Abstract
The elastic modulus of bioengineered materials has a strong influence on the phenotype of many cells including cardiomyocytes. On polyacrylamide (PAA) gels that are laminated with ligands for integrins, cardiac myocytes develop well organized sarcomeres only when cultured on substrates with elastic moduli in the range of 10 kPa to 30 kPa, near those of the healthy tissue. On stiffer substrates (>60 kPa) approximating the damaged heart, myocytes form stress fiber-like filament bundles but lack organized sarcomeres or an elongated shape. On soft (<1 kPa) PAA gels myocytes exhibit disorganized actin networks and sarcomeres. However, when the polyacrylamide matrix is replaced by hyaluronic acid (HA) as the gel network to which integrin ligands are attached, robust development of functional neonatal rat ventricular myocytes occurs on gels with elastic moduli of 200 Pa, a stiffness far below that of the neonatal heart and on which myocytes would be amorphous and dysfunctional when cultured on polyacrylamide-based gels. The HA matrix by itself is not adhesive for myocytes, and the myocyte phenotype depends on the type of integrin ligand that is incorporated within the HA gel, with fibronectin, gelatin, or fibrinogen being more effective than collagen 1. These results show that HA alters the integrin-dependent stiffness response of cells in vitro and suggests that expression of HA within the extracellular matrix (ECM) in vivo might similarly alter the response of cells that bind the ECM through integrins. The integration of HA with integrin-specific ECM signaling proteins provides a rationale for engineering a new class of soft hybrid hydrogels that can be used in therapeutic strategies to reverse the remodeling of the injured myocardium.
Keywords: cardiac myocyte, hyaluronic acid, elastic modulus, sarcomere, mechanosensing
Introduction
Cardiac remodeling during development (Jacot et al., 2011) or disease (Borbely et al., 2005; Leopoldo et al., 2011) is accompanied by alterations in the composition and mechanical properties of the extracellular matrix (ECM). The altered viscoelastic and bioactive properties of the ECM in diseased heart disrupts the structure and function of myocardial cells. Cardiac tissue engineering strategies often involve the replacement of the diseased myocardium with new cells and /or bioactive materials. The stiffness of the matrix or the tissue in which potentially cardiogenic stem cells are introduced is increasingly recognized to be an important determinant of the success of restoring normal function (Berry et al., 2006; Engler et al., 2008; Zhang et al., 2009). The elastic modulus of damaged cardiac tissue (>60 kPa) is sufficient to transform functional cardiomyocytes to a de-differentiated state in vitro (Bajaj et al., 2010; Bhana et al., 2010; Chopra et al., 2011; Curtis and Russell, 2011; Engler et al., 2008; Shi et al., 2011) and to prevent the differentiation of stem cells to a myogenic phenotype (Engler et al., 2006). Such high matrix rigidities also induce formation of large actin filament bundles often called stress fibers and lead to well-defined changes in fibroblast phenotype that culminates in the formation of myofibroblasts, a cell type that has been associated with fibrotic disease (Hinz, 2009; Wang et al., 2003).
A consensus among studies using polyacrylamide hydrogels and other soft substrates as inert matrices on which specific ligands for cardiac myocyte integrins can be attached shows that there is a relatively narrow range of substrate stiffness, between approximately 10 kPa and 30 kPa on which embryonic or neonatal cardiomyocytes retain or regain a normal appearing phenotype characterized by a highly elongated shape, formation of striated sarcomeres, and the ability to contract in a rhythmic manner (Bajaj et al., 2010; Bhana et al., 2010; Chopra et al., 2011; Curtis and Russell, 2011; Engler et al., 2008; Shi et al., 2011). Similar studies using polyacrylamide gels or micro pillars laminated with N-cadherin to mimic cell-cell attachments also suggest that there is a narrow range of stiffness, slightly lower than that for integrins, on which sarcomeres form and myocytes appear functional (Chopra et al., 2011; Ganz et al., 2006). When neonatal cardiomyocytes or cardiac fibroblasts are cultured on substrates that are stiffer than this range, the cells survive, but develop stress fibers and a polygonal shape. When they are cultured on substrates softer than 2 kPa, they lose all forms of large actin-containing bundles and develop a round morphology with disperse actin filaments.
One inference from studies of substrate stiffness effects on cell morphology is that cells develop specialized structures such as stress fibers or sarcomeres partly as the result of well defined forces that are exerted at the cell-matrix or cell-cell junction. With insufficient mechanical resistance from the substrate sarcomeres fail to form, but when the resistance is so great that the force generated within the sarcomere cannot deform the substrate then the actin cytoskeleton remodels to produce larger, less organized actin bundles often called stress fibers. The range of stiffness over which different cell types respond and the manner in which they respond is highly cell type and ligand specific (Georges and Janmey, 2005), implying that the response to matrix mechanics is tightly controlled by signaling pathways presumably similar to those that are triggered by chemical signals. Therefore in principle, altered signaling through activation of receptors has the potential to override or alter mechanical signals, but evidence for such interplay between signaling and mechanosensing is scarce. Here we show that crosslinked hyaluronic acid, when present in the same matrix as ligands for integrins, strongly alters the response of neonatal cardiac myocytes and fibroblasts to soft substrates and allows formation of large myocytes with well-developed and functional sarcomeres at substrate stiffness’s far below those of the mature heart. The present studies are restricted to neonatal maturing myocytes and the response in adult phenotype may be attenuated or different and will need to be explored in future studies.
The studies presented here have implications for the use of injectable acellular/cellular ECM protein functionalized hyaluronan gels for cardiac repair. Injecting non-functionalized hyaluronic acid gels into infracted cardiac tissue has already been shown to have cardiac positive inotropic effects (Ifkovits et al., 2010; Yoon et al., 2009). Hyaluronan has also been used as a component to enhance embryonic and mesenchymal stem cell differentiation towards the cardiac lineage through the SMAD signaling pathway, although the exact mechanisms are not yet clear (Maioli et al., 2010). Functionalizing hyaluronan gels with adhesion proteins and/or growth factors can have added benefits of recruiting/differentiating stem cells and enhancing angiogenesis at the sites of myocardial injury.
Materials and methods
Myocyte Cell isolation, plating and culture
Neonatal ventricular rat myocytes (NVRM) were harvested from the hearts of 1- to 3-day-old euthanized Sprague-Dawley rat pups using a cell isolation kit (Cellutron Life Technology, Baltimore, MD) as described previously (Chopra et al., 2011). Briefly, cells were isolated from the muscle tissue of the two ventricles. The cardiac myocytes were pre-plated for 1 to 2 h to purify the myocyte population. The cells were cultured at a density of 7,000 cells/cm2 in high serum (10% fetal bovine serum) medium (Cellutron) on the various gel substrates for 24 h at 5% CO2 and 37°C. The medium was changed to low serum (2% fetal bovine serum) and maintained for another 24 h. This time period proved sufficient to allow the cells to attach and spread completely after the isolation procedure. For long term culture of the cells the medium was replaced with fresh low serum media every 2 days. Bromodeoxyuridine (BrdU) was added to myocyte medium at a concentration of 200 μM to prevent fibroblast proliferation. The fibroblast population was restricted to approximately 5-10% in short term cultures whereas they are approximately 20-30% of the total cell population in long term.
Gel preparation
Polyacrylamide (PAA) gels of desired stiffness were made by varying acrylamide and bisacrylamide stock solution (Bio-Rad Laboratories, Hercules, CA) concentrations as described previously (Yeung et al., 2005). The method for PAA gel formation and covalent attachment of proteins is essentially as described elsewhere (Chopra et al., 2011). Briefly, the acrylamide solutions are polymerized using TEMED (Fisher BioReagents, Fairlawn NJ) and 10% ammonium persulfate (Fisher BioReagents). The solution was deposited on a 18-mm square glass coverslip pretreated with 3-aminopropyltrimethoxysilane (Sigma-Aldrich, St. Louis, MO) and glutaraldehyde (Sigma-Aldrich). The gels were coated with 0.1mg/ml of either fibronectin (Fn) (Sigma-Aldrich) or collagen type I (Sigma-Aldrich) through the cross-linker N-Sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino) hexanoate (0.5 mg/ml in 50 mM HEPES buffer pH 8) (Thermo Fisher Scientific, Waltham, MA).
Semi-synthetic hyaluronan based hydrogels (HyStem, Glycosan BioSystems) were prepared by reacting the thiol-modified HA (HyStem) with poly(ethyleneglycol) diacrylate (PEGDA Mw=3400 Da, Extralink, Glycosan BioSystems). The hydrogels were formed in combination with either fibronectin, collagen type I, fibrinogen or mixtures of these proteins. Modification of these proteins to covalently link them throughout the hydrogel network was performed using Maleimide-dPEG8-N-HydroxySuccinimide ester (M-dPEG-NHS, Quanta BioDesign). Briefly, HyStem was dissolved with degassed water (DG Water, Glycosan BioSystems) to result in a final gel concentration of 0.8 wt %. The protein was activated by reacting in 1:10 (wt/wt) ratio with the M-dPEG-NHS in a sterile aqueous solution for 30 minutes. This solution was then added to the previously dissolved HyStem, allowed to react for an additional 30 minutes, and finally crosslinked with Extralink using a 1vol:4vol Extralink to HyStem ratio. Protein concentrations varied between 100 ug/mL up to 1 mg/mL and Extralink concentrations varied between 5 mg/mL up to 40 mg/mL. The stiffest hydrogels were prepared using poly(ethyleneglycol) tetra-acrylate (4-arm acrylated PEG, Mw 10,000 Da) at 40 mg/mL.
Surface labeling of FN-containing PAA and HA gels by immunofluorescence using anti-fibronectin antibodies confirmed that the surface coverage of the PAA gels was saturated by Fn and that the surface densities of PAA-Fn and HA-Fn gels were indistinguishable (supplemental Figure 1).
Rheology measurements
The shear modulus for each gel formulation was determined from rheological measurements conducted on either a stress-controlled Bohlin CS-10 rheometer (Malvern Instruments, Westborough MA) or a strain-controlled Rheometrics RFS3 rheometer. In some cases, such as for PAA and freshly prepared HyStem (Figs 3A and 3B), gels were made between the rheometer plates and subjected to a range of strains and frequencies. In other cases, when the elastic moduli of pre-formed gels or gels that had been incubated with cells or media were measured, the dish containing the gel was glued to the bottom plate of the rheometer and the top rheometer plate was gently lowered to form a tight contact with no more than a 5% indentation of the top surface. This method of measurement is essentially similar to that used to measure soft tissue rheology (Georges et al., 2007; Georges et al., 2006). No systematic differences were observed between the varied methods of measurement.
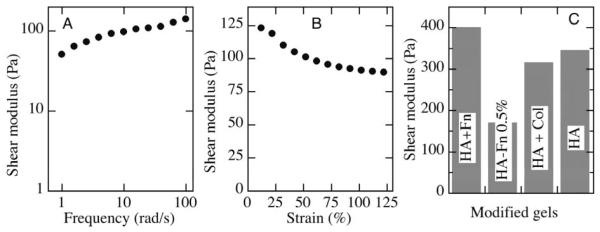
Figure 3.
Shear moduli of derivitized crosslinked HA gels. A. Frequency dependence of shear modulus of PEG diacrylate-crosslinked HA (Hystem) measured at 2% maximal strain. B. Strain dependence of similar Hystem sample measured at 10 rad/s. C. Shear moduli measured at 5% strain and 1 rad/s of HA gels covalently modified with Fn or collagen and incubated with myocytes for 1 weeks in culture before measurement. Crosslinker concentration was 2% except for the HA-Fn gel with 0.5% crosslinker, as shown.
Using atomic force microscopy, no differences were noted in the local substrate elasticity between 7 day old collagen-I and fibronectin coated hyaluronan gels (supplemental Figure 2).
Morphology of cells on PAA and HA gels of varying stiffness
To visualize myocyte or fibroblast cytoskeletons, cells were fixed with 4% paraformaldehyde (Sigma) for 10 min and then washed with 1xPBS. Fixed gel substrates were permeabilized with 0.1% triton X in TBS for 10 min and washed 3 times for 5 min in TBS. Following permeabilization substrates were then incubated for 1 h at room temperature with mouse monoclonal anti-alpha actinin antibody to visualize sarcomere definition and assembly (1:400; Sigma). The gel substrates were washed 3 times with TBS before secondary antibody was applied. Secondary antibody, anti-mouse Alexa 488 (1:500; Invitrogen), was prepared in TBS solution containing 1% BSA. Phalloidin–tetramethylrhodamine B isothiocyanate (1 μg/ml; Sigma) was used to visualize F-actin formation, and bisbenzimide (1 μg/ml; Sigma) to visualize the cell nucleus. Substrates were incubated in secondary antibody for 1 h and washed three times with TBS containing 0.1% Tween. The cells were visualized under a conventional microscope (Carl Zeiss, Thornwood, NY) at x20 and x63. Images were acquired using proprietary software (Axiovision; Carl Zeiss).
Cell area was computed using a dedicated MATLAB program (The MathWorks, Natick, MA) that quantifies (in pixel intensity units) the contrast between the fluorescent cells and the dark background. For each image, the degree of contrast at the cell border was defined and outlined by an unbiased observer. Fast Fourier Transform was used to evaluate repeated striation patterns 10kPa PAA gels using the image J software (National Institutes of Health, Bethesda, MD). Myocyte F-actin alignment was measured using an edge detection Matlab program as described previously (Kemeny and Clyne, 2011). The percentage of myocyte striation area was confined to areas displaying sarcomere lengths ranging from ~1.7 to 1.8μm normalized to the entire cell.
Analysis of myocyte contraction
To calculate the percentage of beating cells, myocytes on various gels were observed under 20x magnification for a period of 1 minute and the total numbers of single beating or non-beating myocytes were counted in 4 regions of each gel substrate. To compute the area change of myocyte contraction, time lapse phase contrast images at x40 of beating myocytes were acquired at 5-frames/second. The area of myocytes before contraction and in the contracted state was computed manually using the image J software (National Institutes of Health, Bethesda, MD). All live cell imaging was conducted at 37°C with 5% CO2 (Zeiss Observer Z1 Microscope).
Statistical analysis
ANOVA followed by a post hoc Fisher’s LSD test was performed to show a significant statistical variation for experiments (where P < 0.05 was considered significant unless otherwise specified).
Error bars indicate standard error unless otherwise specified.
Results
Myocyte shape and myofibrillar assembly respond differently to collagen I and fibronectin(Fn)-coated PAA gels of varying matrix rigidity
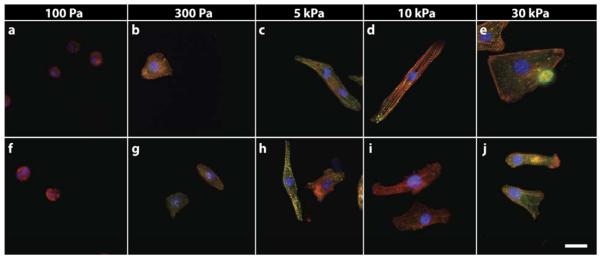
Previous studies have extensively used PAA gels with collagen I (Engler et al., 2008) or mixtures of collagen I and fibronectin (Chopra et al., 2011) to culture cardiac myocytes, and the differential mechanical response between the two ligand types has not been evaluated. The response of neonatal rat cardiomyocytes (NVRM) to changes in substrate stiffness when cultured on polyacrylamide gels laminated with either fibronectin or collagen I is shown in Figure 1. Both of these integrin ligands allow adhesion of the cell to the otherwise non-adhesive gels, but the typical cardiomyocyte morphology characterized by a large aspect ratio and actin fibers with striated appearance of alpha actinin staining occurs only when the substrate shear modulus is in the range from 5 to 10 kPa. Fibronectin and collagen engage different sets of integrins, and the morphologies are similar but not identical on each level of substrate stiffness. Myocytes cultured on collagen and fibronectin gels of 100Pa or 300 Pa (soft) displayed a rounded morphology, scarce F-actin assembly and little or no visible striation (Fig 1 a,b & f,g). As the stiffness of the PAA gel was increased to 5-10 kPa (intermediate/physiological), myocytes on both ECM ligands increased their spread area and took on an elongated shape (Fig 1 c,d,h,i).
Figure 1.
NVRM myofibrillar assembly is sensitive to matrix rigidity and adhesive ligand type. Cardiac myocytes cultured on PAA gels of varying rigidity coated with Fn (a-e) and collagen (f-j), cultured for 48 hours and stained for f-actin (red), α-actinin (green) and nucleus (blue). Myocytes on intermediate Fn gels reassembled myofibrils whereas cells on relatively soft and hard matrices exhibited lower myofibril assembly. Myocytes on collagen I matrices were less efficient at reassembling myofibril. Scale bar indicates 20μm
Myocytes cultured on fibronectin coated PAA gels developed well-organized and polarized myofibrillar assemblies whereas on collagen this response was highly attenuated (Fig.1c,d vs. h.i and Fig.2). On stiffer fibronectin coated PAA substrates (>30 kPa, shear modulus) approximating the pathologic heart, cells increased their spread area and displayed prominent F-actin stress fiber-like filaments but lacked organized myofibrillar assembly or an elongated shape (Fig. 1). In contrast, on stiff collagen-coated PAA substrates the cells did not further increase their spread area and continued to show an attenuated myofibrillar assembly (Fig.1j). These results indicate that the myocyte cytoskeleton remodels in response to substrate rigidity and is strongly influenced by the adhesive ligand to which it binds.
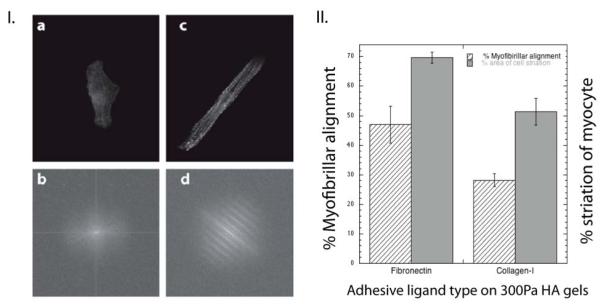
Figure 2.
Cardiac myocyte myofibrillar assembly is sensitive to ECM ligand type.
(I.) Fourier transform spectra of single myocytes cultured for 48 hours on 1 kPa Col-I (a,b) and Fn (c,d) coated PAA gels. F-actin staining was used to identify repeating patterns/striated myofibrils. Myocytes on collagen-PAA gels (b) did not show patterned Fourier transform spectra when compared to Fn (d). (II.) Percentage of aligned fibers and estimated striated area was calculated for myocytes on fibronectin and collagen-I coated 10kPa PAA gels; myofibrils within 15° from the long axis of the cell were considered to be ‘aligned’. Myocyte striation area having sarcomeres of ~ 1.7 to 1.8 μm were used to estimate the total ‘striation area’(± S.E. for >15 cells).
Hyaluronan (HA) gels enhance myofibrillar assembly on soft substrates
When polyacrylamide is replaced by hyaluronic acid as the scaffold on which integrin ligands are attached, the dependence of myocyte morphology on substrate stiffness is radically altered. Hyaluronan gels of varying rigidity were made by the incorporation of different amounts of the crosslinker polyethylene glycol diacrylate to create gels with shear moduli of 50-350 Pa. To achieve a higher stiffness of 1.8 kPa, polyethylene glycol tetra-acrylate was incorporated in the HA solution. To test whether myocytes bind directly to hyaluronan under our culture conditions, HA gels without incorporated adhesive ligands were made, but myocytes and accompanying cardiac fibroblasts cells did not bind these substrates. Therefore, HA by itself is an inert substrate for myocyte attachment. In contrast, when adhesive ligands such as fibronectin, collagen I, gelatin, or fibrinogen were covalently incorporated within the HA gels these composite gels were excellent adhesive substrates for both myocytes and fibroblasts.
Rheological measurements showed that crosslinked HA gels had shear moduli typically in the range of 100-350 Pa, depending on their extent of crosslinking and partly on their age, as disulfide bonds can form very slowly within the networks. These values of shear modulus are in agreement with those measured previously for similar crosslinked HA gels (Vanderhooft et al., 2009). The shear modulus also depended only weakly on frequency (3A) and strain amplitude (3B), consistent with expectations for gels formed by crosslinked flexible polymers (Chen et al., 2010). In particular, they did not exhibit the large degree of strain stiffening seen in gels formed by filamentous biopolymers such as collagen or fibrin. Incorporation of proteins into the HA gels and prolonged incubation with cells and culture media also did not significantly alter gel stiffness (Fig 3C).
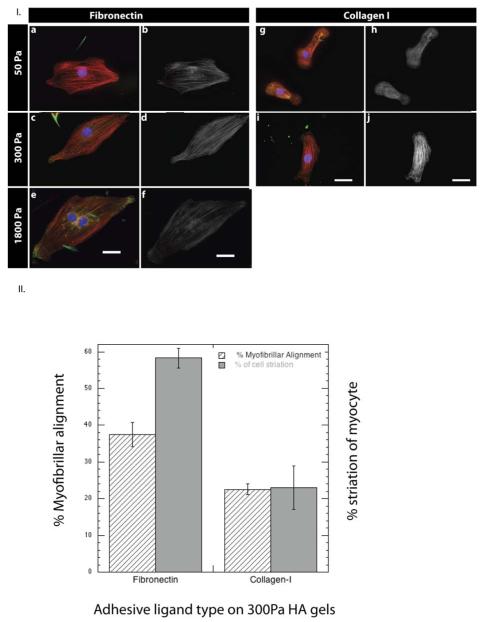
Myocytes cultured on fibronectin-incorporated soft HA gels of varying rigidity (50-1800 Pa) displayed a remarkably different response when compared to Fn-coated PAA gels of similar rigidity (Figure 4). On soft Fn-HA gels (<300Pa) myocytes spread to areas that were comparable to or higher than those seen on even rigid (>30kPa) PAA or glass (GPa) substrates. The increased spreading of myocytes on HA gels was not accompanied by the stress fiber assembly and disorganized myofibrillar assembly that occurs when cells spread on stiff Fn-PAA gels; rather, the myocytes displayed striated F-actin assembly and organized myofibrils (Fig 4 a-f)., Altering the rigidity of HA substrates beyond 1 kPa did not appreciably affect the myofibrillar assembly suggesting that stiffness responses are either overridden or saturated at this range of stiffness on hyaluronan. To examine myocyte response to relatively stiffer HA environments we performed additional experiments by plating cells on 10 μm thin gels. The response to glass like stiffness of myocyte cytoskeletal organization was disrupted and consistent with the results on stiff PAA gels/ tissue culture plastic (supplemental Figure 3). Myocytes cultured on HA gels with covalently incorporated collagen I were more comparable to those seen on collagen I-coated PAA and did not show the same myofibrillar assembly as cells on Fn-HA (Fig. 4 g-j). Incorporation of gelatin, the denatured form of collagen I-HA gels resulted in a myofibril assembly response comparative to that seen on fibronectin, but not intact collagen I. These results are consistent with other findings that gelatin engages the fibronectin receptor and suggests that the ability of hyaluronan to activate myofibril formation and organization is integrin type specific.
Figure 4.
Cardiac myocytes plated on fibronectin/collagen-containing HA gels of varying stiffness. (I.) Myocyte myofibrillar assembly on soft 50 Pa-1800 Pa hyaluronan gels and its sensitivity to the incorporated ECM ligand type. Cardiac myocytes plated on Fn-HA gels (a-f) and col-I-HA (g-j) of varying matrix rigidity, stained for F-actin (red), α-actinin (green) and nucleus (blue) (a,c,e,g,i) and F-actin only (b,d,f,h,j). Myocytes on Fn-HA matrices reassemble myofibrils whereas on collagen myofibrillar assembly is attenuated. Scale bar 20μm. (II.) Percentage of aligned fibers and estimated striated area was calculated for myocytes on fibronectin and collagen-I coated 300Pa HA gels; myofibrils within 15° from the long axis of the cell were considered to be aligned. Myocyte striation areas having sarcomeres of ~ 1.7 to 1.8 μm were used to estimate the total ‘striation area’(± S.E. for >15 cells).
Long term culture of cardiac myocytes and fibroblasts
Cardiac myocytes generally atrophy or differentiate to an altered phenotype when cultured for a long period on rigid substrates. Moreover myocytes either undergo apoptosis or their cultures are predominantly taken over by fibroblasts. On HA gels, however, myocytes cultured for 7 days showed an even higher spread area and hypertrophied appearance (~3300 μm2) with remarkably well formed sarcomeres and organized myofibrils (Fig. 5 i a-c). This result suggests that the engagement of hyaluronan enhances myocyte growth without altering its phenotype.
Figure 5.
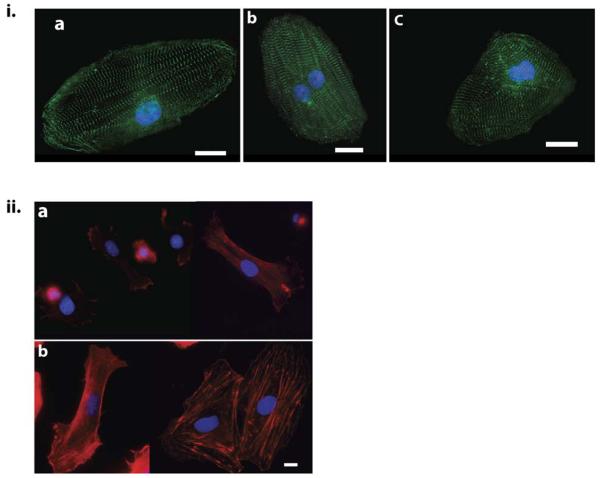
Myocytes hypertrophy is progressive showing enhanced myofibrillar assembly on HA matrices when cultured for prolonged periods. (I.) Cardiac myocytes cultured for 7 days on 300 Pa HA-Fn (a), HA-fibrinogen (b), HA-gelatin (c) displaying well formed sarcomeres stained for α-actinin (green). Scale bar 20μm (II.) Fibroblasts present in NVRM cultures plated on 300 Pa Fn-PAA gels (a) and Fn-HA gels (b), imaged after 48 hours and stained for f-actin (red). Scale Bar 10 μm. Fibroblasts on HA gels increase their spread area and display prominent actin fiber bundles when compared to PAA gels of the same rigidity.
The hypertrophic phenotype and similar myocyte cytoskeleton response was observed for HA gels with incorporated fibronectin, fibrinogen or gelatin, all of which are ligands for similar integrins e.g. α5β1, but was not observed when intact collagen I was the only adhesive anchor.
Non-muscle cells like fibroblasts present in these cultures also displayed a similar response to soft hyaluronan gels. Fibroblasts on soft PAA gels (300Pa) were devoid of stress fibers and had a low spread area (Fig. 5 ii a). On HA gels coated with fibronectin, fibroblasts increased their spread area and displayed prominent F-actin fibers (Fig. 4ii b). These results suggest that hyaluronan also alters the phenotype of non-muscle cells bound to integrin ligands.
Hyaluronan increases the spread area of myocytes
The magnitude of spread area for myocytes on PAA gels coated with fibronectin was higher than that seen on collagen I gels or the mixture of the two (Fig.5). On fibronectin, myocyte area increased with increasing substrate rigidity, whereas for collagen the magnitude of cell spreading plateaued after 10 kPa. These results suggest that a change in cell spreading behavior is a function of substrate rigidity and is in part specific to the integrin type mediating this phenomenon.
On soft hyaluronan gels, the spread area response of myocytes was remarkably altered. Compared to soft PAA gels the magnitude of cell spreading on gelatin HA gels was much higher; as the stiffness of HA gels were increased from 50Pa to 300Pa the magnitude of cell spreading also increased and plateaued thereafter (Fig.5). Changing the ECM ligand type to fibronectin (Fn) on hyaluronan (HA) resulted in a greater magnitude of spreading on soft HA gels, even on the softest Fn-HA gel (50Pa) the spread area magnitude was higher than that seen for the most rigid (30kPa) PAA gel (Fig.5). Cell spread area for myocytes on Fn-HA gels increased initially with substrate rigidity, but it plateaued after 300 Pa. This result indicates a reprogramming of cell spreading response to substrate rigidity on HA gels towards the softer end.
The altered spreading response of myocytes to soft HA gels was specific to the ECM protein incorporated in these gels. As mentioned previously, the magnitude of spreading was much higher for 300 Pa Fn-HA (~2500 μm2) compared to 300 Pa Fn-PAA (~500 μm2), however no difference in the magnitude of myocyte spreading was observed between PAA and HA gels when collagen type I was incorporated. This difference in spread area between HA and PAA was in part rescued by incorporating a mixture of fibronectin and collagen-I to HA gels. Therefore spreading on HA gels is in part dependent on the integrin type receptor engaged and the magnitude appears to be enhanced specifically with Fn-related integrin receptors.
Hyaluronan enhances the beating population of single myocyte cultures
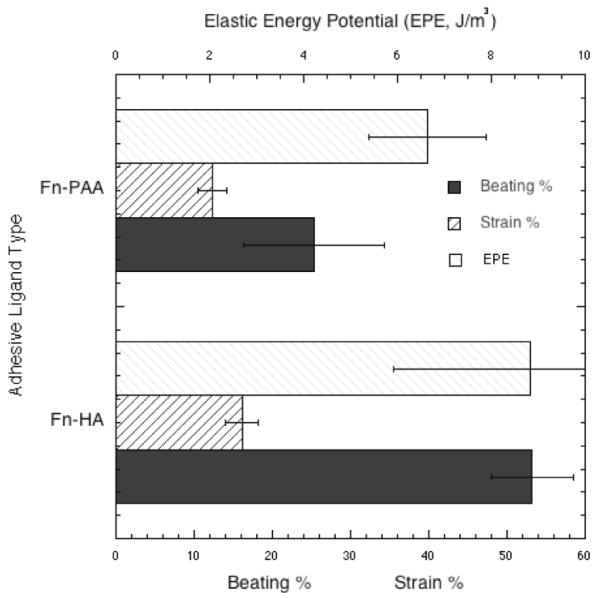
The percentage of beating cells was also affected by HA in the matrix. The beating percentage of myocyte populations was higher (p<0.05, determined using Fisher’s LSD) on 300 Pa Fn-HA gels as compared to 300 Pa and 10 kPa FN-coated PAA gels. The fraction of total cell area displaced during a contractile cycle was also higher for 300 Pa Fn-HA gels. This result reflects the enhanced sarcomere assembly on Fn-HA gels. As expected, the percentage area changes for soft PAA and HA gels (~16%) were higher than those on 10 kPa gels (~6%), indicating that the imposed load/resistance experienced by myocytes on soft HA gels is exceedingly low.
The contractile strain (є) of myocytes was estimated by measuring the change in the major axis of the cell fitting an ellipse to phase contrast images of myocytes during a contractile cycle.
Contractile work per volume was computed as the elastic potential energy density , where Y is the Young’s modulus of the matrix. On soft PAA and HA substrates the contractile work done was much lower (Fig.7) when compared to 10 kPa PAA gels (~83 J/m3). These results confirm that myocytes on HA gels experience a low contractile load resistance. Remarkably, even at these low elastic loads myocytes can reassemble highly robust sarcomeres and myofibrillar structures. The contractile work done by myocytes on Fn-HA gels was minimally increased when compared to Fn-PAA gels of the same rigidity despite the noted large differences in the cell contractile cytoskeleton apparatus.
Figure 7.
Percentage of observed beating cells on 300Pa HA and PAA gels (±S.E. for n>10 cells); Myocyte contractile work measured as potential elastic energy J/m3 on 300 Pa HA and PAA gels (±S.E. n>14 cells); Percentage change in area/area strain of contractile myocytes on soft HA and PAA gels (±S.E. n>14 cells).
Discussion
Engineered biomaterials based on hyaluronic acid have many potential applications in wound repair and tissue engineering and novel methods to derivitize HA with cell adhesion proteins have been devised (Burdick and Prestwich, 2011). The glycosaminoglycan HA is an important component of the basement membrane of tissues. Although cardiac myocytes in culture express CD44, a receptor for HA, they cannot anchor to HA scaffolds solely through this receptor. However, if the HA scaffold also contains ligands for integrins, then myocytes adhere strongly but do not respond to matrix stiffness in the same way they do on other fibronectin- or collagen-coated soft materials like polydimethyl siloxane, silicone films, or polyacrylamide gels. Our results also show that myocytes respond differently to fibronectin and collagen I on PAA as well as HA gels within the given time frame of these experiments. An explanation for this difference could be the involvement of a different subset of integrin types, which can regulate signalling molecules e.g. protein kinase C differentially, affecting myofibrillar assembly (Bullard et al., 2005). Experiments have shown that myocytes tend to spread more on basement membrane proteins when compared to structural proteins like collagen I&III (Hilenski et al., 1992). Even in an in vivo setting these ECM proteins have different functions; fibronectin is found to be a part of the basement membrane which also harbours other proteins like laminin, collagen type IV, serving as an interface for collagen I which is essentially a structural protein providing mechanical support (Fomovsky et al., 2010).
Cells can bind HA (Jiang et al., 2007) through receptors such as CD44 (Goodison et al., 1999) and RHAMM (Slevin et al., 2007). Through mechanisms that are not well understood, cells have differential responses to varying molecular weight hyaluronan polymers (Morrison et al., 2001; Pure and Assoian, 2009; Stamenkovic and Yu, 2010). In most cases, low molecular weight polymers are indicative of injury or disease, while high molecular weight polymers signal homeostasis. Because the HA in the gels studied here is permanently crosslinked into a matrix, cells presumably respond to it as though it was of a high molecular weight.
Cells produce hyaluronan through three hyaluronan synthase proteins – HAS1, HAS2, and HAS3 – which are located at the inner surface of the plasma membrane. HAS2-null mice are embryonic lethal with cardiac and blood vessel abnormalities (Camenisch et al., 2000). By day E9.5, these animals completely lack formation of endocardial cushions (Camenisch et al., 2000).
The simplest explanation for the effect of HA appears to be that although receptors for HA alone cannot anchor myocytes to HA-containing gels, when integrins also engage the matrix through fibronectin, they activate HA receptors that then signal to help orchestrate the cellular response. Other possibilities include a conformational change in Fn caused by its binding to HA, as suggested by biochemical evidence (Isemura et al., 1982). The ability of immobilized HA to sequester growth factors in media and present them to adherent cells might also contribute to the synergistic effects between HA and Fn on myocyte adhesion and morphogenesis The presence of non-muscle cells which show a similar response in terms of spreading to HA gels might also release paracrine signals contributing to altered myocyte spreading and myofibrillar assembly on hyaluronan.
Injecting non-functionalized hyaluronan hydrogels of varying mechanical strength in myocardial infarction models has been shown to improve cardiac function and reduce infarct size (Ifkovits et al., 2010; Yoon et al., 2009).The ability to reset the mechanical response of myocytes using a relatively soft, easily deliverable and biologically compatible hydrogel such as HA is potentially a clinically translatable finding. For example, an injectable RGD peptide-modified form of HA hydrogel may prove to have therapeutic application in the area of cardiac cell-based repair, attenuation of infarct expansion, stabilization and biophysical alteration (cardiomyoplasty) of the affected tissue. Because of its active surface chemistry, HA is amenable to conjugation and delivery of growth factors (e.g. bFGF, PDGF, VEGF) for promoting myogenesis, neovascularization and local remodeling or reverse-remodeling.
Supplementary Material
Figure 6.
Myocyte spreading is sensitive to both substrate rigidity and ligand type. NVRM were cultured for 48 hours on HA or PAA hydrogels of different rigidity that were derivitized with various adhesion proteins as shown. Myocytes cultured on Fn-bound HA gels showed the highest spreading ( ± S.E. for >150 cells).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bajaj P, Tang X, Saif TA, Bashir R. Stiffness of the substrate influences the phenotype of embryonic chicken cardiac myocytes. J Biomed Mater Res A. 2010;95:1261–1269. doi: 10.1002/jbm.a.32951. [DOI] [PubMed] [Google Scholar]
- Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105:1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111:774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- Bullard TA, Borg TK, Price RL. The expression and role of protein kinase C in neonatal cardiac myocyte attachment, cell volume, and myofibril formation is dependent on the composition of the extracellular matrix. Microsc Microanal. 2005;11:224–234. doi: 10.1017/S1431927605050476. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Prestwich GD. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv Mater. 2011 doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, Janmey PA. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J. 2009;96:5095–5102. doi: 10.1016/j.bpj.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr., Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Wen Q, Janmey P, Crocker J, Yodh A. Rheology of soft materials. Annual Review of Condensed Matter Physics. 2010;1:301–322. [Google Scholar]
- Chopra A, Tabdanov E, Patel H, Janmey PA, Kresh JY. Cardiac myocyte remodeling mediated by N-cadherin-dependent mechanosensing. Am J Physiol Heart Circ Physiol. 2011;300:H1252–1266. doi: 10.1152/ajpheart.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MW, Russell B. Micromechanical regulation in cardiac myocytes and fibroblasts: implications for tissue remodeling. Pflugers Arch. 2011 doi: 10.1007/s00424-011-0931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fomovsky GM, Thomopoulos S, Holmes JW. Contribution of extracellular matrix to the mechanical properties of the heart. J Mol Cell Cardiol. 2010;48:490–496. doi: 10.1016/j.yjmcc.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz A, Lambert M, Saez A, Silberzan P, Buguin A, Mege RM, Ladoux B. Traction forces exerted through N-cadherin contacts. Biol Cell. 2006;98:721–730. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol. 2005;98:1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilenski LL, Ma XH, Vinson N, Terracio L, Borg TK. The role of beta 1 integrin in spreading and myofibrillogenesis in neonatal rat cardiomyocytes in vitro. Cell Motil Cytoskeleton. 1992;21:87–100. doi: 10.1002/cm.970210202. [DOI] [PubMed] [Google Scholar]
- Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11:120–126. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH, 3rd, Gorman RC, Burdick JA. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 2010;107:11507–11512. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isemura M, Yosizawa Z, Koide T, Ono T. Interaction of fibronectin and its proteolytic fragments with hyaluronic acid. J Biochem. 1982;91:731–734. doi: 10.1093/oxfordjournals.jbchem.a133746. [DOI] [PubMed] [Google Scholar]
- Jacot JG, Martin JC, Hunt DL. Mechanobiology of cardiomyocyte development. J Biomech. 2011;43:93–98. doi: 10.1016/j.jbiomech.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- Kemeny SF, Clyne AM. A simplified implementation of edge detection in MATLAB is faster and more sensitive than fast fourier transform for actin fiber alignment quantification. Microsc Microanal. 2011;17:156–166. doi: 10.1017/S143192761100002X. [DOI] [PubMed] [Google Scholar]
- Leopoldo AS, Sugizaki MM, Lima-Leopoldo AP, do Nascimento AF, Luvizotto Rde A, de Campos DH, Okoshi K, Dal Pai-Silva M, Padovani CR, Cicogna AC. Cardiac remodeling in a rat model of diet-induced obesity. Can J Cardiol. 2011;26:423–429. doi: 10.1016/s0828-282x(10)70440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maioli M, Santaniello S, Montella A, Bandiera P, Cantoni S, Cavallini C, Bianchi F, Lionetti V, Rizzolio F, Marchesi I, Bagella L, Ventura C. Hyaluronan esters drive Smad gene expression and signaling enhancing cardiogenesis in mouse embryonic and human mesenchymal stem cells. PLoS One. 2010;5:e15151. doi: 10.1371/journal.pone.0015151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, Gutmann DH, Ponta H, Herrlich P. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–980. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure E, Assoian RK. Rheostatic signaling by CD44 and hyaluronan. Cell Signal. 2009;21:651–655. doi: 10.1016/j.cellsig.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Qin L, Zhang X, He K, Xiong C, Fang J, Fang X, Zhang Y. Elasticity of cardiac cells on the polymer substrates with different stiffness: an atomic force microscopy study. Phys Chem Chem Phys. 2011 doi: 10.1039/c1cp20154a. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I, Yu Q. Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci. 2010;11:471–484. doi: 10.2174/138920310791824011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhooft JL, Alcoutlabi M, Magda JJ, Prestwich GD. Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromol Biosci. 2009;9:20–28. doi: 10.1002/mabi.200800141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen H, Seth A, McCulloch CA. Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2003;285:H1871–1881. doi: 10.1152/ajpheart.00387.2003. [DOI] [PubMed] [Google Scholar]
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Fang YH, Lim CH, Kim BS, Son HS, Park Y, Sun K. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J Biomed Mater Res B Appl Biomater. 2009;91:163–171. doi: 10.1002/jbm.b.31386. [DOI] [PubMed] [Google Scholar]
- Zhang S, Sun A, Liang Y, Chen Q, Zhang C, Wang K, Zou Y, Ge J. A role of myocardial stiffness in cell-based cardiac repair: a hypothesis. J Cell Mol Med. 2009;13:660–663. doi: 10.1111/j.1582-4934.2009.00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.