Abstract
Continuing efforts to discover novel means of combating fluconazole resistance in Candida albicans have led to the identification of an indole derivative capable of sensitizing strains demonstrating resistance to fluconazole. This tetracycle (2, ML229) does not appear to act through established Hsp90 or calcineurin pathways to chemosensitize C. albicans, as determined in S. cerevisiae models, and may be a useful probe to uncover alternative resistance pathways.
Keywords: Candida albicans, Fluconazole, Antifungal, Chemosensitizer, Molecular Libraries Probe Production Center, Network (MLPCN)
Candida albicans is a pathogenic yeast responsible for the most frequent superficial and systemic fungal infections in humans.1 Treatment for C. albicans infections generally relies upon topical, oral, or intravenous administration of triazole antimycotics, with fluconazole being the oldest and most widely prescribed member of this class. Like all widely prescribed antimicrobials, fluconazole is susceptible to the development of drug resistance by its target pathogens, and this problem poses a rapidly increasing challenge.2–4 Efforts to prolong the utility of fluconazole have primarily focused on combination therapies with proven antifungal medications,5–9 but research into chemosensitizers affords a promising alternative to combat acquired drug resistance.10–15
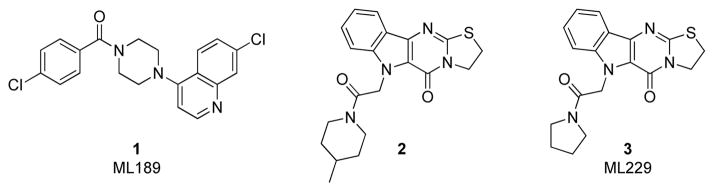
We have recently described our efforts in screening approximately 300,000 compounds from the National Institutes of Health – Molecular Libraries Small Molecule Repository (NIH-MLSMR) to identify potential chemosensitizers of fluconazole-resistant Candida albicans isolates.16,17 This work resulted in the development of piperazinyl quinoline 1 (ML189, Figure 1) that attenuated fluconazole resistance in two C. albicans isolates obtained from an HIV-positive patient.16 In addition to quinoline 1, several other potentiators were identified, including the novel tetracyclic compound 2 (Figure 1).17 In this Letter we report our structure-activity relationship (SAR) studies around hit compound 2 that resulted in development of the new chemical probe ML229 (3). Similar to ML189 (1), the polycyclic indole ML229 (3) reverses C. albicans’s resistance to fluconazole but does not possess any inherent antifungal activity.
Figure 1.
Small molecule probes ML189 and ML229 for reversing fluconazole resistance in Candida albicans clinical isolates. Compounds 1 and 2 were identified by screening the NIH-MLSMR collection.
Dry powder samples of hit compound 2 were tested against C. albicans clinical isolates.18 The weakly resistant strain CaCi-2 and a more resistant CaCi-8 line were both treated enough fluconazole to impair, but not fully eliminate, overall growth.19 Under these conditions, low micromolar concentrations of 2 was observed to effectively suppress the residual, trailing growth that these strains maintained in the presence of fluconazole (Table 1). Toxicity counterscreens revealed that 2 exhibited no detrimental effects on mammalian fibroblasts (3T3 line, IC50 > 26 uM) and was not inherently mycotoxic over the concentration ranges examined (0.2 – 26 uM). The combination of these attributes validated indole 2 as a reasonable starting point for SAR studies.
Table 1.
Structural modifications to the tetracycle scaffolda
| Compound | Structure | IC50 (μM)b
|
|
|---|---|---|---|
| CaCi-2 | CaCi-8 | ||
| 3 |
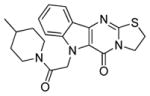
|
3.2 ± 1.0 | 5.5 ± 3.4 |
| 11 |
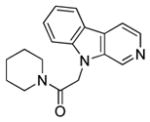
|
>26 | >26 |
| 7a |
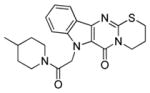
|
12.1 ± 3.2 | 21.3 ± 4.3 |
| 7b |
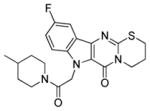
|
2.7 ± 0.5 | 11.7 ± 11.6 |
CaCi-2 and CaCi-8 cells were incubated at 37 °C for 48 hours with test compound and 8 μg/mL (26 μM) fluconazole. Growth inhibition was measured with Alamar Blue fluorescence detection.
Average of at least three runs
The tetracyclic core was synthesized based on the methodology of Sauter and co-workers.20 Beginning with suitably functionalized indoles,21 the core scaffold of 2 could be obtained in a single, convenient transformation (Scheme 1). Commercially available 2-(methylthio)-4,5-dihydrothiazole 5a or 2-(methylthio)-5,6-dihydro-4H-1,3-thiazine 5b was condensed with the appropriate indoles to assemble tetracyclic systems 6. After obtaining various heterocycle cores, the indole nitrogen was capped with substituted alkyl halides to afford the desired analogs 7–10. The structure of the tetracyclic core was elucidated with X-ray crystallography of analog 10k and confirmed our synthetic route could successfully construct these unusual hetereocycles.22
Scheme 1.
General synthesis of tetracyclic indole alkaloids. Reagents and conditions: a) AcOH, 175 °C; b) R2CH2Cl, KOH, DMSO.
SAR exploration began with modifications to the parent ring system. Beta-carboline 11 was prepared and tested in an unsuccessful attempt to truncate the tetracycle (Table 1). In a complimentary approach, an additional carbon was inserted into the dihydrothiazole ring, but expansion of this region generally attenuated activity (7a). It was discovered, however, that incorporation of a fluoro substituent at the 5-position of the indole system (7b) could partially restore activity.
A number of amides were then prepared using the fluorinated scaffold (Table 2). Stronger inhibition of CaCi-2 growth in the presence of fluconazole was noted for this series, with multiple compounds possessing IC50 values in the nanomolar range. It appeared that the fluoro indole core was primarily responsible for the increased potency, as the various amide side chains evaluated appeared to exert little influence. Cyclic (8a–f) and acyclic amides (8g) investigated showed comparable activity against CaCi-2. However, the corresponding esters (8h) and carboxylic acid (8i) were less active. When the entire side chain was removed, the resulting tetracycle 6b was as effective as hit compound 3 in sensitizing CaCi-2 to fluconazole. This series of compounds was then evaluated against the more resistant CaCi-8 strain, but only limited activity was observed in this assay. Despite their potency against CaCi-2, most of the 5-fluoro analogs did not potentiate fluconazole activity in the CaCi-8 isolates. There were a few compounds (e.g. 8f and 8h) that exhibited activity against CaCi-8, but careful examination of their activity profile revealed some of these compounds, such as 8f, actually lost effectiveness as test concentrations increased. This paradoxical dose-response trend, known as the Eagle effect,23 is an established phenomenon with antimycotics24,25 and was observed in a small number of fluorinated indole analogs. Other substituents positioned at C-9, such as methoxy groups (e.g. 9a–b) were investigated, but effective alternatives could not be found. Despite the effectiveness of the 9-fluoro series against CaCi-2, it was ultimately determined that the unmodified indole core would be a more suitable scaffold for further investigation (Table 3).
Table 2.
Activity of substituted cores with various side chainsa
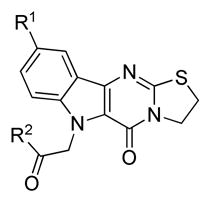
| ||||
|---|---|---|---|---|
| Compound | R1 | R2 | IC50 (μM)b
|
|
| CaCi-2 | CaCi-8 | |||
| 8a | F |
|
0.5 ± 0.1 | >26 |
| 8b | F |
|
0.7 ± 0.2 | >26 |
| 8c | F |
|
2.2 ± 1.2 | >26 |
| 8d | F |
|
0.6 ± 0.2 | 18.9 ± 12.2 |
| 8e | F |
|
0.5 ± 0.1 | >26 |
| 8f | F |

|
1.1 ± 0.3 | 1.1 ± 0.1c |
| 8g | F |
|
0.5 ± 0.2 | >26 |
| 8h | F |
|
3.5 ± 0.9 | 6.0 ± 2.0 |
| 8i | F |
|
>26 | >26 |
| 6b | - |

|
3.8 ± 0.5 | 7.1 ± 5.1 |
| 9a | OMe |
|
>26 | >26 |
| 9b | OMe |
|
>26 | >26 |
CaCi-2 and CaCi-8 cells were incubated at 37 °C for 48 hours with test compound and 8 μg/mL (26 μM) fluconazole. Growth inhibition was measured with Alamar Blue fluorescence detection.
Average of at least three runs
The Eagle effect was observed for this compound. The higher concentrations at which activity was lost were not included when calculating the reported IC50 values.
Table 3.
Activity of the unfunctionalized core and alternative side chainsa
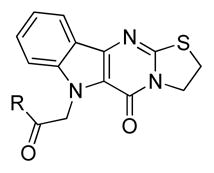
| |||
|---|---|---|---|
| Compound | R | IC50 (μM)b
|
|
| CaCi-2 | CaCi-8 | ||
| 3 |
|
3.2 ± 1.0 | 4.6 ± 3.3 |
| 10a |
|
1.9 ± 0.7 | 2.9 ± 1.2 |
| 10b |
|
>10 | >26 |
| 10c |
|
4.3 ± 1.1 | 6.6 ± 1.0 |
| 10d |
|
7.1 ± 1.6 | 22.5 ± 11.2 |
| 2 |
|
1.1 ± 0.2 | 1.9 ± 0.9 |
| 10e |
|
1.8 ± 0.6 | 3.1 ± 0.7 |
| 10f |

|
5.9 ± 1.5 | 10.2 ± 2.4 |
| 10g |
|
>10 | >26 |
| 10h |
|
2.3 ± 0.2 | >26 |
| 10i |

|
>10 | 23.4 ± 4.4 |
| 10j |

|
>10 | >26 |
| 10k |
|
7.9 ± 4.4 | 13.3 ± 5.3 |
| 10l |
|
>10 | >26 |
| 10m |
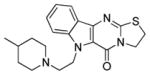
|
>10 | >26 |
| 6a |

|
>10 | 15.7 ± 5.9 |
CaCi-2 and CaCi-8 cells were incubated at 37 °C for 48 hours with test compound and 8 μg/mL (26 μM) fluconazole. Growth inhibition was measured with Alamar Blue fluorescence detection.
Average of at least three runs
Re-evaluation of the side chain showed that the identity of the amide has a small, discernible effect upon cellular potency. The 4-methyl substituent of the piperidinyl amide could be removed (10a) with no significant loss in activity, but replacement with a gem-difluoro group (10b) was not tolerated. Exchanging the piperidine for analogous morpholine (10c) or N-methyl piperazine (10d) did not produce any measureable improvement. A reduction in IC50 values was noted for the smaller pyrrolidine (3) or dihydropyrrole ring (10e), but the inactivity of azetidine amides (10g) suggested that five-membered rings were ideal. Substitution of the pyrrolidine, such as with 3,3-difluoropyrrolidine (10f), typically lowered the effectiveness of these compounds as chemosensitizers. Acyclic amides (e.g. 10h) were less potent than their more constrained cyclic counterparts, and several secondary amides (10i–j) had poor activities. As previously noted with the fluorinated series (vide supra), esters and carboxylic acids were not desirable (10k–l). The lower activity of amine 10m and unsubstituted indole (6a) re-affirmed the acetamide as the preferred appendage.
From this initial SAR investigation, pyrrolidine amide 3 was selected for further examination. This compound demonstrated the ability to sensitize CaCi-2 and CaCi-8 to fluconazole with reasonable potency (IC50 = ~1 and 2 μM, respectively) and did not exhibit the paradoxical Eagle effect associated with some of the fluorinated analogs. After evaluating 3 in several yeast-reporter assays, it was determined that this chemosensitizer did not interfere with Hsp90 or calcineurin-dependant pathways. Both of these signaling mechanisms had been previously implicated in the acquisition of drug resistance by C. albicans.26,27 While the specific cellular target of 3 is currently unknown, it appears to be restricted to fungi as selectivity profiling established that 3 did not inhibit proliferation or survival of murine fibroblasts (IC50 > 26 μM). Additional tests against C. albicans isolates demonstrated that 3 was an ineffective growth inhibitor in the absence of fluconazole (IC50 >26 uM).
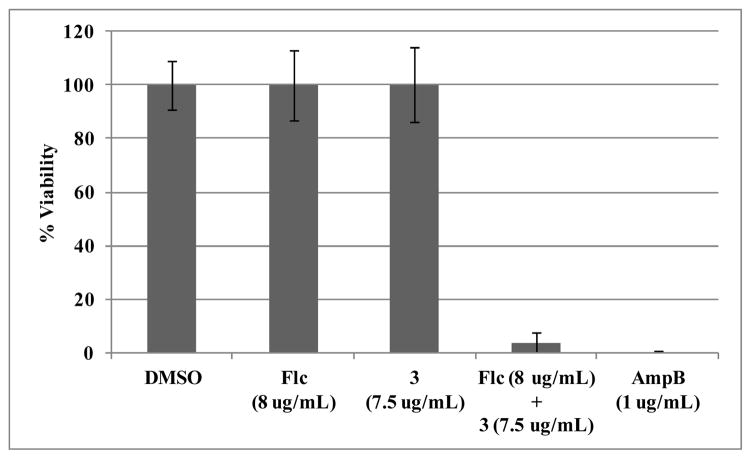
While fluconazole is primarily fungistatic, co-administration with various compounds has been known to produce fungicidal combinations, even when the individual additive possesses only minimal antifungal properties.28–31 To determine if the combination of fluconazole and 3 was fungistatic or fungicidal, the viability of treated cells in liquid culture was examined. The fluconazole-sensitive reference strain C. albicans SC5314 was incubated for 24 hours with fluconazole, tetracycle 3, or both compounds simultaneously. Viable cells were then counted after plating on rich media (Figure 2). Alone, neither fluconazole nor indole 3 decreased viability, but the combination of both agents killed 96% of the initial inoculum. This effectively mirrors the potency of amphotericin B which eradicated all treated fungal cultures. These results suggest that 3 induces chemosensitization by decreasing the survival of fluconazole-treated cells and not through blockade of resistance mechanisms.
Figure 2.
Viability of C. albicans SC5314 inoculums after 24 hour treatment with fluconazole (Flc), ML229 (3), and amphotericin B (AmpB). Average of 4 replicates.
We previously reported primary screening of the NIH-MLSMR collection, and identified a chemical probe class represented by 1 (ML189) that sensitizes Candida albicans clinical isolates to fluconazole.16 In this Letter, we report initial SAR studies on the structurally distinct compound 2 that was also identified as a chemosensitizer of C. albicans in the primary screen. The resulting small molecule probe 3 (ML229), along with 1 (ML189), will be useful tools for interrogating the molecular mechanism by which C. albicans acquires resistance against azole antifungals. To assist such efforts, tetracyclic indole 3 has been registered with the NIH Molecular Libraries Program as probe ML229 and is available upon request.32
Supplementary Material
Acknowledgments
The authors are grateful to Ms. Patti Aha for assistance in manuscript preparation. X-ray crystallography services were provided by Dr. Peter Mueller and the Massachusetts Institute of Technology X-ray diffraction facility. This work was funded by the NIH-MLPCN program (1 U54 HG005032-1 awarded to S.L.S.). BV, LW, and SL are grateful for funding from the NIH (1 R03 MH086456-01).
Footnotes
Crystallographic data for compound 10k, experimental protocols for the preparation of 2, and associated spectroscopic data can be found, in the online version, at doi:
References and notes
- 1.MacCallum D. In: New Insights in Medical Mycology. Kavanagh K, editor. Springer-Netherlands; Dordrecht: 2007. pp. 99–129. [Google Scholar]
- 2.Levy SB, Marshall B. Nat Med. 2004;10:S122. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. Clin Microbiol Rev. 2007;20:133. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanglard D, Odds FC. Lancet Infect Dis. 2002;2:73. doi: 10.1016/s1473-3099(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 5.Kalkanci A, Dizbay M, Sari N, Yalcin B, Fidan I, Arman D, Kustimur S. Cent Eur J Med. 2010;5:194. doi: 10.3109/00365540903321572. [DOI] [PubMed] [Google Scholar]
- 6.Nishi I, Sunada A, Toyokawa M, Asari S, Iwatani Y. J Infect Chemother. 2009;15:1. doi: 10.1007/s10156-008-0653-9. [DOI] [PubMed] [Google Scholar]
- 7.Meletiadis J, Verweij P, Te Dorsthorst D, Meis J, Mouton JW. Med Mycol. 2005;43:133. doi: 10.1080/13693780410001731547. [DOI] [PubMed] [Google Scholar]
- 8.Roling EE, Klepser ME, Wasson A, Lewis RE, Ernst EJ, Pfaller MA. Diagn Micr Infec Dis. 2002;43:13. doi: 10.1016/s0732-8893(02)00361-9. [DOI] [PubMed] [Google Scholar]
- 9.Louie A, Liu W, Miller DA, Sucke AC, Liu QF, Drusano GL, Mayers M, Miller MH. Antimicrob Agents Chemother. 1999;43:2831. doi: 10.1128/aac.43.12.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiGirolamo JA, Li XC, Jacob MR, Clark AM, Ferreira D. J Nat Prod. 2009;72:1524. doi: 10.1021/np900177m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cernicka J, Kozovska Z, Hnatova M, Valachovic M, Hapala I, Riedl Z, Hajós G, Subik J. Int J Antimicro Ag. 2007;29:170. doi: 10.1016/j.ijantimicag.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Gamarra S, Rocha EM, Zhang YQ, Park S, Rao R, Perlin DS. Antimicrob Agents Chemother. 2010;54:1753. doi: 10.1128/AAC.01728-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo XL, Leng P, Yang Y, Yu LG, Lou HX. J Appl Microbiol. 2008;104:831. doi: 10.1111/j.1365-2672.2007.03617.x. [DOI] [PubMed] [Google Scholar]
- 14.Courchesne WE. J Pharmacol Exp Ther. 2002;300:195. doi: 10.1124/jpet.300.1.195. [DOI] [PubMed] [Google Scholar]
- 15.Mai A, Rotili D, Massa S, Brosch G, Simonetti G, Passariello C, Palamara AT. Bioorg Med Chem Lett. 2007;17:1221. doi: 10.1016/j.bmcl.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Youngsaye W, Vincent B, Hartland CL, Morgan BJ, Buhrlage SJ, Johnston S, Bittker JA, MacPherson L, Dandapani S, Palmer M, Whitesell L, Lindquist S, Schreiber SL, Munoz B. Bioorg Med Chem Lett. 2011;21:5502. doi: 10.1016/j.bmcl.2011.06.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The results of the HTS campaign can be viewed free of charge at http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=1979
- 18.Redding S, Smith J, Farinacci G, Rinaldi M, Fothergill A, Rhine-Chalberg J, Pfaller M. Clin Infect Dis. 1994;18:240. doi: 10.1093/clinids/18.2.240. [DOI] [PubMed] [Google Scholar]
- 19.The fluconazole MIC for CaCi-2 and CaCi-8 was determined to be 2 μg/mL (6.5 μM) and 8 μg/mL (26 μM), respectively. For the CaCi-2 and CaCi-8 growth inhibition assays, a fixed fluconazole concentration of 8 μg/mL (26 μM) was used. In CaCi-2, this corresponded to a baseline growth inhibition of 60–80% and was insufficient to suppress the trailing growth associated with this strain. Complete details on the assays performed are provided in reference 16
- 20.Sauter F, Fröhlich J, Chowdhury AZMS, Hametner C. Monatsh Chem. 1997;128:503. [Google Scholar]
- 21.Unangst PC. J Heterocycl Chem. 1983;20:495. [Google Scholar]
- 22.CCDC 858013 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif
- 23.Eagle H, Musselman AD. J Exp Med. 1948;88:99. doi: 10.1084/jem.88.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleischhacker M, Radecke C, Schulz B, Ruhnke M. Eur J Clin Microbiol Infect Dis. 2008;27:127. doi: 10.1007/s10096-007-0411-4. [DOI] [PubMed] [Google Scholar]
- 25.Wiederhold NP. Curr Opin Infect Dis. 2007;20:574. doi: 10.1097/QCO.0b013e3282f1be7f. [DOI] [PubMed] [Google Scholar]
- 26.Cowen LE, Lindquist S. Science. 2005;309:2185. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 27.Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, Cardenas ME, Perfect JR, McCusker JH, Heitman J. EMBO J. 2002;21:546. doi: 10.1093/emboj/21.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vu K, Gelli A. Med Mycol. 2010;48:255. doi: 10.1080/13693780903081968. [DOI] [PubMed] [Google Scholar]
- 29.Zhai B, Zhou H, Yang L, Zhang J, Jung K, Giam CZ, Xiang X, Lin X. J Antimicrob Chemother. 2010;65:931. doi: 10.1093/jac/dkq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma M, Manoharlal R, Negi AS, Prasad R. FEMS Yeast Res. 2010;10:570. doi: 10.1111/j.1567-1364.2010.00637.x. [DOI] [PubMed] [Google Scholar]
- 31.Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D. Antimicrob Agents Chemother. 2000;44:2373. doi: 10.1128/aac.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Compound 2 (probe ML229) is also available from commercial vendors.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.