Abstract
A paradox of vertebrate brain evolution is the unexplained variability in the size of the olfactory bulb (OB), in contrast to other brain regions, which scale predictably with brain size. Such variability appears to be the result of selection for olfactory function, yet there is no obvious concordance that would predict the causal relationship between OB size and behavior. This discordance may derive from assuming the primary function of olfaction is odorant discrimination and acuity. If instead the primary function of olfaction is navigation, i.e., predicting odorant distributions in time and space, variability in absolute OB size could be ascribed and explained by variability in navigational demand. This olfactory spatial hypothesis offers a single functional explanation to account for patterns of olfactory system scaling in vertebrates, the primacy of olfaction in spatial navigation, even in visual specialists, and proposes an evolutionary scenario to account for the convergence in olfactory structure and function across protostomes and deuterostomes. In addition, the unique percepts of olfaction may organize odorant information in a parallel map structure. This could have served as a scaffold for the evolution of the parallel map structure of the mammalian hippocampus, and possibly the arthropod mushroom body, and offers an explanation for similar flexible spatial navigation strategies in arthropods and vertebrates.
Keywords: sensory, memory, orientation, limbic
Why Is the Size of the Olfactory Bulb So Variable?
In 1995, Barbara Finlay and Richard Darlington launched a series of studies that supplied an answer to the fundamental question of why sizes of brain regions vary (1). Proposed initially for mammals but extended to basal vertebrates (e.g., sharks) and evolution by artificial selection (e.g., domestication), it supplied the missing link between the constraints of development and allometry. The “late equals large” principle has one important exception: the olfactory bulb (OB). The size of this forebrain structure, within species, order, or class, does not scale with the rest, and indeed the entire olfactory limbic system (LI), including the hippocampus and amygdala, does not conform to this otherwise universal scaling law (2–4).
Why this should be the case is not yet clear. In their most recent analysis, Finlay et al. suggest: “we speculate that the independent variation of olfactory bulb from the rest of the brain may be not so much selection for olfactory variability, but rather selection for tighter coupling of the other sensory systems that must share thalamic projections and neocortical representations” (2). I would like to propose instead that such selection for olfactory variability exists. The commonly conceived function for olfaction is the ability to detect and discriminate odorants (5–7). A second function, spatial orientation to odorants, is seen as an application of olfactory discrimination. Reversing the primacy of these two functions turns many assumptions and interpretations of olfaction on their heads. What I will call the olfactory spatial (OS) hypothesis offers a unique explanation for the independent scaling of the vertebrate OB: that the scaling reflects directional selection on animals to decode and map patterns of odorants for the purpose of spatial navigation.
Convergence in Olfactory System Structure and Function
The need to orient in space to maximize fitness by acquiring resources and avoiding competition and predation is universal. Indeed it is a defining archetype of what it means to be an animal, most of which are mobile. Olfaction is also universal: “chemicals are probably the original stimuli, since they can participate directly in biochemical reactions without needing a sensory transduction step. This may be the reason that chemicals seem to be the most universal of stimuli. Indeed, it is possible that all organisms make use of chemical stimuli” (8).
Not only do all animals use chemical stimuli, but they do so by using similar mechanisms (5, 9) (Fig. S1). Eisthen documents four convergences in the olfactory system in insects, crustaceans, nematodes, mollusks, and vertebrates: odorant binding proteins in the fluid overlying olfactory receptor (OR) neurons, G protein-coupled receptors as odorant receptors, a two-step pathway in the transduction of odorant signals, and the presence of glomerular neuropils in the first central target of the axons of OR cells (10).
Such structural similarities in olfactory systems remain a remarkable and somewhat mysterious phenomenon. The olfactory system presents other problems: OR projections segregate and project to receptor-specific glomeruli, but beyond the glomerulus, there is no obvious topography (11). The unpredictable variation in the number of OR genes across species is also mysterious. The numbers must be significant, as OR genes represent the largest multigene family in mammals, representing 4% to 5% of the entire proteome (12). At present, there is no accepted hypothesis to explain this variation, which can range from 1,500 chemosensory receptors in the nematode worm (Caenorhabditis elegans), 130 in Drosophila melanogaster, 900 in the laboratory mouse, to 350 in humans (5).
Thus, the study of olfaction is a world of paradoxes: the independent scaling of the OB, the function of convergent neuroarchitectures, and the diversity of OR genes. However, perhaps these paradoxes arise from the assumption that the primary function is discrimination. If instead the OS hypothesis is correct, the structural similarities may be explained by convergent cognitive processes for spatial navigation. Likewise, variability in OB size and OR gene number could reflect the species’s use of odorants in spatial navigation. To explore this proposal, first it is necessary to consider how olfaction differs from other senses.
The Peculiar Case of Olfactory Perception
By its physical properties, the chemical world must be encoded differently. As Bargmann concluded, “the visual system and auditory system are stable because light and sound are immutable physical entities. By contrast, the olfactory system, like the immune system, tracks a moving world of cues generated by other organisms, and must constantly generate, test, and discard receptor genes and coding strategies over evolutionary time” (5). Olfaction’s genius for tracking moving targets has important implications. As Osorio et al. concluded in 1994: “the mammalian neocortex with its protean powers has evolved from the olfactory forebrain of primitive vertebrates [13]. Perhaps because olfaction demands a neural architecture preadapted to learning complex input patterns.” (14).
There is a rich literature on olfactory perception in humans and other animals, including insects, crustaceans, and rodents (15). A primary finding is that the percept of an odorant is nonlinearly intensity dependent. Low and high concentrations of the same odorant can be perceived as dissimilar and unrelated (table 4.1 in ref. 15). A second finding is that an odorant mixture can be perceived as a mixture of its elemental components (i.e., individual odorants) or as a synthetic odor object, which cannot be decomposed. Studies pitting different histories and rewards for different configurations, both in invertebrate and vertebrate taxa, demonstrate that the ability to switch from the elemental to the synthetic percept is widespread (15). The mechanism for this allocation of perception and attention is not yet understood, however (16, 17).
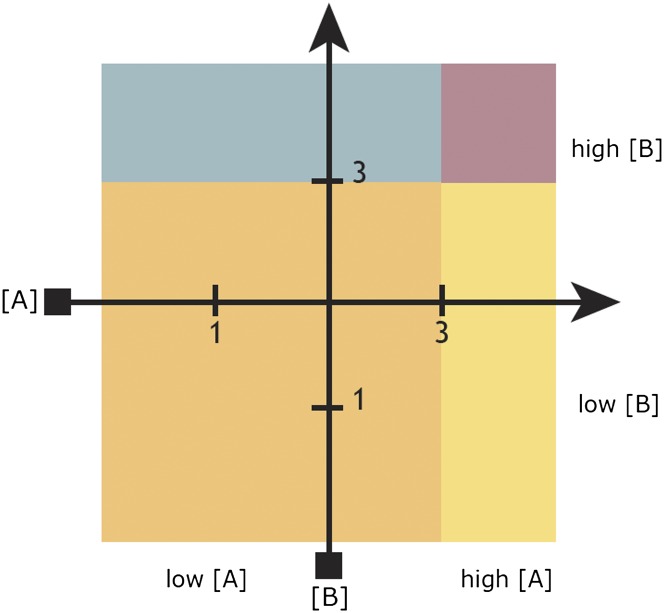
Nonetheless, these observations have implications for the problem of higher-level organization in the olfactory system, as it may be possible to construct a spatial logic from these rules. As seen in Fig. 1, if the percept changes abruptly with intensity, a uniform intensity gradient acquires demarcations. A navigator could use this pattern to confirm its direction or speed of movement along the gradient. If two demarcated gradients intersect, their conjunction could be organized by this principle into local areas of odorant mixtures, which herein will be called neighborhoods. A neighborhood organization could be used to learn the geometrical relationships among odorants, i.e., the olfactory space, which is a mental map of the spatial relationships among odorant distributions in the physical world.
Fig. 1.
Schematic predictions of the spatial olfaction hypothesis. A hypothetical orthogonal grid created by plumes from two odorants, A and B, which increase in concentration from one to three arbitrary units. With increasing intensity, there is a qualitative shift in percept (indicated by color). This further divides the hypothetical olfactory space into subregions known as neighborhoods (see text).
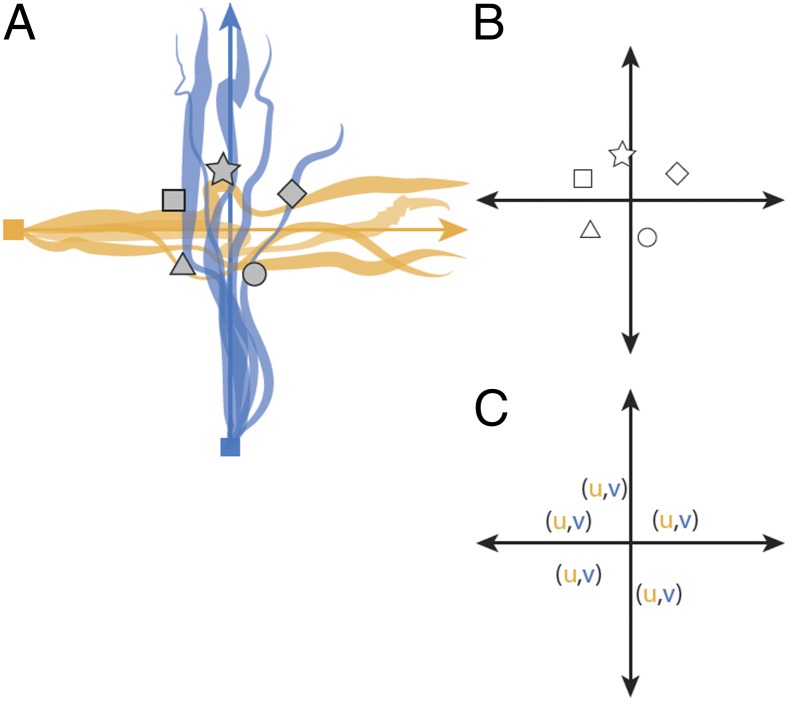
The addition of synthetic odor objects would increase the spatial resolution of the olfactory space (Fig. 2). Now, in addition to the low resolution neighborhoods, the olfactory space could also have high-resolution locations. These synthetic object landmarks could be associated with a neighborhood as well as with other objects in the same neighborhood.
Fig. 2.
Schematic predictions of the spatial olfaction hypothesis. The distribution of synthetic odor objects are landmarks in a dynamic olfactory space. (A) Encoding of odorant ratios as synthetic odor object percepts. (B) Synthetic objects occur at known locations, as defined by odorant ratios, and therefore are landmarks in olfactory space. (C) The coordinate of a synthetic object can therefore be computed from its elemental components. The coordinate system variables (u, v) are adopted from meteorology, where u designates streamwise direction and v crosswind direction (38).
Such an olfactory space would allow a navigator to extract new information from learned odorants. Knowing its speed and rate of sampling, a navigator could extrapolate into the future, predicting the percept farther up the gradient, i.e., both in space and time. If the prediction was correct, the navigator would have confirmed its location in olfactory space. If wrong, the navigator could recalibrate its position by searching for neighborhoods and/or synthetic objects. These two mapping systems for olfactory space would differ in other ways as well. The neighborhood system could be used to quickly form a low-resolution map, on which the navigator deduces direction and general location from changes in intensity and the order of neighborhoods. The synthetic object map would have higher spatial resolution but would also be slower to construct, with the navigator having to learn the location of unique synthetic objects. However, by encoding an odorant ratio in two ways, a navigator could use this information to shortcut between synthetic object locations along elemental gradients (Fig. 2C). By such novel mapping, the navigator could deduce new relationships among these synthetic objects. These new relationships could be used to simulate trajectories in physical space linking two locations and they could also be used to create higher-level categorizations of the original synthetic objects.
Obviously, the question of turbulence looms large, yet animals are highly adapted to decode turbulence (18–20), and odorant distributions may be stable, even in air (21). Olfactory systems are also notably integrated with mechanosensory systems to measure turbulence, such as vibrissae (mammals), antennae (insects), antennules (crustaceans), and lateral lines (fish) (22, 23). Thus, theoretically animals could collect the necessary mechanosensory data to decode the spatial relationships of odorants suspended in a dynamic medium (i.e., air or water).
Parallel Map Solution
If the primary function of olfaction is navigation, the parallel function hypothesis proposed earlier is one solution to this problem, although not the only one. I propose it for two reasons: first, it is a hypothesis that incorporates the known oddities of olfactory perception. Second, Françoise Schenk and I have proposed a similar parallel structure for the hippocampal cognitive map (24). If the OS hypothesis is correct, it suggests that the hippocampal parallel map evolved from the olfactory parallel map, as the mammalian instantiation of a bilaterian cognitive architecture, as discussed later.
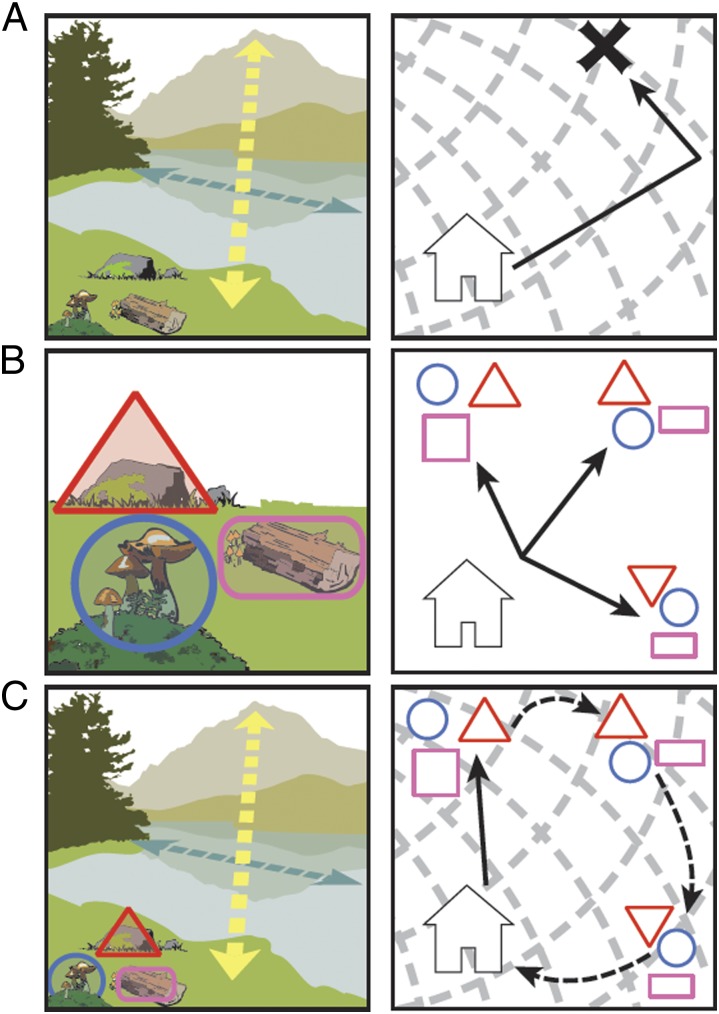
The parallel map theory (PMT), illustrated in Fig. 3, was first proposed as a cognitive mechanism for true navigation in vertebrates, and second, to explain the evolution and function of the mammalian hippocampus (24–26). In PMT, the bearing map (BE) is analogous to the olfactory elemental map, whereas the sketch map (SK) is analogous to the olfactory synthetic object map. The BE (Fig. 3A) is constructed by the navigator as it actively moves in space, comparing successive samples along gradients of graded stimuli, i.e., directional cues. With just a BE, a navigator can extrapolate and predict a future location, even in unexplored territory. In mammals, the proposed neural substrate of the BE is the dentate gyrus. In contrast, the SK encodes constellations of memorized positional cues (i.e., local landmarks; Fig. 3B). The SK encodes the topological arrangement of positional cues to derive relational and temporal order information, and its proposed substrate is the CA1 subfield of Ammon's horn. The BE and SK are brought into register on the integrated map, subserved by subfield CA3, in which objects on the SK are recoded in BE coordinates (Fig. 3C). In concordance with PMT predictions, Manahan-Vaughn and coworkers have recently shown that directional cues facilitate long-term depression (LTD) in the dentate gyrus whereas positional cues facilitate LTD in CA1, and both cue types facilitate LTD in CA3 (27, 28).
Fig. 3.
The parallel map theory of navigation, illustrated with real-world examples and with abstract schematics. (A) BE: colored arrows indicate the vector information extracted from two directional cues, a distant mountain (yellow) and the polarized shape of an oblong body of water (blue). The schematic shows the abstract bicoordinate map and movements of a navigator. (B) SKs: colored shapes outline three unique positional cues. The schematic represents three SKs near the home base of the navigator, with each SK differing not in the number or characteristics of the cues but in the topology of the array. (C) Integrated map: by encoding the location of positional cues (i.e., SKs) on a bicoordinate map (i.e., BE), the navigator can compute novel vectors between two known points, i.e., cognitively map.
As with olfactory space, the hippocampal parallel map provides a powerful tool for mapping spatial relations, with global generalization (i.e., BE) and local specificity (i.e., SK), and the ability to move between these representations in the fully encoded integrated map. In olfactory space, the map is based on chemosensory and mechanosensory inputs. In the BE, chemosensory, mechanosensory inputs as well as other sensory (e.g., visual, auditory, electrosensory) inputs are integrated to create a robust, multisensory representation of space. Such multimodal integration allows information from multiple directional cues to be calibrated. This calibration is critical to spatial navigation under natural conditions (29).
The close relationship between the olfactory system and the hippocampus in mammals has long been recognized; indeed, olfaction was once believed to be the primary function of the hippocampus (13). Thus, the OS hypothesis is not necessarily radical or new, but is instead the revisiting of an old idea in light of new evidence about olfaction and new insights from evolutionary neuroscience.
Predictions of the OS Hypothesis
If the function of olfaction is navigation, perhaps using a parallel map geometry, olfactory structure size should scale with navigational demand. At the same time, the impairment of olfactory structures should impair olfactory discrimination and olfactory navigation. Discrimination of odorants is a separate function of the olfactory system and a component of navigation. It is possible and even likely that these two functions, discrimination and navigation, will be found to segregate in olfactory systems by anatomical locus, physiological mechanism, and/or genetic encoding. However, at present, the genetic code for olfactory perception remains unbroken, and most olfaction research focuses on the discrimination of static odorants, not spatial orientation to changing odorant distributions (6, 7). What is needed to test the OS hypothesis are behavioral and physiological disassociations of the two functions in animals navigating under natural conditions, or laboratory conditions designed to simulate the natural complexity of odorant distributions.
With the exception of studies on homing pigeons, such data are mostly lacking. There is not sufficient space here to review the pertinent scientific literatures (e.g., physiology of animal olfaction, the hippocampus and spatial navigation). Instead, the studies most relevant to the question of the scaling of the OB in vertebrates are mentioned. Even in vertebrates, scaling of the vomeronasal and accessory olfactory systems, or the question of patterns in OR gene number, cannot be assessed here, although an OS-based analysis of these structures and gene families is underway.
If the olfactory system encodes spatial maps of odorants, the absolute size of the OB should covary with the need to make maps of high spatial resolution. It should not scale with demand for the fine discrimination of odorants, e.g., those used in social interactions or discriminating foods by taste. Such discrimination should be accomplished via physiological plasticity in response to the experiences of the individual (30, 31). Therefore, absolute OB size should be predicted by navigational demand. Further, it should be that form of navigation subserved by the BE: first creating vectors from graded stimuli, then combining these into bicoordinate maps for short-cutting and extrapolation (Fig. 3). Thus, the OS hypothesis also predicts that olfactory impairment should impair the BE, and thereby the integrated map and cognitive mapping. Evidence across vertebrates is reviewed later, with a short foray into arthropods, and the article concludes with a proposed scenario for the evolution of the OS system.
Mammals
Although the primacy of olfactory inputs for mammals is widely accepted (32), there are surprisingly few experimental studies of the use of air- or waterborne odorants for navigation. Studies of olfactory search by rescue dogs are one exception but are few in number (33). Most studies are those of laboratory rats orienting to discrete sources of odors in a laboratory maze. Under these conditions, rats will track an odor trail to a goal (34), even underwater (35). They can also orient to an array of odorant sources and will do so in the absence of visual cues (36). As they mature, however, rats require visual cues to orient in a lighted maze, even in the presence of learned olfactory cues. This accords with PMT, which predicts an ontogenetic change from the gradient-based BE to the object-based SK (24, 37). In the laboratory, such effects might be stronger if the static atmospheric conditions could be redesigned to capture the complexity of a natural windscape, the evolved context for olfactory navigation (38).
Nonetheless, impairment of the OB in laboratory rats orienting in the Morris water maze suggests that the OB is necessary for navigation, even in the presence of visual cues. Rats deprived of olfaction via peripheral anosmia showed no impairment, relying instead on visual cues. In contrast, rats with olfactory bulbectomy showed a severe and long-lasting (6 wk) impairment (39). This suggests that the OS system acts as a necessary scaffold for visual navigation, i.e., the same scaffolding function originally proposed for the BE (24). It illustrates a basic tenet of the OS hypothesis: that the function of the OB is spatial navigation, not simply odorant discrimination, as the lesion of the olfactory epithelium impaired discrimination but not navigation.
Comparative studies pointing to the navigational function of the OB in mammals began with a study of terrestrial carnivores by Gittleman, which showed that relative OB size increased with home range size (40). More recently, Reep et al., in 2007, examined the relationship between isocortex (IS) and the LI (OB, olfactory cortex, subicular cortices, hippocampus, septum) in diverse mammalian groups (carnivores, ungulates, xenarthrans, and sirenians) (3). Overall, they found the absolute size of the OB covaried with that of the hippocampus, but was inversely related to the absolute size of the IS, as was the size of the LI to the IS. However, when comparing LI and IS in relation to “brain core” volume [defined as striatum, diencephalon, medulla, and mesencephalon (41)], different patterns emerged. These included high IS plus high LI in carnivores, high IS plus low LI in simians, low IS plus low LI in microbats, and low IS plus high LI in insectivores. Megabats (pteropids) had intermediate IS plus intermediate LI, and ungulates and marine mammals had intermediate IS and low LI (Fig. S2). The authors made the case that such patterns emerged from developmental constraints (3).
Effects of Predatory Strategy
The OS hypothesis would predict that the size of the LI should increase in predators whose prey are predictable in time and space and who can be tracked by their odorants. Likewise, the size of the multisensory IS might be related to planning ability, with an IS increasing in size if prey are predictable but wily and difficult to capture. To apply this corollary of the OS hypothesis, I divide the world into foragers that are “detectors” or “predictors.” Detectors eat prey that are easy to find (e.g., grasses) or impossible to find (e.g., aerial insect clouds) and should thus not invest in brain space for a spatial tracking system. Predictors eat prey the locations of which can be predicted with sufficient data and should therefore invest as needed in a spatial tracking system, whether olfactory (i.e., LI) or not.
Such predictions are confirmed in the results of Reep et al. (3): low LI plus low IS should be found in detectors. Indeed, this is the pattern for grazing ungulates and sirenians and the echolocating microbats, many of which feed on aerial insects (Fig. S2). In contrast, the ancestral mammal was probably an olfactory predator eating small prey, such as invertebrates. Less encephalized prey should engage in fewer spatial counterploys to thwart an olfactory predator (38). This should be reflected in a predictor pattern of high LI plus low IS. This pattern is indeed seen in insectivores and prosimians (Fig. S2). If, however, predictors also face the challenge of eating prey that can map and avoid their movements (38), they must not only invest heavily in LI for mapping odorants in space but also in IS for predicting prey movements. This high LI/high IS pattern is found in terrestrial carnivores. Finally, among predictors, if prey are best detected by using a nonolfactory modality (e.g., vision), investment should decrease in LI but increase in IS; this pattern is seen in the low LI/high IS in simians (Fig. S2).
The pinnipeds present a quandary at first, as they are carnivores, and therefore should be predictors, with a high IS, whereas theirs is only intermediate. Olfaction must be jettisoned, however, in terrestrial species that return to the water, because of its incompatibility with respiration (22). However, as Reep et al. conclude, “the reduction of volume in the hippocampus, which gets only a minor olfactory projection compared to other sources of input, is suspiciously high for an explanation based on denervation” (3).
An alternative hypothesis is that pinnipeds are detectors, not predictors. Such a hypothesis is surprisingly tenable: unlike odontocetes such as dolphins, pinnipeds do not echolocate. Instead, they detect prey with specialized underwater visual systems and mechanoreception by using specialized vibrissae. Some pinnipeds use their mobile vibrissae to haptically search the benthic sea floor for stationary prey, and others use the vibrissae to track the hydrodynamic trails of prey such as fish (23). Schools of highly mobile prey may represent an ephemeral food source that is easier to find than predict in the absence of olfaction, the main sensory modality of other marine carnivores, such as sharks (42), and even aerial marine piscivores, such as albatrosses (43). The pinniped loss of olfaction, combined with low predictability in prey movements, would decrease selection for spatial tracking (44) and pinnipeds may have deinvested in predicting and reinvested in detecting. Again, this is highly speculative but offers a unitary explanation for the data.
Chiropterans are interesting because of the divergence in predatory behavior between the microbats, specialized for echolocation, and megabats (pteropids), who use simple or no echolocation, relying on vision and olfaction to detect prey, e.g., fruit. As predicted by the OS hypothesis, microbats show the low LI/low IS pattern. In contrast, megabats show an intermediate LI/intermediate IS pattern (Fig. S2), which is consistent with their use of olfaction to find their prey.
Hippocampal plasticity, which should also reflect OS function, also differs between microbats and megabats. Adult neurogenesis is found widely in animals but in vertebrates it is always found in the OB and the medial pallium (hippocampus in mammals) (45, 46). Thus, the two structures necessary for the OS system are also the only locations in which adult neurogenesis is found in all vertebrates, including mammals. OB neurogenesis increases with new odorant presentation (47), whereas hippocampal neurogenesis increases with spatial exploration (46). This vertebrate pattern of neurogenesis suggests its ancestral function was related to mapping and encoding the spatial distributions of novel odorants (24).
However, microbats present the exception to this vertebrate rule, despite showing normal hippocampal function, including hippocampal place cells (48). A study of 12 microbat species found no hippocampal neurogenesis in nine species and greatly reduced levels in the others; measures of neurogenesis even varied among species in a genus (49). The OS interpretation of this labile pattern is that detector microbats, relying heavily on spatial audition, have fundamentally replaced their OS system and now require less plasticity in BE components (e.g., OB, dentate gyrus). This hypothesis is supported by new data from the same group on megabats, which show a much higher level of hippocampal neurogenesis than microbats, but lower than that seen in laboratory rodents (50). This, too, would be predicted by the OS hypothesis, as megabats appear to be the predictors of the chiropterans. As with fruit-eating simians, these bats forage for a food resource that can be tracked in space and time. Cognitive mapping has also been demonstrated in a wild megabat, the Egyptian fruit bat (51), as have medial entorhinal grid cells (52). Concordant with this proposed predictor status, megabats show an intermediate LI/intermediate IS pattern (3). Further evidence comes from a comparative study of relative OB size, hippocampal size, and wing size in bats (53), in which wing size is a proxy for navigational ability, increasing in cluttered environments. Wing size increased with relative hippocampal size in microbats, but was unrelated to relative OB size. In contrast, relative OB size and wing size were positively correlated in megabats (53), again supporting the hypothesis that megabats are olfactory predictors whereas microbats are auditory detectors.
In summary, scaling analyses of mammalian LI and IS show distinct patterns of covariation (3). The OS hypothesis offers a unified explanation for these patterns, by proposing an increase in OS structures in predictors and a decrease in detectors. Decreases in LI size occur with shifts in sensory ecology (e.g., pinniped return to water, primate shift to diurnal frugivory, microchiropteran shift to aerial echolocator). Likewise, when prey are mobile and encephalized, the predator’s need to predict their movements drives an increased investment in LI and IS.
Such processes, hypothesized for extant mammals, may also shed light on macroevolutionary patterns in mammalian brain evolution. A recent study that used high-resolution X-ray computed tomography was able to identify three transitions in which early Jurassic mammals showed a significant and sudden increase in absolute brain size (54). At all three transitions, the increase in brain size could be ascribed primarily to increases in absolute OB and olfactory cortex size. The authors conclude, “but at its start, the brain in the ancestral mammal differed from even its closest extinct relatives specifically in its degree of high-resolution olfaction, as it exploited a world of information dominated to an unprecedented degree by odors and scents” (54). The alternative OS explanation is that this is evidence of mammals evolving more sophisticated spatial cognitive abilities, with increases in OB size accompanied by increases in hippocampal size and olfactory cortex size with eventual increases in IS. The mammalian brain may thus have evolved first via mosaic evolution for olfaction, then via concerted isocortical evolution.
Birds
New imaging studies of the relatives of modern birds, the theropod dinosaurs, have shown that OB size was larger in active predators, relative to cerebral size and corrected for phylogenetic independence. Moreover an analysis of phylogenetic trends showed that the direct ancestors of modern birds did not show the modern bird’s reduction in relative OB size, which must therefore be a secondary adaptation (55). This implies that carnivorous predators, whether diurnal theropods or nocturnal terrestrial mammals (40), are olfactory predictors, and require an enhanced OS system to track mobile, dispersed prey.
Finding this pattern in the diurnal ancestor of modern birds is concordant with the observation that despite their visual acuity, many bird species still require olfaction for spatial navigation (56). For example, procellariform (tube-nosed) seabirds, the “fishes of the air,” use olfaction to track unpredictable distributions of prey-related odors (43). When vision is reduced, however, as in secondarily nocturnal species, there is an increase in relative OB size in birds; this has evolved independently multiple times in modern birds (57).
The strongest evidence among vertebrates, however, for the OS hypothesis comes from the homing pigeon. This domesticated strain of the rock dove has been artificially selected for its ability to home from unknown locales for many centuries. Compared with nonhoming strains, the homing pigeon has in absolute size both a larger OB and a larger hippocampus (58). Originally proposed by Papi and later developed by Wallraff, it has now been well established that homing pigeons rely heavily on olfaction for navigation. As reviewed by Wallraff (59), the olfactory navigation hypothesis has been widely tested, across different laboratories and continents, by using a variety of behavioral and physiological manipulations. Physiological impairments have included blocking nostrils, anesthetizing the olfactory epithelium, transecting the olfactory nerve, and ablating the piriform cortex. Such procedures impair navigation even when visual cues are available (59). Although homing pigeons also orient by using geomagnetic fields (60), this input appears to be weighted less heavily than olfaction in experimentally displaced homing pigeons (61) and in migrating songbirds (62). Such experimental evidence for the primacy of olfactory inputs in navigation, across multiple diurnal bird orders, lends strong credence to the OS hypothesis.
Reptiles
Chemical stimuli play a pivotal role in the behavior of reptiles, but we lack studies addressing the covariation of absolute OB size and navigational ability. There is a correlation, however, between relative medial cortex (medial pallium homologue) size and active predation, whereby medial cortex size is larger in active than in sit-and-wait lizards (63). In snakes, rattlesnakes forced to navigate after experimental displacement have an increased volume of medial, but not dorsal or lateral, cortex (64).
Spatial orientation has been well studied in several species of turtles. The semiaquatic red slider turtle can orient by using true spatial strategies in the laboratory, and this ability is impaired after lesions of the medial cortex (65). Sea turtles orient to magnetic fields and to a map-like representation of such fields, adjusting their heading in response to simulated ocean locations in the laboratory (66, 67). In the field, sea turtles may also use windborne odorants to locate their natal beach by orienting upwind (68), but as secondarily aquatic vertebrates, sea turtles have a smaller relative OB size and fewer OR genes than land turtles (69). Thus, living and extinct reptiles appear to show predictable heterogeneity and plasticity in the components of the OS system, in concordance with the OS hypothesis.
Fish
Chemical stimuli are a primary source of information for spatial orientation in fish, from short reorientations to long-distance homing of salmon. Across all spatial scales, fish orient to odorants by calibrating odor sampling to their lateral line perception of hydrodynamic trails (56). The smooth dogfish not only requires intact lateral lines to use odorant sources for orientation (18), but uses the internostril time delay to determine its location relative to the plume (42). Experimental studies of navigation in goldfish demonstrate that it is mediated by the medial pallium homologue in teleosts, the dorsolateral ventral region of the telencephalon (70). As in birds and mammals (71), mating system predicts sex differences in the relative size of this region (72).
A recent analysis of brain scaling in cartilaginous fish has shown that, as in mammals, OB size variance is unrelated to phylogeny. Instead, as in the analysis of LI and IS in mammals (3), the patterns of absolute telencephalon and OB size admitted of no ready explanation (4). However, some of the observed patterns may be addressed with the OS hypothesis. For example, telencephalon and OB absolute size are larger in deep-water than reef-associated species. The shark in deep water may face the same challenge as a nocturnal carnivore on land. In both cases, the predator must predict prey movements and locations by using an olfactory BE, as the positional cues for the SK are absent (deep water) or ambiguous (low light). Therefore, sharks in deep water, but not in reefs, may orient to prey as olfactory predictors. If so, the OS hypothesis may offer insights about basal vertebrate clades as well as tetrapods.
Arthropods
It may be possible to apply the implications of the OS hypothesis even further back in evolutionary time. Tomer et al. have reported that similar highly conserved gene networks are found in the vertebrate pallium and the mushroom body of a marine annelid. They conclude that this ancestral gene network could underlie the evolution and development of complex brains in vertebrates and annelids (73).
This result is particularly timely in light of new studies showing arthropod species, lacking a hippocampus, can demonstrate cognitive mapping. Orienting to laboratory simulations of local geomagnetic fields, Caribbean spiny lobsters can accurately orient toward their home den (74). Studies of cognitive mapping in honey bees by Menzel et al. have shown that displaced honeybees can initiate homing flights from any location within the explored area along novel shortcuts and can choose among at least three goals (75, 76). Honeybees can also shortcut between vectors learned from exploration and those learned from the waggle dance (77).
Applying the same OS logic to arthropods, navigational demand should predict larger investment in the olfactory glomerular structure (i.e., OB in vertebrates) and the multisensory associational structure (i.e., hippocampus). In insects, this is the antennal lobe and mushroom body (78, 79). Antennal lobe size should covary with the use of olfaction in navigation, whereas the multisensory mushroom body, encoding visual, mechanosensory, and olfactory information, should covary with antennal lobe size when navigation is primarily in relation to odorants. There are some indications that this could be the case. As in pinnipeds and sea turtles, secondarily aquatic insects, such as hemipteran water striders, have reduced antennal lobes but large mushroom bodies. Like audition in microbats, the olfactory inputs may have been replaced by mechanosensory encoding of surface ripples. The question of “what the lobes do that causes them to be retained when olfaction is lost?” (78) may therefore have the same answer as in mammals. To understand these potential adaptive radiations in olfactory systems across such diverse taxa, I next consider how the OS system might have evolved in their common ancestor.
Evolution of Olfaction and Evolution of Navigation
Molecular clock and geological evidence agree that the history of bilateria began in the Ediacaran Period, 635 to 542 Myr ago (80). This fauna lived on or just below the tough, erosion-resistant biomat surface, supporting lifestyles such as mat encrusters, mat scratchers, mat stickers, and undermat miners (81). There was no evidence for spatial sensory organs, such as paired eyes for spatial vision, or paired antennae for spatial olfaction (82). The situation changed dramatically as 2D Precambrian matgrounds transformed to 3D Phanerozoic mixgrounds (81). The increasing energy content of prey could have fueled the Cambrian arms race, resulting in ever bigger and more complex predators (82) and associative learning (83). Nonassociative learning processes, such as habituation, were likely present before the evolution of the brain, even of neurons (84, 85). However, it was the challenge of the transition from the peaceful “Garden of Edicara” (81) to the Cambrian bloodbath of predator eating predator that probably supplied the selective force necessary for the evolution of the first brains.
In a highly competitive regime, active prey demand active predators. It is possible that the Cambrian arms race began with the evolution of spatial olfaction and the selective advantage this would give mobile predators. Spatial representation therefore would have evolved as a concrete and specific adaptation for this purpose, exapted from the primitive building blocks of chemotaxis and chemoreception. It would function to encode, organize, and predict the locations of prey, first in olfactory space. As the arms race accelerated, predators with new sensory modalities, such as vision, could detect prey hiding in olfactory refugia, such as turbulent eddies (38). Adding visual cues to the olfactory space would create a robust, multisensory BE. This could then be calibrated and anchored to other reliable environmental features, such as benthic algal mats, rock formations, and magnetic fields. At this point in time, the ancestors of deuterostomes and protostomes, using the common genetic toolkit (73), could have diverged in the details of their OS system, according to developmental constraints. However, all would retain the primacy of olfaction, i.e., olfactory-guided navigation, as the ancestral function of the forebrain (Fig. S3), and they would for this reason eventually converge on a similar neuroarchitecture and similar cognitive mechanisms, such as cognitive mapping.
Built on the olfactory integrated map, this forebrain could encode inputs and memories at both global (i.e., BE) and local (i.e., SK) frames of reference. These frames could be used to organize new data by their similarity to old data and to make supracategorical concepts, by linking local neighborhoods via common vectors. Now the forebrain would not only encode and recall data, it could also extract new relationships de novo—relationships, like the cognitive map shortcut, that had not yet been experienced. By making this construction first in olfactory space, then in a multisensory BE, olfaction may have laid the foundation for the evolution of memory organization in the bilaterian brain.
Conclusions
The OB is a troublesome structure, one that does not scale predictably with the rest of the brain, regardless of taxonomic level of analysis, whether order, family, species, or even individual (2). At present, there is no accepted functional hypothesis to explain this pattern of variation. The OS hypothesis offers a possible solution to this problem by proposing that olfaction evolved for the primary purpose of navigating in a chemical world. From this beginning, I propose that it developed specializations not just for the discrimination of odorants but for organizing the stimuli into functional associative memory structures. I suggest that olfactory percepts may bear evidence that this organization is a parallel map structure.
If the OS hypothesis is correct, the implications are profound. First, the primary function of olfaction would be navigation and its organization explained not by its ability to discriminate but to map odorants in space. Second, the OS system would represent the first and primary driving force in the evolution of associative learning, instantiated by the hippocampus in vertebrates and the mushroom body in arthropods and other protostomes. Not least, the hypothesis lays out a broad research program in “cognitive evo devo,” an enterprise to identify the primitives of cognition hand-in-hand with the primitives of the nervous system (Fig. S3). The peculiar properties of olfaction, as an optimal substrate for combinatorial associative learning, may supply a foundation for this enterprise and thereby inform our understanding not just of the limbic system but of the isocortex as well.
Supplementary Material
Acknowledgments
The author thanks Georg Striedter, Francisco Ayala, and John Avise for organizing the Sackler Colloquium; Leslie Kay, Randolf Menzel, Rachel Herz, and Françoise Schenk for their insights; Georg Striedter and two anonymous reviewers for comments on the manuscript; and the following for their discussion and contributions: Dan Koditschek, Bob Full, C.J. Taylor, Paul Roosin, April Gornik, Eric Fischl, Barbara Meyer, Tom Cline, Cori Bargmann, Mikel Delgado, Scott Bradley, Zoe Burr, Patrick Slattery, Dillon Niederhut, Katia Altschuller Jacobs, John Kedzie Jacobs, and finally Jeff Bitterman (1921–2011), to whom this work is dedicated. This work was supported by funding from National Science Foundation Electrical, Communications and Cyber Systems Grant 1028319.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VI: Brain and Behavior,” held January 19-21, 2012, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/evolution_vi.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201880109/-/DCSupplemental.
References
- 1.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 2.Finlay BL, Hinz F, Darlington RB. Mapping behavioural evolution onto brain evolution: the strategic roles of conserved organization in individuals and species. Philos Trans R Soc Lond B Biol Sci. 2011;366:2111–2123. doi: 10.1098/rstb.2010.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reep RL, Finlay BL, Darlington RB. The limbic system in mammalian brain evolution. Brain Behav Evol. 2007;70:57–70. doi: 10.1159/000101491. [DOI] [PubMed] [Google Scholar]
- 4.Yopak KE, et al. A conserved pattern of brain scaling from sharks to primates. Proc Natl Acad Sci USA. 2010;107:12946–12951. doi: 10.1073/pnas.1002195107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- 6.Murthy VN. Olfactory maps in the brain. Annu Rev Neurosci. 2011;34:233–258. doi: 10.1146/annurev-neuro-061010-113738. [DOI] [PubMed] [Google Scholar]
- 7.Arzi A, Sobel N. Olfactory perception as a compass for olfactory neural maps. Trends Cogn Sci. 2011;15:537–545. doi: 10.1016/j.tics.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Dusenbery DB. Sensory Ecology. New York: W.H. Freeman; 1992. [Google Scholar]
- 9.Ache BW, Young JM. Olfaction: Diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Eisthen HL. Why are olfactory systems of different animals so similar? Brain Behav Evol. 2002;59:273–293. doi: 10.1159/000063564. [DOI] [PubMed] [Google Scholar]
- 11.Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niimura Y. Evolutionary dynamics of olfactory receptor genes in chordates: interaction between environments and genomic contents. Hum Genomics. 2009;4:107–118. doi: 10.1186/1479-7364-4-2-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarnat HB, Netsky MG. Evolution of the Nervous System. 2nd Ed. Oxford, UK: Oxford Univ Press; 1981. [Google Scholar]
- 14.Osorio D, Getz WM, Rybak J. Insect vision and olfaction: different neural architectures for different kinds of sensory signal? In: Clift D, Husbands P, Meyer J-A, Wilson SW, editors. From Animals to Animats 3: Proceedings of the Third International Conference on Simulation of Adaptive Behavior. Cambridge, MA: MIT Press; 1994. pp. 73–81. [Google Scholar]
- 15.Wilson DA, Stevenson RJ. Learning to Smell: Olfactory Perception from Neurobiology to Behavior. Baltimore: Johns Hopkins Univ Press; 2006. [Google Scholar]
- 16.Frederick DE, Barlas L, Ievins A, Kay LM. A critical test of the overlap hypothesis for odor mixture perception. Behav Neurosci. 2009;123:430–437. doi: 10.1037/a0014729. [DOI] [PubMed] [Google Scholar]
- 17.Kay LM, Crk T, Thorngate J. A redefinition of odor mixture quality. Behav Neurosci. 2005;119:726–733. doi: 10.1037/0735-7044.119.3.726. [DOI] [PubMed] [Google Scholar]
- 18.Gardiner JM, Atema J. Sharks need the lateral line to locate odor sources: Rheotaxis and eddy chemotaxis. J Exp Biol. 2007;210:1925–1934. doi: 10.1242/jeb.000075. [DOI] [PubMed] [Google Scholar]
- 19.Atema J. Eddy chemotaxis and odor landscapes: Exploration of nature with animal sensors. Biol Bull. 1996;191:129–138. doi: 10.2307/1543074. [DOI] [PubMed] [Google Scholar]
- 20.Koehl MAR. The fluid mechanics of arthropod sniffing in turbulent odor plumes. Chem Senses. 2006;31:93–105. doi: 10.1093/chemse/bjj009. [DOI] [PubMed] [Google Scholar]
- 21.Wallraff HG. Avian olfactory navigation: Its empirical foundation and conceptual state. Anim Behav. 2004;67:189–204. [Google Scholar]
- 22.Thewissen JGM, Nummela S. Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates. Berkeley: Univ California Press; 2008. [Google Scholar]
- 23.Dehnhardt G, Mauck B. Mechanoreception in secondarily aquatic vertebrates. In: Thewissen JGM, Nummela S, editors. Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates. Berkeley: Univ California Press; 2008. pp. 295–314. [Google Scholar]
- 24.Jacobs LF, Schenk F. Unpacking the cognitive map: The parallel map theory of hippocampal function. Psychol Rev. 2003;110:285–315. doi: 10.1037/0033-295x.110.2.285. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs LF. The evolution of the cognitive map. Brain Behav Evol. 2003;62:128–139. doi: 10.1159/000072443. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs LF. From movement to transitivity: The role of hippocampal parallel maps in configural learning. Rev Neurosci. 2006;17:99–109. doi: 10.1515/revneuro.2006.17.1-2.99. [DOI] [PubMed] [Google Scholar]
- 27.Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 2008;18:968–977. doi: 10.1093/cercor/bhm136. [DOI] [PubMed] [Google Scholar]
- 28.Hagena H, Manahan-Vaughan D. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cereb Cortex. 2011;21:2442–2449. doi: 10.1093/cercor/bhq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freake MJ, Muheim R, Phillips JB. Magnetic maps in animals: A theory comes of age? Q Rev Biol. 2006;81:327–347. doi: 10.1086/511528. [DOI] [PubMed] [Google Scholar]
- 30.Kay LM, et al. Olfactory oscillations: The what, how and what for. Trends Neurosci. 2009;32:207–214. doi: 10.1016/j.tins.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci. 2007;27:8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis JL, Eichenbaum H, editors. Olfaction: A Model System for Computational Neuroscience. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- 33.Hepper PG, Wells DL. How many footsteps do dogs need to determine the direction of an odour trail? Chem Senses. 2005;30:291–298. doi: 10.1093/chemse/bji023. [DOI] [PubMed] [Google Scholar]
- 34.Wallace DG, Gorny B, Whishaw IQ. Rats can track odors, other rats, and themselves: Implications for the study of spatial behavior. Behav Brain Res. 2002;131:185–192. doi: 10.1016/s0166-4328(01)00384-9. [DOI] [PubMed] [Google Scholar]
- 35.Means LW, Alexander SR, O’Neal MF. Those cheating rats: Male and female rats use odor trails in a water-escape “working memory” task. Behav Neural Biol. 1992;58:144–151. doi: 10.1016/0163-1047(92)90387-j. [DOI] [PubMed] [Google Scholar]
- 36.Lavenex P, Schenk F. Integration of olfactory information in a spatial representation enabling accurate arm choice in the radial arm maze. Learn Mem. 1996;2:299–319. doi: 10.1101/lm.2.6.299. [DOI] [PubMed] [Google Scholar]
- 37.Rossier J, Schenk F. Olfactory and/or visual cues for spatial navigation through ontogeny: Olfactory cues enable the use of visual cues. Behav Neurosci. 2003;117:412–425. doi: 10.1037/0735-7044.117.3.412. [DOI] [PubMed] [Google Scholar]
- 38.Conover MR. Predator-Prey Dynamics: the Role of Olfaction. Boca Raton, FL: CRC; 2007. p. 248. [Google Scholar]
- 39.van Rijzingen IM, Gispen WH, Spruijt BM. Olfactory bulbectomy temporarily impairs Morris maze performance: An ACTH(4-9) analog accellerates return of function. Physiol Behav. 1995;58:147–152. doi: 10.1016/0031-9384(95)00032-e. [DOI] [PubMed] [Google Scholar]
- 40.Gittleman JL. Carnivore olfactory bulb size: Allometry, phylogeny and ecology. J Zool (Lond) 1991;225:253–272. [Google Scholar]
- 41.Finlay BL, Darlington RB, Nicastro N. Developmental structure in brain evolution. Behav Brain Sci. 2001;24:263–278. [PubMed] [Google Scholar]
- 42.Gardiner JM, Atema J. The function of bilateral odor arrival time differences in olfactory orientation of sharks. Curr Biol. 2010;20:1187–1191. doi: 10.1016/j.cub.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 43.Nevitt GA. Sensory ecology on the high seas: The odor world of the procellariiform seabirds. J Exp Biol. 2008;211:1706–1713. doi: 10.1242/jeb.015412. [DOI] [PubMed] [Google Scholar]
- 44.Stephens DW. Change, regularity, and value in the evolution of animal learning. Behav Ecol. 1991;2:77–89. [Google Scholar]
- 45.Derby CD. Why have neurogenesis in adult olfactory systems? The Presidential Symposium at the 2006 AChemS Conference. Chem Senses. 2007;32:361–363. doi: 10.1093/chemse/bjm011. [DOI] [PubMed] [Google Scholar]
- 46.Lledo P-M, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 47.Mouret A, Lepousez G, Gras J, Gabellec MM, Lledo PM. Turnover of newborn olfactory bulb neurons optimizes olfaction. J Neurosci. 2009;29:12302–12314. doi: 10.1523/JNEUROSCI.3383-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci. 2007;10:224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- 49.Amrein I, Dechmann DKN, Winter Y, Lipp H-P. Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera) PLoS ONE. 2007;2:e455. doi: 10.1371/journal.pone.0000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gatome CW, Mwangi DK, Lipp HP, Amrein I. Hippocampal neurogenesis and cortical cellular plasticity in Wahlberg’s epauletted fruit bat: A qualitative and quantitative study. Brain Behav Evol. 2010;76:116–127. doi: 10.1159/000320210. [DOI] [PubMed] [Google Scholar]
- 51.Tsoar A, et al. Large-scale navigational map in a mammal. Proc Natl Acad Sci USA. 2011;108:E718–E724. doi: 10.1073/pnas.1107365108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yartsev MM, Witter MP, Ulanovsky N. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature. 2011;479:103–107. doi: 10.1038/nature10583. [DOI] [PubMed] [Google Scholar]
- 53.Safi K, Dechmann DK. Adaptation of brain regions to habitat complexity: A comparative analysis in bats (Chiroptera) Proc Biol Sci. 2005;272:179–186. doi: 10.1098/rspb.2004.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowe TB, Macrini TE, Luo ZX. Fossil evidence on origin of the mammalian brain. Science. 2011;332:955–957. doi: 10.1126/science.1203117. [DOI] [PubMed] [Google Scholar]
- 55.Zelenitsky DK, Therrien F, Ridgely RC, McGee AR, Witmer LM. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proc Biol Sci. 2011;278:3625–3634. doi: 10.1098/rspb.2011.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeBose JL, Nevitt GA. The use of odors at different spatial scales: Comparing birds with fish. J Chem Ecol. 2008;34:867–881. doi: 10.1007/s10886-008-9493-4. [DOI] [PubMed] [Google Scholar]
- 57.Healy S, Guilford T. Olfactory-bulb size and nocturnality in birds. Evolution. 1990;44:339–346. doi: 10.1111/j.1558-5646.1990.tb05203.x. [DOI] [PubMed] [Google Scholar]
- 58.Rehkämper G, Haase E, Frahm HD. Allometric comparison of brain weight and brain structure volumes in different breeds of the domestic pigeon, Columba livia f.d. (fantails, homing pigeons, strassers) Brain Behav Evol. 1988;31:141–149. doi: 10.1159/000116581. [DOI] [PubMed] [Google Scholar]
- 59.Wallraff HG. Avian Navigation: Pigeon Homing as a Paradigm. Berlin: Springer-Verlag; 2005. [Google Scholar]
- 60.Wiltschko W, Wiltschko R. Magnetic orientation and magnetoreception in birds and other animals. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:675–693. doi: 10.1007/s00359-005-0627-7. [DOI] [PubMed] [Google Scholar]
- 61.Gagliardo A, Ioalè P, Savini M, Wild JM. Having the nerve to home: Trigeminal magnetoreceptor versus olfactory mediation of homing in pigeons. J Exp Biol. 2006;209:2888–2892. doi: 10.1242/jeb.02313. [DOI] [PubMed] [Google Scholar]
- 62.Holland RA, et al. Testing the role of sensory systems in the migratory heading of a songbird. J Exp Biol. 2009;212:4065–4071. doi: 10.1242/jeb.034504. [DOI] [PubMed] [Google Scholar]
- 63.Day LB, Crews D, Wilczynski W. Relative medial and dorsal cortex volume in relation to foraging ecology in congeneric lizards. Brain Behav Evol. 1999;54:314–322. doi: 10.1159/000006631. [DOI] [PubMed] [Google Scholar]
- 64.Holding ML, Frazier JA, Taylor EN, Strand CR. Experimentally altered navigational demands induce changes in the cortical forebrain of free-ranging Northern Pacific Rattlesnakes (Crotalus o. oreganus) Brain Behav Evol. 2012;79:144–154. doi: 10.1159/000335034. [DOI] [PubMed] [Google Scholar]
- 65.López JC, Vargas JP, Gómez Y, Salas C. Spatial and non-spatial learning in turtles: The role of medial cortex. Behav Brain Res. 2003;143:109–120. doi: 10.1016/s0166-4328(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 66.Lohmann KJ, Lohmann CMF. Orientation and open-sea navigation in sea turtles. J Exp Biol. 1996;199:73–81. doi: 10.1242/jeb.199.1.73. [DOI] [PubMed] [Google Scholar]
- 67.Putman NF, Endres CS, Lohmann CMF, Lohmann KJ. Longitude perception and bicoordinate magnetic maps in sea turtles. Curr Biol. 2011;21:463–466. doi: 10.1016/j.cub.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 68.Hays GC, et al. Island-finding ability of marine turtles. Proc Biol Sci. 2003;270(Suppl 1):S5–S7. doi: 10.1098/rsbl.2003.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vieyra M. Olfactory receptor genes in terrestrial, freshwater, and sea turtles: evidence for a reduction in the number of functional genes in aquatic species. Chelonian Conserv Biol. 2011;10:181–187. [Google Scholar]
- 70.Salas C, et al. Neuropsychology of learning and memory in teleost fish. Zebrafish. 2006;3:157–171. doi: 10.1089/zeb.2006.3.157. [DOI] [PubMed] [Google Scholar]
- 71.Jacobs LF. The role of social selection in the evolution of hippocampal specialization. In: Tomassi L, Nadel L, Peterson M, editors. Cognitive Biology: Evolutionary and Developmental Perspectives on Mind, Brain and Behavior. Cambridge, MA: MIT Press; 2009. [Google Scholar]
- 72.Costa SS, et al. Sex differences in the dorsolateral telencephalon correlate with home range size in blenniid fish. Brain Behav Evol. 2011;77:55–64. doi: 10.1159/000323668. [DOI] [PubMed] [Google Scholar]
- 73.Tomer R, Denes AS, Tessmar-Raible K, Arendt D. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell. 2010;142:800–809. doi: 10.1016/j.cell.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 74.Boles LC, Lohmann KJ. True navigation and magnetic maps in spiny lobsters. Nature. 2003;421:60–63. doi: 10.1038/nature01226. [DOI] [PubMed] [Google Scholar]
- 75.Menzel R, et al. Honey bees navigate according to a map-like spatial memory. Proc Natl Acad Sci USA. 2005;102:3040–3045. doi: 10.1073/pnas.0408550102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Menzel R, Lehmann K, Manz G, Fuchs J. Vector integration and novel shortcutting in honeybee navigation. Apidologie (Celle) 2012 doi: 10.1007/s13592-012-0127-z. [DOI] [Google Scholar]
- 77.Menzel R, et al. A common frame of reference for learned and communicated vectors in honeybee navigation. Curr Biol. 2011;21:645–650. doi: 10.1016/j.cub.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 78.Strausfeld NJ, Sinakevitch I, Brown SM, Farris SM. Ground plan of the insect mushroom body: functional and evolutionary implications. J Comp Neurol. 2009;513:265–291. doi: 10.1002/cne.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strausfeld NJ. Arthropod Brains: Evolution, Functional Elegance, and Historical Significance. Cambridge, MA: Harvard Univ Press; 2012. [Google Scholar]
- 80.Peterson KJ, Cotton JA, Gehling JG, Pisani D. The Ediacaran emergence of bilaterians: Congruence between the genetic and the geological fossil records. Philos Trans R Soc Lond B Biol Sci. 2008;363:1435–1443. doi: 10.1098/rstb.2007.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seilacher A. Biomat-related lifestyles in the Precambrian. Palaios. 1999;14:86–93. [Google Scholar]
- 82.Plotnick RE, Dornbos SQ, Chen J. Information landscapes and sensory ecology of the Cambrian Radiation. Paleobiology. 2010;36:303–317. [Google Scholar]
- 83.Ginsburg S, Jablonka E. The evolution of associative learning: A factor in the Cambrian explosion. J Theor Biol. 2010;266:11–20. doi: 10.1016/j.jtbi.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 84.Corning WC, Dyal JA, Willows AOD. Invertebrate Learning: Protozoans Through Annelids. New York: Plenum; 1973. [Google Scholar]
- 85.Moroz LL. On the independent origins of complex brains and neurons. Brain Behav Evol. 2009;74:177–190. doi: 10.1159/000258665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.