Abstract
The specialized regions of neocortex of mammals, called areas, have been divided into smaller functional units called minicolumns, columns, modules, and domains. Here we describe some of these functional subdivisions of areas in primates and suggest when they emerged in mammalian evolution. We distinguish several types of these smaller subdivisions. Minicolumns, vertical arrays of neurons that are more densely interconnected with each other than with laterally neighboring neurons, are present in all cortical areas. Classic columns are defined by a repeating pattern of two or more types of cortex distinguished by having different inputs and neurons with different response properties. Sensory stimuli that continuously vary along a stimulus dimension may activate groups of neurons that vary continuously in location, producing “columns” without specific boundaries. Other groups or columns of cortical neurons are separated by narrow septa of fibers that reflect discontinuities in the receptor sheet. Larger regions of posterior parietal cortex and frontal motor cortex are parts of networks devoted to producing different sequences of movements. We distinguish these larger functionally distinct regions as domains. Columns of several types have evolved independently a number of times. Some of the columns found in primates likely emerged with the first primates, whereas others likely were present in earlier ancestors. The sizes and shapes of columns seem to depend on the balance of neuron activation patterns and molecular signals during development.
Keywords: barrels, hypercolumns, visual cortex
Neocortex is an important part of the brain that varies in size from a small cap on the rest of the forebrain (1) to approximately 80% of the brain in humans (2). The varied functions of neocortex depend on the cortical areas, the so-called “organs of the brain” (3) that are specialized for processing different inputs and providing different outputs. Cortical areas can be hard to define and identify, and their exact number in any species is uncertain. However, it is clear that the number of cortical areas varies across extant taxa, from approximately 20–30 or so to perhaps more than 200 in humans (4). Because the first mammals had little neocortex and likely few cortical areas, interest in the evolution of neocortex across the great radiation of mammals has largely focused on the issue of modifying and adding cortical areas. Some of the cortical areas proposed for primates are shown in Fig. 1. However, areas are often composed of smaller subdivisions, the cortical columns or modules, and these subdivisions within areas modify and expand the functions of areas. Thus, an understanding of how different types of neocortex evolved depends not only on determining the numbers and types of cortical areas that exist but also on the modifications of the internal organization of areas that occur in the various lines of evolution, including modifications in columnar organization. Here we review the types of columnar subdivisions of cortical areas that have been proposed (5–8) and then consider how and when such modules might have evolved. The phyletic distributions of the types of columns in extant mammals allow one to infer when such columns evolved (9, 10). Primates, rodents, tree shrews, and lagomorphs are all placed within the superorder Euarchontoglires. Thus, we are especially interested in how types of columns are distributed within the primate radiation, but also whether they are present in the closest relatives of primates. Because the shapes of columns are not always columnar, they also are called modules.
Fig. 1.
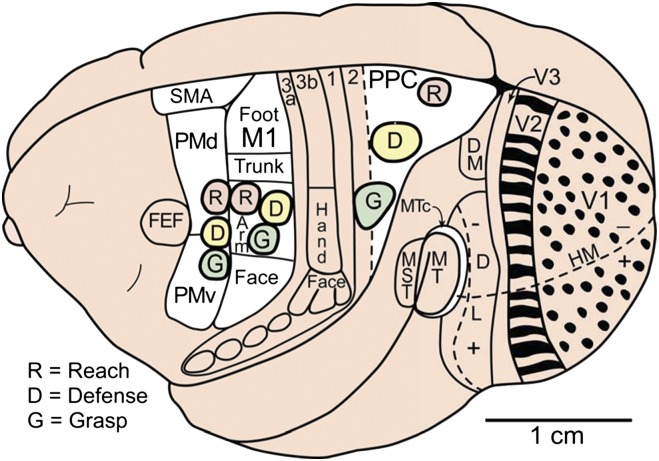
Some of the proposed cortical areas of primates shown on a dorsolateral view of the left cerebral hemisphere. Modular subdivisions of some of these areas are discussed in the text. Visual areas include the first, second, and third area (V1, V2, V3), dorsomedial (DM or V3a) and dorsolateral visual areas (DL or V4), the middle temporal area (MT), the MT crescent (MTc), and the medial superior temporal area (MST). The representation of the zero horizontal meridian (HM) divides the representation of the upper (+) and lower (-) visual hemifields. Motor areas include primary motor cortex (M1), ventral (PMv), and dorsal (PMd) premotor cortex, the supplementary motor area (SMA), and the frontal eye field (FEF). Somatosensory areas include the four areas of anterior parietal cortex (3a, 3b, 1, 2), with the region representing tactile inputs from the hand indicated in area 3b (S1). Modular subdivisions in V1 (dots) and V2 (bands) are shown in black (see text). Ovals mark the locations of proposed reach, defense, and grasp domains in motor and posterior parietal cortex (PPC). Based on Gharbawie et al. (75).
Minicolumns
One of the defining features of neocortex is that it consists of layers and various sublayers of neurons specialized for different steps in processing; neurons in radial (vertical) arrays across the layers are more densely interconnected than neurons along the layers (11–13). As a result, neurons in narrow vertical arrays share many response properties, especially the location of the receptor fields of neurons on the sensory receptor surface. This arrangement has great functional importance, and it is likely responsible for the impressive flexibility and powers of neocortex. Developmentally, minicolumns reflect the radial migration of clones of excitatory neurons from progenitors in the ventricular and subventricular zones (14), as radially arranged sister neurons preferentially develop synapses with each other (15). These vertical arrays of interconnected neurons across the cortical layers have been called minicolumns (16, 17). Minicolumns are sometimes visible as vertical arrays of neurons separated somewhat by neuropil (18, 19). Minicolumns are thought to be 30–50 μm in diameter, although functional boundaries between them are not likely to be sharp owing to the spread of apical dendrites of pyramidal cells and the extents of axon arbors of cortical neurons and subcortical activating inputs. Because minicolumns are clearly visible in a number of cortical areas, and across mammalian species, including monotremes, they may have originated when the ancestors of all extant mammals with a cortex of six layers emerged.
Classic Columns
Mountcastle (1957) (16) introduced the concept of cortical columns after reporting that recordings along microelectrodee trajectories tangential to the surface of somatosensory cortex encountered short sequences of neurons that responded either to light touch on the skin (superficial skin receptors) or touch with pressure (deep receptors). This grouping of cortical neurons according to how they respond to sensory stimuli led to the concept of a patchwork of alternating columns of neurons that extend across all cortical layers, with each type of column activated by a different somatosensory input. The subsequent evidence for such alternating patches of neurons activated by either deep or superficial receptors of the skin and deeper tissue has been limited, and they do not seem to exist in area 3b (S1 proper) of somatosensory cortex of monkeys. Instead, there is evidence for a modular arrangement of groups of neurons in layer 4 that responds to activation by inputs relayed from either slowly adapting or rapidly adapting cutaneous receptors of the skin (20, 21). There is also evidence for at least a partial segregation of territories activated by slowly adapting and rapidly adapting receptors in area 1 of somatosensory cortex of monkeys (22). However, given these limited observations, we can say little about the phyletic distribution of slowly adapting and rapidly adapting cortical columns, or their evolution, even in primates.
More can be said about the blob and interblob surround organization of primary visual cortex (V1) in primates (Fig. 2). All primates seem to have a pattern of cytochrome oxidase (CO)-rich blobs (reflecting high metabolic activity) within interblob surrounds of lower CO levels (23–25). Neurons in the blobs respond to color, are less selective for stimulus orientation, and have higher firing rates than neurons between the blobs (7, 26–29). However, blobs and interblob regions are found not only in primates with trichromatic or dichromatic color systems but also in nocturnal primates with only one functional type of cone in the retina (30). The blobs and interblobs are also distinguished by different patterns of inputs from the visual thalamus, intrinsic connections, and connections with other visual areas (12, 26). In macaque monkeys, most of these connections are well developed in newborns (31, 32). The segregation of groups of neurons by differences in response characteristics that are mediated by differences in activating inputs fits the classic definition of cortical columns, although the blobs and interblobs do not occupy equal territories, and the interblob territory is continuous. The blob and surround pattern evolved in the immediate ancestors of primates, or in archaic primates, given that none of the close relatives of primates, tree shrews, rodents, and lagomorphs, have blobs in V1.
Fig. 2.
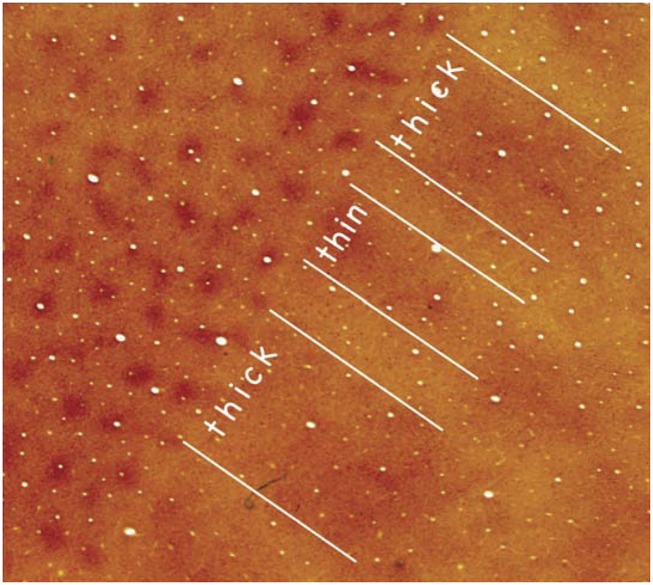
Anatomically defined columns in visual cortex of primates. Sections of primary visual cortex (V1) and the adjoining second visual area (V2) of a macaque monkey have been cut parallel to the brain surface and processed for CO, a marker of neurons with high metabolic requirements. The brain sections provide a “surface view” of parts of V1 and V2. In V1, there is a pattern of CO-rich “blobs” (also called “puffs” or “patches”) surrounded by cortex that expresses less CO, the interblob territory. In V2 an alternating pattern of CO-dark bands, separated by CO-light bands, cross the width of V2. The CO-dark bands are of two types, thick and thin. Thus, there are three types of bank-like structures in V2 that can be anatomically distinguished. Because the CO blobs and interblobs, as well as the CO-dense thick, thin, and interbands have neurons that differ in response properties, they can be considered classic columns. A pattern of CO-dense and CO-light bands is also present in the third cortical visual area, V3, along the outer border (on the right) of V2. Compare with Fig. 1.
Classic columns are also found in the second visual area, V2, of most primates, where V2 is characterized by a repeating series of CO-dense thick stripes and CO-dense thin stripes separated by CO-pale interstripes. These band-like stripes cross the narrow width of V2, and they seem to exist in all anthropoid primates (33). The three types of stripes differ in anatomical connections and have neurons with different response properties. The stripes and differences in connections are apparent in newborn macaques (31, 32). Although the CO-dense stripes are not consistently distinguishable as thick or thin, they can be identified by functional differences, with neurons in the thick stripes sensitive to binocular disparities and stimulus orientation, the neurons in the thin stripes sensitive to luminance and color, and neurons in pale stripes sensitive to stimulus orientation (27, 34–37). The thick stripes project to visual area MT, whereas the other bands project to DL (V4). In prosimian primates, CO stripes in V2 are only weakly apparent, and such stripes are not present in V2 of tree shrews and rodents (33). Thus, aspects of the stripe pattern may have evolved in early primates, whereas such stripes became fully developed as anthropoid primates emerged.
Although the V1 blob and interblob regions, as well as the V2 stripes, do not look like cylindrical pillars, they otherwise conform to the expectations of classic cortical columns. Other such classic columns undoubtedly exist (8), but they largely remain to be explored. One such example is in the MT crescent, MTc, a visual area that forms a belt around the middle temporal visual area, MT (Fig. 1). This poorly understood visual area is composed of a series of CO-dense puffs in a single row, like beads on a string in a belt of CO-pale tissue (38). The significance of these puffs and surrounds in MTc, which have different connections with other visual areas, remains to be determined.
Unbounded Columns That Represent Sectors of a Continuous Stimulus Dimension
Several cortical areas have repeating representations of stimulus orientations for different portions of the visual field (39). Most notably, primary visual cortex of primates, carnivores, and tree shrews have repeating “pinwheel” patterns of cortex, in which stimulus orientation is systematically represented from vertical to horizontal lines and edges and back again (40–42). Groups of neurons most sensitive to one stimulus orientation or another can be selectively activated, the activity pattern optically imaged, and regions of cortex sensitive to different orientations color coded to produce colorful illustrations of arrays of orientation “columns.” These “columns” differ from classic columns in that they have no borders because the orientations of stimuli change continuously without disruption. Thus, the illustrated “borders” between orientation columns are arbitrary. In addition, all “orientation columns” are selective for the same stimulus features, and thus these columns are not of the classic type, which are segregated by different classes of activating inputs. However, each entire array of orientation selective neurons, the pinwheel for a given location in the visual field, can be considered as a larger domain or hypercolumn (43, 44). Orientation hypercolumns are widespread in visual cortex of primates: they also have been identified in V2 stripes, V3, V4 (DL), and MT (34, 45, 46). Neurons in orientation-selective hypercolumns may be divided for each orientation column into halves, preferring one or the other direction of motion perpendicular to the preferred orientation (45). The grouping of neurons by their preferences for stimulus orientation seems to be a trait that emerged first in V1 in the common ancestors of tree shrews and primates, because tree shrews also have orientation hypercolumns. However, the more distant relatives, rodents and rabbits, have orientation-selective neurons in visual cortex but not orientation-selective columns (40). Carnivores have independently evolved orientation hypercolumns in V1. Possibly, the presence of orientation hypercolumns in V1 is a prerequisite for the evolution of such hypercolumns in other visual areas, as found in primates. Orientation hypercolumns have not been reported for areas of extrastriate cortex of tree shrews. Thus, the extrastriate hypercolumns for stimulus orientation may have emerged with the first primates.
Other proposed modules of V2 in primates include subregions of thin stripes selective for different hues (47, 48). These hue-selective subregions are not classic columns because they are not separated by columns that are most sensitive to another stimulus feature, and they have arbitrary boundaries.
There is only limited evidence for the existence of classic columns in auditory cortex. All mammals seem to have primary cortical auditory areas that represent the receptors of the cochlea in a linear manner so that neurons are arranged in one dimension across a cortical area from being most sensitive to low frequency sounds on one end, to high frequency sounds on the other (49). Thus, there are no modular divisions based on sound frequency, although isofrequency bands with arbitrary borders have been described. However, bands of primary auditory cortex where neurons that are excited from both ears (EE bands) alternate with bands of cortex with neurons that are excited by the contralateral ear and inhibited by the ipsilateral ear (EI bands) have been reported for cats (50). The EE and EI bands extend across the isofrequency contours. Because EE and EI bands have neurons of differing functional properties, they qualify as classic columns (although shaped like bands). Such bands have not been identified in auditory cortex of primates.
Modules Representing Separated Parts of Sensory Surfaces
Another type of module, one that also would not qualify as a classic column, concerns separations of groups of neurons in somatotopic maps of the body surface, or retinotopic maps of the two eyes, in areas of cortex. The most well-known example is the rows and columns of “barrels” in primary somatosensory cortex of rats and mice, where a barrel-like structure represents each of the large sensory whiskers on the side of the face (51). The digits and pads of the feet also relate to separated groups of neurons (52).
The many studies of the “barrel field” of mice and rats have revealed that differences in neural activity are important in the formation of barrels, such that the number of barrels varies with the number of facial whiskers. Molecular factors also alter the formation of barrels, as revealed in mutant mice (53). Such segregations of cortical neurons by body part are found in primary somatosensory cortex of many species, but are perhaps most apparent in the somatosensory cortex of the star-nosed mole, where the highly innervated tactile rays of the nose are each separately represented in three areas of somatosensory cortex (54). In primary somatosensory cortex of New and Old World monkeys (55, 56), and possibly other anthropoid primates, the representations of the digits are separated from each other by narrow cell-poor septa, with a more conspicuous septum separating the representation of digit 1 (thumb) from that of the face. Such separated representations of digits in area 3b of primates are variable and have not been described in prosimian primates. Septa that separate representations of digits are more apparent in macaque monkeys than in New World owl monkeys and squirrel monkeys.
It could be argued that the narrow septal regions that separate the cortical barrels, bands, and other modules related to body parts do have neurons that differ in connections, such as having corpus callosum connections, and thus there is an alteration of functional types of columns in the classical sense. However, the septa are cell poor, narrow regions that are primarily there to reflect disruptions of the receptor sheet. Yet, these narrow septa may be opportunistically occupied by late-developing sources of input. Because the septa that form module borders reflect junctions in neuron activity patterns during sensory activation, these septa are most apparent early in sensory hierarchies where short response latencies to sensory stimuli are maintained.
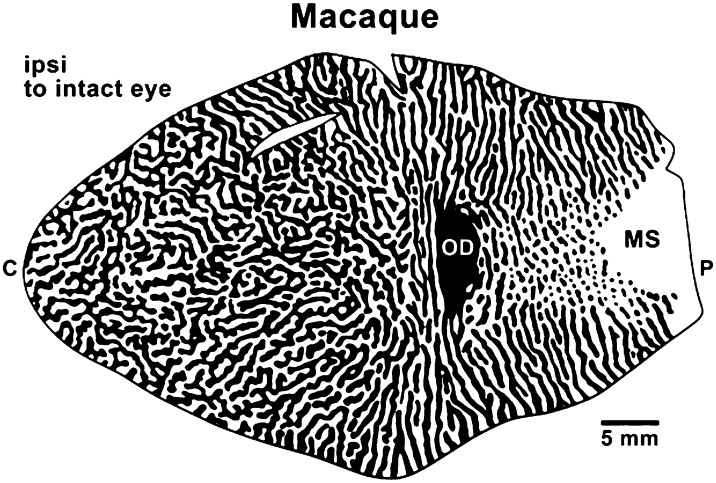
The retina of each eye is a continuous sensory surface, except for the nerve head and a narrow septum corresponding to the nerve head, which disrupts layers of the lateral geniculate nucleus that receive projections from the contralateral eye (57). In cortex, the ocular dominance “columns” in primary visual cortex of primates fall into the category of modules based on disruptions of the sensory surface, because the retina of the two eyes have independent activity patterns prenatally. Thus, the afferents from the hemiretina of each eye terminate in separate layers in the lateral geniculate nucleus of the visual thalamus, and then these layers project in retinotopically matched patterns to primary visual cortex to either congruently overlap or to separate locally in variable patchy-to-banding patterns in layer IV while maintaining some level of retinotopy, depending on species (58, 59). Ocular dominance columns, first revealed in microelectrode recordings (60), and axon termination patterns from layers of the lateral geniculate nucleus (61), can also be demonstrated by differences in activity levels after blocking activity from one eye (25, 62). The segregation of eye-related afferents is very weak in some primates, such as nocturnal prosimian galagos and owl monkeys (63, 64), and highly variable patterns exist in New World monkeys, even across individuals within a species (59). Ocular dominance patterns may reflect a high degree of segregation of thalamic afferents in layer 4 of primary visual cortex, as in Old World monkeys (Fig. 3), apes, and humans, or reflect such a low level of separation that they are anatomically cryptic and only revealed by relative differences in neural responses to each eye as revealed in optical imaging experiments (63) or the expression pattern of activity-dependent genes (65). Ocular dominance “columns” are absent in the closest relatives of primates, tree shrews, rodents, and rabbits, and thus are a feature of visual cortex that evolved in early primates but became more pronounced in Old World monkeys, apes, and humans. Obvious ocular dominance columns have evolved independently in carnivores (66), and they likely exist in other taxa.
Fig. 3.
Ocular dominance columns (bands) in a flat surface view of primary visual cortex (V1) of an Old World macaque monkey as reflected by distribution of terminations of lateral geniculate axons related to each eye in cortical layer 4. Regions of black receive inputs from the ipsilateral eye, including the region of the optic disk of the retina that produces a gap in the projection of the hemiretina of the contralateral eye (OD in cortex). The monocular segment (MS) of V1 is activated by the monocular segment of the contralateral visual hemifield that is seen only by the contralateral eye. Foveal and central vision is represented to the left, and the extreme of peripheral vision is represented to the right. The ocular dominance bands break up into a dot and surround pattern in the part of V1 that represents peripheral vision as the inputs from the contralateral eye (white) become proportionately greater, and form the larger surrounds. Modified from Florence and Kaas (58).
Domains: Larger Functional Divisions of Cortical Areas
Primary motor cortex and dorsal and ventral premotor areas are widely recognized as valid cortical areas, and each of these areas has a somatotopic representation of small movements of body parts that are revealed by brief trains of near-threshold pulses of electrical current. However, cortical motor areas representing major body parts, such as the forelimb, have a locally fractured somatotopy so that different movement zones, roughly the size of minicolumns, are mixed and repeated (Fig. 4). Thus, the forelimb region mixes zones for digit, wrist, elbow, and shoulder movements in a puzzling arrangement (67–69) that is unlike that of primary sensory representations, which closely reflect the organization of the sensory sheet. However, the somatosensory representation of tactile projections to the cerebellar cortex forms a fractured representation of the body surface (70), much like the representations in motor cortex. The explanation for these adjoining patches of cerebellar cortex devoted to various nonadjacent body parts was that neurons in groups of such patches could interact to form “action-involved structures” for directing movement patterns.
Fig. 4.
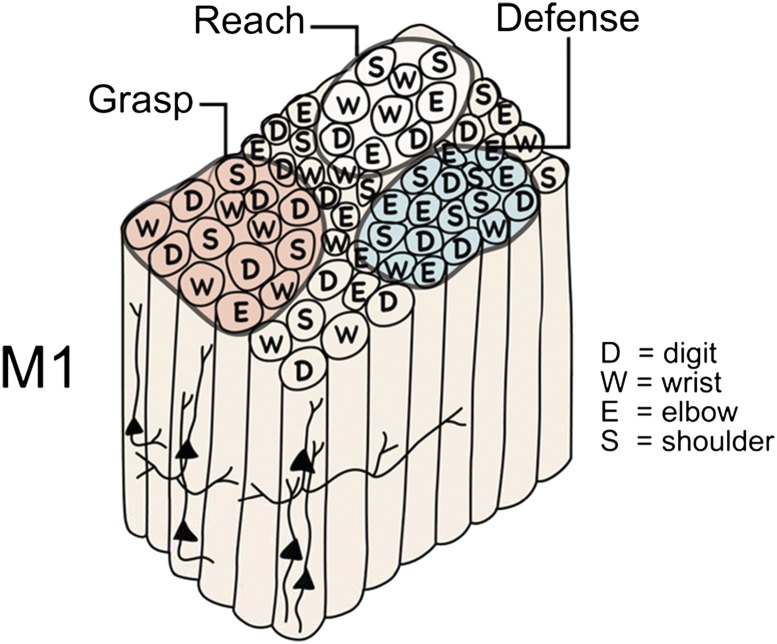
Proposed functional organization of the hand–forearm segment of primary motor cortex (M1) in monkeys and other primates. Although M1 has an overall somatotopy, the local somatotopy is fractured to form a mosaic of radial rows of neurons that evoke small, specific movements when electrically stimulated with brief trains of electrical pulses at threshold levels of current. Thus, neuron arrays or minicolumns for digit movement may adjoin those for wrist, elbow, or shoulder movements. Subsets of these minicolumns seem to be grouped to function in the production of more complex, ethologically relevant movement sequences, such as grasping, reaching, or defending the head against a blow. We refer to these larger divisions of motor, premotor, and posterior parietal cortex (Fig. 1) as domains (75).
It has long been known that longer trains of electrical pulses at higher current levels evoke more complex movement sequences from motor cortex than do short trains at threshold levels (71). More recently, Graziano et al. (72) have used longer (0.5 s) trains of electrical pulses to define different regions or domains (Fig. 1) in motor cortex where different ethologically relevant movement can be evoked (climbing, reaching, grasping, defense of the head, hand-to-mouth). Matching movement domains have been identified in posterior parietal cortex (73–76). In primary motor cortex, several different domains for functionally distinct movement patterns are found in separate parts of the forelimb representation, perhaps offering some explanation for the mosaic of minicolumns for different but related small movements and muscle twitches that are revealed by short trains of pulses at threshold levels of stimulating current (Fig. 4). Thus, circuits within a domain may evoke sequences of movements involving the different body parts represented within the domain.
Functionally matched domains for at least some of the complex movement patterns of primary motor cortex also exist in premotor cortex and in posterior parietal cortex. The domains in posterior parietal cortex may be parts of larger cortical areas. The domains in frontal and posterior parietal cortex have similar spatial arrangements in prosimian galagos, two species of New World monkeys, and Old World macaque monkeys, and there is indirect evidence for them in humans (77). Thus, they likely exist in all primates. Such domains for complex movements may also exist in motor cortex of the relatives of primates, tree shrews and rodents, where M1 also has a fractured somatotopy (78, 79). However, posterior parietal cortex is no more than a narrow strip of cortex in tree shrews and rodents and is unlikely to contain a series of primate-like domains.
Other areas of cortex may also have larger functionally distinct regions within cortical areas. For example, some of the face-selective and object-selective regions of temporal cortex in macaque monkeys and humans resemble domains (80–83). Likewise, the large visual area termed V4 or DL has been divided into large regions or domains of neurons that are either color selective or orientation selective (46), although these large regions might also be considered separate cortical areas (84, 85).
How Do Columns and Modules Emerge in Development?
A number of factors likely contribute to the functional organization of cortex, but at the modular level, activity-dependent selection of coactive afferents together with cellular signals that are position dependent probably are two of the most important variables (53, 86, 87). There is considerable evidence to support this conclusion, but some of the most impressive evidence comes from studies that created three-eyed frogs (88, 89). In frogs, each optic tectum normally receives inputs from only the contralateral eye, but when a third eye is added experimentally to one side of the head during embryonic development, both eyes of that side compete for territory in the same contralateral optic tectum. The projections from each of these eyes respond to molecular signals that tend to produce the same retinotopic pattern in the optic tectum, but local groups of tectal neurons favor inputs from one eye or the other. The result is that the afferents from the two eyes form alternating bands or stripes that resemble the ocular dominance bands in cats and anthropoid primates. The borders between these bands in the optic tectum and visual cortex correspond to locations where abrupt differences in activity patterns occur, and they do not develop or they degrade when activity is blocked (90). Obviously, the ability to form ocular dominance bands did not evolve via natural selection in the optic tectum of frogs for some future function. Instead, the developmental factors that produced these columns were present for other reasons that are not clear but apparently are widely important in nervous system development (89). The capacity for module formation seems to be inherent in all cortical tissue, as well as in other tissue such as the optic tectum or superior colliculus, where inputs of different activation patterns compete for location with an overall global map. Thus, ocular dominance bands and other configurations, as well as orientation modules and other types of columns, including those based on discontinuities of the receptor sheet, have emerged independently in several lines of mammalian evolution. For some of these types of modules, asking what they do (59) may be the wrong question. Instead, we might ask, what else is achieved in neural tissue by the mix of activity-dependent and position-dependent factors that select and group synaptic contacts when these factors coexist at particular developmental times? Purves et al. (6) have suggested that some of the columns that have been described in cortex are “by-products” of synaptic development. If so, what is the product?
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VI: Brain and Behavior,” held January 19–21, 2012, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/evolution_vi.
This article is a PNAS Direct Submission.
References
- 1.Kaas JH. Reconstructing the organization of the neocortex of the first mammals and subsequent modifications. In: Kaas JH, Krubitzer LA, editors. Evolution of Nervous Systems. London: Elsevier; 2007. pp. 27–48. [Google Scholar]
- 2.Azevedo FAC, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 3.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrhinde. Leipzig: Barth; 1909. [Google Scholar]
- 4.Kaas JH, Preuss TM. Human brain evolution. In: Squire LR, editor. Fundamental Neuroscience. San Diego: Academic Press; 2008. pp. 1027–1035. [Google Scholar]
- 5.da Costa NM, Martin KAC. Whose cortical columns would that be? Front Neuroanat. 2010;4:1–10. doi: 10.3389/fnana.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purves D, Riddle DR, LaMantia AS. Iterated patterns of brain circuitry (or how the cortex gets its spots) Trends Neurosci. 1992;15:362–368. doi: 10.1016/0166-2236(92)90180-g. [DOI] [PubMed] [Google Scholar]
- 7.Hendrickson AE. Dots, stripes and columns in monkey visual cortex. Trends Neurosci. 1985;8:406–410. [Google Scholar]
- 8.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- 9.Hennig W. Phylogenetic Systematics. Urbana, IL: Univ of Illinois Press; 1966. [Google Scholar]
- 10.Striedter GF. Principles of Brain Evolution. Sunderland, MA: Sinauer; 2005. [Google Scholar]
- 11.Nieuwenhuys R. The neocortex. An overview of its evolutionary development, structural organization and synaptology. Anat Embryol (Berl) 1994;190:307–337. doi: 10.1007/BF00187291. [DOI] [PubMed] [Google Scholar]
- 12.Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters A, Rockland K, editors. Cerebral Cortex. New York: Plenum Press; 1994. pp. 201–259. [Google Scholar]
- 13.Kaas JH. Cortical circuits: Consistency and variability across cortical areas and species. In: Philips WA, Singer W, editors. Dynamic Coordination in the Brain: From Neurons to Mind. 2010 Strügmann Forum Report. Cambridge, MA: MIT Press; 2010. pp. 25–34. [Google Scholar]
- 14.Rakic P. Radial versus tangential migration of neuronal clones in the developing cerebral cortex. Proc Natl Acad Sci USA. 1995;92:11323–11327. doi: 10.1073/pnas.92.25.11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458:501–504. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mountcastle VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol. 1957;20:408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- 17.Mountcastle VB. Introduction. Computation in cortical columns. Cereb Cortex. 2003;13:2–4. doi: 10.1093/cercor/13.1.2. [DOI] [PubMed] [Google Scholar]
- 18.Buxhoeveden DP, Casanova MF. The minicolumn hypothesis in neuroscience. Brain. 2002;125:935–951. doi: 10.1093/brain/awf110. [DOI] [PubMed] [Google Scholar]
- 19.DeFelipe J, Alonso-Nanclares L, Arellano JI. Microstructure of the neocortex: Comparative aspects. J Neurocytol. 2002;31:299–316. doi: 10.1023/a:1024130211265. [DOI] [PubMed] [Google Scholar]
- 20.Sur M, Wall JT, Kaas JH. Modular distribution of neurons with slowly adapting and rapidly adapting responses in area 3b of somatosensory cortex in monkeys. J Neurophysiol. 1984;51:724–744. doi: 10.1152/jn.1984.51.4.724. [DOI] [PubMed] [Google Scholar]
- 21.Sur M, Wall JT, Kaas JH. Modular segregation of functional cell classes within the postcentral somatosensory cortex of monkeys. Science. 1981;212:1059–1061. doi: 10.1126/science.7233199. [DOI] [PubMed] [Google Scholar]
- 22.Friedman RM, Chen LM, Roe AW. Modality maps within primate somatosensory cortex. Proc Natl Acad Sci USA. 2004;101:12724–12729. doi: 10.1073/pnas.0404884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preuss TM, Kaas JH. Cytochrome oxidase ‘blobs’ and other characteristics of primary visual cortex in a lemuroid primate, Cheirogaleus medius. Brain Behav Evol. 1996;47:103–112. doi: 10.1159/000113231. [DOI] [PubMed] [Google Scholar]
- 24.Horton JC, Hedley-Whyte ET. Mapping of cytochrome oxidase patches and ocular dominance columns in human visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984;304:255–272. doi: 10.1098/rstb.1984.0022. [DOI] [PubMed] [Google Scholar]
- 25.Horton JC. Cytochrome oxidase patches: A new cytoarchitectonic feature of monkey visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984;304:199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- 26.Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984;4:309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felleman DJ. Functional maps in visual cortex: Topographic, modular, and column organizations. In: Masland RH, Albright TA, editors. The Senses. London: Elsevier; 2008. pp. 577–593. [Google Scholar]
- 28.Lu HD, Roe AW. Functional organization of color domains in V1 and V2 of macaque monkey revealed by optical imaging. Cereb Cortex. 2008;18:516–533. doi: 10.1093/cercor/bhm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Economides JR, Sincich LC, Adams DL, Horton JC. Orientation tuning of cytochrome oxidase patches in macaque primary visual cortex. Nat Neurosci. 2011;14:1574–1580. doi: 10.1038/nn.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wikler KC, Rakic P. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J Neurosci. 1990;10:3390–3401. doi: 10.1523/JNEUROSCI.10-10-03390.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barone P, Dehay C, Berland M, Kennedy H. Role of directed growth and target selection in the formation of cortical pathways: Prenatal development of the projection of area V2 to area V4 in the monkey. J Comp Neurol. 1996;374:1–20. doi: 10.1002/(SICI)1096-9861(19961007)374:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin MKL, Kaskan PM, Zhang B, Chino YM, Kaas JH. Cortical and subcortical connections of V1 and V2 in early postnatal macaque monkeys. J Comp Neurol. 2012;520:544–569. doi: 10.1002/cne.22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaas JH. The evolution of the visual system in primates. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge, MA: MIT Press; 2003. pp. 1563–1572. [Google Scholar]
- 34.Kaskan PM, Lu HD, Dillenburger BC, Kaas JH, Roe AW. The organization of orientation-selective, luminance-change and binocular-preference domains in the second (V2) and third (V3) visual areas of New World owl monkeys as revealed by intrinsic signal optical imaging. Cereb Cortex. 2009;19:1394–1407. doi: 10.1093/cercor/bhn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubel DH, Livingstone MS. Segregation of form, color, and stereopsis in primate area 18. J Neurosci. 1987;7:3378–3415. doi: 10.1523/JNEUROSCI.07-11-03378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu HD, Roe AW. Optical imaging of contrast response in Macaque monkey V1 and V2. Cereb Cortex. 2007;17:2675–2695. doi: 10.1093/cercor/bhl177. [DOI] [PubMed] [Google Scholar]
- 37.Livingstone M, Hubel D. Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 38.Kaas JH, Morel A. Connections of visual areas of the upper temporal lobe of owl monkeys: The MT crescent and dorsal and ventral subdivisions of FST. J Neurosci. 1993;13:534–546. doi: 10.1523/JNEUROSCI.13-02-00534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hubel DH, Wiesel TN. Shape and arrangement of columns in cat’s striate cortex. J Physiol. 1963;165:559–568. doi: 10.1113/jphysiol.1963.sp007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaschube M, et al. Universality in the evolution of orientation columns in the visual cortex. Science. 2010;330:1113–1116. doi: 10.1126/science.1194869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzpatrick D. The functional organization of local circuits in visual cortex: insights from the study of tree shrew striate cortex. Cereb Cortex. 1996;6:329–341. doi: 10.1093/cercor/6.3.329. [DOI] [PubMed] [Google Scholar]
- 42.Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991;353:429–431. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
- 43.Hubel DH, Wiesel TN. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972;146:421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- 44.Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- 45.Kaskan PM, Dillenburger BC, Lu HD, Roe AW, Kaas JH. Orientation and direction-of-motion response in the middle temporal visual area (MT) of New World owl monkeys as revealed by intrinsic-signal optical imaging. Front Neuroanat. 2010;4:1–12. doi: 10.3389/fnana.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanigawa H, Lu HD, Roe AW. Functional organization for color and orientation in macaque V4. Nat Neurosci. 2010;13:1542–1548. doi: 10.1038/nn.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao Y, Wang Y, Felleman DJ. A spatially organized representation of colour in macaque cortical area V2. Nature. 2003;421:535–539. doi: 10.1038/nature01372. [DOI] [PubMed] [Google Scholar]
- 48.Roe AW. Modular complexity of area V2 in the macaque monkey. In: Kaas JH, Collins CE, editors. The Primate Visual System. Boca Raton, FL: CRC Press; 2004. pp. 109–138. [Google Scholar]
- 49.Kaas JH. The evolution of auditory cortex: The core areas. In: Winer JA, Schreiner CE, editors. The Auditory Cortex. New York: Springer-Verlag; 2011. pp. 407–427. [Google Scholar]
- 50.Merzenich MM, Kaas JH. Principles of organization of sensory-perceptual systems in mammals. In: Sprague JM, Epstein AN, editors. Progress in Psychobiology and Physiological Psychology. New York: Academic Press; 1980. pp. 1–42. [Google Scholar]
- 51.Woolsey TA, Welker C, Schwartz RH. Comparative anatomical studies of the SmL face cortex with special reference to the occurrence of “barrels” in layer IV. J Comp Neurol. 1975;164:79–94. doi: 10.1002/cne.901640107. [DOI] [PubMed] [Google Scholar]
- 52.Dawson DR, Killackey HP. The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat. J Comp Neurol. 1987;256:246–256. doi: 10.1002/cne.902560205. [DOI] [PubMed] [Google Scholar]
- 53.Erzurumlu RS, Kind PC. Neural activity: Sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Catania KC, Kaas JH. The unusual nose and brain of the star-nosed mole. Biosci. 1996;46:578–586. [Google Scholar]
- 55.Jain N, Catania KC, Kaas JH. A histologically visible representation of the fingers and palm in primate area 3b and its immutability following long-term deafferentations. Cereb Cortex. 1998;8:227–236. doi: 10.1093/cercor/8.3.227. [DOI] [PubMed] [Google Scholar]
- 56.Qi HX, Kaas JH. Myelin stains reveal an anatomical framework for the representation of the digits in somatosensory area 3b of macaque monkeys. J Comp Neurol. 2004;477:172–187. doi: 10.1002/cne.20247. [DOI] [PubMed] [Google Scholar]
- 57.Kaas JH, Guillery RW, Allman JM. The representation of the optic disc in the dorsal lateral geniculate nucleus: A comparative study. J Comp Neurol. 1973;147:163–180. doi: 10.1002/cne.901470203. [DOI] [PubMed] [Google Scholar]
- 58.Florence SL, Kaas JH. Ocular dominance columns in area 17 of Old World macaque and talapoin monkeys: Complete reconstructions and quantitative analyses. Vis Neurosci. 1992;8:449–462. doi: 10.1017/s0952523800004958. [DOI] [PubMed] [Google Scholar]
- 59.Horton JC, Adams DL. The cortical column: A structure without a function. Philos Trans R Soc Lond B Biol Sci. 2005;360:837–862. doi: 10.1098/rstb.2005.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiesel TN, Hubel DH, Lam DMK. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974;79:273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- 62.Takahata T, Higo N, Kaas JH, Yamamori T. Expression of immediate-early genes reveals functional compartments within ocular dominance columns after brief monocular inactivation. Proc Natl Acad Sci USA. 2009;106:12151–12155. doi: 10.1073/pnas.0905092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaskan PM, Lu HD, Dillenburger BC, Roe AW, Kaas JH. Intrinsic-signal optical imaging reveals cryptic ocular dominance columns in primary visual cortex of New World owl monkeys. Front Neurosci. 2007;1:67–75. doi: 10.3389/neuro.01/1.1.005.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takahata T, Shukla R, Yamamori T, Kaas JH. Differential expression patterns of striate cortex enriched genes among Old World, New World, and prosimian primates. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takahata T, et al. Differential expression patterns of occ1-related genes in adult monkey visual cortex. Cereb Cortex. 2009;19:1937–1951. doi: 10.1093/cercor/bhn220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson PA, Olavarria J, Van Sluyters RC. The overall pattern of ocular dominance bands in cat visual cortex. J Neurosci. 1988;8:2183–2200. doi: 10.1523/JNEUROSCI.08-06-02183.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gould HJ., 3rd Body surface maps in the somatosensory cortex of rabbit. J Comp Neurol. 1986;243:207–233. doi: 10.1002/cne.902430206. [DOI] [PubMed] [Google Scholar]
- 68.Donoghue JP, Leibovic S, Sanes JN. Organization of the forelimb area in squirrel monkey motor cortex: Representation of digit, wrist, and elbow muscles. Exp Brain Res. 1992;89:1–19. doi: 10.1007/BF00228996. [DOI] [PubMed] [Google Scholar]
- 69.Qi HX, Stepniewska I, Kaas JH. Reorganization of primary motor cortex in adult macaque monkeys with long-standing amputations. J Neurophysiol. 2000;84:2133–2147. doi: 10.1152/jn.2000.84.4.2133. [DOI] [PubMed] [Google Scholar]
- 70.Shambes GM, Gibson JM, Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol. 1978;15:94–140. doi: 10.1159/000123774. [DOI] [PubMed] [Google Scholar]
- 71.Leyton ASF, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orang-utan, and gorilla. Q J Exp Physiol. 1917;11:137–222. [Google Scholar]
- 72.Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- 73.Stepniewska I, Fang PC, Kaas JH. Microstimulation reveals specialized subregions for different complex movements in posterior parietal cortex of prosimian galagos. Proc Natl Acad Sci USA. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooke DF, Taylor CS, Moore T, Graziano MSA. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci USA. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gharbawie OA, Stepniewska I, Kaas JH. Cortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in new world monkeys. Cereb Cortex. 2011;21:1981–2002. doi: 10.1093/cercor/bhq260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gharbawie OA, Stepniewska I, Qi HX, Kaas JH. Multiple parietal-frontal pathways mediate grasping in macaque monkeys. J Neurosci. 2011;31:11660–11677. doi: 10.1523/JNEUROSCI.1777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaas JH, Gharbawie OA, Stepniewska I. The organization and evolution of dorsal stream multisensory motor pathways in primates. Front Neuroanat. 2011;5:1–7. doi: 10.3389/fnana.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cooke DF, Padberg J, Zahner T, Krubitzer L. The functional organization and cortical connections of motor cortex in squirrels. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr228. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Remple MS, Reed JL, Stepniewska I, Lyon DC, Kaas JH. The organization of frontoparietal cortex in the tree shrew (Tupaia belangeri): II. Connectional evidence for a frontal-posterior parietal network. J Comp Neurol. 2007;501:121–149. doi: 10.1002/cne.21226. [DOI] [PubMed] [Google Scholar]
- 80.Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci USA. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pinsk MA, DeSimone K, Moore T, Gross CG, Kastner S. Representations of faces and body parts in macaque temporal cortex: A functional MRI study. Proc Natl Acad Sci USA. 2005;102:6996–7001. doi: 10.1073/pnas.0502605102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rajimehr R, Young JC, Tootell RB. An anterior temporal face patch in human cortex, predicted by macaque maps. Proc Natl Acad Sci USA. 2009;106:1995–2000. doi: 10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cusick CG, Kaas JH. Cortical connections of area 18 and dorsolateral visual cortex in squirrel monkeys. Vis Neurosci. 1988;1:211–237. doi: 10.1017/s0952523800001486. [DOI] [PubMed] [Google Scholar]
- 85.Stepniewska I, Kaas JH. Topographic patterns of V2 cortical connections in macaque monkeys. J Comp Neurol. 1996;371:129–152. doi: 10.1002/(SICI)1096-9861(19960715)371:1<129::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 86.Kaas JH, Catania KC. How do features of sensory representations develop? Bioessays. 2002;24:334–343. doi: 10.1002/bies.10076. [DOI] [PubMed] [Google Scholar]
- 87.Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci. 2001;2:251–262. doi: 10.1038/35067562. [DOI] [PubMed] [Google Scholar]
- 88.Constantine-Paton M, Law MI. Eye-specific termination bands in tecta of three-eyed frogs. Science. 1978;202:639–641. doi: 10.1126/science.309179. [DOI] [PubMed] [Google Scholar]
- 89.Katz LC, Constantine-Paton M. Relationships between segregated afferents and postsynaptic neurones in the optic tectum of three-eyed frogs. J Neurosci. 1988;8:3160–3180. doi: 10.1523/JNEUROSCI.08-09-03160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cline HT, Debski EA, Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci USA. 1987;84:4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]