Abstract
The prostate requires androgens for development and homeostasis. Prostate cancer shares this dependence, however progression to androgen-independence is common after androgen deprivation. There is considerable interest in achieving therapeutic gene expression after androgen ablation using prostate-specific promoters. Paradoxically, known prostate-restricted cis-regulatory elements are androgen dependent. Hoxb13 expression is restricted in adults to the prostate and colon, and robust Hoxb13 expression persists after castration. To locate regulatory elements conferring this expression pattern, a lacZ reporter was inserted into the Hoxb13 locus on a mouse genomic bacterial artificial chromosome. In transgenic mice, this construct recapitulated the Hoxb13 expression pattern, including expression after castration. Reporter gene activity was maintained during carcinogenesis in a prostate cancer model. Hoxb13 cis-regulatory elements provide a powerful tool to achieve androgen-independent transgene expression in the prostate and distal colon-specific expression in the gastrointestinal tract. These data establish a framework for high-resolution analyses of factors regulating Hoxb13.
Keywords: Colon, Hoxb13, Prostate, TRAMP, Transgenic Mouse
Introduction
HOX genes are largely responsible for anterior/posterior patterning during development in all metazoans and show remarkable conservation in structure and function from nematodes to vertebrates (Krumlauf, 1994). During mammalian evolution, expansion in the number of genes within each Hox cluster has lead to the generation of 13 paralogous groups. Large-scale duplication events also occurred to create four unlinked clusters approximately 100 kb in length (Duboule, 2007). In addition to a clustered organization, HOX genes display spatial and temporal colinearity of expression. Paralogs assigned lower numbers (i.e. Hoxb1) are expressed earlier in development and more anterior in the organism, while those assigned higher numbers are expressed progressively later in development and in a more posterior position (Krumlauf, 1994).
Although much of the research on Hox genes to date has focused on their roles in early embryonic development, Hox gene expression often persists during the period of organogenesis and afterwards in differentiated organs (Krumlauf, 1994; Hombria and Lovegrove, 2003). It is becoming increasingly apparent that a subset of Hox genes continues to play roles in normal and malignant adult cells long after anterior/posterior body patterning has occurred (Cillo et al., 2001). This continued expression may maintain homeostasis by controlling cell differentiation during replenishment of those lost by apoptosis, and by regulating general cellular processes throughout adulthood (Cillo et al., 2001).
Several Abdominal B (AbdB) family Hox genes are reported to play roles in prostate organogenesis, including Hoxa10, Hoxa13, Hoxb13, and Hoxd13. Hoxb13 is unique as it is the only AbdB family member whose expression perdures throughout organogenesis and continues in the mature prostate of adult mice (Sreenath et al., 1999). In adult animals, Hoxb13 expression is restricted to the prostate, distal colon, and rectum (Sreenath et al., 1999). Prostatic expression is highest in the ventral lobe, with decreasing levels in the lateral, dorsal, and anterior lobes (Sreenath et al., 1999). Accumulation of Hoxb13 mRNA is not diminished by castration, indicating testicular androgens are not required for normal levels of expression (Sreenath et al., 1999). Hoxb13 expression is remarkable in that it is highly restricted to the prostate, yet unlike all other prostate-restricted genes, it does not require testicular androgens for high-level expression.
Promoters of several genes with a prostate-restricted expression pattern have been identified. The regulatory regions of PSA (Cleutjens et al., 1996; Schuur et al., 1996; Cleutjens et al., 1997a; Cleutjens et al., 1997b), Pb (Greenberg et al., 1994; Yan et al., 1997), C3(1) (Allison et al., 1989), PSCA (Watabe et al., 2002), and Nkx3.1 (Chen et al., 2005) have been functionally analyzed in transgenic mice. All five genes require androgens for high-level expression in the mature prostate, and all but Nkx3.1 and PSCA are not expressed until, at earliest, the second week of postnatal life. Unlike these genes, Hoxb13 transcriptional activity is robust in the adult prostate in the absence of testicular androgens. In addition, Hoxb13 is also expressed in early stages of prostate organogenesis. The androgen-independent expression of Hoxb13 in the prostate makes it an interesting candidate for an analysis of transcriptional regulation, and the cis-regulatory elements conferring this expression could be employed in a diverse array of transgenic and, potentially, therapeutic applications.
In this study, we employ a reporter gene approach to create a transgene in mice that contains the Hoxb13 cis-regulatory elements required to faithfully mimic the expression pattern of the endogenous gene from development to adulthood, following castration, and in the Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) model. We also report evidence, contradictory to existing literature, that Hoxb13 is expressed during the earliest stages of prostate organogenesis. The identification of these cis-regulatory elements provides a much needed novel reagent for the manipulation of gene expression in the developing, mature, and androgen-deprived prostate gland. It also provides a foundation for a detailed deletion analysis to isolate minimal cis-regulatory elements and to identify the interacting transcription factors that control this unique expression pattern.
Results
The full complement of elements necessary to confer spatial and temporal expression at a level equivalent to the endogenous gene can be dispersed in broad regions that lie both upstream and downstream of the transcriptional start site. Methods for identification of these regulatory elements can be technically challenging. Cloning by traditional restriction digestion methods is often complicated by the lack of endogenous restriction sites within the genomic sequence. Also, the size of genomic fragments necessary to recapitulate the spatial and temporal gene expression patterns can exceed the cloning capacity of standard vectors.
To create a large transgene to survey the Hoxb13 genomic locus for cis-regulatory elements, an E. coli-based homologous recombination approach was undertaken (Lee et al., 2001). Bacterial Artificial Chromosomes (BACs) containing mouse Hoxb13 were identified by end sequence homology using the NCBI Clonefinder program (see Materials and Methods). The clone RP23-335O22 was chosen as the initial BAC for recombination as it contained large regions both upstream (−158,454 bp) and downstream (+60,101 bp) from the Hoxb13 start codon. To fuse the lacZ reporter gene in-frame after the first 8 amino acids of the Hoxb13 locus, a reporter/selection cassette (lacZ-kanr) within the vector pLZKAN was flanked with regions surrounding the start codon of Hoxb13 to create pLZKAN-Hoxb13. Following recombination (Lee et al., 2001), recombinant BACs were purified, subjected to Restriction Fragment Length Polymorphism (RFLP) analysis to determine the integrity of the BAC, and sequenced across the recombination junctions to confirm correct insertion of the lacZ reporter gene. A recombinant BAC with a correctly inserted reporter and without evidence of rearrangements was identified and designated RP23-335O22Hoxb13-lacZ. RP23-335O22Hoxb13-lacZ was linearized with PI-SceI and used to generate transgenic mice. Southern Blot analysis identified six independent transgenic founders carrying the RP23-335O22Hoxb13-lacZ transgene. Founders were either directly analyzed for lacZ expression or mated to FVB/N mice to derive transgenic F1 offspring.
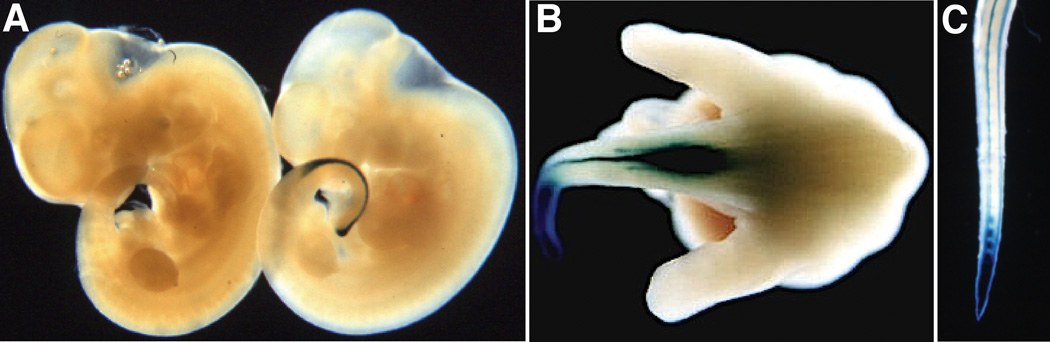
Whole mount microscopic analysis of transgenic embryos from two lines was conducted from 9.0 to 18.5 dpc (days post coitum). Strong expression in somites in the posterior tailbud, a predicted site of Hoxb13 promoter activity, was first detected at 10.5 dpc (Fig.1A). By 12.5 dpc, β-gal activity was observed in the tail bud mesenchyme, neural tube, and caudal extremity of the spinal cord (Fig. 1B). At 18.5, in addition to reporter gene expression in the caudal tail bud and prevertebrae, it was also observed in four parallel muscles tracts that run the length of the tail (Fig. 1C). These observations closely parallel the previously reported expression of endogenous Hoxb13 detected by in situ hybridization and a lacZ knock-in allele (Zeltser et al., 1996; Economides et al., 2003).
Figure 1. Expression of RP23-335O22Hoxb13-lacZ in the caudal region of embryos.
A: Whole mount X-gal stained 10.5 dpc negative control (left) and transgenic (right) embryos. B: Dorsal posterior view of a whole mount X-gal stained 12.5 dpc transgenic embryo. C: Whole mount X-gal stained 18.5 dpc transgenic tail.
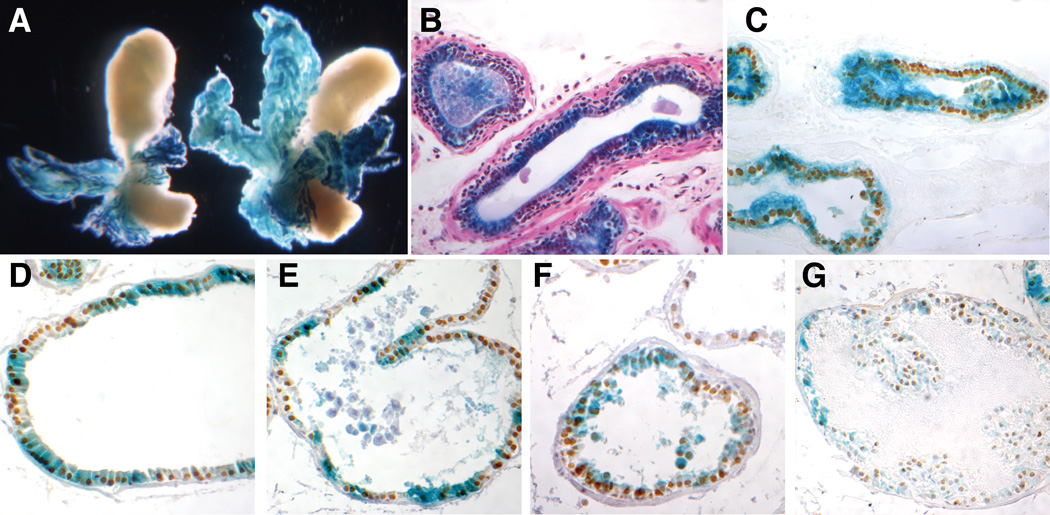
To determine the tissue distribution of lacZ expression in adults, RP23-335O22Hoxb13-lacZ transgenic animals were analyzed for reporter gene activity. Whole mount microscopic analyses of transgenic adult prostates revealed strong reporter gene expression in all six lines (Fig. 2A). Reporter gene expression followed the predicted pattern for Hoxb13 with the strongest staining evident in the ventral lobe, lower expression in the dorsolateral lobes, and weakest in the anterior lobe. Along the length of the ducts, reporter gene expression was strongest in the distal tips and weakest in the most proximal regions near the junction with the urethra. Additional tissues, including the brain, lung, heart, liver, spleen, kidney, stomach, skin, bladder, epididymis, bulbourethral gland, testes, and seminal vesicle were assayed for lacZ expression, and all were found to be negative (data not shown).
Figure 2. Hoxb13 and reporter gene expression in the normal and castrated prostate.
A: Whole mount X-gal stained prostates from castrated (left) and normal (right) 6-week old transgenic littermates. B,C: Paraffin section of X-gal stained ventral lobe from a castrated transgenic mouse with either (B) Hematoxylin and Eosin counterstaining or (C) Hoxb13 immunohistochemistry. D–G: Paraffin section of X-gal stained (D) ventral, (E) lateral, (F) dorsal, and (G) anterior lobes from normal transgenic mouse with Hoxb13 immunohistochemistry.
Histological analyses of Hoxb13 immunohistochemistry in adult prostate lobes revealed strong, uniform Hoxb13 expression throughout the luminal secretory epithelium (Fig. 2D–G). Expression was not detected in the stroma. X-gal staining paralleled the sites of Hoxb13 expression in all four prostate lobes. However, reporter gene expression was intermittent in the epithelium whereas Hoxb13 immunohistochemical staining appeared uniform.
The prostate-restricted regulatory elements characterized to date in transgenic mice require testicular androgens for robust transcriptional activity. In contrast, Hoxb13 mRNA continues to accumulate after castration (Sreenath et al., 1999). To determine whether the RP23-335O22Hoxb13-lacZ transgene is also robustly transcribed in the absence of testicular androgens, transgenic animals were castrated to remove the major source of circulating androgens. As expected, after two weeks, the prostatic lobes from castrated mice were drastically reduced in size compared to the non-castrated control mice (Fig. 2A). Comparison of lacZ activity in whole mount non-castrated and castrated RP23-335O22Hoxb13-lacZ transgenic prostates revealed equally strong staining in both. These data are consistent with previously reported analyses of Hoxb13 mRNA accumulation after castration (Sreenath et al., 1999). At the histological level, the reduction in cytoplasmic volume resulting from castration led to a concomitant reduction in inter-nuclear distance along the epithelium (Fig. 2B). However, the one-to-two cell thickness of the secretory epithelium was largely unchanged. RP23-335O22Hoxb13-lacZ reporter gene activity also remained strong in all prostate lobes of castrates. The reduced inter-nuclear distances lead to an overall more uniform pattern of β-gal activity (Fig. 3B–C ventral lobes shown). Areas of epithelium where reporter gene activity was below detection were interspersed within areas of robust activity in a pattern similar to that observed in non-castrated RP23-335O22Hoxb13-lacZ mice (Fig. 2D–G). To analyze the cellular distribution of endogenous Hoxb13 protein and RP23-335O22Hoxb13-lacZ-driven reporter gene activity in individual lobes, immunohistochemistry was performed on X-gal stained castrated RP23-335O22Hoxb13-lacZ prostates using an anti-Hoxb13 polyclonal antibody. Hoxb13 protein was detected in all lobes in a pattern that closely paralleled that seen in non-castrated mice, with the vast majority of luminal cells throughout the atrophic secretory epithelium showing strong nuclear staining (Fig. 2C–G). The proximal-distal distribution gradient of Hoxb13 along the length of the ducts also appeared to be preserved upon castration.
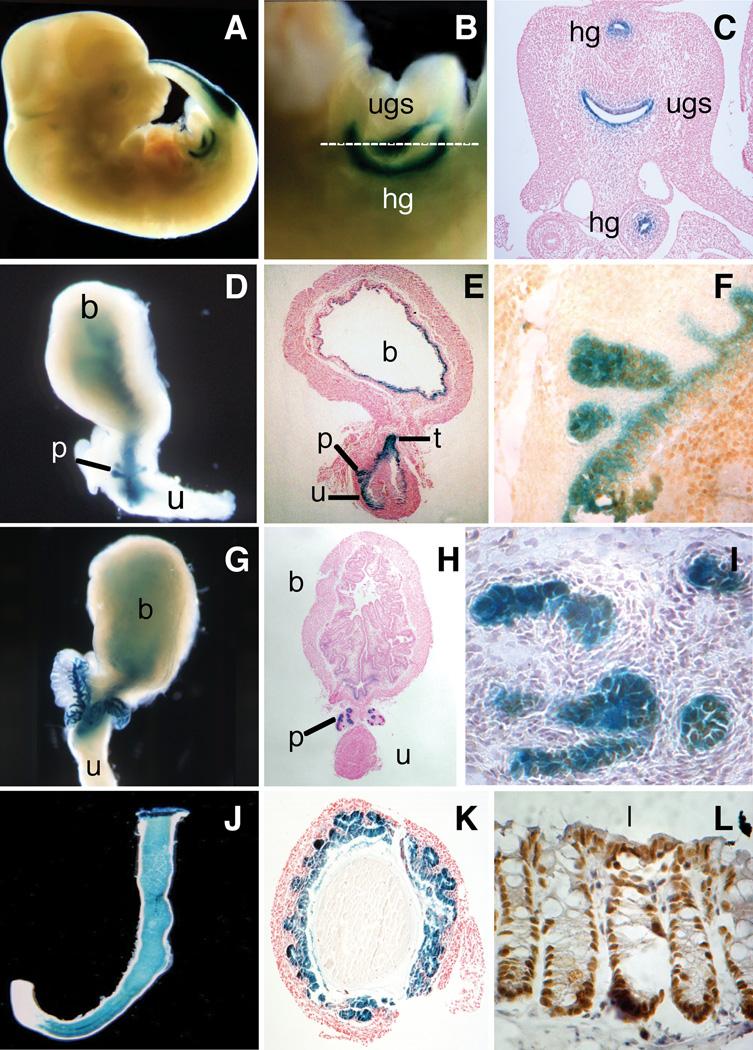
Figure 3. Hoxb13 and Reporter gene expression during urogenital and hindgut development.
A: Whole mount 12.5 dpc embryo with X-gal staining in urogenital sinus, hindgut, and the tailbud. B: Close-up of urogenital sinus (above) and hindgut (below). Dotted line indicates plane of sectioning for 3C. C: Section through embryo showing hindgut (upper, lower X-gal staining) and urogenital sinus (middle). D: Whole mount 18.5 dpc X-gal stained bladder, prostate buds, and urethra. E: Sagittal section of 18.5 dpc bladder, prostate, and urethra counterstained with nuclear fast red. t, trigone region of bladder. F: Hoxb13 immunohistochemistry demonstrating protein expression in X-gal stained 18.5 dpc prostate bud and urethral epithelia. G: Whole mount X-gal stained PND6 bladder, prostate, and urethra. H: Coronal section of X-gal stained bladder, prostate, and urethra counterstained with nuclear fast red. I: Hoxb13 immunohistochemistry of PND6 prostate sections counterstained with hematoxylin. J: Whole mount X-gal stained transgenic 18 dpc colon and rectum. K: Cross section of X-gal-stained adult transgenic colon counterstained with Nuclear Fast Red. L: Immunochemical detection of Hoxb13 in colon cross sections. l, lumen of colon.
Reporter Gene Activity During Urogenital and Hindgut Development
Two RP23-335O22Hoxb13-lacZ transgenic lines were chosen for detailed temporal and spatial analysis of reporter gene expression from 12.5 days post-coitum to sexual maturity. Reporter gene activity was first detected in the urogenital sinus and hindgut epithelium at 12.5 dpc (Fig. 3A–C), consistent with Hoxb13 in situ hybridization (Zeltser et al., 1996). Reporter gene expression that was initiated in the hindgut at 12.5 dpc continued into adulthood.
The expression of lacZ continued in the urogenital sinus at 17.5 dpc, when it was detected in the epithelium of the bladder and urethra. By 18.5 dpc, expression was restricted to the epithelium of urogenital sinus-derived tissue (Fig. 3D). X-gal staining in the primitive bladder was strongest in the trigone region and became progressively weaker towards the apex (Fig. 3E). β-gal activity was also detected in the prostatic urethra and strongly in the newly emerged epithelial buds of all four prostate lobes (Fig. 3E,F).
X-gal staining remained strong during branching morphogenesis in the postnatal day 6 (PND6) prostate (Fig. 3G), and became progressively weaker in the bladder and urethral epithelium after birth (Fig. 3H). By adulthood, reporter gene expression was extinguished in the bladder and urethral epithelium. Hoxb13 was also not detected in adult bladder and urethral epithelium by immunohistochemical staining. However, reporter gene activity in early postnatal life remained strong along the length of the emerging prostatic ducts during the process of branching morphogenesis, paralleling Hoxb13 expression (Fig. 3I).
All transgenic lines showed robust expression in the colon beginning at the junction between the ascending and descending colon and continuing through the rectum (Fig. 3J). This anterior expression boundary was maintained throughout adulthood. Histological analyses revealed that Hoxb13 was restricted to the colonic epithelium and was found throughout the crypts (Fig. 3K). X-gal staining appeared uniform throughout the crypts and paralleled Hoxb13 expression (Fig. 3L).
Reporter Gene Expression in the TRAMP Model of Prostate Cancer
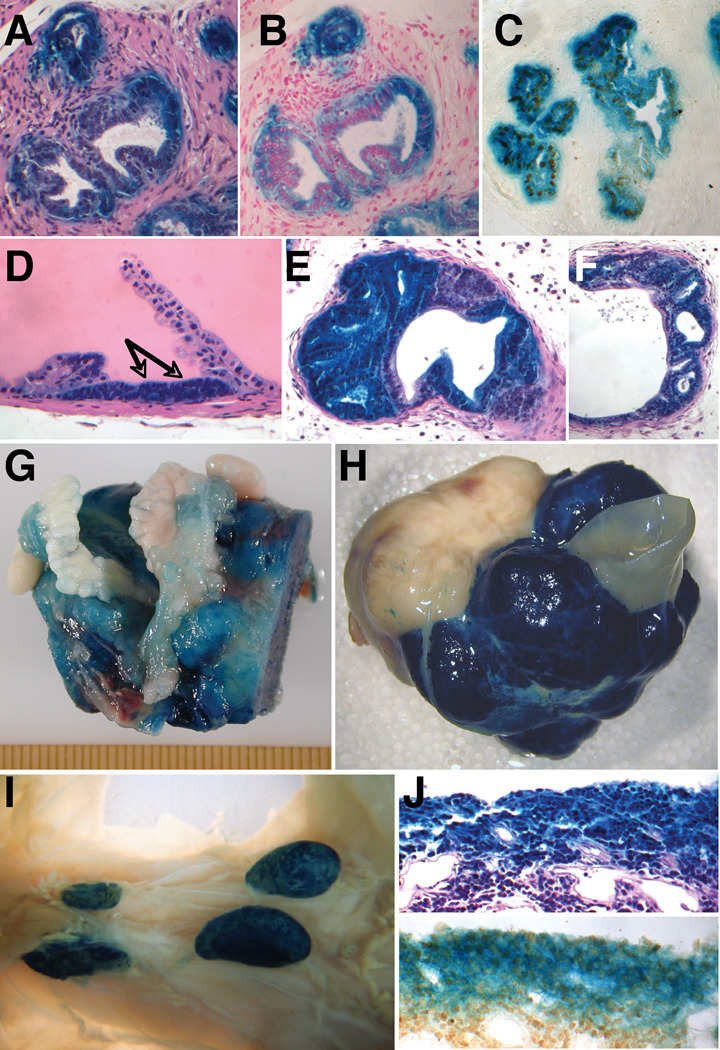
To determine whether the Hoxb13 cis-regulatory elements present in the RP23-335O22Hoxb13-lacZ transgene would be active during disease progression in a model of prostate carcinogenesis, RP23-335O22Hoxb13-lacZ transgenic mice were mated to the C57Bl/6-TRAMP strain (Greenberg et al., 1995). Double transgenic mice carrying both the Hoxb13 reporter transgene and the SV40 large and small T-antigen driven by the rat Pbsn promoter were identified. The TRAMP model was chosen for these analyses given its well-characterized and predictable progression from prostatic intraepithelial neoplasia (mPIN) to metastatic disease. We were particularly interested in assessing the transcriptional activity of the RP23-335O22Hoxb13-lacZ reporter gene in metastatic prostate cancer, since an overarching goal of this work was to identify regulatory elements that might be of value in gene therapy settings. By 16 weeks of age in the TRAMP model, a range of pathologies are typically present to varying degrees in all four mouse prostate lobes (Gingrich et al., 1999). To analyze endogenous Hoxb13 protein levels and RP23-335O22Hoxb13-lacZ reporter gene activity in the TRAMP model, a double transgenic male was subjected to whole-body X-gal staining. All major organs were dissected at necropsy and size-reduced to allow fixative and X-gal penetration. Visual inspection of X-gal stained whole mount organs revealed strong reporter gene activity in the prostate and colon. X-gal staining was also observed in axially located lymph nodes in the sacral and upper lumbar regions (data not shown). Histological analyses of the 16 week-old RP23-335O22Hoxb13-lacZ double transgenic prostate revealed the spectrum of epithelial and stromal abnormalities anticipated in TRAMP/FVB F1 hybrids including microinvasive carcinoma (Fig. 4A–C), mPIN (Fig. 4D), well-differentiated prostate adenocarcinoma (Fig. 4E–F), and phyllodes lesions (data not shown).
Figure 4. Hoxb13 and Reporter gene expression in the TRAMP model of prostate carcinogenesis.
A–C: Progressive sections of a X-gal stained dorsal lobe of a 16 week old double transgenic showing microinvasion. (A) Hematoxylin and Eosin stained, (B) Nuclear Fast red stained, (C) Hoxb13 immunohistochemistry. D: mPIN (indicated by arrows) in a 16 week old double transgenic anterior lobe. E,F: Well-differentiated carcinoma in the ventral lobe of a 16 week old double transgenic. G,H: Whole mount X-gal stained organ-confined tumors from 25 week old double transgenics. (G) anterior view, (H) ventral view. I: X-gal stained metastases to axially located lymph nodes in the sacral and upper lumbar regions of a 25 week old double transgenic mouse. J: Section of tumor periphery from a 25 week old X-gal stained double transgenic showing positive Hoxb13 immunohistochemistry in the absence of X-gal staining. (Upper panel) Hematoxylin and Eosin staining, (Lower panel) Hoxb13 immunohistochemistry.
To assess Hoxb13 levels and RP23-335O22Hoxb13-lacZ reporter gene activity in late-stage disease, double transgenic males were analyzed at 25 weeks. Three individuals with abdominal distension and approaching a moribund state were characterized by both whole mount X-gal staining and Hoxb13 immunohistochemistry. All three had large tumors in the prostatic region (Fig. 4G–H). Following transcardial perfusion with fixative, a partial urogenital axis including the tumor mass was dissected and stained en bloc with X-gal. Other major organs and the remaining carcass were also analyzed for β–gal activity. The large tumor masses in the prostatic region of all three showed strong but variable reporter gene activity. In one, the entire tumor mass showed robust reporter gene expression (Fig. 4G). In a second tumor mass, strong reporter gene activity was observed in ~75% of the surface area. In the remaining ~25%, scattered X–gal staining regions were dispersed within a region that appeared to be largely devoid of staining (Fig. 4H). The third large tumor mass stained in a similar manner, with scattered positive cells in a largely unstained tumor (data not shown).
As expected, reporter gene expression in other organs was confined to the colon and rectum of all 3 late-stage TRAMP tumor-bearers. However, areas of X–gal staining were observed in commonly reported sites of TRAMP metastasis, including axial lymph nodes, spleen, and lung (Fig. 4I, data not shown). In one animal, clumps of β-gal expressing cells were observed traveling within lymphatic ducts (data not shown). Histological and immunohistochemical analyses of the large tumor masses revealed a neuroendocrine phenotype that typifies late-stage disease in the TRAMP model (Huss et al., 2007; Chiaverotti et al., 2008). Nuclear Hoxb13 expression was observed regardless of the extent of X–gal staining in each tumor (Fig. 4J). Strong β–gal activity also correlated closely with Hoxb13 immunoreactivity.
Discussion
Using a reporter gene approach in transgenic mice, we have identified a genomic region containing cis-regulatory elements that faithfully recapitulate the temporal and spatial expression pattern of Hoxb13. A 218 kb BAC containing a lacZ reporter gene recombined in the Hoxb13 locus was sufficient to direct an expression pattern nearly identical to that reported for the endogenous Hoxb13 gene. Reporter gene activity was also characterized in detail in the prostate gland under conditions of androgen deprivation and during the course of carcinogenesis in the TRAMP model. In addition, we define the cellular distribution of Hoxb13 protein accumulation in adult prostate gland and colon.
Reporter gene expression was observed in the caudal extent of the tail bud beginning between 9.5 and 10.5 dpc, in good agreement with previous reports which showed similar location and time of onset (Zeltser et al., 1996; Economides et al., 2003). In agreement with previous reports of endogenous Hoxb13 expression, reporter gene activity was maintained in the derivatives of the tail bud including the developing secondary neural tube, the caudal spinal ganglia, and the caudal prevertebrae (Zeltser et al., 1996; Economides et al., 2003). The concordance of expression between the reporter gene and the reported pattern of endogenous Hoxb13 suggests that the 218 kb BAC contains all the necessary cis-regulatory elements to recapitulate Hoxb13 expression during anterior/posterior body patterning.
Reporter gene expression was detected in the hindgut, which was previously observed by in situ hybridization, but was not reported in knock-in Hoxb13+/lacZ mice (Zeltser et al., 1996; Economides et al., 2003). Furthermore, reporter gene expression in the hindgut was found to continue through embryogenesis and into adulthood. Endogenous Hoxb13 expression has previously been described in the mature mouse distal colon (Sreenath et al., 1999), and is reported to be commonly down-regulated in cases of human colorectal cancer (Jung et al., 2005).
To our knowledge, our analysis is the first to report that after the primitive hindgut is partitioned from the cloaca, Hoxb13 expression persists throughout development of the colon and rectum. In the mature gastrointestinal tract, reporter gene expression is restricted to the epithelium. Staining is throughout the entire crypt, with the strongest staining observed near the base. This region corresponds with the location of the putative stem cells (Radtke and Clevers, 2005), creating the intriguing prospect that Hoxb13 provides positional information to maintain identity in the caudal extent of the intestinal tract.
Given the restricted and robust expression of the RP23-335O22Hoxb13-lacZ transgene in the descending colon and rectum, it may be useful for transgenic applications targeted specifically to that gastrointestinal region. The regulatory elements of Vil1 are widely used to manipulate gene expression for studying colorectal development and cancer, however Vil1 regulatory elements direct gene expression within a broader region of the intestinal tract rather than exclusively to the colon and rectum (Pinto et al., 1999; el Marjou et al., 2004).
The activity of our reporter gene closely correlated with previous reports of Hoxb13 mRNA expression in the urogenital sinus at 12.5 dpc (Zeltser et al., 1996; Economides et al., 2003). Extant literature describing Hoxb13 expression within the urogenital sinus does not detail what occurs after 12.5 dpc. Hoxb13 expression in the urogenital sinus or its derivatives has been reported to initiate at PND10 in the mouse prostate (Economides and Capecchi, 2003). In the rat ventral prostate lobe Hoxb13 expression has been observed at birth (Huang et al., 2007). However, our analyses demonstrate that Hoxb13 expression initiates in the urogenital sinus, and continues in the prostate into adulthood. These discrepancies may be due to limits in sensitivity of the methods previously used for detection, although it is possible that the mouse and rat have different temporal activation of Hoxb13. Alternatively, PND10 expression of a lacZ reporter gene knocked into the endogenous Hoxb13 locus may reflect possible loss of autoregulation during early stages of prostate development due to insufficient Hoxb13 levels caused by haploinsufficiency.
Before prostatic buds are clearly visible at ~17.5 dpc, Hoxb13 is detected in the epithelium of the bladder and in the prostatic region of the urethra. At 18.5 dpc, strong Hoxb13 expression is observed in the emerging prostatic epithelial buds. Although no defects were seen in Hoxb13 knockout mice at early stages of prostate development, this does not rule out a potential role for Hoxb13 during prostate development as other Hox genes are expressed at this stage and are known to have compensatory effects (Warot et al., 1997; Economides and Capecchi, 2003). In good agreement with previous reports, castration did not diminish reporter gene expression in the adult prostate (Sreenath et al., 1999). Additionally, Hoxb13 protein was observed in the prostate of castrated mice at levels comparable to those seen in the normal prostate.
Both Hoxb13 and lacZ were expressed in the prostate pathologies manifested by the TRAMP model. This is consistent with reports of HOXB13 mRNA expression in human prostate cancers, and further suggests that HOXB13 protein may be present and functional. Although there are no known roles for HOXB13 in promoting prostate malignancy, there is evidence that HOXB13 increases invasiveness of breast and endometrial cancers (Ma et al., 2004; Zhao et al., 2005), and it also promotes the progression of ovarian cancer (Miao et al., 2007).
Although the X-gal staining appeared robust by whole mount microscopic analysis in the mature normal, castrated and neoplastic prostate, histological analyses revealed variegated lacZ expression that is not consistent with the uniform immunohistochemical staining for Hoxb13. While it is possible that this inconsistent lacZ expression may be due to lack of sufficient cis-regulatory elements in the RP23-335O22Hoxb13-lacZ transgene, there are other potential factors that may account for these observations. Restrictive access of fixative or X-gal solution would result in a lack of staining, and increased growth due to neoplasia could contribute to this limitation. Additionally, the stochastic nature of transgenic lacZ expression in postnatal tissue is well documented (Montoliu et al., 2000). This variable staining could be explained by the innate ability of lacZ to be silenced by CpG methylation. This methylation-induced silencing of lacZ has been demonstrated by reducing the CpG content by varying degrees, which resulted in dramatic increases in staining intensity and consistency (Chevalier-Mariette et al., 2003). Additionally, CpG methylation increases during pathogenesis in the prostate (Nelson et al., 2007).
Taken together, these reporter gene analyses suggest that the 218 kb BAC contains the necessary elements to recapitulate the expression of endogenous Hoxb13 in the mouse. Although the reporter gene expression in the prostate is histologically non-uniform, it otherwise parallels the expression of endogenous Hoxb13 in the normal, atrophic, and neoplastic prostate. These regulatory elements are valuable for applications of transgenic technology including generating genetically engineered models of prostate and colorectal cancer, manipulating gene expression in vivo, creating tissue-restricted knockout or gain of function models, and possibly developing gene therapy strategies to treat androgen-independent prostate cancer.
Methods
Construction of pLZKAN-Hoxb13
pLZKAN was constructed from pLZURA (Bradshaw et al., 1995) by replacing the URA3 cassette contained on an XbaI fragment with a kanr cassette. The kanr cassette was amplified from pKD4 (Datsenko and Wanner, 2000) by standard PCR with the oligos 5’CGCGGCTAGCGTGTAGGCTGGATCTGCTTC and 5’CGCGGCTAGCCATATGAATATCCTCCTTA, digested with NheI to produce complementary ends, and ligated into the XbaI-digested pLZURA vector to create pLZKAN. pLZKAN-Hoxb13 was then constructed by inserting sequences homologous to the Hoxb13 locus both upstream and downstream of the lacZ/kanr cassette. The upstream flanking sequence was a 197 bp fragment homologous to the upstream region and first eight codons of Hoxb13 (−174 bp to +23 bp) that was amplified by PCR with the oligos 5’ATATCCCGGGGGTGGCATAATTGCCGGG and 5’ATATAAGCTTGCTCTTGCGTCTCTTGCG. The amplified product contained a HindIII site on the 5’ end and a SmaI site on the 3’ end. The amplified product was digested with HindIII and SmaI and cloned in-frame with lacZ into HindIII and SmaI sites in the pLZKAN vector. The downstream flanking sequence was a 255 bp fragment homologous to the Hoxb13 coding region (+124 bp to +378 bp) that was amplified by PCR with the oligos 5’ATATGCGGCCGCTGATGCCAACTGTCA and 5’TTATCCGCGGTGGGACGGCTGGGATA to generate a NotI site on the 5’ end and a SacII site on the 3’ end. The amplified product was digested with NotI and SacII and cloned into the NotI and SacII sites in the pLZKAN vector
For recombination, pLZKAN-Hoxb13 was digested with NotI and HindIII and then fractionated by sucrose gradient centrifugation. Fractions containing the reporter gene/selectable marker cassette were finally prepared for recombination by dialysis against deionized water.
Generation of RP23-335O22Hoxb13-lacZ
The BAC RP23-335O22, which contained 218,555 bp of the mouse genome, was identified by homology to the Hoxb13 coding region using Clonefinder (http://www.ncbi.nlm.nih.gov/genome/clone/clonefinder/CloneFinder.html) and was obtained from BACPAC Resources (http://bacpac.chori.org/). RP23-335O22 was then electroporated into the E. coli strain DY380, with transformants being confirmed by RFLP analysis (Lee et al., 2001). Homologous recombination into RP23-335O22 was performed using the cassette purified from pLZKAN-Hoxb13 according to protocol (Lee et al., 2001). Transformants were selected on plates containing chloramphenicol and kanamycin. Potential recombinants were prepared with the Qiagen Large-Construct kit and digested with enzymes to confirm recombination and BAC integrity. The resulting transgenic construct was named RP23-335O22Hoxb13-lacZ.
Transgenic Mouse Generation and Genotyping
RP23-335O22Hoxb13-lacZ was linearized with PI-Sce I (New England Biolab, Beverly, MA), then purified by sucrose gradient centrifugation and dialyzed as described (Bieberich et al., 1990). The fragments were injected into FVB single cell embryos and transgenic mice were generated as described (Gordon and Ruddle, 1983). Founders or transgenic offspring were identified by Southern Blot analyses. Genomic DNA was isolated using the Gentra Puregene Mouse Tail Kit (Qiagen Inc., Valencia, CA). 10 mg of extracted DNA was digested with EcoR I and EcoR V, electrophoresed on a 0.8% agarose gel, then transferred to a Nytran-N membrane (Whatman Inc, Florham Park, NJ). The vector pLZKAN-Hoxb13 was linearized with Sac II, then the SP6 universal primer was used to create a probe for Southern Blot with the Ambion Strip-EZ PCR kit (Applied Biosystems, Foster City, CA). Blot hybridization was performed using the Ambion Ultra-Hyb Solution (Applied Biosystems, Foster City, CA), washed according to manufacturer’s instructions, and exposed to X-ray film.
C57BL/6 TRAMP males (Jackson Laboratory, Bar Harbor, MA) were bred to non-transgenic C57BL/6 nontransgenic females to derive hemizygous males. Transgenic offspring were identified by PCR as described (Greenberg et al., 1995). All animal procedures were approved by the UMBC Institutional Animal Care and Use Committee.
Detection of β-Gal Activity
Embryos or tissues were dissected and kept on cold PBS until fixed with 4% paraformaldehyde for 30 to 60 minutes. The staining was performed as described (Bieberich et al., 1990) until the desired intensity was achieved as compared to a non-transgenic negative control.
Generation of the Hoxb13 Antibody
Exon I of human HOXB13 was fused in-frame to a 6xHis tag. The recombinant protein was expressed in E. coli and purified by Ni+ column chromatography. The recombinant protein was used to immunize rabbits (Covance Inc., Princeton, NJ). Antiserum was purified by a HOXB13 affinity column.
Immunohistochemistry on Paraffin Sections
Tissues were fixed with 4% paraformaldehyde 4 to 16 hours, then dehydrated and embedded in paraffin for sectioning. Paraffin sections on standard glass slides were deparrafinized and rehydrated. For antigen retrieval, slides were microwaved for 15 minutes in 1 mM EDTA pH 8.0. Immunohistochemistry was performed using the Envision+ System-HRP (DAB) kit (DakoCytomation, Carpentaria, CA), with the rabbit polyclonal anti-human HOXB13 antibody (1:250 or 1:500 dilution for 1–2 hours at room temperature). Tissues were then dehydrated and mounted for examination.
Acknowledgment
Supported by: (NIH: DK59152) (Congressionally Directed Medical Research Program: DAMD17-98-1-8477)
References
- Allison J, Zhang YL, Parker MG. Tissue-specific and hormonal regulation of the gene for rat prostatic steroid-binding protein in transgenic mice. Mol Cell Biol. 1989;9:2254–2257. doi: 10.1128/mcb.9.5.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich CJ, Utset MF, Awgulewitsch A, Ruddle FH. Evidence for positive and negative regulation of the Hox-3.1 gene. Proc Natl Acad Sci U S A. 1990;87:8462–8466. doi: 10.1073/pnas.87.21.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw MS, Bollekens JA, Ruddle FH. A new vector for recombination-based cloning of large DNA fragments from yeast artificial chromosomes. Nucleic Acids Res. 1995;23:4850–4856. doi: 10.1093/nar/23.23.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Mutton LN, Prins GS, Bieberich CJ. Distinct regulatory elements mediate the dynamic expression pattern of Nkx3.1. Dev Dyn. 2005;234:961–973. doi: 10.1002/dvdy.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier-Mariette C, Henry I, Montfort L, Capgras S, Forlani S, Muschler J, Nicolas JF. CpG content affects gene silencing in mice: evidence from novel transgenes. Genome Biol. 2003;4:R53. doi: 10.1186/gb-2003-4-9-r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaverotti T, Couto SS, Donjacour A, Mao JH, Nagase H, Cardiff RD, Cunha GR, Balmain A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol. 2008;172:236–246. doi: 10.2353/ajpath.2008.070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillo C, Cantile M, Faiella A, Boncinelli E. Homeobox genes in normal and malignant cells. J Cell Physiol. 2001;188:161–169. doi: 10.1002/jcp.1115. [DOI] [PubMed] [Google Scholar]
- Cleutjens KB, van der Korput HA, Ehren-van Eekelen CC, Sikes RA, Fasciana C, Chung LW, Trapman J. A 6-kb promoter fragment mimics in transgenic mice the prostate-specific and androgen-regulated expression of the endogenous prostate-specific antigen gene in humans. Mol Endocrinol. 1997a;11:1256–1265. doi: 10.1210/mend.11.9.9974. [DOI] [PubMed] [Google Scholar]
- Cleutjens KB, van der Korput HA, van Eekelen CC, van Rooij HC, Faber PW, Trapman J. An androgen response element in a far upstream enhancer region is essential for high, androgen-regulated activity of the prostate-specific antigen promoter. Mol Endocrinol. 1997b;11:148–161. doi: 10.1210/mend.11.2.9883. [DOI] [PubMed] [Google Scholar]
- Cleutjens KB, van Eekelen CC, van der Korput HA, Brinkmann AO, Trapman J. Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J Biol Chem. 1996;271:6379–6388. doi: 10.1074/jbc.271.11.6379. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134:2549–2560. doi: 10.1242/dev.001065. [DOI] [PubMed] [Google Scholar]
- Economides KD, Capecchi MR. Hoxb13 is required for normal differentiation and secretory function of the ventral prostate. Development. 2003;130:2061–2069. doi: 10.1242/dev.00432. [DOI] [PubMed] [Google Scholar]
- Economides KD, Zeltser L, Capecchi MR. Hoxb13 mutations cause overgrowth of caudal spinal cord and tail vertebrae. Dev Biol. 2003;256:317–330. doi: 10.1016/s0012-1606(02)00137-9. [DOI] [PubMed] [Google Scholar]
- el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Foster BA, Greenberg NM. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 1999;2:70–75. doi: 10.1038/sj.pcan.4500296. [DOI] [PubMed] [Google Scholar]
- Gordon JW, Ruddle FH. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, Angelopoulou R, Dodd JG, Duckworth ML, Rosen JM, et al. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- Hombria JC, Lovegrove B. Beyond homeosis--HOX function in morphogenesis and organogenesis. Differentiation. 2003;71:461–476. doi: 10.1046/j.1432-0436.2003.7108004.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Pu Y, Hepps D, Danielpour D, Prins GS. Posterior Hox gene expression and differential androgen regulation in the developing and adult rat prostate lobes. Endocrinology. 2007;148:1235–1245. doi: 10.1210/en.2006-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss WJ, Gray DR, Tavakoli K, Marmillion ME, Durham LE, Johnson MA, Greenberg NM, Smith GJ. Origin of androgen-insensitive poorly differentiated tumors in the transgenic adenocarcinoma of mouse prostate model. Neoplasia. 2007;9:938–950. doi: 10.1593/neo.07562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Kim RS, Zhang H, Lee SJ, Sheng H, Loehrer PJ, Gardner TA, Jeng MH, Kao C. HOXB13 is downregulated in colorectal cancer to confer TCF4-mediated transactivation. Br J Cancer. 2005;92:2233–2239. doi: 10.1038/sj.bjc.6602631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Ma XJ, Wang Z, Ryan PD, Isakoff SJ, Barmettler A, Fuller A, Muir B, Mohapatra G, Salunga R, Tuggle JT, Tran Y, Tran D, Tassin A, Amon P, Wang W, Enright E, Stecker K, Estepa-Sabal E, Smith B, Younger J, Balis U, Michaelson J, Bhan A, Habin K, Baer TM, Brugge J, Haber DA, Erlander MG, Sgroi DC. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5:607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Miao J, Wang Z, Provencher H, Muir B, Dahiya S, Carney E, Leong CO, Sgroi DC, Orsulic S. HOXB13 promotes ovarian cancer progression. Proc Natl Acad Sci U S A. 2007;104:17093–17098. doi: 10.1073/pnas.0707938104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoliu L, Chavez S, Vidal M. Variegation associated with lacZ in transgenic animals: a warning note. Transgenic Res. 2000;9:237–239. doi: 10.1023/a:1008995730285. [DOI] [PubMed] [Google Scholar]
- Nelson WG, Yegnasubramanian S, Agoston AT, Bastian PJ, Lee BH, Nakayama M, De Marzo AM. Abnormal DNA methylation, epigenetics, and prostate cancer. Front Biosci. 2007;12:4254–4266. doi: 10.2741/2385. [DOI] [PubMed] [Google Scholar]
- Pinto D, Robine S, Jaisser F, El Marjou FE, Louvard D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem. 1999;274:6476–6482. doi: 10.1074/jbc.274.10.6476. [DOI] [PubMed] [Google Scholar]
- Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- Schuur ER, Henderson GA, Kmetec LA, Miller JD, Lamparski HG, Henderson DR. Prostate-specific antigen expression is regulated by an upstream enhancer. J Biol Chem. 1996;271:7043–7051. doi: 10.1074/jbc.271.12.7043. [DOI] [PubMed] [Google Scholar]
- Sreenath T, Orosz A, Fujita K, Bieberich CJ. Androgen-independent expression of hoxb-13 in the mouse prostate. Prostate. 1999;41:203–207. doi: 10.1002/(sici)1097-0045(19991101)41:3<203::aid-pros8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dolle P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- Watabe T, Lin M, Ide H, Donjacour AA, Cunha GR, Witte ON, Reiter RE. Growth, regeneration, and tumorigenesis of the prostate activates the PSCA promoter. Proc Natl Acad Sci U S A. 2002;99:401–406. doi: 10.1073/pnas.012574899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Sheppard PC, Kasper S, Lin L, Hoare S, Kapoor A, Dodd JG, Duckworth ML, Matusik RJ. Large fragment of the probasin promoter targets high levels of transgene expression to the prostate of transgenic mice. Prostate. 1997;32:129–139. doi: 10.1002/(sici)1097-0045(19970701)32:2<129::aid-pros8>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Zeltser L, Desplan C, Heintz N. Hoxb-13: a new Hox gene in a distant region of the HOXB cluster maintains colinearity. Development. 1996;122:2475–2484. doi: 10.1242/dev.122.8.2475. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yamashita T, Ishikawa M. Regulation of tumor invasion by HOXB13 gene overexpressed in human endometrial cancer. Oncol Rep. 2005;13:721–726. [PubMed] [Google Scholar]