Abstract
Many bird species exhibit dramatic seasonal switches between territoriality and flocking, but whereas neuroendocrine mechanisms of territorial aggression have been extensively studied, those of seasonal flocking are unknown. We collected brains in spring and winter from male field sparrows (Spizella pusilla), which seasonally flock, and male song sparrows (Melospiza melodia), which are territorial year-round in much of their range. Spring collections were preceded by field-based assessments of aggression. Tissue series were immunofluorescently multilabeled for vasotocin, mesotocin (MT), corticotropin-releasing hormone (CRH), vasoactive intestinal polypeptide, tyrosine hydroxylase, and aromatase, and labeling densities were measured in many socially relevant brain areas. Extensive seasonal differences are shared by both species. Many measures correlate significantly with both individual and species differences in aggression, likely reflecting evolved mechanisms that differentiate the less aggressive field sparrow from the more aggressive song sparrow. Winter-specific species differences include a substantial increase of MT and CRH immunoreactivity in the dorsal lateral septum (LS) and medial amygdala of field sparrows but not song sparrows. These species differences likely relate to flocking rather than the suppression of winter aggression in field sparrows, because similar winter differences were found for two other emberizids that are not territorial in winter—dark-eyed juncos (Junco hyemalis), which seasonally flock, and eastern towhees (Pipilo erythropthalmus), which do not flock. MT signaling in the dorsal LS is also associated with year-round species differences in grouping in estrildid finches, suggesting that common mechanisms are targeted during the evolution of different life histories.
Keywords: seasonality, sociality, songbird
At the termination of the breeding season, many bird species leave their exclusive territories and join flocks that range from small parties to thousands of individuals. This dramatic seasonal shift in behavioral phenotype undoubtedly has profound fitness implications, but to our knowledge no studies have addressed the neural or endocrine mechanisms that promote seasonal flocking. In contrast, mechanistic studies of avian territorial aggression are relatively extensive and have inarguably revolutionized the field of behavioral endocrinology (1, 2). However, few of these studies explore the brain mechanisms of territoriality (1, 3). Using four emberizid songbird species that have evolved divergent life-history strategies, we here examine seasonal variation and evolutionary diversity in six neurochemical systems and demonstrate links of those systems to both winter flocking and territorial aggression.
On the basis of the immediate early gene responses of (i) male rodents to resident–intruder encounters, and (ii) male song sparrows (Melospiza melodia) to simulated territorial intrusion (playback of song and presentation of a caged male decoy), it seems that the neural substrates of territorial aggression are extensively comparable in birds and mammals. Thus, in both taxa significant activation is observed in the medial bed nucleus of the stria terminalis (BSTm), lateral septum (LS), paraventricular nucleus of the hypothalamus (PVN), anterior hypothalamus (AH), lateral portion of the ventromedial hypothalamus (VMH), and midbrain central gray (4–7; also see ref. 8). For the year-round territorial song sparrow, immediate early gene results are largely comparable in winter and summer (4, 5), although microarray data suggest that hypothalamic responses to simulated intrusion are very different in winter and summer, perhaps reflecting the fact that luteinizing hormone is released during territorial challenges only in the breeding season (9). Conversely, neurons that produce steroidogenic enzymes such as aromatase (ARO) may show greater activity in winter, given that territoriality in song sparrows shifts from reliance on gonadal steroids during the breeding season to nongonadal hormone production during the fall and winter (1, 2).
Remarkably, neural mechanisms that influence group-size decisions have received very little attention, although recent studies have begun to address this topic using five estrildid finch species that exhibit relatively stable group sizes year-round. These studies show that multiple neurochemical systems have evolved in relation to grouping behavior, particularly within the LS and associated subnuclei of the posterior septum. Receptor densities for vasotocin (VT; homolog of the mammalian nonapeptide vasopressin), mesotocin (MT; homolog of the mammalian nonapeptide oxytocin), corticotropin-releasing hormone (CRH), and vasoactive intestinal polypeptide (VIP) all exhibit patterns of parallel and divergent evolution that closely track species-typical group size (10, 11). Furthermore, VT neurons in the BSTm that project to the LS are sensitive to social valence and exhibit differential Fos responses in territorial and flocking species (12). Antisense knockdown of VT production in those cells potently reduces gregariousness in the highly social zebra finch (Taeniopygia guttata) (13), and antagonism of V1a-like and oxytocic receptors in the septum likewise reduces preferred group sizes (11, 13). The relative distribution of nonapeptide receptors across LS subnuclei may also be relevant to species differences in grouping, because flocking species have proportionally higher receptor binding in the dorsal (pallial) LS, whereas territorial species exhibit proportionally more binding in the subpallial LS (10, 11). Consistent with these findings, septal VT infusions reduce territorial aggression in emberizid sparrows and estrildid finches (14, 15). Finally, dopamine circuits are likely also relevant to grouping behavior, because gregarious finch species exhibit significantly more tyrosine hydroxylase-immunoreactive (TH-ir) neurons in the caudal ventral tegmental area (VTA) than do territorial species (16). The activity of these neurons is tightly coupled to courtship behavior, and perhaps to other aspects of affiliation as well (16).
These prior studies of avian sociality have focused exclusively on species that exhibit stable, year-round variation in species-typical group sizes (17). We hypothesize that the same neurochemical systems have evolved to mediate seasonal transitions between territoriality and flocking, but this remains to be determined. As a first approach to this hypothesis, we here quantify the neurochemical innervation of numerous brain areas in emberizid species that (i) alternate between gregarious and territorial phenotypes (field sparrow, Spizella pusilla, and dark-eyed junco, Junco hyemalis) (18, 19), (ii) are territorial year-round in much of their range (song sparrow) (20), or (iii) switch from breeding territoriality to loose distributions in fall and winter, without flocking (Eastern towhee, Pipilo erythropthalmus) (21). The four clades giving rise to these species diverged at approximately the same time, relatively early in emberizid phylogeny (22). Our focus is on males, given that breeding territoriality is typically most intense in males. Complete datasets from spring and winter birds are reported for song and field sparrows, including correlations with spring aggression. Winter differences that may reflect flocking in field sparrows were further explored in comparisons of winter juncos and towhees. Given that winter differences in neurochemistry between field and song sparrows potentially reflect differences in either winter aggression or winter flocking, the junco–towhee comparison is particularly useful. Specifically, we hypothesize that if winter differences between field and song sparrows reflect flocking, then juncos and towhees should exhibit a comparable winter difference. If winter differences between field and song sparrows reflect a lack of aggression in field sparrows, then juncos and towhees should not differ, because neither is territorial in winter.
We hypothesized that flocking-related changes in neurochemistry would be evidenced in one of two ways. Most obvious would be a winter increase in field sparrows (which flock in winter) that is not exhibited by song sparrows (which are territorial year-round). Alternatively, given that neurochemical circuits that promote winter flocking may also be involved in other affiliation behaviors that are expressed in the breeding season, such as pair bonding and caring for young, we hypothesized that field sparrows may maintain some neuroendocrine systems year-round that show a winter collapse in song sparrows. Both patterns are observed and are strongly supported by follow-up comparisons of juncos and towhees.
Finally, all of the substances examined here are made in multiple cell groups in the brain and may be relevant to a wide variety of behaviors, including both flocking and territoriality, dependent upon the brain area. For instance, whereas VT neurons in the BSTm respond primarily to affiliation-related social stimuli, those in the PVN are responsive to a diversity of stressors (17). TH cell groups likewise show great variation in response profiles (16, 23, 24). We therefore do not combine analyses across all brain areas for each neurochemical, given that each neurochemical is not a unitary “system.”
Results
General Approach.
Tissue from field and song sparrows (n = 6 males per species and season; 24 total) was immunofluorescently multilabeled for VT, VIP, and TH (series 1), and MT, CRH, and ARO (series 2). Representative photomicrographs are shown in Fig. 1. We were not uniformly satisfied with the quality of TH labeling in series 1, and therefore labeled a third series for TH using an antibody that yielded robust labeling in all subjects (Methods; a third series was not available for two spring subjects, one field and one song sparrow, because of earlier processing errors). We followed up on significant winter differences by labeling a single series of junco and towhee tissue for MT, CRH and TH; and labeled a limited amount of tissue from a second junco–towhee series for VT and VIP. Note that for logistical purposes related to antibody line-ups, most antigens were labeled using different fluorophores in the field–song and junco–towhee datasets, and thus labeling densities can only be compared within each species pair, not across.
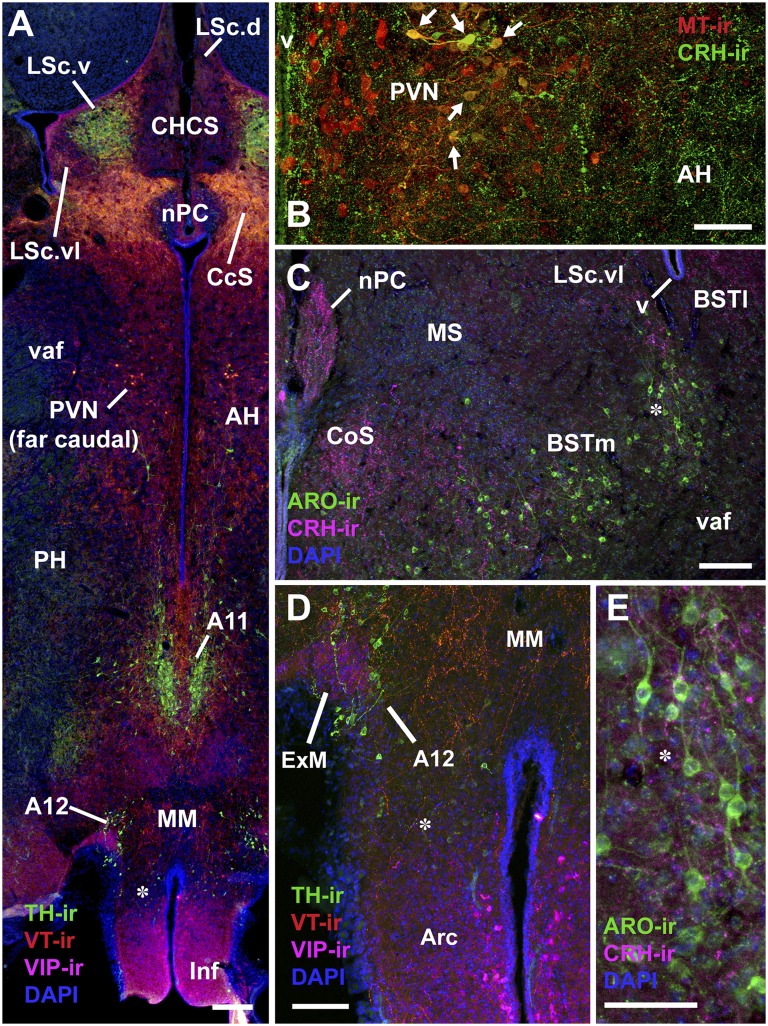
Fig. 1.
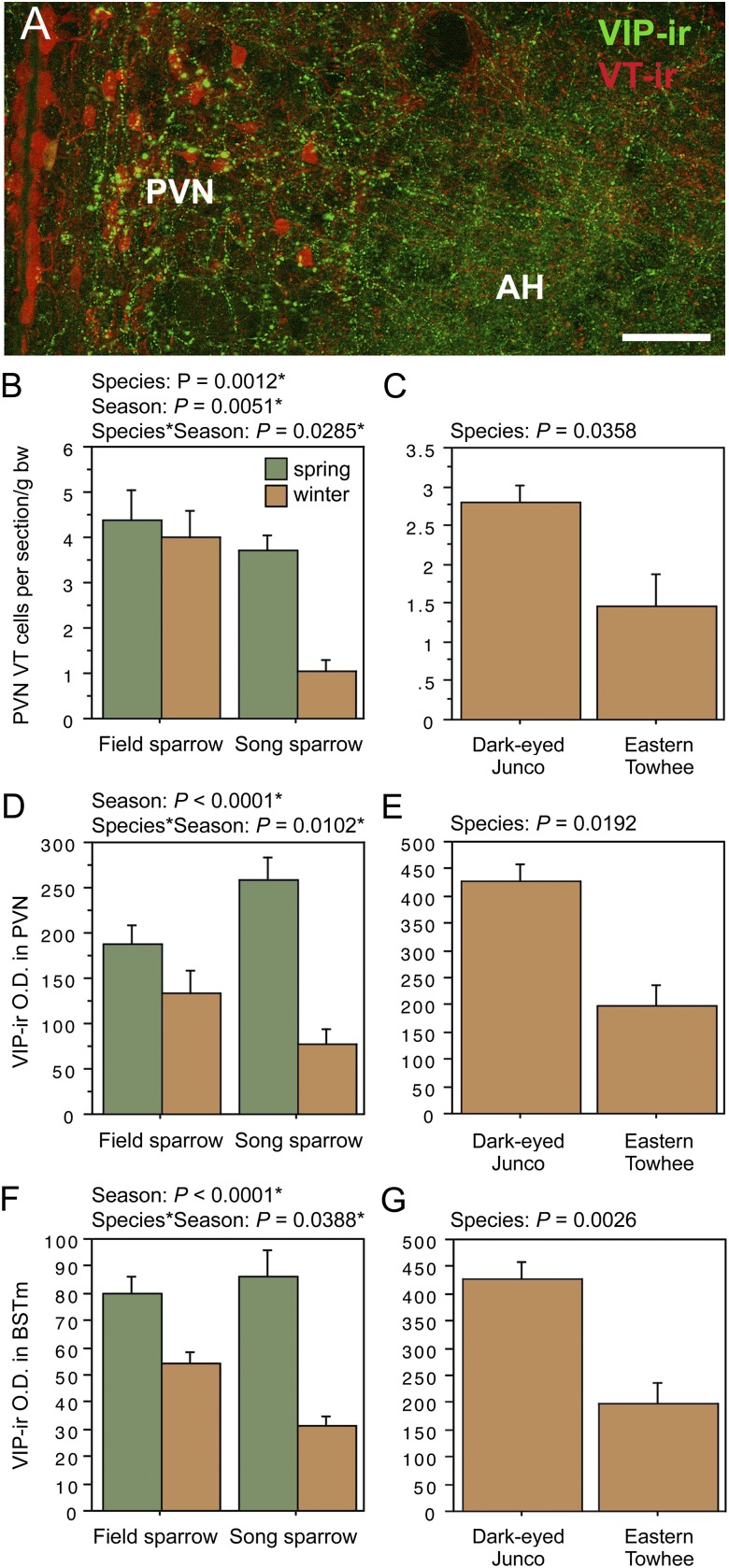
Representative immunolabeling in field sparrows. B is from a winter field sparrow; all others are from spring subjects. (A) TH, VIP, and VT immunolabeling in the caudal septum and posterior hypothalamus. Asterisk corresponds to asterisk in D. (B) MT and CRH immunolabeling in the PVN and AH. Note the extensive colabeling in the lateral subpopulation of neurons (arrows). (C) ARO and CRH immunolabeling in the BSTm and adjacent structures. Asterisk corresponds to asterisk in E. (Scale bars, 100 μm in A, C, and D; 50 μm in B and E.) Arc, arcuate nucleus; CHCS, cortico-habenular and cortico-septal tract (fimbria); CoS, commissural septal nucleus; ExM, external mammillary nucleus; Inf, infundibulum; LSc, caudal division of the lateral septum (.d, dorsal; .v, ventral; .vl, ventrolateral); MM, medial mammillary area; MS, medial septum; nPC, nucleus of the pallial commissure; PH, posterior hypothalamic area; v, lateral ventricle; vaf, ventral amygdalofugal tract.
Optical densities (ODs) of immunolabeling were measured in the medial preoptic nucleus, several hypothalamic areas (PVN, AH, and lateral and medial divisions of the VMH); anterior and posterior medial amygdala (MeA); BSTm; lateral BST; central gray; nucleus intercollicularis; rostral and caudal VTA; and nucleus accumbens. In addition, we quantified labeling in subnuclei of the septal complex that are differentiated on the basis of chemoarchitecture, peptide receptor distributions, and/or transcriptional responses to social stimuli (4, 10, 11, 25, 26). These are the nucleus of the pallial commissure; caudocentral septum (CcS); rostral LS subdivision (LSr); and both pallial and subpallial portions of the caudal LS subdivision, which are denoted here as LSc.d and subpallial LSc (includes both ventral and ventrolateral subnuclei). The LSc.d and subpallial LSc were analyzed at rostral and caudal levels. In addition to OD, we conducted counts of TH-ir cells in the VTA (A10 cell group), central gray (A11), dorsolateral tuberomammillary area (external mammillary nucleus; A12), and subparaventricular area (A14). VIP-ir cells were counted in the tuberal hypothalamus, and CRH, VT, and MT cells were counted in the PVN. Alpha values after Benjamini-Hochberg corrections for the false discovery rate (27) are reported in the figure captions and tables for the field and song sparrows, for which we collected full datasets (Methods). Results of Species × Season ANOVAs and within-species regressions with aggression are reported in SI Appendix, Tables S1–S6 (ANOVAs) and S7–S12 (regressions) (corresponding to MT, CRH, TH, VT, VIP, and ARO, respectively, for ANOVAs and regressions).
Neurochemical Signatures of Seasonal Flocking.
As described in the Introduction, we hypothesized that flocking-related changes in neurochemistry would take the form of either (i) a winter increase in flocking field sparrows that is not exhibited by song sparrows, or (ii) the maintenance of some neuroendocrine systems year-round in field sparrows that show a winter collapse in song sparrows.
The first pattern is observed for both MT-ir and CRH-ir fiber densities in the anterior and posterior MeA (“nucleus taeniae”) and the rostral LSc.d (SI Appendix, Tables S1 and S2). CRH is additionally increased in the LSr. As shown in Fig. 2, the LS innervation consists of extremely fine-caliber processes that arborize most extensively in the pallial LS. In winter field sparrows, MT-ir processes form numerous, light pericellular baskets. Similarly fine processes are observed in the MeA.
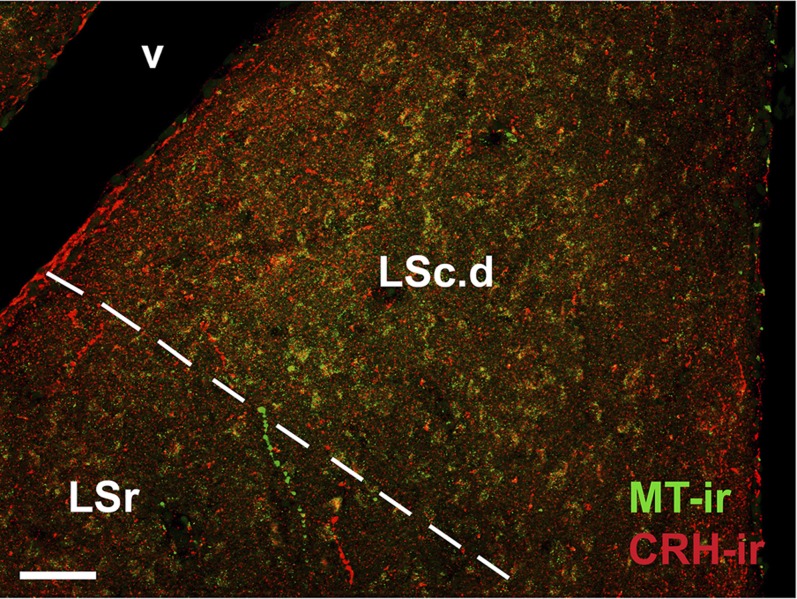
Fig. 2.
Fine-caliber MT-ir and CRH-ir fibers in the LSc.d of a winter field sparrow. The approximate pallial–subpallial boundary is indicated by the dashed line, highlighting the greater arborization within the pallial LS (LSc.d). (Scale bar, 50 μm.) v, lateral ventricle.
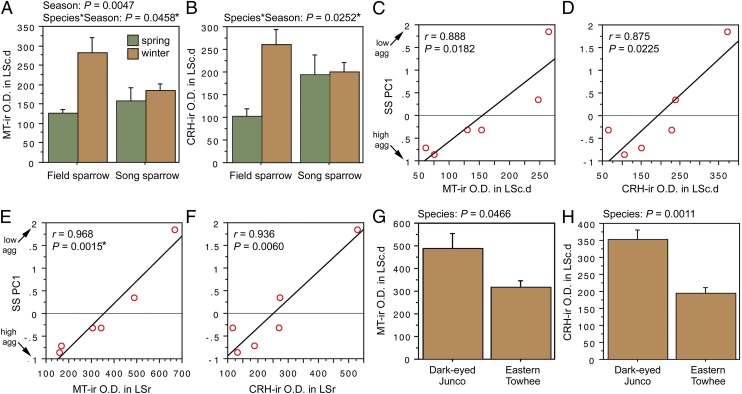
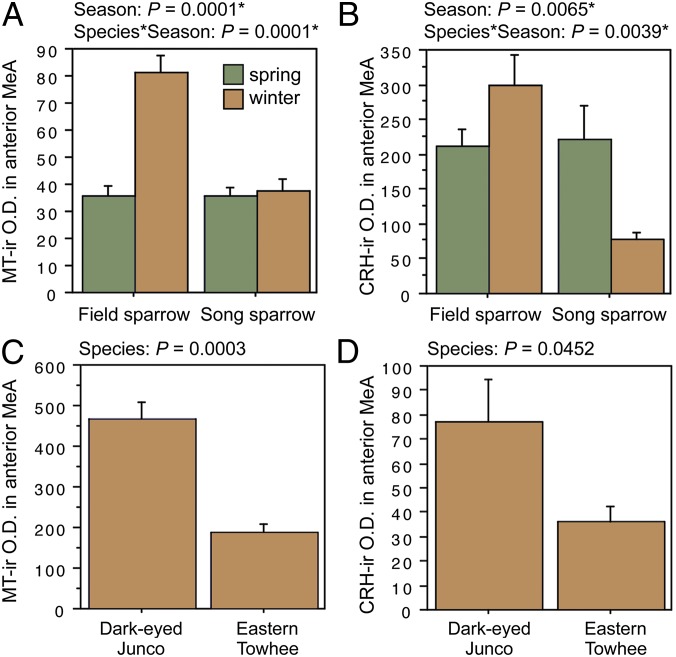
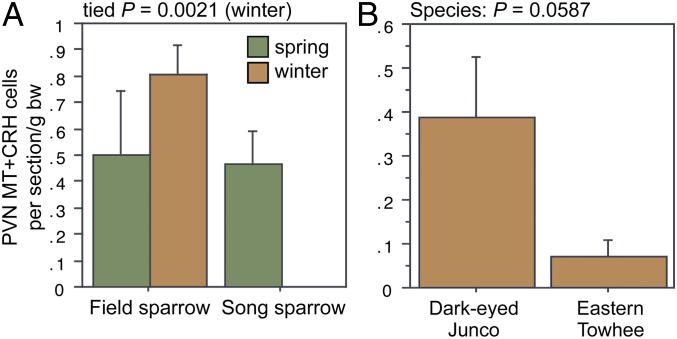
ANOVA results for the LSc.d are shown in Fig. 3 A and B. Importantly, both MT-ir and CRH-ir fiber densities in the rostral LSc.d and LSr correlate negatively with multiple measures of aggression (Fig. 3 C–F), and thus the increased densities in winter field sparrows may serve to suppress aggression rather than promote flocking. To address this issue, we quantified MT and CRH immunolabeling in wintering dark-eyed juncos, which flock, and eastern towhees, which loosely distribute in winter and do not flock. This comparison reveals significantly higher MT-ir and CRH-ir fiber densities in the rostral LSc.d of juncos relative to towhees (Fig. 3 G and H) but no differences in CRH OD in the LSr (P = 0.07). A parallel set of results is obtained for MT and CRH OD in the anterior MeA (field > song; junco > towhee; Fig. 4), but juncos and towhees do not differ in the posterior MeA (MT, P = 0.28; CRH, P = 0.71). Notably, colocalization of CRH and MT in PVN neurons is significantly greater in winter field sparrows than song sparrows (Fig. 5A), and winter juncos likewise tend to show more colocalization than towhees (P < 0.06; Fig. 5B). Double-labeling does not correlate with measures of aggression (all P > 0.10).
Fig. 3.
OD (in arbitrary units) of (A) MT-ir fibers and (B) CRH-ir fibers in the LSc.d of field and song sparrows collected in spring and winter, showing increased innervation density in winter field sparrows. (C–F) MT-ir and CRH-ir fiber densities correlate negatively with song sparrow aggression (SS PC1) in both the LSc.d (C and D) and LSr (E and F), suggesting that the increased innervation in winter field sparrows may suppress aggression rather than promote flocking. (G and H) However, comparisons of two species that are not territorial in winter show that MT-ir and CRH-ir fiber densities are greater in the flocking species (dark-eyed junco) than in the nonflocking species (eastern towhee). Data are shown as means ± SEM *Significant after Benjamini-Hochberg corrections (sparrows).
Fig. 4.
OD (in arbitrary units) of (A) MT-ir fibers and (B) CRH-ir fibers in the anterior MeA of field and song sparrows collected in spring and winter, showing increased innervation density in winter field sparrows. (C and D) MT-ir and CRH-ir fiber densities are greater in the flocking dark-eyed junco than in the nonflocking eastern towhee. Data are shown as means ± SEM *Significant after Benjamini-Hochberg corrections (sparrows).
Fig. 5.
(A) Number of PVN neurons double-labeled for MT and CRH in field and song sparrows. Because of a lack of variance in winter song sparrows, winter data were analyzed using Mann-Whitney tests. (B) A similar trend is observed for winter juncos and towhees.
The second pattern described above, in which field sparrows maintain circuitry year-round that collapses during winter in song sparrows, is observed for VT-ir cell number in the PVN; and VIP OD in the PVN, AH, rostral subpallial LSc, CcS, and BSTm (in some cases field sparrows maintain relatively more but show a slight decline from spring). Fig. 6A shows representative VT and VIP immunolabeling in the PVN and AH, and as shown in Fig. 6 B and C, the field–song difference in VT neurons is matched by a similar difference between winter juncos and towhees, indicating a relationship to flocking. However, with the exception of VIP OD in the BSTm, the Species × Season effects for VIP are complex, with species differences in both winter and spring, but in different directions. That is, spring VIP OD measures in the PVN, AH, and septal areas are actually higher in song than in field sparrows. Furthermore, as described in the following section, AH and CcS measures correlate positively with spring aggression, which we did not anticipate for variables that promote flocking. Despite these complexities, we conducted follow-up comparisons in juncos and towhees, and although no differences are observed for VIP OD in the AH (P = 0.14) or CcS (P = 0.85; areas where VIP immunolabeling correlates positively with aggression), juncos do show greater VIP OD in the PVN and BSTm, following the pattern of higher fiber density in winter field sparrows relative to song sparrows. Relevant data are shown in Fig. 6 D–G.
Fig. 6.
(A) Representative immunolabeling for VT and VIP in the PVN and AH of a winter field sparrow. (B, D, and F) VT-ir cell number in the PVN, VIP-ir OD (in arbitrary units) in the PVN, and VIP-ir OD in the BSTm of field and song sparrows. (C, E, and G) Corresponding data for juncos and towhees. Data are shown as means ± SEM. *Significant after Benjamini-Hochberg corrections (sparrows).
In addition to the patterns described above, one other finding initially suggested a possible relationship to flocking. This is a main effect of Species for TH immunolabeling in the rostral and caudal VTA, where field sparrows exhibit significantly higher TH-ir cell numbers and OD year-round relative to song sparrows (SI Appendix, Table S3). Cell numbers also correlate negatively with aggression (next section). However, comparable differences are not exhibited by winter juncos and towhees, suggesting that the year-round difference between field and song sparrows reflects their year-round differences in aggression, as presented below.
Finally, no winter differences are exhibited for VT OD in the BSTm (as would be predicted from estrildids), although VT-ir fiber density in spring is significantly higher in field sparrows than in song sparrows (SI Appendix, Fig. S1). Again, as described in the next section, this is associated with species differences in aggression.
Neurochemical Signatures of Species-Specific Territorial Behavior.
Before collections in the breeding season, we took three measures of territorial behavior during 3 min of song playback: latency to respond (by song, fly-by, or flyover), flights (defined as close fly-bys and flyovers), and songs. We then erected a mist net, began another round of playback, and took a second measure of response latency. Many measures of neurochemistry correlate significantly with these behavioral measures on a within-species level (next section). However, relevant to our focus on divergent life histories, we were particularly interested in determining whether measures of neurochemistry predicted species differences in aggression, given that that field sparrows are substantially less aggressive during the breeding season than are song sparrows.
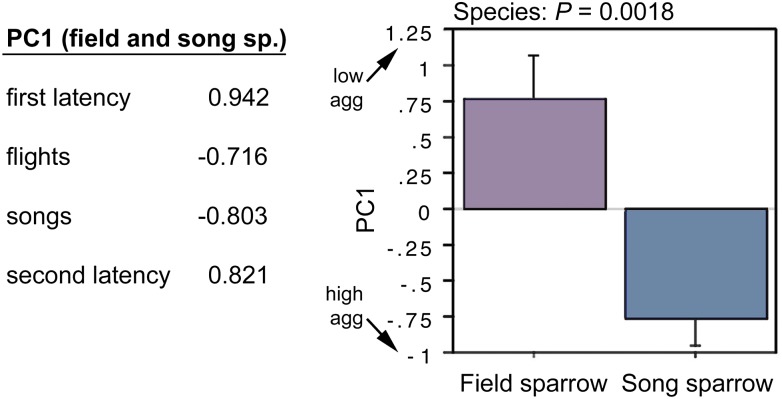
To quantify the species differences in aggression, we conducted a principle component (PC) analysis of the four behavioral measures, combining data for both species (P = 0.0029). This yields a single component (PC1) that strongly loads all four measures (Fig. 7) and explains 68% of the behavioral variance. A t test of PC scores confirms that song sparrows are more aggressive than field sparrows during the breeding season (Fig. 7), and more striking, PC scores for the two species are nonoverlapping. Thus, neurochemical measures that correlate with PC1 are strong candidates as mechanisms underlying evolutionary divergence in territoriality (although experience of aggression may also be a factor; Discussion).
Fig. 7.
PC loadings from a combined analysis of field and song sparrow aggression (Left) and a comparison of PC scores by species (Right). PC1 explains 68% of the variance and yields nonoverlapping values for field and song sparrows. Data are shown as means ± SEM.
Note that because of the strong loadings of latency measures, the direction of PC1 values is counterintuitive (i.e., higher PC scores reflect lower aggression). The PC1 score for one of the field sparrows was 2.8 SDs above the mean, and thus this subject was excluded from the regressions.
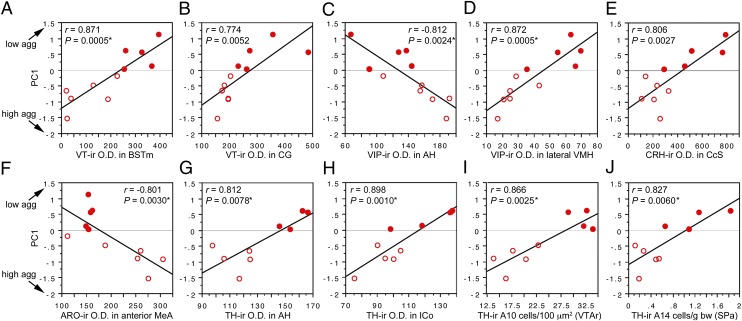
Regression analyses reveal significant negative correlations with PC1 (and thus positive correlations with aggression) for VIP OD in the AH and CcS; ARO OD in the posterior MeA (with a strong trend in the anterior MeA, as well); CRH OD in the posterior MeA and nucleus accumbens; and MT OD in the caudal subpallial LSc. In contrast, regression analyses reveal positive correlations with PC1 (and thus negative correlations with aggression) for VIP OD in the medial and lateral VMH; VT OD in the BSTm, central gray, and nucleus intercollicularis; CRH OD in the CcS; and TH OD in the medial preoptic nucleus, AH, LSr, and nucleus intercollicularis. In addition, TH-ir cell numbers in the rostral VTA, tuberomammillary hypothalamus, and subparaventricular area correlate positively with PC1. All regression results are shown in SI Appendix, Table S13, and 10 of the strongest correlations are shown in Fig. 8. Note that significance is not obtained solely on the basis of large species differences, because data points within each species tend to follow the overall slope.
Fig. 8.
(A–J) Regressions of neurochemical measures (OD, A–H; cell counts, I–J) and an index of aggression (PC1; Fig. 7) in field and song sparrows (closed and open circles, respectively). See x-axes for neurochemical variable and brain area. *Significant after Benjamini-Hochberg corrections (sparrows). CG, central gray; ICo, nucleus intercollicularis; SPa, subparaventricular area.
Individual Differences in Aggression.
As just described, many neurochemical measures correlate with both individual and species differences in aggression. However, neurochemical variables may relate to individual differences within a given species without also relating to differences in aggression across species. We therefore conducted behavioral PC analyses for field and song sparrows independently. However, whereas a significant matrix is obtained for song sparrows (P = 0.0318), this is not the case for field sparrows (P = 0.60), likely because the field sparrows displayed few flights and songs, and little variation in those measures. Thus, we conducted regressions for field sparrows based on the average of their two latency measures, and for song sparrows based on a single-species PC (SS PC1), that explains 64% of the variance and exhibits strong loadings for flights (−0.913) and both latencies (0.901 and 0.928, respectively), but a weak loading for songs (−0.234). Results of these analyses are reported in SI Appendix, Tables S7–S12.
Discussion
Although neuroendocrine mechanisms of seasonal territoriality have been extensively described (1–3), those of seasonal flocking have not, and brain mechanisms that evolve in relation to species differences in the intensity of territorial aggression are likewise unknown. We now show that in emberizid songbirds, several neurochemical variables reflect seasonal shifts from territoriality to flocking, whereas numerous other variables correlate with both individual and species differences in territorial aggression. Given that the relevant neurochemical systems may be influenced by social interactions (e.g., via altered hormone levels), we must be cautious in our interpretations, because neurochemical variation may be the product of species differences in behavior rather than the drivers of it. However, as expounded upon in the following sections, other relevant findings suggest that many of the species differences are indeed products of evolution and mechanistic drivers of behavioral variation. Finally, our results reveal a remarkable degree of seasonal, neurochemical plasticity within socially relevant brain areas that is far more extensive than previously appreciated.
Neurochemical Profiles of Seasonal Flockers.
Estrildid finches that are gregarious year-round exhibit nonapeptide binding sites in the rostral LSc.d (pallial LS) at much higher densities than do territorial estrildids (10, 11). The relevance of these binding sites to flocking is supported by the demonstrations that intraventricular and intraseptal infusions of nonapeptide receptor antagonists (V1a and oxytocin receptor antagonists) reduce preferences for larger groups in the highly gregarious zebra finch (11, 13), as does antisense knockdown of VT-ir neurons in the BSTm (13)—neurons that seem to provide the majority of VT-ir innervation to the LS (28, 29). Conversely, preferences for larger groups are facilitated by intraventricular infusions of MT (11). The present findings are strongly consistent with those in estrildids: field sparrows show a significant increase in MT-ir fiber density in the LSc.d during winter, when they form flocks, whereas the year-round territorial song sparrow does not. Flocking dark-eyed juncos likewise show a higher MT-ir fiber density in the LSc.d during winter than do nonflocking, nonterritorial eastern towhees. This pattern of MT results is replicated in the anterior MeA, and a very similar pattern of CRH innervation is observed in both the rostral LSc.d and anterior MeA.
Social affiliation in rodents is also linked to nonapeptide signaling in the LS. For instance, nonapeptide receptor densities in the LS increase in response to communal rearing (30), promote pair bonding (31), and correlate positively with both social investigation (32) and maternal behaviors [and in the pallial LS specifically (33)]. Although the specific significance of peptide action in the pallial LS remains to be directly demonstrated, recent findings in mice demonstrate that the pallial LS plays an important role in linking contextual stimulus information to the activation of the mesolimbic dopamine system, which influences incentive motivational processes and reward (34). The functional properties of the anterior MeA are relatively less clear. In mammals, the posterior subnuclei have been far more extensively studied, although Newman (35) has suggested that the anterior MeA exerts broad effects on social arousal. Homology of MeA subnuclei in birds and mammals remains to be demonstrated.
The finding that CRH innervation paralleled the MT innervation was unexpected but is consistent with the fact that these two peptides are produced in many of the same neurons in the PVN (Fig. 1B) and that colocalization is greater in winter flockers (Fig. 5). CRH is generally linked to anxiety-like processes and stress (36), which may be the connection to flocking, given that thermoregulatory and foraging challenges lead to facultative grouping in many vertebrate species (37, 38). Thus, we might hypothesize that winter flockers are in some sense hyperresponsive to the challenges of winter. This hypothesis also fits well with the observation that flocking birds exhibit significantly greater numbers of VT-ir PVN neurons in the winter than do nonflocking birds. Given that VT-ir fiber density collapses during winter in almost every brain area that we examined, it seems likely that these “extra” PVN neurons in flocking species project to the anterior pituitary, where VT acts as a secretagogue for adrenocorticopic hormone (39) and thereby contribute to a higher glucocorticoid tone.
Finally, we observed complex patterns of VIP-ir fiber densities, some of which correlate positively with aggression (next section). However, winter flocking (and not aggression) is associated with higher densities of VIP-ir fibers in the PVN and BSTm. Similarly, gregarious finch species exhibit higher densities of VIP binding sites in the BSTm than do territorial species (10), providing additional evidence that VIP signaling in the BSTm promotes grouping.
Species Differences in Territorial Aggression.
As shown here, field sparrows are significantly less aggressive than are song sparrows. Thus, the present dataset allows us to identify neurochemical mechanisms that may have evolved in relation to territorial behavior, because we are able to correlate measures of neurochemistry with aggressive behavior across both individuals and species. As a caveat to this approach, we observed widespread winter decreases in immunolabeling, suggesting the likelihood of positive relationships between gonadal hormones and labeling density. Thus, because male–male interactions typically elevate levels of testosterone (2), we must consider that any positive correlations between neurochemistry and behavior may be the product of male–male interactions and not the cause of it. For instance, ARO gene expression correlates positively with both aggression and plasma T in juncos (40). Nonetheless, most of the strongest relationships described here for neurochemistry and aggression are negative.
For instance, VT-ir fiber density in the BSTm collapses in winter, yet we also see that it correlates negatively with individual and species differences in aggression. This observation is consistent with the findings that (i) gregarious estrildids exhibit relatively more VT-ir neurons in the BSTm than do territorial species (12), (ii) those neurons respond selectively to affiliation-related stimuli (12), and (iii) infusions of VT into the septum (a major recipient of BSTm VT projections) reduce overt territorial aggression in both field sparrows and territorial finches (14, 15).
Similarly, VIP immunolabeling correlates negatively with sparrow aggression in the lateral VMH and tuberal hypothalamus, but also positively in the AH and caudal septum. These results are strongly consistent with a variety of findings in territorial finches. For instance, intraseptal VIP infusions facilitate offensive aggression (14), whereas antisense knockdown of VIP production in the AH virtually abolishes it (note that VIP-ir cells in the AH are only detectable after colchicine pretreatment and were thus not examined here). VIP-ir cell numbers in the AH of control finches correlate positively with aggression, but consistent with our present findings, VIP-ir cell numbers relate negatively to aggression in the tuberal hypothalamus (SI Appendix, Fig. S2). These finch data were obtained from birds in nonbreeding condition, suggesting that the positive relationship between AH VIP and aggression is not dependent upon gonadal steroids. Hence, VIP circuitries in the AH-CcS and mediobasal hypothalamus, which bear positive and negative relationships to aggression, respectively, are likely both relevant to behavioral evolution in sparrows.
We observed many other correlations across species that cannot be as readily interpreted, because of a lack of direct functional data, but those findings nonetheless provide the basis for many hypothesis-driven experiments on the evolution of aggression.
Widespread Seasonal Plasticity.
Although the present study was designed to focus on aggression and flocking, the analyses in field and song sparrows reveal a remarkable and unanticipated amount of seasonal plasticity, including all six neurochemical systems and 21 brain areas that we examined. Most remarkable are CRH and VIP. Seasonal plasticity has been shown for VIP within the septum and infundibulum (41, 42), but to our knowledge no such plasticity has been shown for the CRH innervation of the brain. However, we observed significant seasonal variation in 13 of the sampling areas for CRH and 11 of the sampling areas for VIP. Seasonal plasticity for both peptides is exhibited in the MeA, BST, septal complex, medial preoptic nucleus, hypothalamic nuclei, and midbrain. Even in the case of VT, for which extensive seasonal and hormone-mediated plasticity is already known (as with VP in mammals) (29, 39), the extent of seasonal remodeling came as a surprise. Interestingly, the most extensive plasticity known for mammals comes from jerboas (Jaculus orientalis) that were collected in the field (43), as were the animals in the present study, suggesting that exposure to a full range of seasonal cues is necessary to reveal the natural extent of seasonal plasticity.
Conclusions
We here hypothesized that flocking-related changes in neurochemistry take the form of either (i) a winter increase in flockers that is not exhibited by nonflocking species, or (ii) the maintenance of some neuroendocrine systems year-round in flockers that show a winter collapse in nonflockers. The first pattern is exhibited in the MT and CRH innervation of the pallial LS and anterior MeA, and in the colocalization of MT and CRH in the PVN. The second pattern is observed for VT-ir cell numbers in the PVN, and VIP innervation of the PVN and BSTm. A much larger number of neurochemical variables seem to evolve in relation to territorial aggression, and all neurochemicals and brain areas examined here exhibit remarkable seasonal plasticity.
Methods
Animals.
Spring field and song sparrows were caught April through May 2009 in the vicinity of Bloomington, IN. Wintering sparrows were caught in the vicinity of Bloomington, IN and in Davidson County, TN, between December 2008 and February 2009. Juncos and towhees were collected in the vicinity of Bloomington, IN in January 2010. Collections were made under applicable state and federal permits, and all procedures were in accordance with guidelines established by the National Institutes of Health for the ethical treatment of animals.
Tissue Processing and Image Analysis.
Subjects were killed within 30 min of capture. Perfusions, tissue processing, and immunofluorescent labeling followed standard protocols (16, 25, 44). All Alexa Fluor (A.F.) conjugates were purchased from Invitrogen. Secondaries were raised in donkey. Sparrow series 1 was labeled using sheep anti-TH (Novus Biologicals) guinea pig anti-VP (Bachem), and rabbit anti-VIP (Bachem), with A.F. 488, biotin followed with streptavidin-A.F. 594, and A.F. 680 secondaries, respectively. Sparrow series 2 was labeled using custom sheep anti-ARO, rabbit anti-MT (VA10; a kind gift of H. Gainer, National Institute of Neurological Disorders and Stroke, Bethesda, MD), and guinea pig anti-CRH (Bachem), using A.F. 488, 594, and 680 secondaries, respectively. Sparrow series 3 was labeled using mouse anti-TH (Immunostar) and A.F. 594 secondary. The specificity of all antibodies has been addressed [(25, 44); see company datasheets for TH]. Each processing run contained a mixture of species and seasons. Junco and towhee series 1 was labeled using rabbit anti-MT, mouse anti-TH, and guinea pig anti-CRH, with A.F. 488, 594 and 680 secondaries, respectively. Additional junco and towhee tissue was labeled using guinea pig anti-VP and rabbit anti-VIP, with A.F. 594 and 680 secondaries, respectively.
Although some larger areas with robust labeling were captured at 5×, most photomicrographs were obtained at 10× using a Zeiss AxioImager microscope outfitted with a Z-drive and optical dissector (Apotome; Carl Zeiss). OD of label and background was measured in Adobe Photoshop CS5 (Adobe Systems) from monochrome images, and background values were subtracted for statistical analysis. Cell counts were conducted as previously described (12, 16). All cells were counted in each relevant section for smaller cell groups and are represented as number of cells per section/gram body weight. TH-ir cells in the VTA were counted within a standardized box and are represented as number of cells per 100 μm2.
Statistics.
All ANOVAs, regressions, and PC analyses described in Results were conducted using Statview 5.0 for Macintosh. Given the large number of analyses, some concern arises with regard to type I error, although all brain areas and neurochemicals examined here are known a priori to be relevant to social behavior (although not in all possible combinations). Corrections for multiple comparisons in such instances are usually too conservative and not appropriate (45), and we therefore do not emphasize them in our interpretations. However, they may still provide a useful metric for evaluation, thus each of our data tables and figure panels provides information on significance relative to Benjamini-Hochberg corrections for the false discovery rate (27). Corrections were applied to each set of ANOVAs (e.g., for VT measures across all brain areas) and to each corresponding set of regressions. Again, although not emphasized in Results, the robustness of our findings is notable; for example, 73 of 78 ANOVAs that yield P values <0.05 were significant after corrections. Note that although the Benjamini-Hochberg correction initially applies a Bonferroni criterion, it adjusts α in a stepwise manner for remaining tests as long as P values continue to be significant at each step.
Supplementary Material
Acknowledgments
We thank Francisco Ayala, John Avise, and Georg Striedter for inviting this contribution; Jacob Callis, Brian Gress, Alexis Howard, Aubrey Kelly, Melissa Knisley, and Brittany Welsh for assistance with immunocytochemistry and/or cell counts; Ellen Ketterson, Dawn O’Neal, and Ryan Kiley for assistance with collections; Drew King and Meredith West for property access; and Harold Gainer for the donation of antiserum. Support for this study was provided by Indiana University.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VI: Brain and Behavior,” held January 19–21, 2012, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/evolution_vi.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203394109/-/DCSupplemental.
References
- 1.Soma KK. Testosterone and aggression: Berthold, birds and beyond. J Neuroendocrinol. 2006;18:543–551. doi: 10.1111/j.1365-2826.2006.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingfield JC. Historical contributions of research on birds to behavioral neuroendocrinology. Horm Behav. 2005;48:395–402. doi: 10.1016/j.yhbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Maney DL, Goodson JL. Neurogenomic mechanisms of aggression in songbirds. Adv Genet. 2011;75:83–119. doi: 10.1016/B978-0-12-380858-5.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: Segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Goodson JL, Evans AK, Soma KK. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport. 2005;16:1719–1723. doi: 10.1097/01.wnr.0000183898.47160.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: Defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- 8.Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS ONE. 2011;6:e20720. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukai M, et al. Seasonal differences of gene expression profiles in song sparrow (Melospiza melodia) hypothalamus in relation to territorial aggression. PLoS ONE. 2009;4:e8182. doi: 10.1371/journal.pone.0008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci USA. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly AM, et al. Vasotocin neurons and septal V1a-like receptors potently modulate songbird flocking and responses to novelty. Horm Behav. 2011;60:12–21. doi: 10.1016/j.yhbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- 15.Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- 16.Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci USA. 2009;106:8737–8742. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodson JL, Kingsbury MA. Nonapeptides and the evolution of social group sizes in birds. Front Neuroanat. 2011;5:13. doi: 10.3389/fnana.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey M, Burhans DE, Nelson DA. Field sparrow. Birds North Am. 1994;103:1–19. [Google Scholar]
- 19.Nolan V, Jr, et al. Dark-eyed Junco (Junco hyemalis) Birds North Am. 2002;716:1–44. [Google Scholar]
- 20.Arcese P, Sogge MK, Marr AB, Patten MA. Song sparrow (Melospiza melodia) Birds North Am. 2002;704:1–40. [Google Scholar]
- 21.Greenlaw JS. Eastern towhee (Pipilo erythropthalmus) Birds North Am. 1996;262:1–32. [Google Scholar]
- 22.Carson RJ, Spicer GS. A phylogenetic analysis of the emberizid sparrows based on three mitochondrial genes. Mol Phylogenet Evol. 2003;29:43–57. doi: 10.1016/s1055-7903(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 23.Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- 25.Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol. 2004;473:293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung CH, et al. Neural distribution of vasotocin receptor mRNA in two species of songbird. Endocrinology. 2011;152:4865–4881. doi: 10.1210/en.2011-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 28.De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 29.De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci. 2009;3:25. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 32.Ophir AG, Zheng DJ, Eans S, Phelps SM. Social investigation in a memory task relates to natural variation in septal expression of oxytocin receptor and vasopressin receptor 1a in prairie voles (Microtus ochrogaster) Behav Neurosci. 2009;123:979–991. doi: 10.1037/a0016663. [DOI] [PubMed] [Google Scholar]
- 33.Curley JP, Jensen CL, Franks B, Champagne FA. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm Behav. 2012;61:454–461. doi: 10.1016/j.yhbeh.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: A functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 36.Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol. 1999;115:1–22. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert C, et al. One for all and all for one: The energetic benefits of huddling in endotherms. Biol Rev Camb Philos Soc. 2010;85:545–569. doi: 10.1111/j.1469-185X.2009.00115.x. [DOI] [PubMed] [Google Scholar]
- 38.Davies NB. Food, flocking and territorial behaviour of pied wagtails (Motacilla alba yarellii Gould) in winter. J Anim Ecol. 1976;45:235–253. [Google Scholar]
- 39.Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- 40. Rosvall KA, et al. Neural sensitivity to testosterone predicts individual differences in male and female aggression: Implications for behavioral evolution. Proc Roy Soc B, in press. [DOI] [PMC free article] [PubMed]
- 41.Wacker DW, Schlinger BA, Wingfield JC. Combined effects of DHEA and fadrozole on aggression and neural VIP immunoreactivity in the non-breeding male song sparrow. Horm Behav. 2008;53:287–294. doi: 10.1016/j.yhbeh.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Kosonsiriluk S, et al. Vasoactive intestinal peptide and its role in continuous and seasonal reproduction in birds. Gen Comp Endocrinol. 2008;159:88–97. doi: 10.1016/j.ygcen.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Lakhdar-Ghazal N, et al. Vasopressin in the brain of a desert hibernator, the jerboa (Jaculus orientalis): Presence of sexual dimorphism and seasonal variation. J Comp Neurol. 1995;358:499–517. doi: 10.1002/cne.903580404. [DOI] [PubMed] [Google Scholar]
- 44.Kabelik D, Kelly AM, Goodson JL. Dopaminergic regulation of mate competition aggression and aromatase-Fos colocalization in vasotocin neurons. Neuropharmacology. 2010;58:117–125. doi: 10.1016/j.neuropharm.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.