Abstract
WNT signaling plays a central role in the regulation of cellular growth and differentiation. In this issue of the JCI, Mori et al. link WNT signaling to the oxidative capacity of adipocytes during obesity. They show that secreted frizzled-related protein 5 is an extracellular matrix–residing protein that is highly induced during obesity and inhibits oxidative phosphorylation in a tissue-autonomous manner, possibly by sequestering WNT3a. These results implicate local WNT signaling as an attractive target for combating obesity.
WNTs are secreted proteins that play important roles in the regulation of many different cellular functions, including growth and development. WNTs signal via frizzled receptors to activate intracellular signaling pathways that lead to the stabilization of β-catenin (the so-called canonical pathway), or they stimulate various β-catenin–independent signals, like Ca2+ influx or JNK activation (the noncanonical pathway) (1). Secreted frizzled-related proteins (SFRPs) containing cysteine-rich domains related to those of frizzled receptors negatively regulate WNT signaling by neutralizing WNTs extracellularly (2).
WNT signaling has previously been reported to play an important role in adipocyte differentiation. The activation of β-catenin by WNTs, including WNT6, WNT10a, and WNT10b, blocks adipocyte differentiation (3, 4). In contrast, the effects of the putative noncanonical ligands WNT4 and WNT5a on adipocyte differentiation remain the subject of controversy. It has been shown that knockdown of WNT4 or WNT5a diminishes adipocyte differentiation of 3T3-L1 preadipocytes (5); however, suppressive actions of WNT5a treatment have also been reported (6).
In this issue of the JCI, Mori, MacDougald, and colleagues report an unexpected role of SFRP5 on the oxidative capacity of adipocytes in vivo and ex vivo (7). The authors confirmed previous findings that Sfrp5 mRNA expression is restricted to adipocytes within white adipose tissue (8, 9) and showed that Sfrp5 expression was induced during late stages of adipocyte differentiation. Furthermore, they found that Sfrp5 mRNA expression was upregulated in several mouse models of obesity, with a strong correlation between adipocyte size and Sfrp5 transcript levels.
SFRP5 blocks the fat furnace
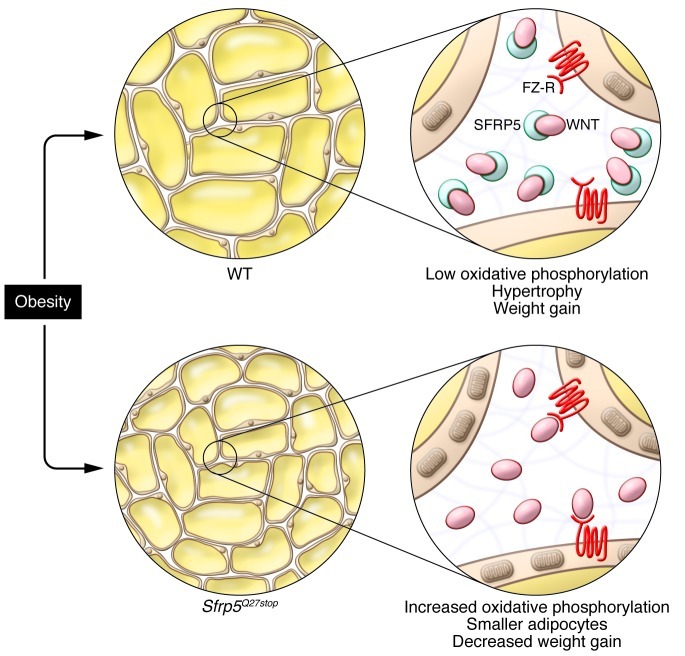
To further investigate the role of SFRP5 in adipose biology, the authors used a mouse model with an N-ethyl-N-nitrosourea–generated point mutation in the Sfrp5 locus that causes a premature stop codon in the first exon (Sfrp5Q27stop mice). These SFRP5-deficient mice were indistinguishable from their WT littermates, and mesenchymal stem cells isolated from Sfrp5Q27stop and WT mice displayed similar adipogenic differentiation (7). However, when challenged with a high-fat diet (HFD), most Sfrp5Q27stop mice gained less weight and were more insulin sensitive than WT littermates. The resistance to obesity was based on decreased appearance of large hypertrophic adipocytes in response to the HFD. This effect is at least primarily tissue autonomous, since it was recapitulated in adipose tissue transplants independent of the genetic background of the host. In support of primarily paracrine/autocrine effects in the adipose tissue, SFRP5 was only detected in the extracellular matrix, not in the supernatant, of adipocyte cultures.
Intriguingly, Mori et al. found that the adipocytes of obese Sfrp5Q27stop mice had a higher oxidative capacity, due to increased number as well as increased oxidative capacity of mitochondria (7). Importantly, this effect must be cell autonomous, since adipocytes differentiated in vitro from mesenchymal precursors of Sfrp5Q27stop mice maintained their oxidative phenotype with respect to gene expression and oxygen consumption. Thus, the loss of SFRP5 leads to an increase in mitochondrial oxidative capacity in adipocytes, which in turn diminishes the accumulation of lipids while on HFD (Figure 1).
Figure 1. SFRP5 is an adipocyte-derived, matrix-residing protein that sequesters WNTs to reduce WNT signaling.
Endogenous WNTs stimulate the oxidative capacity of adipocytes by increasing mitochondrial number and activity. During obesity, SFRP5 levels rise, thereby suppressing the oxidative metabolism of adipocytes, which subsequently leads to adipocyte hypertrophy. In the absence of SFRP5 (Sfrp5Q27stop), adipocytes keep their oxidative potential, which leads to an overall shift to smaller adipocytes and thus limits weight gain. FZ-R, frizzled receptor.
Sfrp5 expression in obesity
High expression levels of Sfrp5 have previously been reported as the best a priori predictor for a large increase in body weight in genetically identical mice (10). In line with this, the present study convincingly demonstrated that in many models of obesity, including db/db, ob/ob, ovariectomized, and HFD-fed mice, increased Sfrp5 transcript levels in adipose tissues correlated with adipocyte size (7). Similar upregulation of Sfrp5 protein was documented after 12 weeks of HFD by Ouchi, Walsh, and colleagues (8). Notably, however, suppression of SFRP5 expression was observed after 24 weeks of HFD (8).
Mori et al. showed that Sfrp5 expression was induced during adipocyte differentiation (7). In addition, Sfrp5 transcript levels in adipose tissue were shown to correlate with adipocyte size (7). Consistent with this, Sfrp5 expression has been previously shown to be induced by refeeding (11).
It is unclear what transcription factors account for the induction during differentiation, and chromatin occupancy analyses of adipogenic transcription factors have not demonstrated binding close to the Sfrp5 locus (12, 13). Similarly, the mechanisms inducing expression of the gene during obesity and in response to refeeding are unknown. It is possible that insulin-activated factors such as sterol-regulatory element binding proteins play a role; however, proinflammatory signals, secondary to the increase in adipocyte size, could also be involved during obesity.
Function of SFRP5 in obesity
The results of the present study differ from those of the Walsh group in the reported effects of genetic ablation of Sfrp5, despite a similar genetic background and a comparable dietary regime. In contrast to the antiobesity phenotype of the Sfrp5Q27stop mice, the Sfrp5–/– mice used by the Walsh group displayed an increase in adiposity, adipocyte size, inflammatory response, and insulin resistance after exposure to HFD (8). Of note, this metabolic phenotype of obese Sfrp5–/– mice was assessed after 12 weeks of HFD, at which time Sfrp5 expression was elevated in WT littermate controls. The reason for the difference between the phenotypes is unknown, since neither of the two targeting strategies is predicted to have off-target effects on other genes. The Sfrp5Q27stop mice were generated by chemical mutagenesis (14) and backcrossed to C57BL/6 for approximately 20 generations; whereas the Sfrp5–/– mice were generated by replacing exon 1 with a PGKneobpAloxA cassette (15).
Moreover, the two studies also differ in their suggested mechanisms of action. Mori et al. demonstrated that ectopically expressed SFRP5 was mainly associated with the extracellular matrix in cell cultures (7). Furthermore, adipose tissue transplants deficient in SFRP5 protein could not be rescued by high levels of host SFRP5 from db/db mice (7). This is consistent with the notion that WNTs/SFRPs primarily act in an autocrine and paracrine manner. Conversely, the Walsh group reported that ectopically expressed SFRP5 is secreted to the medium and serum, where it acts systemically; moreover, adenovirally expressed SFRP5 protein could rescue the phenotype of Sfrp5–/– mice (8). Thus, it appears that the primary direct mechanism of action in the adipose tissue is autocrine/paracrine signaling. However, systemic administration of SFRP5 may also affect adipose tissues, possibly by indirect mechanisms via other tissues. Mori et al. suggest that SFRP5 decreases mitochondrial oxidative capacity in adipocytes and that this defect is amplified during obesity as a result of increased SFRP5 levels (7).
Fishing for the escaping WNT
An important question as to which WNT ligand(s) mediate the effect of SFRP5 deficiency remains to be resolved. Mori et al. report that treatment with purified recombinant WNT3a, a putative canonical WNT, recapitulated the cell-autonomous effects of SFRP5 deficiency on adipocytes, including increased expression of genes involved in oxidative phosphorylation (7). Notably, however, the WNT3a-treated cells showed an increase in basal oxidative metabolism rather than their maximal capacity, which suggests that WNT3a administration leads to induction of slightly different signals than does SFRP5 deficiency. In contrast, the noncanonical WNT5a was reported to account for the effects seen by the Walsh group (8). Both WNTs have been shown to affect mesenchymal stem cell fate by suppressing adipocyte differentiation. WNT3a has been reported to induce COUP-TFII, which represses Pparg expression (16), whereas WNT5a signaling directs corepressor complexes to PPARγ target sites, thereby blunting PPARγ transactivation (6). It is unclear how this repression of PPARγ activity would promote obesity in the study by the Walsh group (8).
Walsh and colleagues reported that SFRP5 counteracts WNT5a-mediated noncanonical activation of JNK, the activity of which is increased by obesity. Consistent with this, Sfrp5–/– mice lose their phenotype on a Jnk1–/– background (8). However, the interpretation is complicated, since HFD-fed Jnk1–/– mice are resistant to developing obesity (17), meaning that these mice would mimic the lean situation, in which ablation of SFRP5 does not affect the metabolic phenotype. To strengthen the argument for a SFRP5-JNK axis, it would be important to determine the cell types involved, as JNK activity in different cell types has been shown to contribute to obesity and insulin resistance (17). Mori et al. demonstrated that SFRP5 loss of function did not alter JNK activity in obese mice, which indicates that SFRP5 is not a major regulator of JNK. Conversely, the authors showed that WNT3a signaling could mimic the effects of loss of SFRP5 (7). Neither the Walsh group nor the MacDougald group has yet demonstrated direct binding of SFRP5 to WNT3a or WNT5a. However, SFRP5 was previously shown to bind WNT5a using overexpression of tagged proteins (18), and SFRP5 is likely to bind WNT3a based on in silico analyses using a human protein-protein interaction prediction database, PIP (19, 20). It has also been shown that WNT3a and WNT5a bind and trigger phosphorylation events at different subsets of coreceptors, LRP5/6 and ROR1/2, respectively (21). Thus, understanding coreceptor availability and activation would provide some insight into the putative WNT signaling pathways affected by SFRP5.
In summary, the link between global Sfrp5 deletion and the action of the putative WNT ligands on adipose tissue is weak. For example, it has not been shown that elevation of WNT3a or WNT5a levels in the adipose tissue causes a phenotype similar to that of SFRP5 loss. It is also possible that the action of SFRP5 on adipocytes is independent of WNT ligands, as has been reported for other SFRPs (22).
Lingering questions
In addition to resolving the conflicting data obtained from different Sfrp5-deficient mouse models, a future challenge will be to obtain insight into the regulation of Sfrp5 expression. It will be particularly interesting to uncover the mechanisms underlying the increased expression in response to obesity and refeeding, conditions that are both associated with high insulin levels. Furthermore, it will be important to determine which WNT signaling pathways are affected by SFRP5 deficiency in adipose tissue and to uncover the mechanisms by which SFRP5 and WNT signaling modulates nuclear and mitochondrial gene expression involved in oxidative metabolism.
Acknowledgments
A. Rauch is supported by an EMBO long-term fellowship.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(7):2349–2352. doi:10.1172/JCI64196.
See the related article beginning on page 2405.
References
- 1.Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf). 2012;204(1):17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 2.Rattner A, et al. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94(7):2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 4.Cawthorn WP, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a beta-catenin-dependent mechanism. Bone. 2012;50(2):477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishizuka M, Koyanagi A, Osada S, Imagawa M. Wnt4 and Wnt5a promote adipocyte differentiation. FEBS Lett. 2008;582(21–22):3201–3205. doi: 10.1016/j.febslet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Takada I, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007;9(11):1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 7.Mori H, et al. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest. 2012;122(7):2405–2416. doi: 10.1172/JCI63604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouchi N, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329(5990):454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulte DM, et al. Pro-inflammatory wnt5a and anti-inflammatory sFRP5 are differentially regulated by nutritional factors in obese human subjects. PLoS One. 2012;7(2):e32437. doi: 10.1371/journal.pone.0032437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koza RA, et al. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2(5):e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lagathu C, et al. Dact1, a nutritionally regulated preadipocyte gene, controls adipogenesis by coordinating the Wnt/beta-catenin signaling network. Diabetes. 2009;58(3):609–619. doi: 10.2337/db08-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen R, et al. Genome-wide profiling of PPARgamma:RXR and RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008;22(21):2953–2967. doi: 10.1101/gad.501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siersbaek R, et al. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 2011;30(8):1459–1472. doi: 10.1038/emboj.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quwailid MM, et al. A gene-driven ENU-based approach to generating an allelic series in any gene. Mamm Genome. 2004;15(8):585–591. doi: 10.1007/s00335-004-2379-z. [DOI] [PubMed] [Google Scholar]
- 15.Satoh W, Gotoh T, Tsunematsu Y, Aizawa S, Shimono A. Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development. 2006;133(6):989–999. doi: 10.1242/dev.02274. [DOI] [PubMed] [Google Scholar]
- 16.Okamura M, et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proc Natl Acad Sci U S A. 2009;106(14):5819–5824. doi: 10.1073/pnas.0901676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6(5):386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22(21):3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott MS, Barton GJ. Probabilistic prediction and ranking of human protein-protein interactions. BMC Bioinformatics. 2007;8:239. doi: 10.1186/1471-2105-8-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDowall MD, Scott MS, Barton GJ. PIPs: human protein-protein interaction prediction database. Nucleic Acids Res. 2009;37(database issue):D651–D656. doi: 10.1093/nar/gkn870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grumolato L, et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24(22):2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121(pt 6):737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]