Abstract
Thirty thousand years ago, humans kept track of numerical quantities by carving slashes on fragments of bone. It took approximately 25,000 y for the first iconic written numerals to emerge among human cultures (e.g., Sumerian cuneiform). Now, children acquire the meanings of verbal counting words, Arabic numerals, written number words, and the procedures of basic arithmetic operations, such as addition and subtraction, in just 6 y (between ages 2 and 8). What cognitive abilities enabled our ancestors to record tallies in the first place? Additionally, what cognitive abilities allow children to rapidly acquire the formal mathematics knowledge that took our ancestors many millennia to invent? Current research aims to discover the origins and organization of numerical information in humans using clues from child development, the organization of the human brain, and animal cognition.
Keywords: analog magnitude, functional MRI, mathematics education, numerical cognition, numerosity
This review traces the origins of numerical processing from “primitive” quantitative abilities to math intelligence quotient (IQ). “Primitive” quantitative abilities are those that many animals use to estimate the value of an object or event, for instance its distance, length, duration, number, amplitude, saturation, or luminance (among others). The constraints on how human and animal minds process these different quantities are similar (1). For example, all of these quantities show cognitive processing limitations that can be predicted by Weber’s law. Weber’s law states that quantity discrimination is determined by the objective ratio between their values. This ratio-based psychological and neural signature of quantity processing indicates that many quantities are represented in an analog format, akin to the way in which a machine represents intensities in currents or voltages (1). I discuss the types of constraints that influence quantity discrimination, using “number” as the initial example, and then consider the psychological and neural relationship between “number” and other quantitative dimensions. Similar constraints on processing across different quantities have been interpreted as evidence that they have a common evolutionary and/or developmental origin and a common foundation in the mind and brain (2–11). The resolution of these issues is important for understanding the inherent organization of our most basic conceptual faculties. The issue is also important for understanding how our formal mathematical abilities originated.
Primitive quantitative abilities play a role in how modern humans learn culture-specific, formal mathematical concepts (1). Preverbal children and nonhuman animals possess a primitive ability to appreciate quantities, such as the approximate number of objects in a set, without counting them verbally. Instead of counting, children and animals can mentally represent quantities approximately, in an analog format. Studies from our group and others have shown that human adults, children, and nonhuman primates share cognitive algorithms for encoding numerical values as analogs, comparing numerical values, and arithmetic (4, 12–14). Developmental studies indicate that these analog numerical representations interact with children’s developing symbolic knowledge of numbers and mathematics (12, 15). Furthermore, the brain regions recruited during approximate number representations are shared by adult humans, nonhuman primates, and young children who cannot yet count to 30 (2, 16, 17). Finally, it has recently been demonstrated that neural regions involved in analog numerical processing are related to the development of math IQ (18). Taken together, current findings implicate continuity in the primitive numerical abilities that are shared by humans and nonhumans, as well as a degree of continuity in human numerical abilities ranging from primitive approximation to complex and sophisticated math.
Oldest Numbers in the World
The fact that humans have been recording tallies with sticks and bones for 30,000 y is impressive, but the critical issue is this: what cognitive abilities enabled them to encode quantities in the first place? To identify the inherent constraints on humans’ ability to process numerical information, it is helpful to consider the evolutionary history of numerical thought. We can look for clues to the evolutionary precursors of numerical cognition by comparing human cognition with nonhuman primate cognition. The degree to which humans and nonhuman primates share numerical abilities is evidence that those abilities might derive from a common ancestor, in the same way that common morphology like the presence of 10 fingers and toes in two different primate species points to a common morphological heritage.
So far, there is evidence that nonhuman primates share three essential numerical processing mechanisms with modern humans: an ability to represent numerical values (17, 19–21), a general mechanism for mental comparison (22), and arithmetic algorithms for performing addition and subtraction (23, 24). These findings compliment and extend a long history of research on the numerical abilities of nonhuman animals (see ref. 25 for review).
Representation.
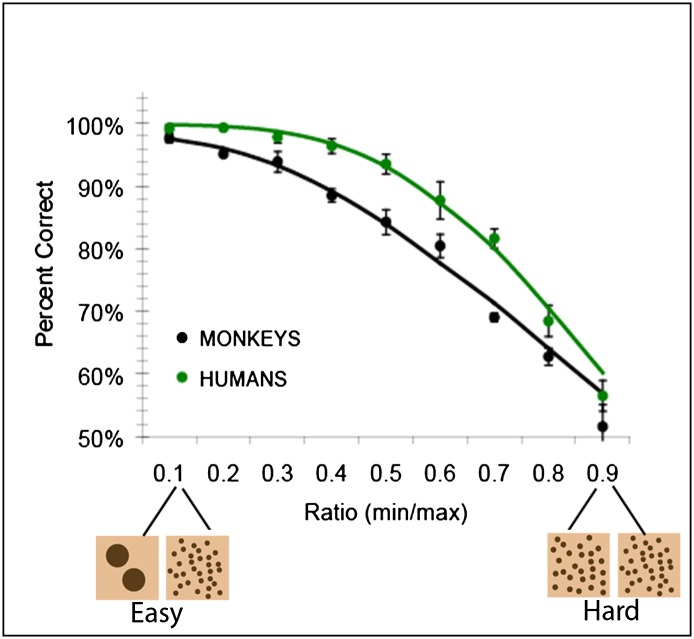
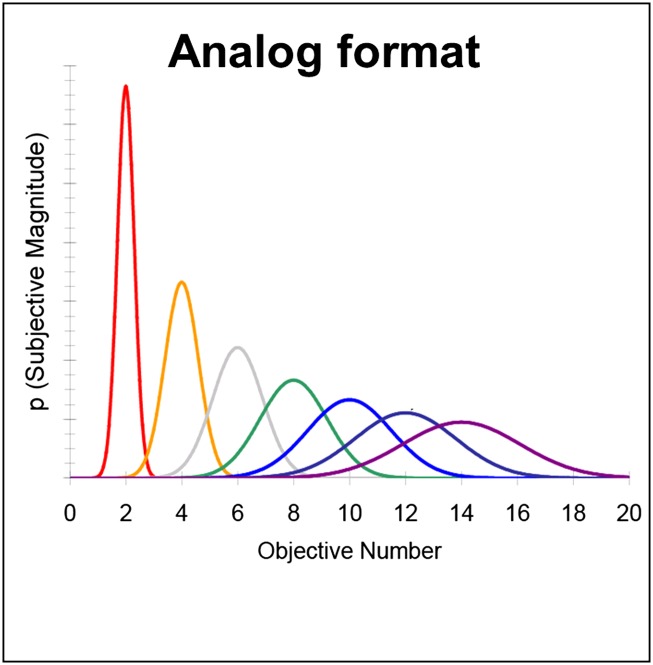
When adult humans and monkeys are given a task in which they have to rapidly compare two visual arrays and touch the array with the smaller numerical value (without counting the dots), their performance reliably yields the pattern shown in Fig. 1: accuracy decreases as the ratio between the numerical values in the two arrays approaches 1 (19; see refs. 1 and 26 for review). The explanation of this performance pattern is that both groups are representing the numerical values in an analog format (Fig. 2).
Fig. 1.
Accuracy on a numerical discrimination task for monkeys and humans plotted by the numerical ratio between the stimuli. From Cantlon and Brannon (19).
Fig. 2.
An analog representation of numerical value represents an objective numerical value with a probability distribution that scales with the size of the objective numerical value. From Cantlon et al. (48). Reprinted with permission from AAAS.
In an analog format, number is represented only approximately, and it is systematically noisy (1, 26). More precisely, the probability of noise (i.e., the spread of the distributions) in the subjective representation of a number increases with the objective number of items that are coded by that representation. Consequently, the probability of confusion (i.e., the overlap between distributions) between any two objective numbers increases as their value increases. This means that the probability of having an accurate subjective representation of a numerical value decreases with its objective value. This relationship can be succinctly quantified by the ratio between the numerical values being compared. Two different pairs of numerical values that have the same ratio (e.g., 2 and 4, 4 and 8) have the same amount of overlap, or the same probability of confusion. As numerical pairs get larger and closer together, their ratio increases and so does the probability that they will be confused (leading to more errors). For example, one might be 80% accurate at choosing the larger number when the numerical choices are 45 vs. 70 (45/70 = a 0.64 ratio) but might perform at chance when the choices are 45 vs. 50 (45/50 = a 0.9 ratio). This effect is known as Weber’s law. The curves in Fig. 1 (from ref. 4) represent predicted data from a model of number representation under Weber’s law (27), and they show that the predictions of this analog numerical model fit the data well.
The empirical data from monkeys and humans and the fit of the analog model demonstrate that although humans have a means of representing numerical values precisely using words and Arabic numerals, they still have an approximate, analog numerical system that functions essentially in the same way as in monkeys.
Comparison.
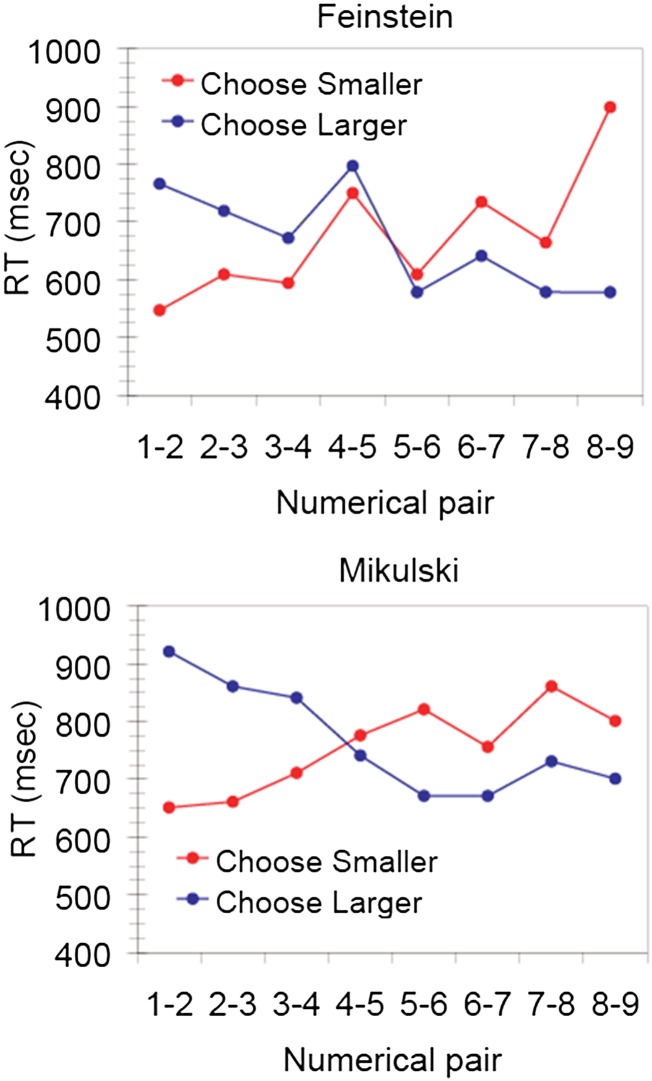
The ratio effect, described by Weber’s law, indicates that numerical values can be represented in an analog format. However, that does not tell us anything about the process by which two numerical values are compared. We have identified a signature of mental comparison in monkeys that is commonly observed when adult humans make judgments of magnitudes: the semantic congruity effect (22, 28). The semantic congruity effect is a response time effect that is observed in adult humans’ response times whenever they have to compare things along a single dimension. For instance, when people are presented with pairs of animal names and asked to identify the larger or smaller animal from memory, they show a semantic congruity effect in their response time: people are faster to choose the smaller of a small pair of items (e.g., ant vs. rat) than they are to choose the larger of that pair. However, for pairs of large items (e.g., horse vs. cow), people are faster to choose the larger item than the smaller item. This effect suggests that the physical size of the animal interacts with the “size” of the question (whether “Which is larger?” or “Which is smaller?”) in subjects’ judgments. In humans, the semantic congruity effect is observed for judgments of many dimensions, including judgments of numerical values, from Arabic numerals. We found that this effect is also observed in monkeys when they compare numerical values from arrays of dots. Monkeys performed a task in which they had to choose the larger numerical value from two visual arrays when the background color of the computer screen was blue, but when the screen background was red, they had to choose the smaller numerical value of the two arrays. As shown in Fig. 3 (from ref 22), both monkeys showed a crossover pattern of faster response times when choosing the smaller of two small values compared with the larger of two small values, and the opposite pattern for large values. The semantic congruity effect is the signature of a mental comparison process wherein context-dependent mental reference points are established (e.g., 1 for “choose smaller” and 9 for “choose larger”), and reaction time is determined by the distance of the test items from the reference points; this has been modeled as the time it takes for evidence to accrue in the comparison of each item to the reference point (28). In humans the semantic congruity effect is observed for a variety of mental comparisons from both perceptual and conceptual stimuli: brightness, size, distance, temperature, ferocity, numerals, etc. Our data from nonhuman primates indicate that the mental comparison process that yields the semantic congruity effect is a primitive, generalized, nonverbal mental comparison process for judging quantities and other one-dimensional properties.
Fig. 3.
Semantic congruity effect in the response times of two different monkeys (Feinstein and Mikulski) on a numerical comparison task in which they sometimes chose the larger numerical value from two arrays (blue) and other times chose the smaller value (red). The cross-over pattern reflects the effect of semantic congruityy. From Cantlon and Brannon (22).
In fact, the ability to compare quantities, and the proposed algorithm underlying that ability, could be so primitive that it extends to nonprimate animals. A recent study by Scarf et al. (29) showed that pigeons can compare numerical values, and in doing so they represent an abstract numerical rule that can be applied to novel numerical values. Pigeons’ accuracy on that ordinal numerical task is comparable to that of monkeys tested on an identical task (21).
Arithmetic.
Arithmetic is the ability to mentally combine values together to create a new value without having directly observed that new value. We have found that monkeys possess a capacity for basic, nonverbal addition that parallels human nonverbal arithmetic in a few key ways (24). First, monkeys and humans show a ratio effect when performing rapid nonverbal addition, similar to the ratio effect described earlier. Monkeys’ and humans’ accuracy during arithmetic depends on the ratio between the values of the choice stimuli. We also observed a classic signature of human arithmetic in monkeys’ performance: the problem size effect. Adult humans typically exhibit a problem size effect wherein performance worsens as the problem outcome value increases (30). Like humans, monkeys exhibited a problem size effect in their addition accuracy (even when controlling for the ratio effect).
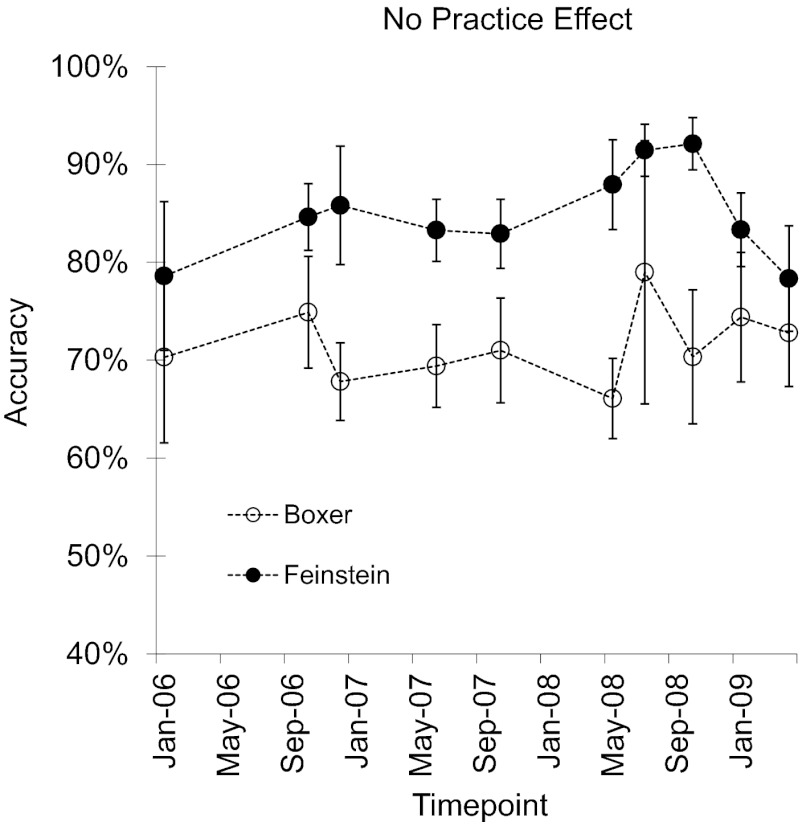
However, there are also important and potentially informative differences between the performance of humans and monkeys. Adult humans and young children show a practice effect in their arithmetic performance wherein performance on a specific problem improves the more that it is practiced (30). Monkeys do not show a practice effect for specific problems. This was the case even over 3 y of practice on a specific problem (Fig. 4 shows performance for two monkeys, over 3 y of testing on 1 + 1, 2 + 2, and 4 + 4). Nonhuman primate arithmetic thus parallels human nonverbal arithmetic in the ratio and problem size effects but not the practice effect, which has been observed primarily in symbolic arithmetic performance in humans. Presumably, discrete symbols are necessary for humans to encode arithmetic problems in a format that is amenable to memorization, which is why monkeys do not show a practice effect.
Fig. 4.
The lack of a practice effect in monkeys’ addition performance over 3 y. Data from Cantlon and Brannon (24).
The overarching conclusion from this line of research is that the abilities to represent, compare, and perform arithmetic computations reflect a cognitive system for numerical reasoning that is primitive and based on analog magnitude representations. However, if analog numerical cognition is truly “primitive” and homologous across primate species, then it should be rooted in the same physical (neural) system in monkeys and humans. In fact, there is evidence from multiple sources that analog numerical processing recruits a common neural substrate in monkeys, adult humans, and young children (Fig. 5).
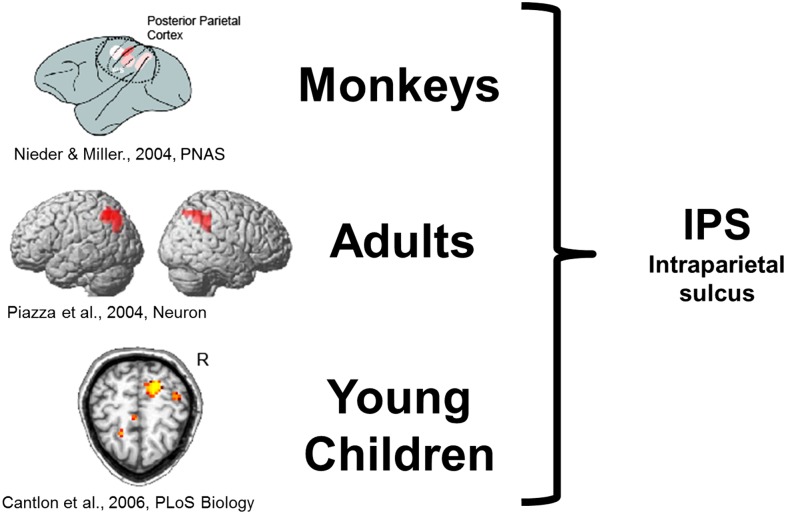
Fig. 5.
Monkeys, human adults, and human children exhibit similar activation in the IPS during analog numerical processing. Redrawn from refs. 31, 32 [Reprinted from Neuron, 44(3), Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S, Tuning curves for approximate numerosity in the human intraparietal sulcus, 547–555, Copyright (2004) with permission from Elsevier], and 33.
In monkeys who are trained to match visual arrays of dots according to number, single neurons along the intraparietal sulcus (IPS) will respond maximally to a preferred numerical value, and their firing rate decreases as the number that is presented gets numerically farther from that preferred value (31). This neural firing pattern has been linked to the behavioral ratio effect and is thought to reflect analog numerical tuning in the IPS. A similar pattern of numerical tuning has been observed with functional MRI in the human IPS. Manuela Piazza et al. (32) found a neural adaptation effect for numerical values in the IPS that depended on the ratio between the adapted numerical value and a deviant numerical value. Our group also observed neural adaptation in the IPS for numerical values ranging from 8 to 64 in preschool children who could not yet verbally count to 30 (33). Together, these studies reflect a common neural source for analog numerical representation that bridges species as well as stages of human development and is thus independent of language and formal mathematics experience. These neural data support the conclusion derived from the behavioral data that there is continuity between humans and nonhuman animals in the mechanisms underlying analog numerical representations.
Then There Were Symbols
A long history of studies with preverbal human infants has shown that they too possess an ability to quantify objects with approximate, analog representations (12). Thus, there is general agreement that the analog system for numerical reasoning is primitive in human development. A fundamental question is how a child’s developing understanding of numerical symbols interfaces with preverbal analog representations of number. Of particular interest is how children initially map numerical meanings to the first few symbolic number words (15, 34–37). There is currently a debate over the types of preverbal numerical representations that form the initial basis of children’s verbal counting. However, regardless of how this initial mapping transpires, behavioral evidence suggests that as children learn words in the counting sequence, they map them to approximate, analog representations of number (38–40). Lipton and Spelke (39) found that 4-y-old children could look at a briefly presented array of 20 dots and, if they could count to 20, they could verbally report (without counting) that there were twenty dots in the array, and their errors were systematically distributed around 20 (i.e., their errors exhibited a numerical ratio effect). If they could not yet count to 20, however, they responded with random number labels. Thus, as soon as children learn a particular verbal count word in the sequence, they know the approximate quantity to which it corresponds without counting, suggesting that number words are attached to the analog numerical code as soon as they are learned. These data have been taken to indicate that analog numerical representations are used to assign semantic meanings to numerical symbols over human development. There is also evidence that children who have learned to count verbally, but have not yet learned to add and subtract, psychologically “piggy back” on analog arithmetic representations as they transition to an understanding of exact symbolic arithmetic (40). The general conclusion that then emerges is that the cognitive faculties that children initially use for nonsymbolic, analog numerical operations (and which they share with nonhuman animals) provide a scaffolding for verbal counting in early childhood.
Is “Number” Alone?
The data from the development of counting in early childhood make the case that a primitive numerical system is conceptually transformed into a system for symbolic numbers. However, how do we know that analog numerical representations are the sole precursors of formal, symbolic numerical cognition? Currently, we do not. Although numerical reasoning seems to be primitive in the sense that it is shared among primate species, other quantitative abilities are just as widespread. For instance, the abilities to judge nonnumerical intensities such as size, time, brightness, height, weight, velocity, pitch, and loudness are as common among animal species as the ability to judge numerical values. Furthermore, all of these quantities can be discriminated by human infants, and discriminations among instances from those continua bear many of the same properties and signatures as numerical discrimination [e.g., ordinality, Weber’s law, the semantic congruity effect, arithmetic transformations (see ref 11 for review)]. In adults, all of these dimensions are effortlessly mapped to numerals. For example, adult humans can represent loudness, handgrip pressure, time, size, and brightness as numerical values. Finally, evidence from the semantic congruity effect (described earlier) suggests that many different quantitative dimensions are mentally compared by a common process. The modularity and taxonomy of analog numerical representations is a central issue for understanding the development and origins of numerical and mathematical cognition. Here I discuss relations between numerical cognition and other quantitative dimensions, such as size, length, duration, brightness, pitch, and loudness.
Until recently, the cognitive and neural mechanisms of numerical cognition were considered to be specialized processes. Neuropsychological and neuroimaging studies of adult humans have shown that numerical knowledge dissociates from other forms of semantic knowledge, and it has been argued from those data that the processes subserving numerical knowledge are domain specific (see ref. 16 for review). For example, individuals with semantic dementia, resulting from left temporal lobe atrophy, exhibit severe impairments on picture and word naming tasks but can be spared for number tasks (41). The opposite disorder of impaired numerical cognition but spared semantic and linguistic knowledge has also been demonstrated (42, 43). Moreover, in cases of developmental dyscalculia, mathematical reasoning can become selectively impaired over development (without impairments to other aspects of reasoning). Furthermore, developmental dyscalculia is coupled with atypical anatomy and functional responses in the IPS (44, 45). The fact that focal brain injuries and developmental impairments, perhaps especially to the IPS, specifically impair numerical reasoning indicates that at some level of cognitive and neural processing, numerical computation is independent. However, it remains unclear what aspects of numerical processing operate independently of other psychophysical and conceptual domains. Most previous neuropsychological and neuroimaging studies controlled for many nonnumerical abilities (eye movements, spatial attention, memory, semantic knowledge), but they did not test performance on continuous dimensions other than number (length, area, brightness, etc.). Thus we cannot know whether other quantitative abilities were simultaneously impaired in many of those neuropsychological patients.
Recently, Marco Zorzi et al. (7) found that representations of spatial and numerical continua can be jointly impaired in patients with right parietal lesions and hemispatial neglect; patients not only neglect the left visual field and place the midpoint of a line right of center in a line bisection task, but they also overestimate the middle value of two numbers in a numerical bisection task. The patients thus neglect both the left side of a line and the left side of their mental representation of the numerical continuum. This finding and several others have led to proposals that concepts of “space” and “number” are interrelated (8, 9).
The degree to which “space” (e.g., size, height, or length) interacts with numerical information is currently being investigated with a range of methods (see refs. 4, 5, and 9 for reviews). One view is that space and number have a biologically privileged psychological relationship (6, 46, 47). Evidence for this view comes from developmental studies of number and space representation (6, 47). In line-bisection tasks, incidental displays of dot arrays presented at the endpoints of the line systematically distort preschoolers’ perception of the line’s midpoint; subjects bisect the line asymmetrically toward the larger number of dots (6). In addition, infants spontaneously map number onto space when habituated to positively correlated number/line-length pairs (47). The fact that infants map number onto space within the first months of life has been used to argue for an innate bias to relate space and number.
Biologically privileged relations between space and number are also indicated by the universality of their association (46). The ability to map numbers onto space (number lines) is widespread among human cultures. The Mundurucu, an Amazonian people who lack a rich linguistic system for discrete number words or symbols, can place sets of objects that vary in numerical value onto horizontal lines in numerical order (just as Western subjects do). That finding supports the conclusion that mapping between space and number is not culturally determined by reading and reciting numerical symbols, because Mundurucu do not generally use such symbols. However, this finding does not necessarily indicate the presence of an innate bias to map numbers to space in humans, but may represent an analogical relation between the ordinal properties of the stimuli or the primacy of “space” alone (48). In support of those alternatives, there is evidence that a similar mapping to space is made with representations of pitch in typical adults from Western cultures (49). If pitch shows the same kind of relation to space as number does, then a biologically “privileged” relation between space and number seems less likely. One possibility is that the relationship is ubiquitous among any of a number of dimensions (e.g.., pitch, number, length, loudness, etc). Alternatively, number and space and pitch and space could be related because of a privileged representation of space alone, which grounds a number of quantitative representations.
Several researchers have suggested deep psychological interactions not just between number and space but among many quantitative dimensions. In their review of behavioral data from humans and other animals, Gallistel and Gelman (50) argued that although number is objectively a discrete property, it should be represented with an analog magnitude code. They argued that animals must combine discrete number with continuous quantities in making decisions. For example, they observed that animals need to combine estimated time and amount of potential food in making foraging decisions (i.e., for “rate”). Because natural numbers are discrete and time is continuous, combining information from these incompatible formats necessitates conversion to a common analog format. The same argument could be applied to “density,” which integrates information about number and surface area. This idea implicates the possibility of common representations and shared computations for multiple quantities.
Studies in young children provide evidence that different quantitative representations have a common foundation, in the sense that they develop together. As described earlier, numerical discriminations are modulated by the ratio between the values, as per Weber’s law. In human infants, the ratio effects for judgments of size, time, and number are refined at a similar rate of development (11, 51, 52). Infants’ discriminations of size, time, and number improve by approximately 30% between 6 and 9 mo of age. Similarly, in children, the precision of numerical discrimination improves from ages 6 to 8 y, and the discrimination of luminance, duration, and length systematically follow the same developmental trajectory (53, 54). Because they develop at the same rate, it is likely that either the same mechanism underlies the different abilities or that different mechanisms are subject to the same constraints. The developmental trajectories of the discrimination of other quantities, such as loudness, pitch, pressure, temperature, density, motion, and saturation, have not been tested. However, there is evidence that young children and even infants can form compatible representations across many of these different dimensions (55–58).
As mentioned earlier, the dimensions of space and number can be related to one another already in infancy (47). One recent study showed that 9-mo-olds were equally likely to transfer an arbitrary, experimentally learned magnitude-to-texture association from one dimension (e.g., number) to another dimension (size or duration) (59). In addition, 9-mo-olds can readily learn pairs of positively (but not negatively) correlated line lengths and tone durations (60), suggesting that infants at least can represent an abstract “more-than” and “less-than” representation that applies to both dimensions. However, 9-mo-old infants do not show equal sensitivity to monotonic pairings between the dimensions of loudness and space as they do for pairing of space and time (60). Those findings suggest that there may be an asymmetry between magnitudes in their intrinsic ordinal associations. It is important to note, however, that asymmetries in relations between magnitudes could arise either through a biologically privileged psychological mapping (6) or through correlational and statistical learning (see ref. 3 for discussion).
Perhaps the best evidence for early-developing psychological relations among quantities is that infants at 4 mo of age spontaneously prefer to look at a ball that is bouncing congruently with the pitch of an auditory stimulus (the ball goes up when the pitch goes up) compared with a ball that is bouncing incongruently with pitch. In addition, they prefer to look at a shape that is getting sharper as the pitch of the auditory stimulus gets higher than the reverse (58). Infants are thus capable of aligning the dimensions of pitch and space (height) as well as pitch and shape (sharpness) early in development. Similarly, 3-y-olds reliably match high-pitched sounds to smaller and brighter balls in a categorization task (56). Those data show that magnitude dimensions beyond the canonical “privileged” dimensions of space and number can be mapped onto each other early in development.
Relations among different quantities also have been found at the neural level in adult humans and nonhuman primates. As mentioned above, individuals with spatial neglect resulting from damage to parietal cortex can exhibit impaired numerical processing. Single-neuron data from neurophysiology studies of monkeys broadly indicate that regions of parietal cortex represent space, time, and number (61). Moreover, some data even suggest that a single parietal neuron can represent more than one type of magnitude. In one study (61), monkeys were trained to perform a line-length matching task and a numerical matching task. During stimulus presentation as well as a subsequent delay, single neurons in the IPS responded selectively to visual stimuli according to their numerosity or length. Although some neurons responded only to numerosity and others only to line length, a subset of cells (∼20%) responded to both magnitudes of line length and numerical value. These and other studies, including functional MRI studies of adults, have led some researchers to argue for a “distributed but overlapping” representation of different magnitudes at the neural level (4, 8, 61). Simply put, different types of magnitude representation, including size, number, and time (and possibly others such as brightness), share some neural resources in parietal cortex but not others. The next section discusses some possible explanations of the origin of the relationship between number and other quantitative dimensions.
How Is Number Linked to Other Quantities?
How do different quantitative dimensions become related in the mind and brain in the first place? We have recently reviewed existing theoretical frameworks for how quantitative relations might originate (3). Here, I briefly sketch five mechanisms for how different quantities could become related in the mind. These hypotheses are not mutually exclusive and may even be complementary.
Correlational and Statistical Associations.
Learning via association and correlation is the classic developmental account of the origins of abstract percepts and concepts (e.g., ref. 62). On this view, integrated representations of information coming from separate senses, modalities, or cognitive domains arise from exposure to correlations in the environment. Under this account, relations among magnitudes would arise from the strength of their correlations in the natural environment. For example, it takes a long time to walk a great distance (time and space are correlated), and a large number of a particular object tends to take up more surface area than a small number of that object (number and space are correlated). In this way, empirical correlations between different quantities can be absorbed through experience.
Analogical Reasoning.
Another possibility is that conceptual alignment of relational information, termed “structural similarity,” mediates mapping among magnitude dimensions (55). On this view, cross-dimensional mapping could be a form of analogy. Relations between magnitudes could develop through conceptual knowledge of how those dimensions are structured (60). For example, knowledge of the conceptual fact that time and number are ordinal and monotonic dimensions (they are organized from small/short to large/long) could serve as the cognitive basis for identifying relations among those dimensions.
Amodal Representations.
A third conceptual framework that could be useful for understanding relations among magnitudes derives from the literature on cross-modal sensory perception. Gibson (63) argued that an abstract, amodal representation of intensity or amount of stimulation is present from birth or very early in infancy. On her view, amodal representations can take one of two forms: (i) intersensory redundancy (e.g., timing information about hammer strikes can be sampled from both the auditory and visual modalities), and (ii) relative intensity (e.g., “sharpness, bluntness, and jerkiness”; ref. 63, p. 219). Under a conceptualization of magnitude representation within this framework, redundancy of information would be the main source of representational overlap. For example, a bright light could be mapped to a loud tone because they both evoke an amodal representation of relatively high intensity.
Automatic Cross-Activation.
A fourth hypothesis is suggested by evidence that infants experience something akin to synesthesia of sensory representations near birth (reviewed in ref. 64). A strong version of this hypothesis claims that a percept experienced in one modality automatically stimulates a percept in another modality. Over the course of the first year of life, these associated percepts become weaker as overabundant neural connections between different functional areas of the brain become pruned or inhibited. Magnitudes, under a similar conceptualization, might be related via automatic cross-activation of dimension representations. This could imply that patterns of associations (mappings) between many magnitudes are initially strong in infancy, then get weaker during the first year(s), and then return to a strong state later in development. Generally speaking, the developmental data from cross-modal perception indicate that patterns of associations among magnitudes might not strengthen straightforwardly over development.
Evolutionary History.
A final possibility is that relations among magnitudes derive from their evolutionary history rather than solely from developmental processes that unfold within an individual lifespan. On this view, one quantitative dimension evolved from another, inheriting functional similarities and potentially mutual dependencies in neural and computational operations. For example, many magnitude representations could have emerged from descent with modification of the functional substrates that code for space, resulting in a common psychological and neural code for dimensions such as space, number, time, loudness, brightness, and pitch (3).
Clearly there is a dense set of possibilities for how different quantities could come to be related in the mind and brain. The five hypotheses sketched above address different levels of influence ranging from ontogeny to phylogeny. They also address different levels of psychological functioning ranging from basic representations of psychophysical values to abstract perceptual and conceptual relations. Different levels of analysis will be important for understanding the full taxonomy of numerical cognition in humans. However, although questions remain as to how primitive numerical representations are organized with respect to other types of quantities (e.g., size, time, loudness), it is clear that human children use those primitive numerical representations to learn the process of verbal counting early in development. Verbal counting (discussed earlier) is the first formal cognitive step toward acquiring the uniquely human capacity for complex symbolic math. In the next section we discuss how the “primitive” analog numerical abilities are related to symbolic math in humans.
Origins of Math IQ
A further issue central to understanding the taxonomy of primitive numerical cognition is the extent to which analog numerical abilities bear a neural relationship with full-blown formal mathematics IQ. Researchers have begun to examine, in humans, how formal math intelligence may be modulated by developments in the “primitive” analog numerical system that is shared by nonhuman primates, adult humans, and children. These studies have largely hinged on analyses of individual differences in numerical and mathematical abilities.
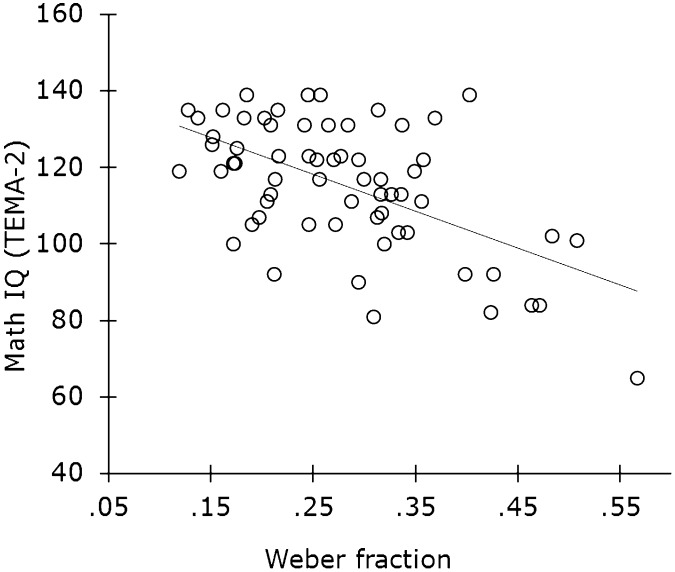
Individual differences in math IQ are predicted by differences in analog numerical sensitivity (18, 65, 66). Studies with children indicate that analog numerical ability correlates with performance on math IQ tests and that formal math ability is more closely correlated with analog numerical abilities than it is with other formal abilities, such as reading. For example, in Fig. 6, adolescents’ analog numerical ability (measured by the Numerical Weber Fraction) correlates with their math IQ from early childhood [measured by the Test of Early Mathematics Ability (TEMA)-2 test score]. This and similar findings indicate that the “primitive” ability to estimate numerical values from sets of objects is related to the development of full-blown math skills. Other studies highlight the role of executive function and working memory in the development of formal mathematical reasoning (67–69). Together, these studies indicate a need to understand the relative contributions of domain-specific and domain-general processes to formal mathematical skill.
Fig. 6.
Childhood math IQ (measured by the TEMA-2) is correlated with the precision of analog numerical discrimination (measured by subjects’ Weber fractions). A higher Weber fraction reflects worse discrimination. Redrawn from Halberda et al. (18).
Behavioral data, like those described earlier, provide evidence of a relationship between the skills required for analog numerical processing and those that are used in formal mathematics by children. Neuroimaging studies of children can provide an independent source of data on whether there is a common foundation for analog numerical abilities and formal math by testing whether a common neural substrate underlies both faculties. As described above, analog quantity judgments recruit regions of the IPS in adult humans, human children, and nonhuman primates. One issue is whether the same neural patterns that are evoked during analog numerical processing are observed when children and adults process the symbolic numbers that are unique to human culture (e.g., numerals, number words). Several studies suggest that they do: regions of the IPS exhibit activity that is greater for numerical symbols compared with control stimuli, and those IPS regions also exhibit the numerical distance and ratio effects in their neural responses (2, 70–73). Research further suggests that the same neural response patterns are elicited for both symbolic and nonsymbolic (analog) numbers in the same subjects (72). Together, these results implicate neural overlap in the substrates underlying symbolic and nonsymbolic (analog) numerical representations in humans.
In humans, a second brain region is often recruited during symbolic numerical tasks: the prefrontal cortex, particularly the inferior frontal gyrus, bordering insular cortex (72–75). Structurally, the prefrontal cortex is thought to be unique in primates compared with other mammals (76). In humans the prefrontal cortex responds during many types of abstract judgments (77), and several studies have noted a unique involvement of the prefrontal cortex in the development of semantic representations, symbols, and rules (see ref. 78 for review). A pattern of greater activation of prefrontal sites in children compared with adults has also been observed for numerical and basic mathematical tasks (73, 74, 79). The role of prefrontal cortex in children’s symbolic numerical processing is related to performance factors such as response time, or “time on task” (75; see also ref. 80), which could reflect the nascent state of children’s abstract, symbolic numerical representations. Studies with nonhuman primates have suggested that they too engage prefrontal cortex during numerical processing (see ref. 78 for review) and that prefrontal regions play a unique role in associating analog numerical values with arbitrary symbols at the level of single neurons in monkeys (81).
Findings that highlight mutual involvement of the IPS and prefrontal cortex in basic numerical tasks have led to the hypothesis that interactions between frontal and parietal regions are important for the development of uniquely human numerical cognition, such as symbolic coding. Specifically, it has been proposed that the IPS computes “primitive” analog numerical representations and the prefrontal cortex facilitates links between those analog numerical computations and symbolic number representations in humans (73, 78). If this hypothesis is correct then network-level neural synchrony between frontal and parietal regions should predict formal mathematics development in humans. That is, individual variability in the strength of correlations between neural responses in frontal and parietal regions, or “functional connectivity,” should be related to individual variability in mathematics performance. We have recently tested this hypothesis and found that number-specific functional connectivity of the fronto-parietal network does predict children’s math IQ test scores (independently of their verbal IQ test scores) (75). The implication is that number-specific changes in the interactions between frontal and parietal regions are related to the development of symbolic, formal math concepts in children. This general conclusion is in line with the hypothesis that interactions between the “primitive” numerical operations of the IPS and the abstract, symbolic operations of frontal cortex give rise to formal mathematics concepts in humans.
Conclusion
The goal of this review has been to examine the origins and organization of numerical abilities ranging from analog quantification to formal arithmetic. The general hypothesis is that the uniquely human ability to perform complex and sophisticated mathematics can be traced back to a simpler computational system that is shared among many animals: the analog numerical system. Humans and nonhuman animals possess a common system for making numerical judgments via analog representations. Throughout development, analog numerical representations interact with the uniquely human ability to represent numerical values symbolically, suggesting a relationship between “primitive” and modern numerical systems in humans. Data from neural analyses of numerical processing support this conclusion and provide independent confirmation that these are in fact related systems. Questions remain regarding the precise taxonomy of the development and organization of numerical information, and its relationship to other domains, such as “space.” However, the general nature of the relationship between “primitive” and modern numbers seems to derive from evolutionary constraints on the structure of numerical concepts in the mind and brain as well as the conceptual and neural foundation that evolution has provided for the development of numerical thinking in humans.
Acknowledgments
I thank Brad Mahon and Vy Vo for comments. Support was received from National Institute of Child Health and Human Development Grant R01HD064636 and the James S. McDonnell Foundation.
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VI: Brain and Behavior,” held January 19–21, 2012, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/evolution_vi.
This article is a PNAS Direct Submission.
References
- 1.Gallistel CR, Gelman R. Preverbal and verbal counting and computation. Cognition. 1992;44:43–74. doi: 10.1016/0010-0277(92)90050-r. [DOI] [PubMed] [Google Scholar]
- 2.Ansari D. Effects of development and enculturation on number representation in the brain. Nat Rev Neurosci. 2008;9:278–291. doi: 10.1038/nrn2334. [DOI] [PubMed] [Google Scholar]
- 3.Bonn C, Cantlon JF. The origins and structure of quantitative concepts. Cognitive Neuropsychology. 2012 doi: 10.1080/02643294.2012.707122. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantlon JF, Platt ML, Brannon EM. Beyond the number domain. Trends Cogn Sci. 2009;13:83–91. doi: 10.1016/j.tics.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lourenco SF, Longo MR. Origins and development of generalized magnitude representation. In: Dehaene S, Brannon E, editors. Space, Time and Number in the Brain: Searching for the Foundations of Mathematical Thought. 1st Ed. London: Academic; 2011. pp. 225–244. [Google Scholar]
- 6.de Hevia MD, Spelke ES. Spontaneous mapping of number and space in adults and young children. Cognition. 2009;110:198–207. doi: 10.1016/j.cognition.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zorzi M, Priftis K, Umiltà C. Brain damage: Neglect disrupts the mental number line. Nature. 2002;417:138–139. doi: 10.1038/417138a. [DOI] [PubMed] [Google Scholar]
- 8.Pinel P, Piazza M, Le Bihan D, Dehaene S. Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron. 2004;41:983–993. doi: 10.1016/s0896-6273(04)00107-2. [DOI] [PubMed] [Google Scholar]
- 9.Walsh V. A theory of magnitude: Common cortical metrics of time, space and quantity. Trends Cogn Sci. 2003;7:483–488. doi: 10.1016/j.tics.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Cohen Kadosh R, Cohen Kadosh K, Henik A. When brightness counts: The neuronal correlate of numerical-luminance interference. Cereb Cortex. 2008;18:337–343. doi: 10.1093/cercor/bhm058. [DOI] [PubMed] [Google Scholar]
- 11.Feigenson L. The equality of quantity. Trends Cogn Sci. 2007;11:185–187. doi: 10.1016/j.tics.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Feigenson L, Dehaene S, Spelke E. Core systems of number. Trends Cogn Sci. 2004;8:307–314. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Gallistel CR. Animal cognition: The representation of space, time and number. Annu Rev Psychol. 1989;40:155–189. doi: 10.1146/annurev.ps.40.020189.001103. [DOI] [PubMed] [Google Scholar]
- 14.Meck WH, Church RM. A mode control model of counting and timing processes. J Exp Psychol Anim Behav Process. 1983;9:320–334. [PubMed] [Google Scholar]
- 15.Gelman R, Gallistel CR. The Child’s Understanding of Number. Cambridge, MA: Harvard Univ Press; 1978. p. xiii. [Google Scholar]
- 16.Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cogn Neuropsychol. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- 17.Nieder A. Counting on neurons: The neurobiology of numerical competence. Nat Rev Neurosci. 2005;6:177–190. doi: 10.1038/nrn1626. [DOI] [PubMed] [Google Scholar]
- 18.Halberda J, Mazzocco MM, Feigenson L. Individual differences in non-verbal number acuity correlate with maths achievement. Nature. 2008;455:665–668. doi: 10.1038/nature07246. [DOI] [PubMed] [Google Scholar]
- 19.Cantlon JF, Brannon EM. Shared system for ordering small and large numbers in monkeys and humans. Psychol Sci. 2006;17:401–406. doi: 10.1111/j.1467-9280.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- 20.Cantlon JF, Brannon EM. How much does number matter to a monkey (Macaca mulatta)? J Exp Psychol Anim Behav Process. 2007;33:32–41. doi: 10.1037/0097-7403.33.1.32. [DOI] [PubMed] [Google Scholar]
- 21.Brannon EM, Terrace HS. Ordering of the numerosities 1 to 9 by monkeys. Science. 1998;282:746–749. doi: 10.1126/science.282.5389.746. [DOI] [PubMed] [Google Scholar]
- 22.Cantlon JF, Brannon EM. Semantic congruity affects numerical judgments similarly in monkeys and humans. Proc Natl Acad Sci USA. 2005;102:16507–16511. doi: 10.1073/pnas.0506463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beran MJ, Beran MM. Chimpanzees remember the results of one-by-one addition of food items to sets over extended time periods. Psychol Sci. 2004;15:94–99. doi: 10.1111/j.0963-7214.2004.01502004.x. [DOI] [PubMed] [Google Scholar]
- 24.Cantlon JF, Brannon EM. Basic math in monkeys and college students. PLoS Biol. 2007;5:e328. doi: 10.1371/journal.pbio.0050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emmerton J. Cook RG, editor. Birds’ judgments of number and quantity. Avian Visual Cognition. 2001. Available at http://www.pigeon.psy.tufts.edu/avc/emmerton/. Accessed April 1, 2012.
- 26.Dehaene S. Varieties of numerical abilities. Cognition. 1992;44:1–42. doi: 10.1016/0010-0277(92)90049-n. [DOI] [PubMed] [Google Scholar]
- 27.Pica P, Lemer C, Izard V, Dehaene S. Exact and approximate arithmetic in an Amazonian indigene group. Science. 2004;306:499–503. doi: 10.1126/science.1102085. [DOI] [PubMed] [Google Scholar]
- 28.Holyoak KJ. Form of analog size information in memory. Cognit Psychol. 1977;9:31–51. [Google Scholar]
- 29.Scarf D, Hayne H, Colombo M. Pigeons on par with primates in numerical competence. Science. 2011;334:1664. doi: 10.1126/science.1213357. [DOI] [PubMed] [Google Scholar]
- 30.Campbell JID. Handbook of Mathematical Cognition. New York: Psychology Press; 2005. [Google Scholar]
- 31.Nieder A, Miller EK. A parieto-frontal network for visual numerical information in the monkey. Proc Natl Acad Sci USA. 2004;101:7457–7462. doi: 10.1073/pnas.0402239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004;44:547–555. doi: 10.1016/j.neuron.2004.10.014. Available at http://www.sciencedirect.com/science/journal/08966273. [DOI] [PubMed] [Google Scholar]
- 33.Cantlon JF, Brannon EM, Carter EJ, Pelphrey KA. Functional imaging of numerical processing in adults and 4-y-old children. PLoS Biol. 2006;4:e125. doi: 10.1371/journal.pbio.0040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wynn K. Children’s understanding of counting. Cognition. 1990;36:155–193. doi: 10.1016/0010-0277(90)90003-3. [DOI] [PubMed] [Google Scholar]
- 35.Gelman R, Butterworth B. Number and language: How are they related? Trends Cogn Sci. 2005;9:6–10. doi: 10.1016/j.tics.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Le Corre M, Carey S. One, two, three, four, nothing more: An investigation of the conceptual sources of the verbal counting principles. Cognition. 2007;105:395–438. doi: 10.1016/j.cognition.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piazza M. Neurocognitive start-up tools for symbolic number representations. Trends Cogn Sci. 2010;14:542–551. doi: 10.1016/j.tics.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Wynn K. Childrens acquisition of the number words and the counting system. Cognit Psychol. 1992;24:220–251. [Google Scholar]
- 39.Lipton JS, Spelke ES. Preschool children’s mapping of number words to nonsymbolic numerosities. Child Dev. 2005;76:978–988. doi: 10.1111/j.1467-8624.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- 40.Gilmore CK, McCarthy SE, Spelke ES. Symbolic arithmetic knowledge without instruction. Nature. 2007;447:589–591. doi: 10.1038/nature05850. [DOI] [PubMed] [Google Scholar]
- 41.Cappelletti M, Butterworth B, Kopelman M. Spared numerical abilities in a case of semantic dementia. Neuropsychologia. 2001;39:1224–1239. doi: 10.1016/s0028-3932(01)00035-5. [DOI] [PubMed] [Google Scholar]
- 42.Cipolotti L, Butterworth B, Denes G. A specific deficit for numbers in a case of dense acalculia. Brain. 1991;114:2619–2637. doi: 10.1093/brain/114.6.2619. [DOI] [PubMed] [Google Scholar]
- 43.Warrington EK. The fractionation of arithmetical skills: A single case study. Q J Exp Psychol A. 1982;34:31–51. doi: 10.1080/14640748208400856. [DOI] [PubMed] [Google Scholar]
- 44.Molko N, et al. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40:847–858. doi: 10.1016/s0896-6273(03)00670-6. [DOI] [PubMed] [Google Scholar]
- 45.Price GR, Holloway I, Räsänen P, Vesterinen M, Ansari D. Impaired parietal magnitude processing in developmental dyscalculia. Curr Biol. 2007;17:R1042–R1043. doi: 10.1016/j.cub.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Dehaene S, Izard V, Spelke E, Pica P. Log or linear? Distinct intuitions of the number scale in Western and Amazonian indigene cultures. Science. 2008;320:1217–1220. doi: 10.1126/science.1156540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Hevia MD, Spelke ES. Number-space mapping in human infants. Psychol Sci. 2010;21:653–660. doi: 10.1177/0956797610366091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantlon JF, Cordes S, Libertus ME, Brannon EM. Comment on “Log or linear? Distinct intuitions of the number scale in Western and Amazonian indigene cultures”. Science. 2009;323:38. doi: 10.1126/science.1164878. author reply 38. Available at http://www.sciencemag.org/content/323/5910/38.2.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rusconi E, Kwan B, Giordano BL, Umiltà C, Butterworth B. Spatial representation of pitch height: The SMARC effect. Cognition. 2006;99:113–129. doi: 10.1016/j.cognition.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Gallistel CR, Gelman II. Non-verbal numerical cognition: From reals to integers. Trends Cogn Sci. 2000;4:59–65. doi: 10.1016/s1364-6613(99)01424-2. [DOI] [PubMed] [Google Scholar]
- 51.Brannon EM, Lutz D, Cordes S. The development of area discrimination and its implications for number representation in infancy. Dev Sci. 2006;9:F59–F64. doi: 10.1111/j.1467-7687.2006.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.vanMarle K, Wynn K. Six-month-old infants use analog magnitudes to represent duration. Dev Sci. 2006;9:F41–F49. doi: 10.1111/j.1467-7687.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 53.Holloway ID, Ansari D. Domain-specific and domain-general changes in children’s development of number comparison. Dev Sci. 2008;11:644–649. doi: 10.1111/j.1467-7687.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 54.Volet SD, Clement A, Fayol M. Time, number and length: Similarities and differences in discrimination in adults and children. Q J Exp Psychol. 2008;61:1827–1846. doi: 10.1080/17470210701743643. [DOI] [PubMed] [Google Scholar]
- 55.Gentner D, Medina J. Similarity and the development of rules. Cognition. 1998;65:263–297. doi: 10.1016/s0010-0277(98)00002-x. [DOI] [PubMed] [Google Scholar]
- 56.Mondloch CJ, Maurer D. Do small white balls squeak? Pitch-object correspondences in young children. Cogn Affect Behav Neurosci. 2004;4:133–136. doi: 10.3758/cabn.4.2.133. [DOI] [PubMed] [Google Scholar]
- 57.Smith LB, Sera MD. A developmental analysis of the polar structure of dimensions. Cognit Psychol. 1992;24:99–142. doi: 10.1016/0010-0285(92)90004-l. [DOI] [PubMed] [Google Scholar]
- 58.Walker P, et al. Preverbal infants’ sensitivity to synaesthetic cross-modality correspondences. Psychol Sci. 2010;21:21–25. doi: 10.1177/0956797609354734. [DOI] [PubMed] [Google Scholar]
- 59.Lourenco SF, Longo MR. General magnitude representation in human infants. Psychol Sci. 2010;21:873–881. doi: 10.1177/0956797610370158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srinivasan M, Carey S. The long and the short of it: On the nature and origin of functional overlap between representations of space and time. Cognition. 2010;116:217–241. doi: 10.1016/j.cognition.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tudusciuc O, Nieder A. Neuronal population coding of continuous and discrete quantity in the primate posterior parietal cortex. Proc Natl Acad Sci USA. 2007;104:14513–14518. doi: 10.1073/pnas.0705495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piaget J. The Child’s Conception of Number. London: Routledge & Paul; 1952. p. ix. [Google Scholar]
- 63.Gibson E. Principles of Perceptual Learning and Development. New York: Appleton-Century-Crofts; 1969. p. viii. [Google Scholar]
- 64.Spector F, Maurer D. Synesthesia: A new approach to understanding the development of perception. Dev Psychol. 2009;45:175–189. doi: 10.1037/a0014171. [DOI] [PubMed] [Google Scholar]
- 65.Bugden S, Ansari D. Individual differences in children’s mathematical competence are related to the intentional but not automatic processing of Arabic numerals. Cognition. 2011;118:32–44. doi: 10.1016/j.cognition.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Holloway ID, Ansari D. Mapping numerical magnitudes onto symbols: The numerical distance effect and individual differences in children’s mathematics achievement. J Exp Child Psychol. 2009;103:17–29. doi: 10.1016/j.jecp.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Bull R, Scerif G. Executive functioning as a predictor of children’s mathematics ability: Inhibition, switching, and working memory. Dev Neuropsychol. 2001;19:273–293. doi: 10.1207/S15326942DN1903_3. [DOI] [PubMed] [Google Scholar]
- 68.Mazzocco MM, Kover ST. A longitudinal assessment of executive function skills and their association with math performance. Child Neuropsychol. 2007;13:18–45. doi: 10.1080/09297040600611346. [DOI] [PubMed] [Google Scholar]
- 69.Mazzocco MMM, Singh Bhatia N, Lesniak-Karpiak K. Visuospatial skills and their association with math performance in girls with fragile X or Turner syndrome. Child Neuropsychol. 2006;12:87–110. doi: 10.1080/09297040500266951. [DOI] [PubMed] [Google Scholar]
- 70.Holloway ID, Ansari D. Developmental specialization in the right intraparietal sulcus for the abstract representation of numerical magnitude. J Cogn Neurosci. 2010;22:2627–2637. doi: 10.1162/jocn.2009.21399. [DOI] [PubMed] [Google Scholar]
- 71.Cohen Kadosh R, Cohen Kadosh K, Kaas A, Henik A, Goebel R. Notation-dependent and -independent representations of numbers in the parietal lobes. Neuron. 2007;53:307–314. doi: 10.1016/j.neuron.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 72.Piazza M, Pinel P, Le Bihan D, Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53:293–305. doi: 10.1016/j.neuron.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 73.Cantlon JF, et al. The neural development of an abstract concept of number. J Cogn Neurosci. 2009;21:2217–2229. doi: 10.1162/jocn.2008.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ansari D, Garcia N, Lucas E, Hamon K, Dhital B. Neural correlates of symbolic number processing in children and adults. Neuroreport. 2005;16:1769–1773. doi: 10.1097/01.wnr.0000183905.23396.f1. [DOI] [PubMed] [Google Scholar]
- 75.Emerson RW, Cantlon JF. Early math achievement and functional connectivity in the fronto-parietal network. Dev Cogn Neurosci. 2012;2:S139–S151. doi: 10.1016/j.dcn.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Preuss TM. Evolutionary specializations of primate brain systems. In: Ravosa MJ, Dagosto M, editors. Primate Origins: Adaptations and Evolution. New York: Springer; 2007. pp. 625–675. [Google Scholar]
- 77.Miller EK, Freedman DJ, Wallis JD. The prefrontal cortex: Categories, concepts and cognition. Philos Trans R Soc Lond B Biol Sci. 2002;357:1123–1136. doi: 10.1098/rstb.2002.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nieder A. Prefrontal cortex and the evolution of symbolic reference. Curr Opin Neurobiol. 2009;19:99–108. doi: 10.1016/j.conb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 79.Rivera SM, Reiss AL, Eckert MA, Menon V. Developmental changes in mental arithmetic: Evidence for increased functional specialization in the left inferior parietal cortex. Cereb Cortex. 2005;15:1779–1790. doi: 10.1093/cercor/bhi055. [DOI] [PubMed] [Google Scholar]
- 80.Schlaggar BL, et al. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- 81.Diester I, Nieder A. Semantic associations between signs and numerical categories in the prefrontal cortex. PLoS Biol. 2007;5:e294. doi: 10.1371/journal.pbio.0050294. [DOI] [PMC free article] [PubMed] [Google Scholar]