Abstract
Aims
Ceragenin CSA-13 is a synthetic mimic of cationic antibacterial peptides, with facial amphiphilic morphology reproduced using a cholic acid scaffold. Previous data have shown that this molecule displays broad-spectrum antibacterial activity, which decreases in the presence of blood plasma. However, at higher concentrations, CSA-13 can cause lysis of erythrocytes. This study was designed to assess in vitro antibacterial and haemolytic activity of CSA-13 in the presence of pluronic F-127.
Methods and Results
CSA-13 bactericidal activity against clinical strains of bacteria associated with topical infections and in an experimental setting relevant to their pathophysiological environment, such as various epithelial tissue fluids and the airway sputum of patients suffering from cystic fibrosis (CF), was evaluated using minimum inhibitory and minimum bactericidal concentration (MIC /MBC) measurements and bacterial killing assays. We found that in the presence of pluronic F-127, CSA-13 antibacterial activity was only slightly decreased, but CSA-13 haemolytic activity was significantly inhibited. CSA-13 exhibits bacterial killing activity against clinical isolates of Staphylococcus aureus, including methicillin-resistant strains, Pseudomonas aeruginosa present in CF sputa, and biofilms formed by different Gram (+) and Gram (−) bacteria. CSA-13 bactericidal action is partially compromised in the presence of plasma, but is maintained in ascites, cerebrospinal fluid, saliva, and bronchoalveolar lavage fluid. The synergistic action of CSA-13, determined by the use of a standard checkerboard assay, reveals an increase in CSA-13 antibacterial activity in the presence of host defence molecules such as the cathelicidin LL-37 peptide, lysozyme, lactoferrin and secretory phospholipase A (sPLA).
Conclusion
These results suggest that CSA-13 may be useful to prevent and treat topical infection.
Significance and Impact of the Study
Combined application of CSA-13 with pluronic F-127 may be beneficial by reducing CSA-13 toxicity.
Keywords: antibacterial agents, microbiological assay, pluronic F-127
Introduction
The ubiquity of natural cationic antibacterial peptides (CAPs) and the variation in amino acid sequences among CAPs suggest that facial amphiphilicity, rather than specific biochemical interactions with a unique bacterial target, defines the mechanisms by which they exert bactericidal effects. This concept has motivated many efforts to design and synthesize steroid conjugate mimics of CAPs that, in addition to having CAP-like biochemical properties, are characterized by lower cost of synthesis, stability under physiological conditions and resistance to protease digestion (Malabarba et al. 1992; Li et al. 1999; Randazzo et al. 2009). CSA-13 is a leading molecule from the ceragenin family tested to date and is relatively simple to prepare and purify (Epand et al. 2008; Lai et al. 2008). CSA-13 displays antimicrobial activity against vancomycin- resistant Staphylococcus aureus (VRSA) (Chin et al. 2007), multidrug-resistant Pseudomonas aeruginosa (Chin et al. 2008), Helicobacter pylori (Leszczynska et al. 2009) and periodontopathic bacteria such as Streptococcus mutans and Porphyromonas species (Isogai et al. 2009). CSA-13 is also active against vaccinia virus (VV) (Howell et al. 2009) and Trypanosoma cruzi (Lara et al. 2009). In animal studies, CSA-13 shows low toxicity, supporting this compound’s possible application in human treatment (Saha et al. 2008). However, similar to CAPs (Wang et al. 1998; Ciornei et al. 2005), CSA-13 antibacterial activity was found to be compromised in the presence of human blood plasma (Bucki et al. 2007b). This finding might partially explain why CSA-13 was not effective when applied as a conjugate in polyurethane foam pads to prevent a peri-implant tissue infection that mimics contaminated osseointegrated implants in a sheep model (Perry et al. 2009).
Apart from being antimicrobial, CSA-13 binds and neutralizes bacterial lipopolysaccharide (Bucki et al. 2007b) and in combination with dioleoylphosphatidylethanolamine (DOPE), facilitates the uptake of a reporter plasmid into various cell lines (Kichler et al. 2005). CSA-13 might therefore have beneficial effects in the treatment of sepsis and has potential for application in gene therapy. The mechanism of CSA-13 action is not fully understood, but it involves direct interaction with negatively charged bacterial membrane molecules such as phosphatidylglycerol, which results in membrane permeabilization and depolarization (Lai et al. 2008; Van Bambeke et al. 2008).
Pluronic F-127 (poloxamer 407) is a nonionic surfactant composed of polyoxyethylene–polyoxypropylene copolymers characterized by low toxicity and good solubilizing capacity (Escobar-Chavez et al. 2006). At a concentration up to 10−4%, pluronic F-127 forms mono-molecular micelles and at a concentration above, multimolecular aggregates consisting of a hydrophobic central core with hydrophilic polyoxyethylene chains facing the external medium (Escobar-Chavez et al. 2006). The unique thermoreversible and drug release characteristics of F-127 show great promise for its application as a pharmaceutical vehicle for drug delivery. Accordingly, effective treatment of chronic otitis caused by methicillin resistant Staph. aureus (MRSA) was achieved using a temperature responsive pluronic F-127 / vancomycin matrix system (Lee et al. 2004). Additionally, in the previous study, pluronic F-127 was shown to increase the incorporation of acetoxymethyl ester dye into cells (Maruyama et al. 1989), the parenteral delivery of peptides and proteins from its co-formulated nanoparticles (Barichello et al. 1999), and was identified as an important component of a bioengineered skin graft which holds great promise for improving the healing of skin defects (Cai et al. 2005).
In this study, we report that CSA-13 is highly active against clinical isolates of bacteria causing different topical infections and bacterial biofilms. CSA-13 haemolytic activity decreases in the presence of pluronic F-127, and its antibacterial activity is potentiated in the presence of host defence antibacterial molecules. The results suggest that cholic acid-based mimics of CAPs, such as CSA-13, have potential for broad-spectrum treatment of bacteria associated with topical infection.
Methods
Materials
Brain heart infusion agar (BHI) was from Emapol (Gdańsk, Poland). Pseudomonas isolation agar, tryptic soy broth (TSB), Brucella broth (BB) and Mueller Hinton Agar (MHA) were purchased from DIFCO (Sparks, MD, USA). An ID 32 STAPH kit to identify staphylococcal isolates and Mannitol salt agar (MSA) were from bioMérieux, (La Balme Les Grottes, France), E-tests to determine susceptibility to methicillin were obtained from AB Biodisk (Solna, Sweden). Beta-lactamase (cefinase) test was from Becton Dickinson (San Jose, CA, USA). Pseudomonas aeruginosa Xen 5 strain engineered through conjugation and transposition of a plasmid carrying the transposon Tn5 luxCDABE was purchased from Caliper Life Science Inc. (Mountain View, CA, USA). LL-37 peptide was purchased from Peptide 2.0 Inc. (Chantilly, VA, USA). Pluronic F-127, lactoferrin, lysozyme and secretory phospholipase A (sPLA) were from Sigma (St Louis, MO, USA). CSA-13 was synthesized as described previously (Ding et al. 2002). Cystic fibrosis (CF) sputum samples were collected from patients attending the University of Pennsylvania Health System Adult Cystic Fibrosis Center at Presbyterian Hospital (PA, USA; IRB-803255).
Haemolytic and bactericidal activities of CSA-13 in the presence of pluronic F-127
CSA-13 haemolytic activity was tested using red blood cells (RBCs) suspended in phosphate-buffered saline (PBS) (haematocrit c. 5%) with and without the addition of pluronic F-127 (1 or 5%). RBCs were incubated for 1 h at 37°C after CSA-13 addition (0–500 μmol l−1). Relative haemoglobin concentration in supernatants after centrifugation at 2000 g was monitored by measuring optical absorbance at 540 nm. Then, 100% haemolysis was taken from samples in which 2% Triton X-100 was added. CSA-13 bactericidal activity against Ps. aeruginosa Xen 5 and clinical isolate Staph. aureus A1 was determined as described previously (Bucki et al. 2004; Bucki and Janmey 2006). Pseudomonas aeruginosa Xen 5 and Staph. aureus A1 were grown to mid-log phase at 37°C (controlled by the evaluation of optical density at 600 nm) and resuspended in PBS. Two different experiments were performed to assess CSA-13 antibacterial activity. In the first, CSA-13 bactericidal activity was evaluated using a bacterial killing assay. When required, pluronic F-127 was added to the bacterial suspension before CSA-13 treatment, and after 1-hour incubation at 37°C bacterial suspensions were placed on ice and diluted 10-to 1000-fold. Then, 10 μl aliquots of each dilution were spotted on Pseudomonas isolation agar or LB agar. The number of colonies at each dilution was counted the following morning. The colony-forming units (CFU ml−1) of the individual samples were determined from the dilution factor. In a second experiment, changes in Ps. aeruginosa Xen 5 chemiluminescence signals during a 5-min period were evaluated after CSA-13 (10 μmol l−1) addition to its TSB suspension (c. 108 CFU ml−1) using a FlexStation 3 platform (Molecular Devices, Sunnyvale, CA, USA) or after 50 s and 16 h using a Fuji Film LAS-300 system.
Antimicrobial activity of CSA-13 against clinical bacterial strains
Bacteria from clinical specimens were grown on MSA. Staphylococcus aureus identification was performed with an ID 32 STAPH kit followed by reading of results using the ATB system bioMérieux, according to the manufacturer’s instructions. Susceptibility to methicillin and vancomycin and the presence of beta-lactamase were determined using E-test and cefinase respectively. Staphylococcus aureus susceptibility to macrolides, lincosamides and streptogramins B was evaluated using diffusion methods on MHA with bacterial inoculum density at 0·5 (McFarland scale) (Fiebelkorn et al. 2003; Standards CLSI 2009). CSA-13 minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) against different strains of Staph. aureus (8 × 105 CFU ml−1) were determined using Mueller–Hinton broth and MHA respectively. The bactericidal activity of CSA-13 against Ps. aeruginosa in sputum samples collected from CF patients with chronic lung infection was measured as previously described (Tang et al. 2005; Bucki et al. 2007a). The CF sputa were diluted 10×, and 100 μl samples were treated with antibacterial agents (10–50 μmol l−1) with and without pluronic F-127 (1 or 5%). After 1 h of incubation at 37°C, the suspensions were placed on ice and diluted 10- to 1000-fold. Then, 10-μl aliquots of each dilution were spotted on Pseudomonas isolation agar plates for overnight culture at 37°C. The number of colonies at each dilution was counted the following morning. The colony-forming units (CFU ml−1) of the individual samples were determined from the dilution factor.
CSA-13 activity against bacterial biofilm
Surface hydrophobicity of Ps. aeruginosa Xen 5, Moraxella catarrhalis, H. pylori, Staph. aureus ATCC43300 and Enterococcus faecalis was estimated using the salt aggregation test (SAT) (Lindahl et al. 1981). Biofilm biomass of bacteria after CSA-13 treatment was evaluated by an optical density reading at 450 nm. Bacterial survival in the biofilm following exposure to the antibiotic was assessed after triphenyltetrazolium chloride (TTC) addition (viable bacteria can reduce the TTC to a red product depending on the level of dehydrogenase activity) (Bartoszewicz et al. 2007). For all bacterial strains (except H. pylori), biofilm formation was performed in aerobic conditions using polystyrene plates with bacterial inocula at c. 108 CFU ml−1 in TSB. Helicobacter pylori biofilm formation was performed in BB suspension under microaerophilic conditions maintained with the use of a Gas Pack-Campylobacter gas generation kit BR60 (Oxoid, Cambridge, UK). For each experiment, bacterial suspensions were placed in polystyrene plates, and a biofilm was allowed to form for 24–72 h. Plate adherence ability was used to differentiate between biofilm and planktonic bacteria that were washed out before adding CSA-13 to individual wells. In the case of Ps. aeruginosa Xen 5, in addition to OD measurement, biofilm biomass was evaluated using crystal violet (CV) staining (0·1%) (Peeters et al. 2008) and chemiluminescence intensity measurements (as an additional determinant of biofilm viability), which was evaluated using a Fuji Film LAS-300 system before and after CSA-13 treatment. Chemiluminescence (densitometry analysis) was performed using Image Gauge (version 4.22) software (Fuji Photo Film Co, Edison, NJ, USA).
Antibacterial activity of CSA-13 in different body fluids
Many factors present at infection sites such as negatively charged glycosaminoglycans, mucins, DNA, and F-actin can compromise host antibacterial peptide activity as well as that of their synthetic mimics (Weiner et al. 2003; Herasimenka et al. 2005). Recently, we found that CSA-13 resists inhibitory action of molecules present in CF sputum (Bucki et al. 2007b) and saliva (Bucki et al. 2008), and CSA-13 activity in other body fluid has not been studied yet. To assess CSA-13 potential to kill bacteria in different environments, we evaluated changes of Ps. aeruginosa Xen 5 luminescence (c. 108 CFU ml−1) in PBS or PBS mixed with 50% blood plasma, ascites, cerebrospinal fluid, saliva or BAL at different time points, up to 6 h after CSA-13 addition (10 and 30 μmol l−1).
CSA-13 antibacterial activity in combination with host defence molecules
Combined antibacterial activity was determined with the use of a standard checkerboard assay (Rahbar et al. 2010; Norden 1982). A serial dilution of two antibacterial agents mixed together in microtiter plates, so that each row and column of the plate contained a fixed amount of one agent, and an increasing concentration of a second one was prepared before bacteria addition. The concentration ranged from approximately the MIC value to an eightfold dilution below this amount. Each plate also contained a row and column in which a serial dilution of each agent was presented alone. A calculation of fractional inhibitory concentration (FIC) index (FCIindex = FICA + FICB = [A] /MICA + [B] /MICB) was used to determine synergy (Kristiansen 1992).
Statistical analysis
Data are reported as mean ± SD from 3 to 6 experiments. Differences between means were evaluated using the unpaired Student’s t-test, with P < 0·05 being taken as the level of significance.
Results
Pluronic F-127 decreases haemolytic but not antibacterial activity of CSA-13
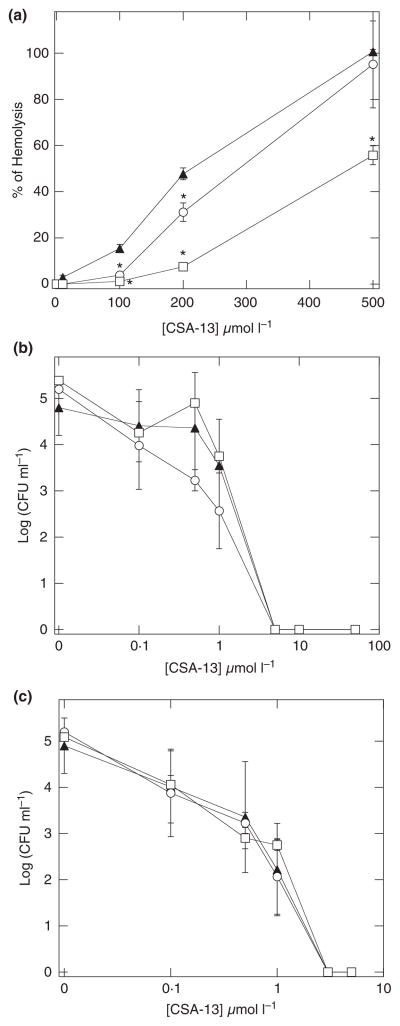
It is generally accepted that CAPs and their mimics possess a mechanism of antibacterial action that involves nonspecific interference with plasma membrane lipid organization, which results in the loss of the membrane’s ability to control homoeostasis of the intracellular environment. This mechanism assures a large spectrum of antibacterial functions mediated by these molecules, but on the other hand, can result in cytotoxicity towards host cells. Evaluation of haemoglobin release from erythrocytes (RBC) provides a simple measure of potential cytotoxicity for this class of molecules. As shown in Fig. 1, CSA-13 does not affect RBC membrane permeability at concentrations sufficient to kill different bacteria (Fig. 1b,c, Tables 1 and 2). However, at a concentration 10× higher than average MBC values, against different clinical strains of Staph. aureus (Table 1), we observed a concentration-dependent increase in haemoglobin release (Fig. 1a). Notably, in the presence of pluronic F-127, CSA-13 haemolytic activity was greatly reduced. More precisely, in combination with 5% pluronic F-127, a concentration of ceragenin CSA-13 of 100 μmol l−1, which by itself had caused 20% of RBC lysis, becomes nonhaemolytic. As shown in Fig. 1b,c, the killing activity of CSA-13 against Ps. aeruginosa Xen 5 and Staph. aureus A1 evaluated in PBS enriched with 1 and 5% pluronic acid F-127 did not show significant differences compared to the killing activity of the control.
Figure 1.
Haemoglobin release from human red blood cells (panel a) after addition of CSA-13 to their phosphate-buffered saline (PBS) suspension without (▲) or with 1% (○) or 5% (□) of pluronic F-127. Antibacterial activity of CSA-13 against Pseudomonas aeruginosa Xen 5 (panel b) and Staphylococcus aureus A1 (panel c) in PBS (▲) or PBS containing 1% (○) or 5% (□) pluronic F-127. Error bars represent standard deviations from three measurements. *Significantly different from control sample.
Table 1.
Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of CSA-13 against different clinical isolates of Staphylococcus aureus
| Bacteria source | Strain number | Strain characteristic | CSA-13 (μmol l−1) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| MRSA | MLSB | β-lactamase | MIC | MBC | ||
| Surgery wound | 1 | + | + | + | 0·8 | 3·125 |
| Surgery wound | 2 | + | + | + | 1·56 | 3·125 |
| Surgery wound | 3 | + | + | + | 0·8 | 3·125 |
| Diabetic foot ulcer | 4 | + | + | + | 0·8 | 1·56 |
| Diabetic foot ulcer | 5 | − | + | + | 0·8 | 3·125 |
| Diabetic foot ulcer | 6 | − | + | + | 1·56 | 3·125 |
| Diabetic foot ulcer | 7 | − | + | + | 1·56 | 6·25 |
| Vaginal swabs | 8 | − | + | + | 1·56 | 6·25 |
| Nasal swab | 9 | − | − | + | 1·56 | 6·25 |
| Pus from acne | 10 | − | − | + | 1·56 | 6·25 |
| Pus from acne | 11 | − | − | + | 0·8 | 3·125 |
| Vaginal swabs | 12 | − | − | + | 0·8 | 3·125 |
| Nasal swab | 13 | − | − | − | 1·56 | 6·25 |
| Pus from acne | 14 | − | − | − | 0·8 | 6·25 |
| Pus from acne | 15 | − | − | − | 0·8 | 6·25 |
| Pus from furunculus | 16 | − | − | − | 3·125 | 6·25 |
| Staph. aureus ATCC29213 | 17 | − | − | + | 0·8 | 3·125 |
Table 2.
Antibacterial activity of CSA-13 against clinical strains of Pseudomonas aeruginosa [log (CFU ml−1)] in the presence of pluronic F-127
| Bacteria in CF sputum samples | CSA-13 (μmol l−1) | Pluronic (%)
|
||
|---|---|---|---|---|
| 0 | 1 | 5 | ||
| Sample 1 | ||||
| Ps. aeruginosa (mucoid) | 0 | 5·18 | 5·20 | 5·31 |
| Ps. aeruginosa (nonmucoid) | 1 | 4·77 | 5·14 | 5·17 |
| MSSA | 5 | 4·74 | 4·95 | 4·96 |
| 10 | 4·86 | 4·91 | 5·09 | |
| 50 | 3·16 | 4·69 | 4·84 | |
| Sample 2 | ||||
| Ps. aeruginosa (mucoid) | 0 | 3·9 | 4·12 | 4·61 |
| Ps. aeruginosa (nonmucoid) | 1 | 4·12 | 4·35 | 4·21 |
| MSSA | 5 | 4·05 | 4·19 | 4·34 |
| 10 | 3·92 | 4·04 | 4·01 | |
| 50 | 2·17 | 3·31 | 3·13 | |
| Sample 3 | ||||
| Ps. aeruginosa (nonmucoid) | 0 | 3·03 | 2·53 | 2·25 |
| 1 | 2·49 | 2·30 | 3·22 | |
| 5 | 2·24 | 2·11 | 2·21 | |
| 10 | 2·32 | 3·12 | 2·74 | |
| 50 | 0 | 0 | 0 | |
| Sample 4 | ||||
| Ps. aeruginosa (nonmucoid) | 0 | 4·58 | 4·76 | 4·81 |
| 1 | 4·82 | 4·86 | 4·98 | |
| 5 | 4·53 | 4·80 | 4·77 | |
| 10 | 4·45 | 4·35 | 4·51 | |
| 50 | 2·33 | 3·74 | 3·13 | |
| Sample 5 | ||||
| Ps. aeruginosa (nonmucoid) | 0 | 5·56 | 5·65 | 5·82 |
| 1 | 5·58 | 5·57 | 5·51 | |
| 5 | 5·56 | 5·44 | 5·39 | |
| 10 | 5·49 | 5·39 | 5·69 | |
| 50 | 3·22 | 2·93 | 4·63 | |
| Sample 6 | ||||
| Ps. aeruginosa (nonmucoid) | 0 | 3·80 | 4·06 | 4·09 |
| 1 | 3·73 | 3·99 | 3·74 | |
| 5 | 3·61 | 3·95 | 3·69 | |
| 10 | 3·64 | 3·61 | 3·78 | |
| 50 | 3·15 | 3·44 | 3·47 | |
CF, cystic fibrosis. MSSA, Methicillin sensitive Staphylococcus aureus.
CSA-13 antibacterial activity against clinical isolates of Staphylococcus aureus
Bacteria from the Staphylococcus group can cause a multitude of diseases as a result of infection in various tissues. However, very often the infections caused by Staph. aureus take place on the surface of skin. Susceptibility testing of different clinical isolates including MRSA, MLSB and β-lactamase (+) Staph. aureus strains (n = 16) demonstrated that the MIC and MBC values were on average 1·27 ± 0·62 and 4·58 ± 1·75 μmol l−1 and range between 0·8–3·125 and 1·56–6·25 μmol l−1 respectively (Table 1). On a molar base, these values were at least two times lower compared to antibacterial activity of cathelicidin LL-37 against the same strains (data not shown).
Outgrowth of Pseudomonas aeruginosa bacteria from cystic fibrosis sputum samples treated with CSA-13 or its combination with pluronic F-127
Chronic airway infection is associated with abbreviated life spans of patients suffering from cystic fibrosis lung disease. A limited number of types of organisms are responsible for these infections, with Ps. aeruginosa and Staph. aureus being of primary importance (Gilligan 1991; Mainz et al. 2009). As shown in Table 2, the number of Ps. aeruginosa colonies outgrowing from CSA-13 (1–50 μmol l−1) treated sputum samples was lower compared to control. Inhibition of bacterial outgrowth was also observed after CSA-13 treatment in combination with pluronic acid F-127. However, in two of six study samples (samples 1, 5; Table 2), 50 μmol l−1 of CSA-13 in combination with 5% pluronic F-127 had a smaller effect than that of CSA-13 by itself. This effect might indicate the ability of pluronic F-127 to promote bacterial growth of some Ps. aeruginosa strains.
CSA-13 activity against bacterial biofilm
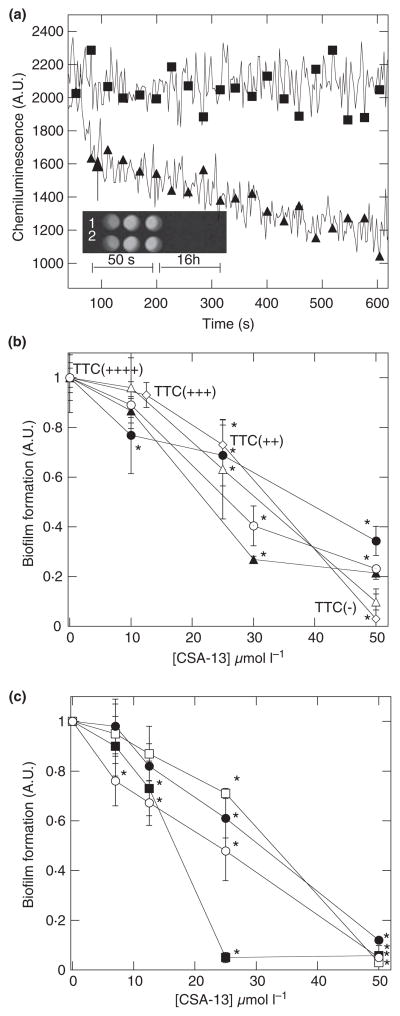
Bacteria in a biofilm state represent a high morbidity risk due to antibiotic resistance. Chemiluminescent signals generated by Ps. aeruginosa Xen 5, CV staining and OD measurements accompanied by the evaluation of bacterial ability to reduce TTC generated data indicating strong antibacterial biofilm activity of ceragenin CSA-13 (Fig. 2). Addition of CSA-13 to a Ps. aeruginosa Xen 5 suspension resulted in a quick (c. 50 s) decrease of chemiluminescent signals, indicating that CSA-13 is able to reach and interfere with the metabolic machinery of bacterial cells on the order of seconds. On the long term, this interference resulted in bacterial death as indicated by the lack of detectable chemiluminescent signal after 16 h of incubation (see inset in panel a of Fig. 2). This effect was confirmed by a control experiment in which there was no bacterial outgrowth from the Ps. aeruginosa Xen 5 samples treated with CSA-13 or polymyxin B after 16 h of incubation (data not shown). As indicated by data in Fig. 2b, CSA-13 is not only active against planktonic Ps. aeruginosa Xen 5, but it can effectively kill bacteria embedded in biofilm. Anti-biofilm activity of CSA-13 is maintained in the presence of pluronic F-127. Additionally, as shown in Fig. 2c, CSA-13 is effective against biofilm formed by Mor. catarrhalis, H. pylori, Staph. aureus ATCC43300 and Ent. faecalis.
Figure 2.
Bactericidal activity against planktonic and biofilm-forming bacteria. Decrease of Pseudomonas aeruginosa Xen 5 luminescence (■) after CSA-13 addition (▲) to Tryptic soy broth (TSB) inoculum with bacterial concentration c. 108 CFU ml−1 (panel a). The insert on panel a represents the luminescence of Ps. aeruginosa Xen 5 (200 μl) at 50 s and 16 h after CSA-13 (line 1) or polymyxin B (line 2) addition (both at concentration 10 μmol l−1). Data from one experiment performed in triplicate are shown. Antibacterial activity of CSA-13 against biofilm of Ps. aeruginosa Xen 5, evaluated 24 h after its administration (panel b). Relative value of optical density (◇), crystal violet absorbance (●), luminescence (△), luminescence in the presence of pluronic F-127 1% (▲) and 5% (⊙) at various concentrations of CSA-13. Antibacterial activity of CSA-13 against biofilm of Moraxella catarrhalis (□), Helicobacter pylori (○), Staphylococcus aureus ATCC43300 (■) and Enterococcus faecalis (●), evaluated 24 h after its administration (panel c). On panels b and c, error bars represent standard deviations from three to five measurements. *Significantly different from control sample.
CSA-13 activity in various body fluids
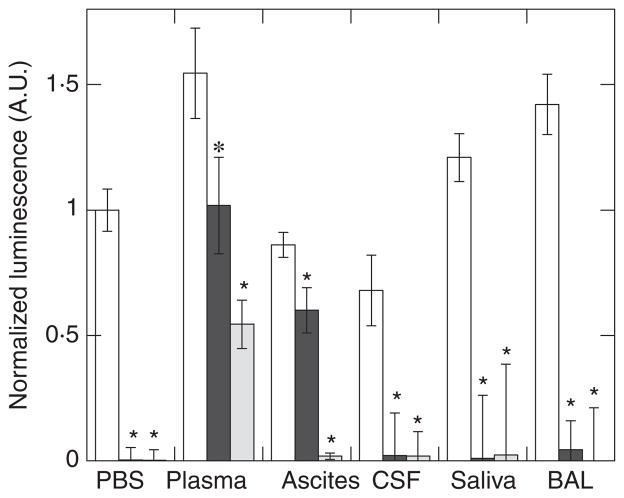
Antibacterial activity of exogenous molecules can be modulated by different host factors present at infection sites. To assess the potential of CSA-13 to kill bacteria in various clinical settings, we evaluated the luminescence of Ps. aeruginosa Xen 5 bacteria in PBS suspensions containing 50% of various body fluids including plasma, ascites, cerebrospinal fluid, saliva and BAL (Fig. 3). All specimen collection was performed in accordance with a protocol approved by the Medical University of Białystok Ethics Committee for Research on Humans and Animals, and written consent was obtained from all subjects. In accordance with the data shown in Fig. 2a, decreased Ps. aeruginosa Xen 5 luminescence by CSA-13 is indicative of bactericidal activity. The increased bacterial luminescence that was observed 6 h after bacterial growth in the presence of different body fluids, compared to luminescence in PBS, indicates differences in bacterial growth that likely reflect the availability of nutrition and the variable concentration of endogenous host immune defence molecules. Under 10 μmol l−1 CSA-13 treatment, a decrease of bacterial luminescence was observed in all evaluated body fluids, but to different extents. The least amount of activity (a drop of 33% in luminescence) was observed in the presence of human plasma. With an increase in CSA-13 concentration from 10 to 30 μmol l−1, luminescence decreased significantly for all body fluids. More precisely, luminescence decrease was 66, 93, 96, 98 and 99% respectively, in plasma, ascites, cerebrospinal fluid, saliva and BAL. These data suggest that the antibacterial activity of CSA-13 is compromised in blood plasma, which might limit CSA-13 use for systemic application, but remains high in other biofluids.
Figure 3.
Antibacterial activity of CSA-13 against Pseudomonas aeruginosa Xen 5 in human body fluids was determined by luminescence readings 6 h after addition of equal amounts of bacteria to an equal volume of phosphate buffered saline (PBS), or PBS supplemented with 50% of plasma, ascites, cerebro-spinal fluid (CSF), saliva or bronchoalveolar lavage (BAL). In each condition, the white column indicates luminescence signal in control samples. Black and grey columns indicate luminescence signal in the presence of 10 and 30 μmol l−1 CSA-13 respectively. Data from one experiment performed in triplicate are shown. Two other experiments with samples obtained from different subjects show similar trends. *Significantly different from control sample.
CSA-13 activity in the presence of LL-37 peptide, lysozyme, lactoferrin and sPLA
The degree of synergy between CSA-13 and different natural antibacterial agents that are usually present at infection sites against three selected bacterial strains was determined by the checkerboard technique as shown in Table 3. Synergy or partial synergy was observed between CSA-13 and all natural antibacterial agents used in this study when tested against Gram (+) Staph. aureus bacteria. In the case of Gram (−) Ps. aeruginosa Xen 5 and H. pylori, partial synergy was observed with the combination of CSA-13 and LL-37, synergy against H. pylori with a combination of CSA-13 and sPLA, and an additive effect against both G (−) bacteria with a combination of CSA-13 and lysozyme. Indifference and partial synergy were observed using CSA-13 with lactoferrin against Ps. aeruginosa Xen 5 and Staph. aureus ATCC 43300 respectively. In a parallel set of experiments, additive effects and synergy were observed between CSA-13 and sPLA.
Table 3.
Interaction between CSA-13 and different host antibacterial agents assessed with fractional inhibitory concentration (FIC) index. FICindex was interpreted as follows: <0·5 (synergy; S), 0·5–0·75 (partial synergy; PS), 0·76–1·0 (additive effect; AE), >1·0–4·0 (indifference; I), >4·0 (antagonism, A)
| Bacterial strain | FIC | CSA-13 with
|
Interpretation 1/2/3/4 | |||
|---|---|---|---|---|---|---|
| 1 LL-37 |
2 lysozyme |
3 lactoferrin |
4 sPLA |
|||
| Pseudomonas aeruginosa Xen 5 | FICA/FICB | 0·368/0·308 | 0·387/0·572 | 0·574/0·574 | 0·472/0·449 | PS/AE/I/AE |
| FIC index | 0·676 | 0·959 | 1·148 | 0·921 | ||
| Staphylococcus aureus ATCC 43300 | FICA/FICB | 0·1/0·163 | 0·104/0·305 | 0·328/0·515 | 0·238/0·469 | S/S/PS/PS |
| FIC index | 0·263 | 0·409 | 0·843 | 0·707 | ||
| Helicobacter pylori | FICA/FICB | 0·465/0·168 | 0·48/0·371 | 0·371/0·379 | 0·304/0·093 | PS/AE/PS/S |
| FIC index | 0·633 | 0·851 | 0·750 | 0·397 | ||
Discussion
Topical infections take place within tissue surrounding bacterial entry areas and are usually located on skin and mucosal surfaces. Despite many advances in diagnosis and treatment, these infections are still an important factor associated with patient mortality. Very often topical infections are caused by resistant bacterial strains and, from a local starting point, may propagate to systemic infection, especially in patients with diabetes mellitus, burn trauma or HIV (Gould et al. 2007; Patel et al. 2008). Furthermore, microbial biofilms are increasingly recognized as important contributors to human disease (Lynch and Robertson 2008). New strategies to prevent and treat these infections, including those associated with biofilms, are needed. One limitation of CAPs and their cationic lipid steroid derivative mimics is nonspecific plasma membrane toxicity that may result in host cell damage. Accordingly, a previous study shows that CSA-13 action at a concentration above c. 50 μmol l−1 can result in haemoglobin and LDH release indicative of host cell membrane damage. We found that RBC membrane disruption but not bacterial killing activity of CSA-13 was significantly decreased by pluronic F-127. This finding may provide a solution to control toxicity of CSA-13 and potentially other cationic peptide mimics by co-formulation with F-127. Although the presence of pluronic F-127 does not increase CSA-13 antibacterial activity directly, it might enable the use of CSA-13 at a higher concentration. In this study, we also provide experimental evidence that CSA-13 has potential for treatment of topical bacterial infection. In agreement with previous reports (Chin et al. 2007), we have shown that CSA-13 kills various clinical isolates of Staph. aureus including MRSA strains and Ps. aeruginosa within cystic fibrosis sputum. In addition to already published information related to activity of this molecule at infection sites, such as CSA-13 resistance to inactivation by anionic polyelectrolytes (Bucki et al. 2007a), or protease (Leszczynska et al. 2009) and synergism with exogenous antibiotics (Chin et al. 2008; Saha et al. 2008), we found that CSA-13 effectively kills different bacterial strains embedded in biofilm and that its antibacterial activity is potentialized by host-secreted antibacterial agents. To investigate CSA-13 activity against Ps. aeruginosa Xen 5 biofilm, we used four different techniques and obtained similar results with all of them. Our biofilm study confirmed that luminescence evaluation of transformed bacteria provides a rapid method for real-time monitoring of biofilms (Kadurugamuwa et al. 2003). Additionally, our results are in agreement with a recent study showing that CSA-13 has bactericidal activity against Ps. aeruginosa even in mature biofilms (Nagant et al. 2010). Over the broad range of sub-MIC concentration, cathelicidin LL-37 peptide, which interferes with bacterial membrane lipid packing, lysozyme, which hydrolyses the cross-linkages between chains of peptidoglycans, lactoferrin, which deprives bacteria of iron, and secretory PLA2, which likely kills bacteria due to its lipolytic activity, all increased the antibacterial activity of CSA-13. These data are in good agreement with findings that a combination of natural host antibacterial molecules acts in an additive or synergistic, but not antagonistic, manner (Singh et al. 2000). Therefore, CSA-13 at the infection site, where the concentration of endogenous antibacterial molecules such as LL-37 increase due to the stimulatory effect of bacterial wall products, may enhance topical host antibacterial defence. In conclusion, we show that CSA-13 has a large spectrum of bactericidal activity against planktonic and biofilm bacterial cells. Given that CSA-13’s strong antibacterial activity was reduced in blood plasma, CSA-13 application has greater potential to prevent or treat topical bacterial infection. Pluronic F-127 represents a promising tool to reduce CSA-13 membrane toxicity towards host cells. Future safety, efficacy and pharmacokinetic studies in vivo should be performed to correlate our in vitro findings.
Acknowledgments
This work was supported by the NIH grant HL67286, the Cystic Fibrosis Foundation and Medical University of Bialystok grants 3-22695L and 3-22477F. We gratefully acknowledge the help of Marianne Ferrin and patients of the Adult Cystic Fibrosis Center of the University of Pennsylvania for providing sputum samples.
Footnotes
Transparency declaration
PAJ and RB in 2008 were involved in a sponsored research agreement with Critical Biologics Inc. in a project directed at evaluating the potential clinical use of gelsolin, but not otherwise related to the present study. None of the research reported in this paper was supported by any corporate entity. Other authors: none to declare.
References
- Barichello JM, Morishita M, Takayama K, Nagai T. Absorption of insulin from pluronic F-127 gels following subcutaneous administration in rats. Int J Pharm. 1999;184:189–198. doi: 10.1016/s0378-5173(99)00119-2. [DOI] [PubMed] [Google Scholar]
- Bartoszewicz M, Rygiel A, Krzeminski M, Przondo-Mordarska A. Penetration of a selected antibiotic and antiseptic into a biofilm formed on orthopedic steel implants. Ortop Traumatol Rehabil. 2007;9:310–318. [PubMed] [Google Scholar]
- Bucki R, Janmey PA. Interaction of the gelsolinderived antibacterial PBP10 peptide with lipid bilayers and cell membranes. Antimicrob Agents Chemother. 2006;50:2932–2940. doi: 10.1128/AAC.00134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucki R, Pastore JJ, Randhawa P, Vegners R, Weiner DJ, Janmey PA. Antibacterial activities of rhodamine B-conjugated gelsolin-derived peptides compared to those of the antimicrobial peptides cathelicidin LL37, magainin II, and melittin. Antimicrob Agents Chemother. 2004;48:1526–1533. doi: 10.1128/AAC.48.5.1526-1533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucki R, Byfield FJ, Janmey PA. Release of the antimicrobial peptide LL-37 from DNA/ F-actin bundles in cystic fibrosis sputum. Eur Respir J. 2007a;29:624–632. doi: 10.1183/09031936.00080806. [DOI] [PubMed] [Google Scholar]
- Bucki R, Sostarecz AG, Byfield FJ, Savage PB, Janmey PA. Resistance of the antibacterial agent ceragenin CSA-13 to inactivation by DNA or F-actin and its activity in cystic fibrosis sputum. J Antimicrob Chemother. 2007b;60:535–545. doi: 10.1093/jac/dkm218. [DOI] [PubMed] [Google Scholar]
- Bucki R, Namiot DB, Namiot Z, Savage PB, Janmey PA. Salivary mucins inhibit antibacterial activity of the cathelicidin-derived LL-37 peptide but not the cationic steroid CSA-13. J Antimicrob Chemother. 2008;62:329–335. doi: 10.1093/jac/dkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Cao YL, Cui L, Liu W, Guan WX. Use of autologous tissue engineered skin to treat porcine full-thickness skin defects. Chin J Traumatol. 2005;8:269–276. [PubMed] [Google Scholar]
- Chin JN, Rybak MJ, Cheung CM, Savage PB. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:1268–1273. doi: 10.1128/AAC.01325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JN, Jones RN, Sader HS, Savage PB, Rybak MJ. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J Antimicrob Chemother. 2008;61:365–370. doi: 10.1093/jac/dkm457. [DOI] [PubMed] [Google Scholar]
- Ciornei CD, Sigurdardottir T, Schmidtchen A, Bodelsson M. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob Agents Chemother. 2005;49:2845–2850. doi: 10.1128/AAC.49.7.2845-2850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Guan Q, Walsh JP, Boswell JS, Winter TW, Winter ES, Boyd SS, Li C, et al. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J Med Chem. 2002;45:663–669. doi: 10.1021/jm0105070. [DOI] [PubMed] [Google Scholar]
- Epand RM, Epand RF, Savage PB. Ceragenins (cationic steroid compounds), a novel class of antimicrobial agents. Drug News Perspect. 2008;21:307–311. doi: 10.1358/dnp.2008.21.6.1246829. [DOI] [PubMed] [Google Scholar]
- Escobar-Chavez JJ, Lopez-Cervantes M, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharm Sci. 2006;9:339–358. [PubMed] [Google Scholar]
- Fiebelkorn KR, Crawford SA, McElmeel ML, Jorgensen JH. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J Clin Microbiol. 2003;41:4740–4744. doi: 10.1128/JCM.41.10.4740-4744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan PH. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould IM, MacKenzie FM, MacLennan G, Pacitti D, Watson EJ, Noble DW. Topical antimicrobials in combination with admission screening and barrier precautions to control endemic methicillin-resistant Staphylococcus aureus in an Intensive Care Unit. Int J Antimicrob Agents. 2007;29:536–543. doi: 10.1016/j.ijantimicag.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Herasimenka Y, Benincasa M, Mattiuzzo M, Cescutti P, Gennaro R, Rizzo R. Interaction of antimicrobial peptides with bacterial polysaccharides from lung pathogens. Peptides. 2005;26:1127–1132. doi: 10.1016/j.peptides.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Howell MD, Streib JE, Kim BE, Lesley LJ, Dunlap AP, Geng D, Feng Y, Savage PB, et al. Ceragenins: a class of antiviral compounds to treat orthopox infections. J Invest Dermatol. 2009;129:2668–2675. doi: 10.1038/jid.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai E, Isogai H, Takahashi K, Okumura K, Savage PB. Ceragenin CSA-13 exhibits antimicrobial activity against cariogenic and periodontopathic bacteria. Oral Microbiol Immunol. 2009;24:170–172. doi: 10.1111/j.1399-302X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- Kadurugamuwa JL, Sin L, Albert E, Yu J, Francis K, DeBoer M, Rubin M, Bellinger-Kawahara C, et al. Direct continuous method for monitoring biofilm infection in a mouse model. Infect Immun. 2003;71:882–890. doi: 10.1128/IAI.71.2.882-890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichler A, Leborgne C, Savage PB, Danos O. Cationic steroid antibiotics demonstrate DNA delivery properties. J Control Release. 2005;107:174–182. doi: 10.1016/j.jconrel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kristiansen JE. The antimicrobial activity of non-antibiotics. Report from a congress on the antimicrobial effect of drugs other than antibiotics on bacteria, viruses, protozoa, and other organisms. APMIS Suppl. 1992;30:7–14. [PubMed] [Google Scholar]
- Lai XZ, Feng Y, Pollard J, Chin JN, Rybak MJ, Bucki R, Epand RF, Epand RM, et al. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc Chem Res. 2008;41:1233–1240. doi: 10.1021/ar700270t. [DOI] [PubMed] [Google Scholar]
- Lara D, Feng Y, Bader J, Savage P, Maldonado R. Anti-trypanosomatid activity of ceragenins. J Parasitol. 2010;96:638–642. doi: 10.1645/GE-2329.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lee JE, Baek WY, Lim JO. Regional delivery of vancomycin using pluronic F-127 to inhibit methicillin resistant Staphylococcus aureus (MRSA) growth in chronic otitis media in vitro and in vivo. J Control Release. 2004;96:1–7. doi: 10.1016/j.jconrel.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Leszczynska K, Namiot A, Fein DE, Wen Q, Namiot Z, Savage PB, Diamond S, Janmey PA, et al. Bactericidal activities of the cationic steroid CSA-13 and the cathelicidin peptide LL-37 against Helicobacter pylori in simulated gastric juice. BMC Microbiol. 2009;9:187. doi: 10.1186/1471-2180-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Lewis MR, Gilbert AB, Noel MD, Scoville DH, Allman GW, Savage PB. Antimicrobial activities of amine- and guanidine-functionalized cholic acid derivatives. Antimicrob Agents Chemother. 1999;43:1347–1349. doi: 10.1128/aac.43.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M, Faris A, Wadstrom T, Hjerten S. A new test based on ‘salting out’ to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981;677:471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Lynch AS, Robertson GT. Bacterial and fungal biofilm infections. Annu Rev Med. 2008;59:415–428. doi: 10.1146/annurev.med.59.110106.132000. [DOI] [PubMed] [Google Scholar]
- Mainz JG, Naehrlich L, Schien M, Kading M, Schiller I, Mayr S, Schneider G, Wiedemann B, et al. Concordant genotype of upper and lower airways P. aeruginosa and S. aureus isolates in cystic fibrosis. Thorax. 2009;64:535–540. doi: 10.1136/thx.2008.104711. [DOI] [PubMed] [Google Scholar]
- Malabarba A, Ciabatti R, Kettenring J, Scotti R, Candiani G, Pallanza R, Berti M, Goldstein BP. Synthesis and antibacterial activity of a series of basic amides of teicoplanin and deglucoteicoplanin with polyamines. J Med Chem. 1992;35:4054–4060. doi: 10.1021/jm00100a010. [DOI] [PubMed] [Google Scholar]
- Maruyama I, Hasegawa T, Yamamoto T, Momose K. Effects of pluronic F-127 on loading of fura 2 /AM into single smooth muscle cells isolated from guinea pig taenia coli. J Toxicol Sci. 1989;14:153–163. doi: 10.2131/jts.14.153. [DOI] [PubMed] [Google Scholar]
- Nagant C, Tré-Hardy M, El-Ouaaliti M, Savage P, Devleeschouwer M, Dehaye JP. Interaction between tobramycin and CSA-13 on clinical isolates of Pseudomonas aeruginosa in a model of young and mature biofilms. Appl Microbiol Biotechnol. 2010;88:251–263. doi: 10.1007/s00253-010-2748-3. [DOI] [PubMed] [Google Scholar]
- Norden CW. Problems in determination of antibiotic synergism in vitro. Rev Infect Dis. 1982;4:276–281. doi: 10.1093/clinids/4.2.276. [DOI] [PubMed] [Google Scholar]
- Patel PP, Vasquez SA, Granick MS, Rhee ST. Topical antimicrobials in pediatric burn wound management. J Craniofac Surg. 2008;19:913–922. doi: 10.1097/SCS.0b013e318175b516. [DOI] [PubMed] [Google Scholar]
- Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods. 2008;72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Perry EL, Beck JP, Williams DL, Bloebaum RD. Assessing peri-implant tissue infection prevention in a percutaneous model. J Biomed Mater Res B Appl Biomater. 2009;92:397–408. doi: 10.1002/jbm.b.31528. [DOI] [PubMed] [Google Scholar]
- Rahbar M, Mehrgan H, Hadji-Nejad S. Enhancement of vancomycin activity by phenothiazines against vancomycin-resistant Enterococcus faecium in vitro. Basic Clin Pharmacol Toxicol. 2010;107:676–679. doi: 10.1111/j.1742-7843.2010.00558.x. [DOI] [PubMed] [Google Scholar]
- Randazzo RA, Bucki R, Janmey PA, Diamond SL. A series of cationic sterol lipids with gene transfer and bactericidal activity. Bioorg Med Chem. 2009;17:3257–3265. doi: 10.1016/j.bmc.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Savage PB, Bal M. Enhancement of the efficacy of erythromycin in multiple antibiotic-resistant gram-negative bacterial pathogens. J Appl Microbiol. 2008;105:822–828. doi: 10.1111/j.1365-2672.2008.03820.x. [DOI] [PubMed] [Google Scholar]
- Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- Standards CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Nineteenth Informational Supplement. Vol. 29. Clinical and Laboratory Standards Institute; 2009. p. M-100-S119. [Google Scholar]
- Tang JX, Wen Q, Bennett A, Kim B, Sheils CA, Bucki R, Janmey PA. Anionic poly(amino acid)s dissolve F-actin and DNA bundles, enhance DNase activity, and reduce the viscosity of cystic fibrosis sputum. Am J Physiol Lung Cell Mol Physiol. 2005;289:L599–L605. doi: 10.1152/ajplung.00061.2005. [DOI] [PubMed] [Google Scholar]
- Van Bambeke F, Mingeot-Leclercq MP, Struelens MJ, Tulkens PM. The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends Pharmacol Sci. 2008;29:124–134. doi: 10.1016/j.tips.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Agerberth B, Lothgren A, Almstedt A, Johansson J. Apolipoprotein A-I binds and inhibits the human antibacterial / cytotoxic peptide LL-37. J Biol Chem. 1998;273:33115–33118. doi: 10.1074/jbc.273.50.33115. [DOI] [PubMed] [Google Scholar]
- Weiner DJ, Bucki R, Janmey PA. The antimicrobial activity of the cathelicidin LL37 is inhibited by F-actin bundles and restored by gelsolin. Am J Respir Cell Mol Biol. 2003;28:738–745. doi: 10.1165/rcmb.2002-0191OC. [DOI] [PubMed] [Google Scholar]