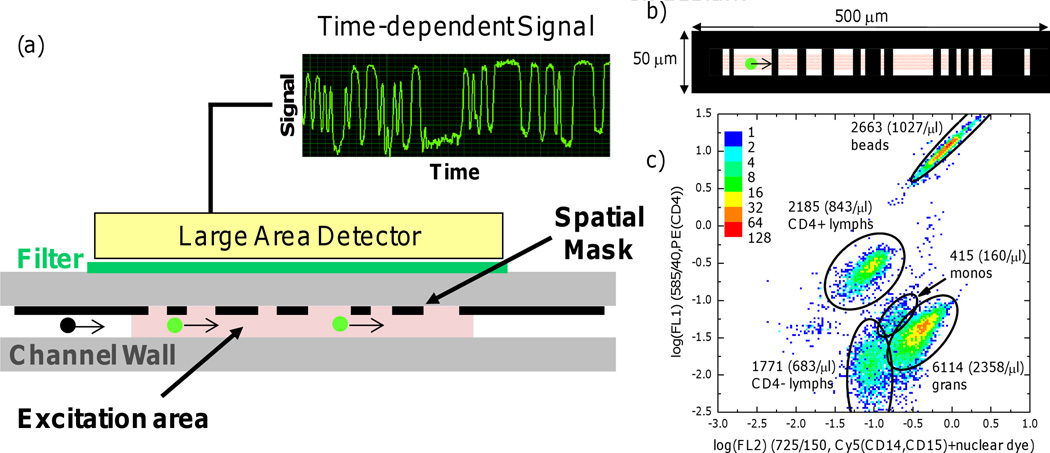
To avoid the detection complexity caused by the intricate optics and precision alignment in conventional cytometry, a ‘spatially modulated emission’ technique to assay labeled particles that pass through a relatively large excitation area was developed (Figure 1) [45]. A spatially-patterned mask modulates the intensity of the fluorescent light directed to a large-area detector [46]. The resulting time-dependent signal is then processed through standard correlation analysis and compared to known calibration patterns to extract the fluorescence intensity and speed of individual cells. Based on this principle, a compact flow cytometer prototype that is low-cost, hand-held, and battery powered has been constructed. Initial evaluation of this prototype for CD4 counting produced results that were in agreement with FACS [46].
Figure 1. Spatially modulated emission eliminates complex optics.
(a) A cross-sectional diagram showing the concept of spatially modulated emission for cell counting [45]. Fluorescently labeled cells (green dots) flow past patterned “windows” in a microchannel to create a characteristic sensogram on a large-area detector based on their size and specific label. (b) Design of the spatial mask used for CD4 counting. Flow direction: 0.5 mm, transverse direction: 50 µm, (c) Density plot of the sensograms obtained from whole blood acquired by the spatial modulation technique with immunolabeling of CD4 and CD14/CD15 with two different fluorophores (adapted from Kiesel et al. [63]).
Electrical and mechanical sensing
The integration of electrical sensing into a cell counting system has great potential for the development of portable and rugged instruments. Imaging equipment relies on lenses and focusing for analysis which increases the size and complexity of the tools. Electrical sensing is derived from solid state components and therefore in principle requires only a sensing geometry, process sensor output and a user interface (test input and result output) [47]. The Coulter counter was developed to detect cell sizes via measuring direct current resistance change to sense particles passing through an aperture between two flow chambers [48]; an impedance pulse is generated every time a cell passes through the aperture. This same principle was used to design a 3D hydrodynamic focusing chip combined with alternating current impedance detection that counts co-cultured lymphoblast cells in suspension (Figure 2) [49]. Hydrodynamic 3D focusing ensures a consistent particle location relative to the sensing microelectrodes; it therefore improves detection sensitivity and facilitates interpretation of cell size. In a follow-up study, the self-referencing microelectrodes were added at the entrance and exit of an immunoaffinity CD4 capture chamber (Figure 2) [47]. The difference between cell counts before and after depleting CD4+ cells in the chamber is considered the absolute CD4 count. Preliminary tests showed excellent correlation with optical measurement in a range from 100 to 700 CD4+ cells per µL.
Figure 2. Impedance sensing before and after immunocapture of cells yields cell-type specific counts.
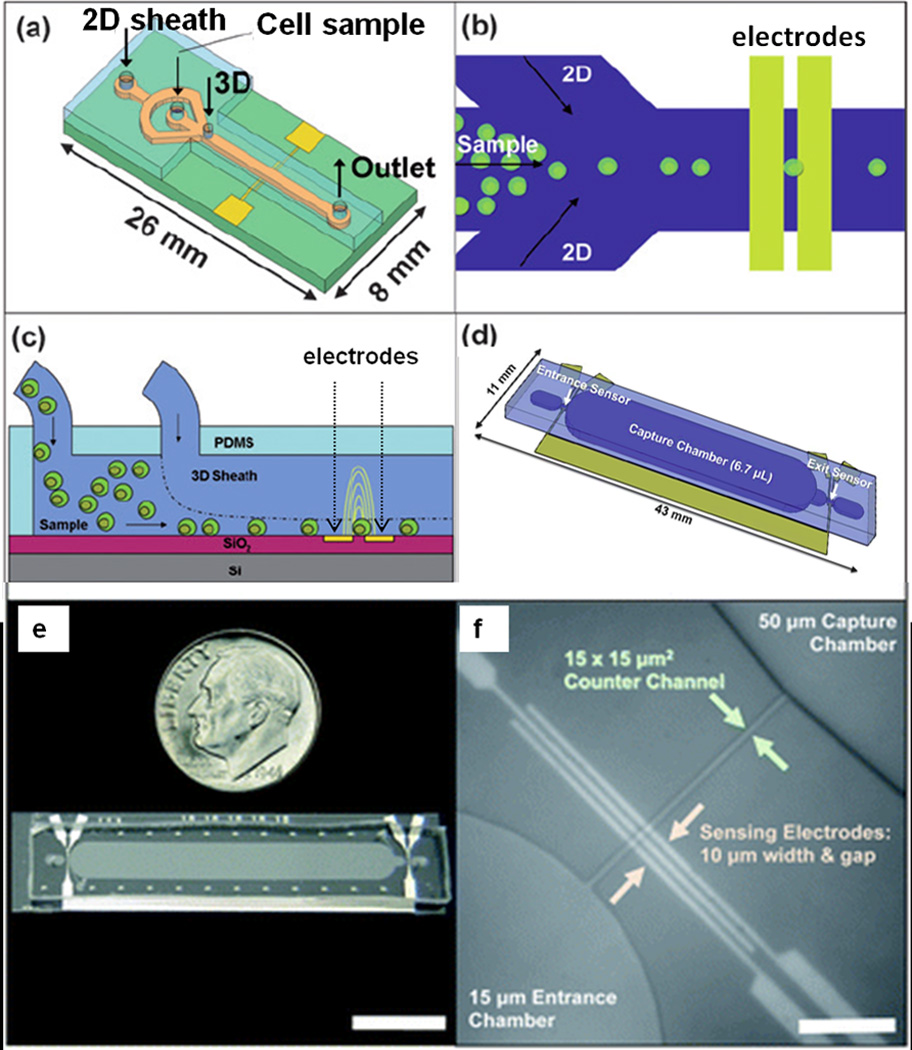
Schematics (2a – 2d) and photograph (2e) of a microchannel Coulter counter with two sets of electrodes at the inlet and outlet, respectively, of a CD4 capture chamber functionalized with antibodies to CD4. Cells flow through capture chamber where they are allowed to react with the surface. Impedance pulses at the inlet count all particles, but pulses at the outlet count only particles not bound by the specific capture surface. (2f) Micrograph of an entrance counter region depicting the three electrodes capable of self-referencing, narrow counter channel, and 50 µm-high capture chamber (scale bar = 100 µm; adapted from Watkins et al [47] Reproduced with permission of The Royal Society of Chemistry).
Another impedance pulse-sensing device uses two pair of microfabricated, parallel-plate electrodes to analyze passing cells at two electrical frequencies [50]. Here, cells of different types are identified by their size and membrane capacitance. The method is enhanced by labeling cells with microspheres with a distinctive electrical signature, such that cell subtypes can be distinguished after specific tagging. CD4+ cells labeled with latex particles (2 µm) from peripheral blood mononuclear cells (PBMCs) have been detected to assess the absolute CD4 count or %CD4 [51].
Electrical detection of substrate-bound cells has also been developed. Methods include anti-hCD4 coated planar microelectrodes to determine the total number of CD4+ cells adhered to the electrodes [52]. Immobilization of cells on anti-hCD4-coated electrodes impedes current flow, which is directly measured through a current amplifier. However, the surface impedance correlates poorly with the number of captured cells, likely owing to confounding signals from nonspecific cell binding. Other limitations of this device include the requirement of PBMCs or lysed blood as the starting sample together with an extended incubation time of 1.5 hours for cell attachment. To correct for non-specificity, an array with 200 electrochemical sensing regions has been built. Each region is the size of a single cell and provides a binary readout of CD4+ cell binding [53]. In this approach, background signal from non-specific binding can be corrected for by defining a signal threshold that corresponds to unequivocal CD4+ cell binding.
Mechanical sensors, such as quartz crystal microbalance sensors, have also being used to detect surface-bound CD4+ cells. Here, the quartz crystal microbalance sensor is functionalized with anti-hCD4. The attached CD4+ cells increase the mass of the quartz crystal, proportionally decreasing the resonant frequency of its oscillation, which can be measured to indicate cell concentration in the original sample [54].
Hybrid devices that combine advances in electrical and optical sensing have also been developed. The gate of a metal oxide semiconductor field effect transistor (MOSFET) is connected to the sensing aperture in the fluid circuit and detects any cell passage as a resistive pulse, with the pulse amplitude proportional to the cell size, while a miniaturized laser-fiber optic detection system determines the number of labeled CD4+ cells [57]. To improve the signal-to-noise ratio in the electrical sensor, the same group has fabricated another hybrid device that replaces the MOSFET with a differential amplification circuit [55,56]. The current throughput of this device is on the order of 10 cells/second. The throughput is two to three orders of magnitude lower than modern flow cytometers and too low to handle clinical samples that contain thousands of leukocytes per microliter. Sensor parallelization may improve throughput [57].
Commercial CD4 counting technologies
Instrumented POC CD4 test devices
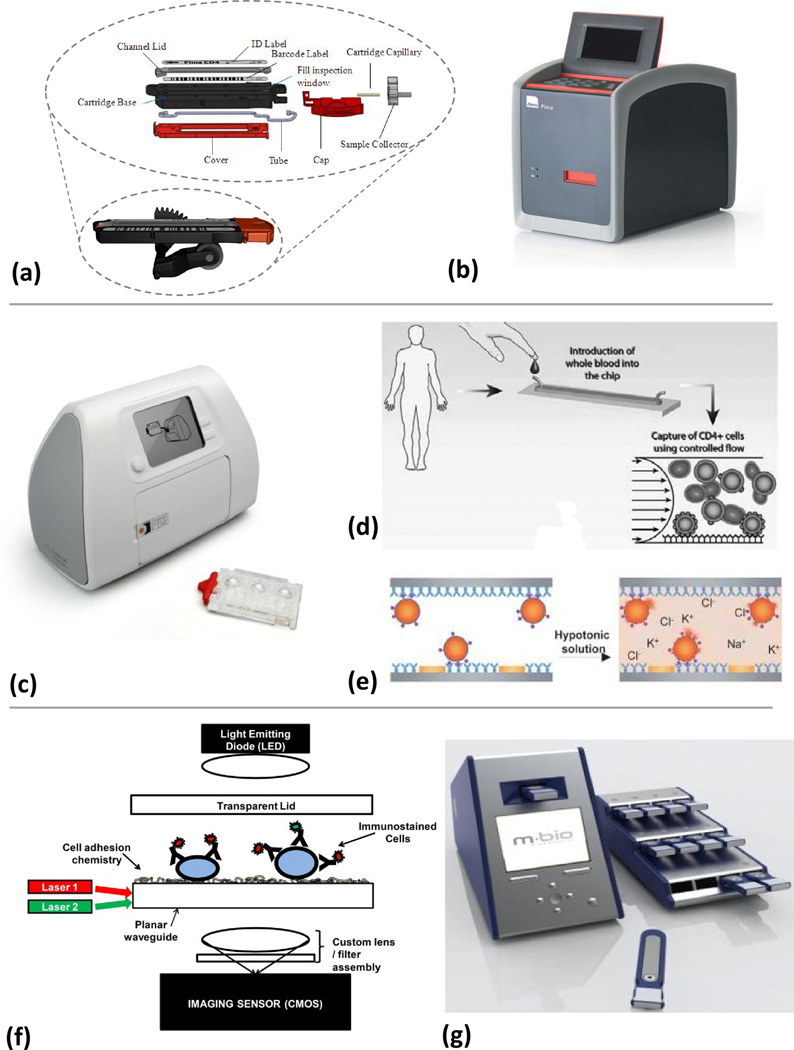
There are several commercial POC CD4 counting devices that are now available and several that are under development (Table 1). Most of these can use fingerstick blood specimens, are rapid, robust, have flexible power options and utilize stable, dried reagents enclosed in a test cassette or device. The devices are designed for minimal operator use with varying though puts, data analyses and result outputs (Table 1). The PointCare NOW™ and the CyFlow® miniPOC (Figure 1) are modified flow cytometers. The PointCare NOW™ is the only POC CD4 device that currently has Food and Drug Administration (FDA) clearance. It should be noted that this system is significantly heavier than the other technologies making it less portable in terms of a user carrying the device and venipuncture is required for the larger specimen volume, which may limit its use in low resource settings where phlebotomists are scarce. However, additional equipment such as pipettors is not needed for operation and so supply of other critical reagents is not a factor.
A CD4 POC test marketed by Alere (Waltham, MA), the Pima™ CD4 test (Table 1 and Figure 3a–3b), is the first commercially available instrumented CD4 counting method that does not use traditional flow cytometric approaches. The Pima™ utilizes dual-fluorescence image analysis to count CD3+ and CD4+ using labeled anti-hCD3 and anti-hCD4. The Pima™ cartridge contains all reagents and specimen during processing. The Pima™ reader supplies the pumping functions and images the results (Figure 3b) to determine co-localization of CD3+ and CD4+ cells. The result as the absolute number of CD4+ T lymphocytes per µL and the CD3+/CD4+ ratio is displayed by the instrument along with quality control results. Cartridges using beads that represent “Normal” and “Low” counts are intended for daily quality control testing of the PIMA™ instrument. Recent evaluations in Zimbabwe and Mozambique have shown good performance in comparison to flow cytometry and user assessment of the test protocols suggest that the Pima™ has the potential for use at the POC [8,58].
Figure 3. Working principles of different analysers.
(a) Alere Pima™ Analyser cassette, shown assembled in position on peristaltic mechanism from reader, and exploded view (b) The Pima™ reader. The disposable, bar-coded (with test ID, expiry date, and channel volume) detection cassette contains stabilized reagents in a flexible tube that is divided into a metering chamber, an incubation/reaction chamber, a reading chamber, and a pumping chamber. The cassette with 5 µL of sample is placed into the reader for processing. Peristaltic pumping moves the sample to an incubation chamber to permit reagent dissolution and antibody binding to the CD3+/CD4+ cells. The stained sample is then moved to the detection chamber, and if process controls are satisfied, images are taken at 20 discrete positions in the channel at two different emission wavelengths (images reproduced with permission from Alere). (c) The Daktari analyser test consists of a reader and a disposable cassette which contains all of the reagents, stabilized in blister packs. (d) The cassette with blood sample is placed in the instrument. Reagents are released from the blister pack and pass into the test chamber. The blood moves at a predefined flow rate through a chamber functionalized with anti-hCD4 monoclonal antibodies (shown here in side view), resulting in shear-gradient (represented by the parabola with flow-velocity arrows) enhanced chromatographic separation from monocytes (adapted from [46]). (e) The retained CD4 cells are lysed and the released ions are detected by impedance spectroscopy, the signal of which correlates to the CD4 count (adapted from [47]). The heavy gray lines represent the electrodes. (Reproduced with permission of The Royal Society of Chemistry). (f) A schematic of the mBio SnapCount™ CD4 analyser and cartridge shows that three images can be taken by the CMOS of each location in the cartridge: A bright field image (illuminated by the LED), and two fluorescence images for the fluorophores on the anti-hCD3+ and the anti-hCD4+ (illuminated by two laser diodes and a proprietary waveguide). Flow into the cartridge is passive. (g) The SnapCount™ analyser, with a single cartridge in front and a work-rack of cartridges along side. The rack facilitates the loading of multiple cartridges for parallel processing (images and schematic reproduced with permission from mBio).
Daktari Diagnostics, Inc. (Boston, MA) is developing a CD4 test that uses a novel microfluidic affinity-chromatography/shear-gradient technique to differentially capture CD4+ cells from whole blood and a unique non-optical detection to count them (Table 1, Figure 3c–3e). The test consists of a reader and a disposable cassette which contains all of the reagents, stabilized in blister packs (Figure 3c). Here, flowing CD4+ cells adhere to an antibody coated chamber while larger-sized monocytes are subject to large shear forces and cannot bind. The captured CD4 cells are then lysed and the release of cellular ions is measured by impedance spectroscopy[59]. The decrease in impedance in the chamber due to CD4+ cell binding has been shown to correlate with cell count over three orders of magnitude, including 350 CD4+/mm3, the threshold for ART [60].
mBio Diagnostics, Inc. (Boulder, CO) is developing a CD4+ cell-counting system, SnapCount™. It is a static two-color fluorescence imaging cytometry system composed of single-use disposable cartridges and a simple reader with on-cartridge immunostaining of whole blood samples (Table 1, Figure 3f–3g). The instrument addresses the high-cost of conventional optical systems by using LightDeck™ technology, a fluorescence assay illumination approach that is a variation on planar waveguide technology (Figure 3f–3g) which uses low-cost lasers, optics, and imaging sensors that are now ubiquitous in cell phones and consumer electronics. As the instrument is only utilized in the reading step, multiple cartridges can be processed in parallel, providing a throughput of eight to ten samples per hour. This relatively high sample throughput might allow remote health care settings to meet their greater demand with fewer instruments.
Non-instrumented CD4 test devices
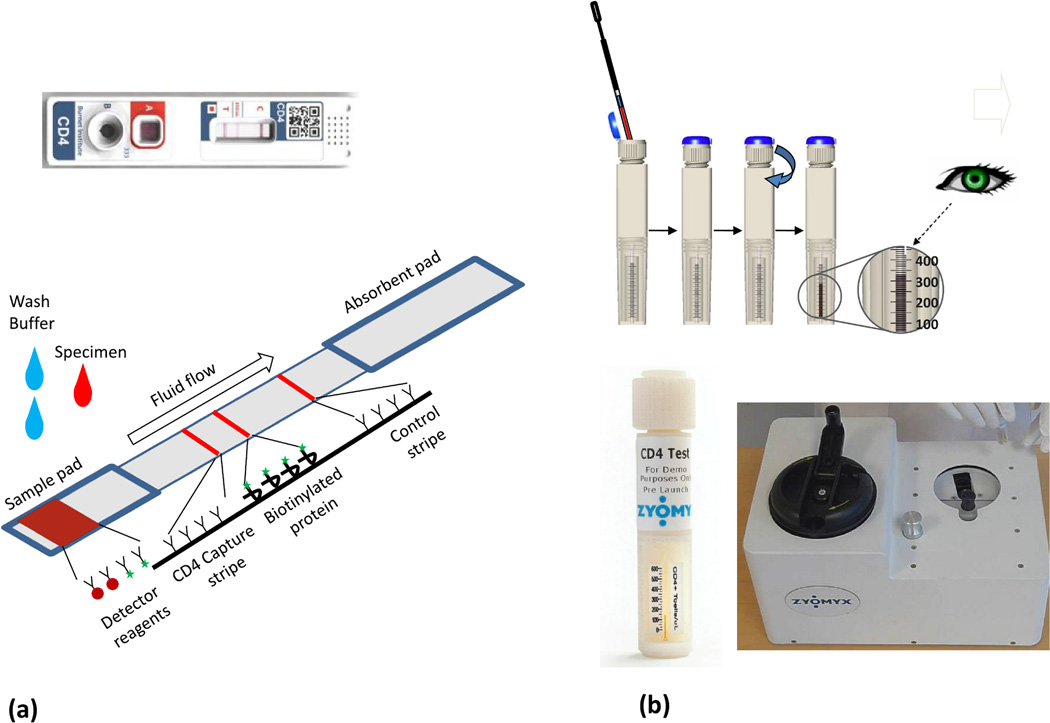
In 2005, the CD4 initiative held by Imperial College, London (funded by the Bill & Melinda Gates Foundation) partnered with industrial and academic teams to develop power-free POC CD4 tests[61] and three devices are now being further developed from this project. The Burnet Institute (www.burnet.edu.au/) CD4 test device is a semiquantitative immunochromatrographic strip (ICS, Figure 4a). Some ICS’ have been shown to be effective tools for POC diagnostics in LRS; they are small, low-cost, have acceptable performance, reagent stability, and have a test format that is familiar to the intended user group. Figure 4a shows a schematic of the Burnet assay. The transmembrane domain of cellular CD4 is recognized by biotin-labeled anti-hCD4 is detected by a colloidal gold-labeled anti-biotin. A capture stripe of anti-CD4 is adjacent to a reference stripe of a biotinylated surrogate protein. The CD4 count is determined by the user to be either greater or lower than the reference stripe. Beckman Coulter has been developing a similar semiquantitative ICS format to enumerate CD4 at the POC and received a recent award from the BMGF to continue the assay development.
Figure 4. Analyzer-independent CD4 counting devices.
(a) The Burnet Institute CD4 test immunochromatrographic strip (ICS); actual device (top, image reproduced with permission from the Burnet Institute) and schematic (bottom). The ICS consists of three major component parts—a sample pad (containing labeled detector analytes, red blood cell and monocyte capture reagents, and cell lysis reagents), a nitrocellulose chromatographic strip with an anti-hCD4 capture stripe, a comparator stripe (a precise deposition of biotinylated protein relating to a given number of CD4 cells) and a control stripe (labeled C on the cassette) that captures excess detector reagent to indicate fluidic flow and binding of the detector reagent. The flow rate is optimized by an absorbent pad at the terminal end of the ICS. The flow of detector analyte is initiated by the addition of a wash buffer after the addition of the blood specimen. The semi-quantitative test result is obtained by comparing the band intensity developed on the capture stripe (T) to the adjacent reference stripe located to the right. (b) The Zyomyx CD4 test principle (sequence of images starting at upper left). The device consist of two chambers, an upper collection and monocyte removal chamber and the lower CD4 test stacking component that together form a single test unit (i) Initially, 100 µL of whole blood is added to rehydrate lyophilized reagents stored in an upper chamber in the test unit (looks like a twist on cap in the image, actual unit is shown at bottom left). The reagents include high-density particles coated with an anti-hCD4 and anti-hCD14 coated magnetic beads for monocyte removal via a magnetic collar. (ii) The sample is mixed in the upper chamber of the test device for 2–3 minutes via a spring loaded mixer/centrifuge (shown at bottom right). (iii) Breaking a seal by twisting causes the monocyte depleted blood to move into a lower chamber that is filled with a separation matrix. (iv). This chamber tapers into an 80 µm-wide capillary tube at the base of the device. The density of the matrix, combined with manually powered centrifugation of the column causes sedimentation of the high-density, bead-associated CD4 T cells and excludes confounding cells and debris. (v) The height of the visible bead/cell column in the capillary is directly proportional to the number of CD4 cells when viewed against a pre-calibrated scale (Images reproduced with permission from Zyomyx).
Zyomyx, Inc. (Hayward, CA) has developed a novel bead sedimentation system to count CD4+ cells (Table 1, Figure 4b) [10]. The reagents include high-density anti-hCD4 particles as well as anti-hCD14 magnetic beads. The device uses sedimentation of high-density, bead-associated CD4+ cells as a measurement principle. The height of the sedimented and visible bead/cell column in a capillary is directly proportional to the number of CD4+ cells when viewed against a pre-calibrated scale, much like reading a thermometer. Preliminary studies suggest a similar performance to FACS across a wide range of CD4 counts from the median 350 cells/mm3 [10].
There is no technology currently in widespread use for counting CD4 at the POC. Most of the technologies described above have incorporated the basic tenets for POC in terms of instrument size, specimen volume, reagent stability, independent power supply, and simple user interface. However, other factors such as throughput, associated consumables and reagents, user training, robustness of design and the method of analysis vary considerably. Three products are available using either scaled flow cytometric methods (PointCareNOW™ and the CyFlow® miniPOC) or novel technology (the Alere Pima™). Flow-based systems have the advantage of established technology and have been thoroughly evaluated in larger scale formats. Thus, evaluation of the new instruments will focus on improving and demonstrating the robustness and the ease of use in LRS. Only some of the instruments address the need for appropriate quality controls (QC) and POC testing is an area where QC is essential t o ensure that specimen preparation, equipment and reagents are functional, and user compliance with the protocol. The Pima™ incorporates QC indicators for specimen collection, reagent and equipment function. The devices being developed by Daktari and mBio offer cassette-based instrumented readers for increased accuracy with a simple user interface and single or no moving parts. This simplified instrumentation is anticipated to cost less than other CD4 instruments in addition to potentially lower failure rates and reliance on maintenance.
Conclusions
Current developments in CD4 counting technology for use at or near the POC are part of an effort to expand access to testing and eventual ARV treatment services for HIV communities in LRS. Innovative new platforms/assay systems have been developed and are commercially available or in late stages of research and development [67]. Newly developed instrumented devices described above use simple, robust, and inexpensive components (e.g., sheathless or microflow, lens free detection and light emitting diodes as a light source), demonstrate the potential for portable, small footprint, low maintenance, cassette-based and low cost readers in decentralized CD4 counting. Some of these devices like non-instrumented devices still require a great deal more performance, operations research, and cost effectiveness data before an informed decision can be made about their uptake at the country level.
We speculate that in the future there may be multiple testing technologies deployed in varying testing contexts within and between high burden countries. Flow cytometry-based CD4 testing may remain a viable and cost effective testing strategy in urban settings. In peri-urban and rural areas, the introduction of new testing approaches should move in conjunction with increased availability of ART drugs. Capital equipment and human resource costs of applying effective CD4 testing at the POC will be a significant factor in adoption and widespread uptake of these emerging products. In summary, the next generation of CD4 testing technology will provide fundamental support to the expansion of HIV treatment and treatment monitoring programs in LRS. Further research is urgently needed to create an actionable evidence base for local policymakers and decision makers who face difficult sets of constraints when making procurement and implementation decisions.
Acknowledgements
The authors acknowledge the kind permission from the Royal Society of Chemists, John Wiley & Sons, Alere, Daktari Diagnostics, mBio Diagnostics, the Burnet Institute, Axxin Ltd., and Zyomyx, Inc. for image reproduction and/or access to detailed descriptions of their technology. PATH acknowledges the National Institutes of H ealth’s National Institute of Biomedical Imaging and Bioengineering Center to Advance Point-of-Care Diagnostics for Global Health (GHDx Center) grant 1U54EB007949-01 and other discretionary donors to PATH. XC acknowledges the NIH NIAID-1R21AI081638 and Pennsylvania Department of Health Cure Formula Fund. We would like to acknowledge the assistance of Maurine Murtagh and Christopher Crudder in critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
XC is on the Scientific Advisory Board for Daktari Diagnostics. The other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. The Program for Appropriate Technology in Health (PATH), the employer of DSB, KRH, MSS, and MS, is currently evaluating CD4 test products from Daktari Diagnostics, the Burnet Institute, and Zyomyx, Inc.
Reference List
- 1.UNAIDS. AIDS Epidemic Update. 2009
- 2.WHO. World Health Organization; 2005. Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS Case Definitions for Surveillance: Africa Region. [Google Scholar]
- 3.Sterne JA, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. World Health Organization; 2010. Antiretroviral Therapy for HIV Infection in Adults and Adolescents. Recommendations for a Public Health Approach. (2010 revision. edn) [PubMed] [Google Scholar]
- 5.Aina O, et al. Reference values of CD4 T lymphocytes in human immunodeficiency virus-negative adult Nigerians. Clin. Diagn. Lab Immunol. 2005;12:525–530. doi: 10.1128/CDLI.12.4.525-530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis KA, et al. Determination of CD4 antigen density on cells: role of antibody valency, avidity, clones, and conjugation. Cytometry. 1998;33:197–205. doi: 10.1002/(sici)1097-0320(19981001)33:2<197::aid-cyto14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Gilks CF, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 8.Mtapuri-Zinyowera S, et al. Evaluation of the PIMA point-of-care CD4 analyzer in VCT clinics in Zimbabwe. J. Acquir. Immune. Defic. Syndr. 2010;55:1–7. doi: 10.1097/QAI.0b013e3181e93071. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DA, et al. Point-of-care testing. Curr. HIV. /AIDS Rep. 2011;8:31–37. doi: 10.1007/s11904-010-0067-z. [DOI] [PubMed] [Google Scholar]
- 10.Zachariah R, et al. Viewpoint: Why do we need a point-of-care CD4 test for low-income countries? Trop. Med. Int. Health. 2011;16:37–41. doi: 10.1111/j.1365-3156.2010.02669.x. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Technical Brief on CD4 Technologies. 2010
- 12.Cassens U, et al. Simplified volumetric flow cytometry allows feasible and accurate determination of CD4 T lymphocytes in immunodeficient patients worldwide. Antivir. Ther. 2004;9:395–405. [PubMed] [Google Scholar]
- 13.Balakrishnan P, et al. An inexpensive, simple, and manual method of CD4 T-cell quantitation in HIV-infected individuals for use in developing countries 25. J Acquir Immune Defic Syndr. 2004;36:1006–1010. doi: 10.1097/00126334-200408150-00002. [DOI] [PubMed] [Google Scholar]
- 14.Diagbouga S, et al. Successful implementation of a low-cost method for enumerating CD4+ T lymphocytes in resource-limited settings: the ANRS 12–26 study. AIDS. 2003;17:2201–2208. doi: 10.1097/00002030-200310170-00008. [DOI] [PubMed] [Google Scholar]
- 15.Didier JM, et al. Comparative assessment of five alternative methods for CD4+ T-lymphocyte enumeration for implementation in developing countries. J. Acquir. Immune. Defic. Syndr. 2001;26:193–195. doi: 10.1097/00042560-200102010-00017. [DOI] [PubMed] [Google Scholar]
- 16.Lutwama F, et al. Evaluation of Dynabeads and Cytospheres compared with flow cytometry to enumerate CD4+ T cells in HIV-infected Ugandans on antiretroviral therapy. J. Acquir. Immune. Defic. Syndr. 2008;48:297–303. doi: 10.1097/QAI.0b013e31817bbc3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyamuya EF, et al. Evaluation of the FACScount, TRAx CD4 and Dynabeads methods for CD4 lymphocyte determination. J. Immunol. Methods. 1996;195:103–112. doi: 10.1016/0022-1759(96)00094-4. [DOI] [PubMed] [Google Scholar]
- 18.Bi X, et al. Modified Dynabeads method for enumerating CD4+ T-lymphocyte count for widespread use in resource-limited situations. J Acquir Immune Defic Syndr. 2005;38:1–4. doi: 10.1097/00126334-200501010-00001. [DOI] [PubMed] [Google Scholar]
- 19.Yari A, et al. SMARThivCD4mos: a complexity-free and cost effective model technology for monitoring HIV patients CD4 number in resource-poor settings. Bioinformation. 2008;2:257–259. doi: 10.6026/97320630002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briggs C, et al. Measurement of CD4+ T cells in point-of-care settings with the Sysmex pocH-100i haematological analyser. Int. J. Lab Hematol. 2009;31:169–179. doi: 10.1111/j.1751-553X.2007.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorslund S, et al. A PDMS-based disposable microfluidic sensor for CD4(+) lymphocyte counting. Biomed Microdevices. 2008:851–857. doi: 10.1007/s10544-008-9199-y. [DOI] [PubMed] [Google Scholar]
- 22.Thorslund S, et al. Bioactive heparin immobilized onto microfluidic channels in poly(dimethylsiloxane) results in hydrophilic surface properties. Colloids Surf. B Biointerfaces. 2005;46:240–247. doi: 10.1016/j.colsurfb.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Thorslund S, et al. Bioactivated PDMS microchannel evaluated as sensor for human CD4+ cells-The concept of a point-of-care method for HIV monitoring. Sensors and Actuators B. 2007;123:847–855. [Google Scholar]
- 24.Lumb R, et al. Not all microscopes are equal. Int. J. Tuberc. Lung Dis. 2006;10:227–229. [PubMed] [Google Scholar]
- 25.Ymeti A, et al. A single platform image cytometer for resource-poor settings to monitor disease progression in HIV infection. Cytometry A. 2007;71:132–142. doi: 10.1002/cyto.a.20375. [DOI] [PubMed] [Google Scholar]
- 26.Li X, et al. An immunomagnetic single-platform image cytometer for cell enumeration based on antibody specificity. Clin Vaccine Immunol. 2007;14:412–419. doi: 10.1128/CVI.00372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, et al. Clinical evaluation of a simple image cytometer for CD4 enumeration on HIV-infected patients. Cytometry B Clin. Cytom. 2010;78:31–36. doi: 10.1002/cyto.b.20488. [DOI] [PubMed] [Google Scholar]
- 28.Bae SY, et al. Absolute CD4+ cell count using a plastic microchip and a microscopic cell counter. Cytometry B Clin. Cytom. 2009;76:345–353. doi: 10.1002/cyto.b.20470. [DOI] [PubMed] [Google Scholar]
- 29.Kiesel P, et al. OPTOFLUIDICS: 'Spatially modulated emission' advances point-of-care diagnostics. 2010 [Google Scholar]
- 30.Janossy G, Shapiro H. Simplified cytometry for routine monitoring of infectious diseases. Cytometry B Clin. Cytom. 2008;74(Suppl 1):S6–S10. doi: 10.1002/cyto.b.20405. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro HM. "Cellular astronomy"--a foreseeable future in cytometry. Cytometry A. 2004;60:115–124. doi: 10.1002/cyto.a.20067. [DOI] [PubMed] [Google Scholar]
- 32.Tibbe AG, et al. Imaging technique implemented in CellTracks system. Cytometry. 2002;47:248–255. doi: 10.1002/cyto.10085. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, et al. Microfluidic CD4+ T-cell counting device using chemiluminescence-based detection. Anal. Chem. 2010;82:36–40. doi: 10.1021/ac902144w. [DOI] [PubMed] [Google Scholar]
- 34.Ozcan A, Demirci U. Ultra wide-field lens-free monitoring of cells on-chip. Lab Chip. 2008;8:98–106. doi: 10.1039/b713695a. [DOI] [PubMed] [Google Scholar]
- 35.Gohring JT, Fan X. Label Free Detection of CD4+ and CD8+ T Cells Using the Optofluidic Ring Resonator. 2010:5798–5808. doi: 10.3390/s100605798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yun H, et al. Simultaneous counting of two subsets of leukocytes using fluorescent silica nanoparticles in a sheathless microchip flow cytometer. Lab Chip. 2010;10:3243–3254. doi: 10.1039/c0lc00041h. [DOI] [PubMed] [Google Scholar]
- 37.Stybayeva G, et al. Lensfree holographic imaging of antibody microarrays for high-throughput detection of leukocyte numbers and function. Anal. Chem. 2010;82:3736–3744. doi: 10.1021/ac100142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thangawng AL, et al. A hard microflow cytometer using groove-generated sheath flow for multiplexed bead and cell assays. Anal. Bioanal. Chem. 2010;398:1871–1881. doi: 10.1007/s00216-010-4019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin YH, Lee GB. Optically induced flow cytometry for continuous microparticle counting and sorting. Biosens. Bioelectron. 2008;24:572–578. doi: 10.1016/j.bios.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Wang MM, et al. Microfluidic sorting of mammalian cells by optical force switching. Nat. Biotechnol. 2005;23:83–87. doi: 10.1038/nbt1050. [DOI] [PubMed] [Google Scholar]
- 41.Mao X, et al. Single-layer planar on-chip flow cytometer using microfluidic drifting based three-dimensional (3D) hydrodynamic focusing. Lab Chip. 2009;9:1583–1589. doi: 10.1039/b820138b. [DOI] [PubMed] [Google Scholar]
- 42.Church C, et al. Electrokinetic focusing and filtration of cells in a serpentine microchannel. Biomicrofluidics. 2009;3:44109. doi: 10.1063/1.3267098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goddard GR, et al. Analytical performance of an ultrasonic particle focusing flow cytometer. Anal. Chem. 2007;79:8740–8746. doi: 10.1021/ac071402t. [DOI] [PubMed] [Google Scholar]
- 44.Wang YN, et al. On-chip counting the number and the percentage of CD4+ T lymphocytes. Lab Chip. 2008;8:309–315. doi: 10.1039/b713932b. [DOI] [PubMed] [Google Scholar]
- 45.Kiesel P, et al. Spatially modulated fluorescence emission from moving particles. Appl. Phys. Lett. 2009;94 041107. [Google Scholar]
- 46.Kiesel P, et al. 'Spatially modulated emission' advances point-of-care diagnostics. 2010 [Google Scholar]
- 47.Watkins NN, et al. A microfabricated electrical differential counter for the selective enumeration of CD4+ T lymphocytes. Lab Chip. 2011 doi: 10.1039/c0lc00556h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coulter W. Proceedings of National Electronics Conference. 1956;12:1034. [Google Scholar]
- 49.Watkins N, et al. A robust electrical microcytometer with 3-dimensional hydrofocusing. Lab Chip. 2009;9:3177–3184. doi: 10.1039/b912214a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmes D, et al. Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry. Lab Chip. 2009;9:2881–2889. doi: 10.1039/b910053a. [DOI] [PubMed] [Google Scholar]
- 51.Holmes D, Morgan H. Single cell impedance cytometry for identification and counting of CD4 T-cells in human blood using impedance labels. Anal. Chem. 2010;82:1455–1461. doi: 10.1021/ac902568p. [DOI] [PubMed] [Google Scholar]
- 52.Mishra NN, et al. On-chip micro-biosensor for the detection of human CD4(+) cells based on AC impedance and optical analysis. Biosens. Bioelectron. 2005;21:696–704. doi: 10.1016/j.bios.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Jiang X, Spencer MG. Electrochemical impedance biosensor with electrode pixels for precise counting of CD4+ cells: a microchip for quantitative diagnosis of HIV infection status of AIDS patients. Biosens. Bioelectron. 2010;25:1622–1628. doi: 10.1016/j.bios.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Bachelder EM, et al. Utilizing a quartz crystal microbalance for quantifying CD4(+) T cell counts. Sensor Letters. 2005;3:211–215. [Google Scholar]
- 55.Wu X, et al. Simultaneous particle counting and detecting on a chip. Lab Chip. 2008;8:1943–1949. doi: 10.1039/b804319a. [DOI] [PubMed] [Google Scholar]
- 56.Wu X, et al. Microfluidic differential resistive pulse sensors. Electrophoresis. 2008;29:2754–2759. doi: 10.1002/elps.200700912. [DOI] [PubMed] [Google Scholar]
- 57.Zhe J, et al. A micromachined high throughput Coulter counter for bioparticle detection and counting. J. of Micromech. and Microeng. 2007;17:304–313. [Google Scholar]
- 58.Jani IV, et al. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS. 2011 doi: 10.1097/QAD.0b013e328344f424. [DOI] [PubMed] [Google Scholar]
- 59.Cheng X, et al. A microchip approach for practical label-free CD4+ T-cell counting of HIV-infected subjects in resource-poor settings. J Acquir Immune Defic Syndr. 2007;45:257–261. doi: 10.1097/QAI.0b013e3180500303. [DOI] [PubMed] [Google Scholar]
- 60.Cheng X, et al. Cell detection and counting through cell lysate impedance spectroscopy in microfluidic devices. Lab Chip. 2007;7:746–755. doi: 10.1039/b705082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willyard C. Simpler tests for immune cells could transform AIDS care in Africa. Nat. Med. 2007;13:1131. doi: 10.1038/nm1007-1131. [DOI] [PubMed] [Google Scholar]
- 62.Redd AD, et al. T-cell enumeration from dried blood spots by quantifying rearranged T-cell receptor-beta genes. J. Immunol. Methods. 2010;354:40–44. doi: 10.1016/j.jim.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kiesel P, et al. Monitoring CD4 in whole blood with an opto-fluidic detector based on spatially modulated fluorescence emission. Cytometry A. 2011;79:317–324. doi: 10.1002/cyto.a.21042. [DOI] [PubMed] [Google Scholar]