Abstract
Acoustic signaling behaviors are widespread among bony vertebrates, which include the majority of living fishes and tetrapods. Developmental studies in sound-producing fishes and tetrapods indicate that central pattern generating networks dedicated to vocalization originate from the same caudal hindbrain rhombomere (rh) 8-spinal compartment. Together, the evidence suggests that vocalization and its morphophysiological basis, including mechanisms of vocal–respiratory coupling that are widespread among tetrapods, are ancestral characters for bony vertebrates. Premotor-motor circuitry for pectoral appendages that function in locomotion and acoustic signaling develops in the same rh8-spinal compartment. Hence, vocal and pectoral phenotypes in fishes share both developmental origins and roles in acoustic communication. These findings lead to the proposal that the coupling of more highly derived vocal and pectoral mechanisms among tetrapods, including those adapted for nonvocal acoustic and gestural signaling, originated in fishes. Comparative studies further show that rh8 premotor populations have distinct neurophysiological properties coding for equally distinct behavioral attributes such as call duration. We conclude that neural network innovations in the spatiotemporal patterning of vocal and pectoral mechanisms of social communication, including forelimb gestural signaling, have their evolutionary origins in the caudal hindbrain of fishes.
Keywords: evolution, vocal communication, pacemaker neurons, speech, language
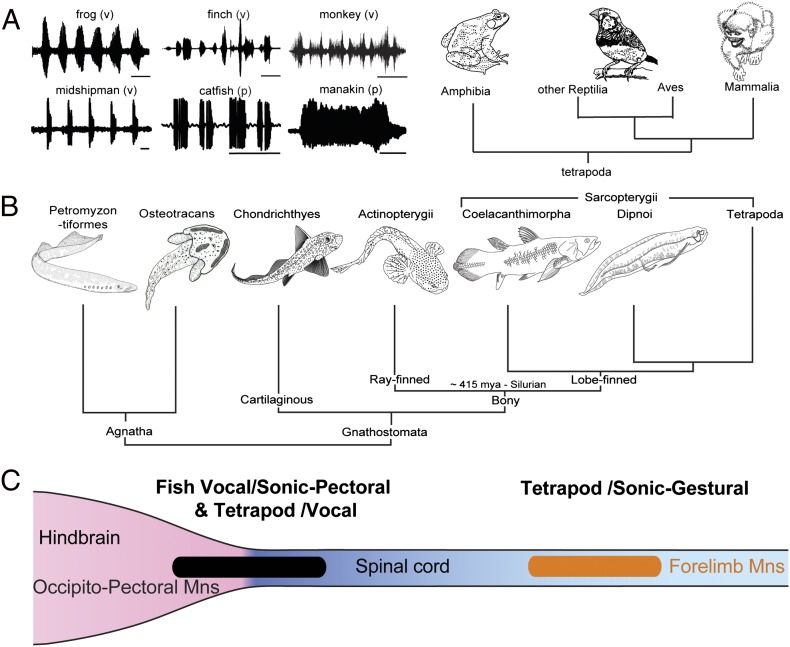
Early hindbrain development in all major vertebrate lineages exhibits a shared anatomical blueprint of cranial motor nuclei and nerves originating in one or more serially arranged segments or rhombomeres (rhs; e.g., refs. 1, 2). Here, we consider the development and evolution of hindbrain circuitry leading to novel innovations in social signaling, integrating information across behavioral, neurophysiological, and morphological levels of analysis. Two neural networks are the focus: the sonic–vocal basis of acoustic signaling (Fig. 1A) and pectoral control of anterior appendages, fins, and forelimbs (Fig. 1B). For context, we first briefly review vertebrate phylogeny and the ancestral “blueprint” for hindbrain motor phenotypes.
Fig. 1.
Evolution of vocal–pectoral motor systems in fishes and tetrapods. (A) Waveforms of representative social vocalizations of bullfrog (time base 1 s), zebra finch (250 ms), squirrel monkey (200 ms), midshipman fish (500 ms), catfish (250 ms), and club-winged manakin (100 ms). Vocal (v) and nonvocal pectoral (p) basis is indicated. (B) Cladogram of vertebrates, including jawless (agnatha) and jawed (gnathostome) radiations (Osteostracans represent an extinct agnathan group with pectoral fins). (C) Summary of location of vocal and sonic motoneurons. Among fishes, the occipitospinal motor column (black) gives rise to motoneurons innervating muscles of vocal organs dedicated to sonic functions (e.g., swim bladder) and pectoral fins that can also serve a sonic function. This same column gives rise to vocal motoneurons in tetrapods. Among tetrapods, forelimb motoneurons (orange) that function in both sonic and gestural signaling are located in the spinal cord. (A adapted from ref. 11; B and C adapted from ref. 13.)
Vertebrate Phylogeny
Living craniates include jawless vertebrates or agnathans and jawed vertebrates or gnathostomes (Fig. 1B; reviewed in ref. 3). Fossil evidence indicates several lineages of extinct agnathans (e.g., Osteostracans; Fig. 1B; e.g., ref. 4). Chondrichthyes (i.e., cartilaginous fishes) are the most basal group of jawed vertebrates and include two subclasses, Elasmobranchii (i.e., sharks, skates, and rays) and Holocephali or chimaeras. Bony vertebrates, the sister group to Chondrichthyes, include Actinopterygii or ray-finned fishes and the Sarcopterygii or lobe-finned fishes. Sarcopterygians include the coelacanth (Latimeria), lungfish (Dipnoi), and tetrapods.
Here, we mainly review recent evidence showing that a caudal hindbrain (rh8)-spinal cord compartment is the developmental origin of premotor-motor circuitry for sonic–vocal and pectoral behavioral phenotypes. Actinopterygians, which include nearly half of living vertebrate species, were the focus of these studies. By integrating these new findings into a single framework, we aim to achieve a more complete understanding of the evolutionary origins of vocal and pectoral motor systems among vertebrates in general, including the more highly derived pectoral systems of tetrapods that serve a range of functions including nonvocal sonic and forelimb gestural signaling.
Hindbrain Segmental Blueprint
Vertebrates have two functional series of hindbrain motor nuclei, somatic and branchiomeric (1, 2), that were likely present in the earliest, pregnathostome vertebrates (5). Somatic nuclei innervate head muscle derived from unsegmented (i.e., prechordal plate) and segmented paraxial mesoderm (i.e., occipital somites); branchiomeric nuclei target derivatives of paraxial mesoderm that migrate into the pharyngeal arches (1, 6, 7). Comparative studies delineate a conserved pattern of hindbrain somatic and branchiomeric motor nuclei spatially segregated along the rostral–caudal axis across eight rhs (1, 8). Most nuclei originate in one or two rhs with little variation in extent or location across taxa (1). Of particular interest for this review is rh8, which has two to three times the longitudinal extent of more anterior segments and can be subdivided into at least two to three subdivisions in teleost fishes and birds (9–11). Additional evidence for hindbrain segmentation, including a distinct rh8-spinal boundary, comes from rh-specific patterns of gene expression (e.g., refs. 12–14).
Evolutionary Developmental “Hotspots” for Novel Pattern Generators
Caudal hindbrain rhs are a developmental and evolutionary “hotspot” (sensu ref. 15) for innovations in neural networks controlling complex motor function. Bass and Baker (16) hypothesized that the appearance of novel respiratory and cardiovascular pumps during the protochordate–vertebrate transition (17, 18) depended upon the evolution of equally novel, genetically specified pattern generating circuits developing in rhs 7 and 8. Rhombomeres 7 and 8 were also proposed as the source of more recently derived premotor-motor networks unique to jawed vertebrates, such as those controlling sound production, that have social signaling functions (16). The development of precerebellar climbing fibers from a distinct rh8 nucleus, the inferior olive (10), underscored a preeminent role for caudal hindbrain nuclei in the spatiotemporal patterning of complex motor behaviors such as vocalization and eye movement.
Sonic–Vocal Pattern Generator
Sonic motor systems in fishes provide excellent models for directly linking neural mechanisms to behavioral outcomes, in part, because the physical attributes of acoustic signals (e.g., interpulse and intercall intervals, duration, amplitude), like its underlying neural activity, are easily quantified (19). Sonic mechanisms vary within and between fish lineages (20–22). Although most species studied so far generate acoustic signals by vibrating the swim bladder, a second well-known set of mechanisms depends on pectoral appendage vibration (23–25). Neuronal patterning of sound production has been most extensively investigated in species using a sonic swim bladder; hence, we first discuss these species. We will then turn our attention to pectoral-dependent mechanisms in the broader context of the motor control of pectoral appendages.
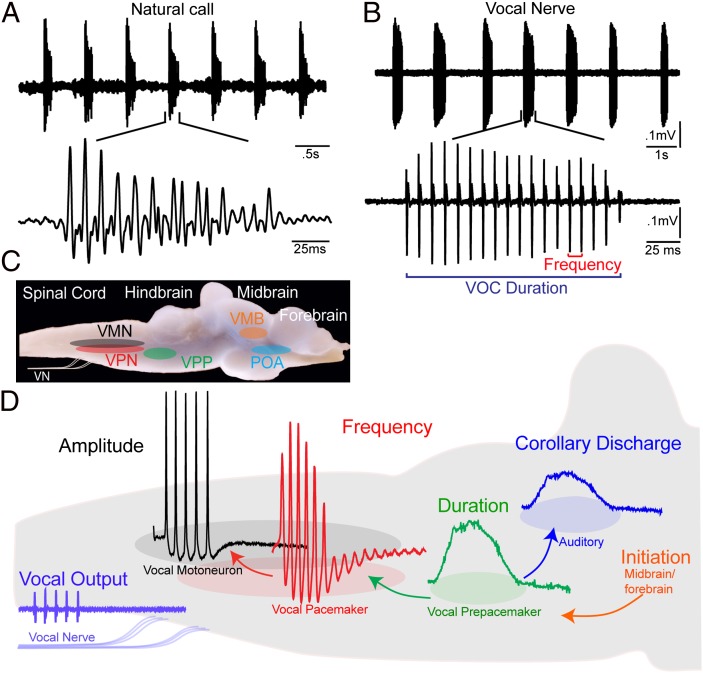
Swim bladder vibration is driven by the contraction of a single pair of muscles attached directly or indirectly to the swim bladder. This biomechanical simplicity has provided a unique opportunity to show how acoustic characters are directly determined by the intrinsic and network properties of a hindbrain central pattern generator controlling one pair of muscles. Toadfishes, a single order and family (Batrachoidiformes, Batrachoididae) of teleost fishes commonly known as toadfish and midshipman fish, have been widely studied as neurobehavioral models for acoustic communication (26–28). Among toadfishes, sonic muscles directly attached to the swim bladder are innervated by paired occipital nerve roots exiting the caudal hindbrain (20, 22). The temporal properties of occipital nerve motor volleys directly set pulse repetition rate (equivalent to fundamental frequency of harmonic calls for fish), duration, and complex patterns of frequency and amplitude modulation of entire calls (29–32). Individual sound pulses are matched 1:1 with each spike-like, occipital nerve potential (Fig. 2 A and B) that results from the synchronous activity of an expansive vocal motor nucleus (VMN) extending from the caudal hindbrain into the rostral spinal cord (Fig. 2 C and D) (29). Paired midline VMNs fire in synchrony (29), with bilaterally synchronous occipital spikes leading to simultaneous contraction of both vocal muscles and one sound pulse (33).
Fig. 2.
Vocal behavior and neural network of plainfin midshipman fish. (A) Oscillogram record of repetitive series of natural calls (“grunt train”) recorded with hydrophone; lower trace shows one call. (B) Spontaneous vocal motor volley recorded from vocal occipital nerve (VOC) with temporal properties like those of natural vocalization; lower trace shows one VOC. VOC duration is time between first and last pulses; frequency is pulse repetition rate. (C) Vocal motor nuclei superimposed on lateral view of intact brain. Indicated are VPN, VPP, and VMN nuclei and vocal nerve (VN). Vocal midbrain (VMB) and forebrain preoptic area (POA) are vocally active sites. (D) Premotor compartmentalization of neurons code for distinct acoustic attributes. Representative intracellular records from vocal nuclei and vocal nerve superimposed on background sagittal image of caudal hindbrain. Descending input from vocal midbrain/forebrain neurons activates vocal hindbrain. Vocal prepacemaker nucleus is source of known corollary discharge informing auditory nuclei about a vocalization’s temporal properties. (Adapted from ref. 37.)
A descending vocal motor pathway in toadfishes extends from forebrain preoptic-anterior hypothalamic to midbrain and caudal hindbrain levels (34–36). Premotor vocal pacemaker neurons (VPNs) densely innervate VMNs and receive input from a more rostral, anatomically separate prepacemaker [vocal prepacemaker (VPP)] nucleus (Fig. 2 C and D) (29, 34, 37). In an in vivo preparation, surgical isolation of the hindbrain-spinal region including the VPP–VPN–VMN network shows this region alone can produce a patterned output matching call temporal properties (30, 31).
Investigations of toadfish known as midshipman, using in vivo intracellular recording and staining, show how the VPP–VPN–VMN network determines natural vocal attributes. Chagnaud et al. (37) demonstrate that precise temporal patterning of natural vocalization (Fig. 2 A and B) depends on extreme network-wide synchrony and distinct intrinsic properties for each vocal nucleus. Sustained depolarizations in VPP, subthreshold membrane oscillations in VPN, and a combination of differential recruitment and low excitability in VMN directly code natural call duration, frequency, and amplitude, respectively (37, 38) (Fig. 2D). In addition to coding duration, prepacemaker neurons are the source of input to a rostral hindbrain nucleus directly innervating the inner ear and lateral line organs that is the anatomical basis for a vocal corollary discharge (Fig. 2D) (39). These new results for fishes, together with nerve recordings and more limited single-neuron recordings in tetrapods, led to the proposal that anatomically separate hindbrain populations code distinct call attributes in fishes and tetrapods (37).
Among tetrapods, the coupling of sound production and respiration leads to airflow-dependent vibration of sonic laryngeal and syringeal membranes (e.g., refs. 19, 40, 41). Despite the close connection between vocal and respiratory pattern generators in tetrapods, evidence for a vocal–respiratory pattern generator in more basal vertebrates like fishes has been missing. Video and sound analysis of advertisement calling (“humming”) by midshipman fish (42) reveals a strong rhythmic correlation between vocal, respiratory and postural (i.e., pectoral) systems (Fig S1). Vocal–respiratory coupling in this case likely reflects the increased oxygen demands of repetitive muscle contractions during the unusually long duration (from minutes to 1 h) hum vocalizations. Pectoral fin motion may stabilize the body during prolonged calling and/or aid in the increased movement of oxygenated water across the gills during humming (e.g., ref. 43).
We propose that vocal–respiratory coupling originated in fishes and was subsequently adopted by tetrapods. Neurophysiological support for this hypothesis comes from studies of the vocal pattern generator in fully aquatic frogs that produce sound independent of airflow and yet exhibit vocal–respiratory coupling in the caudal hindbrain (44). In birds and mammals, nuclei integrating vocal (i.e., laryngeal and syringeal) and respiratory activity are also positioned in the caudal hindbrain, adjacent to vocal motoneurons (45–48). Although a vocal–respiratory integration site has yet to be identified in fishes, it will likely be in the caudal hindbrain as in tetrapods.
Evolutionary Development of Sonic–Vocal Pattern Generator
We originally adopted the term “vocal” to describe occipital-innervated sonic systems in fishes, like the toadfishes discussed earlier, because of multiple characters they share with tetrapods (34): (i) production of social context-dependent signals, e.g., agonistic vs. advertisement; (ii) dedication of muscles, like those of the tetrapod syrinx and larynx, to sound production; (iii) shared origins of sonic muscles in fishes and tetrapods from occipital somites; (iv) likely homology of occipital nerve roots innervating fish sonic muscles and hypoglossal nerve roots innervating avian syringeal muscles; and (v) the same location in caudal hindbrain of fish sonic motor nucleus and avian tracheosyringeal division of hypoglossal motor nucleus innervating syringeal muscles (49).
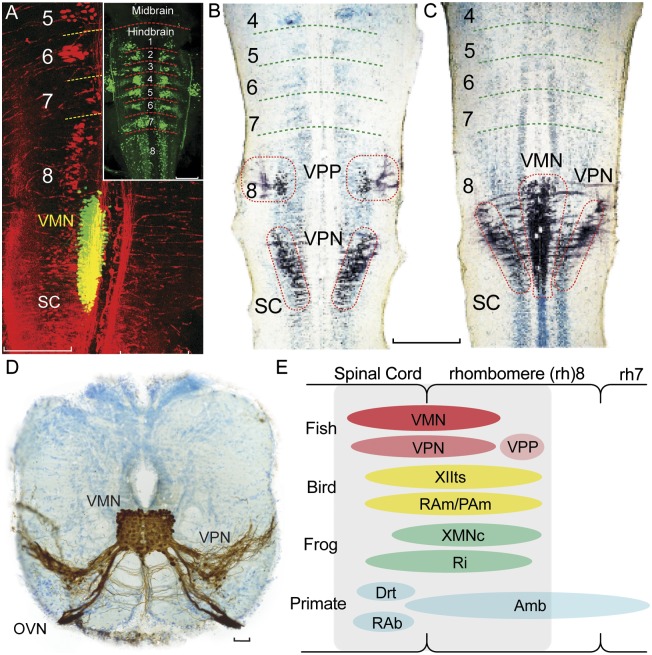
Developmental studies in fishes now support the hypothesis that hindbrain pattern generators for vocalization in the two main clades of bony vertebrates, Actinopterygii and Sarcopterygii (Fig. 1B), share developmental and evolutionary origins. Fluorescent dextran-amine injections into the developing vocal muscle of newly hatched midshipman and toadfish larvae showed a cigar-shaped VMN extending from caudal rh8 into the rostral spinal cord, a longitudinal extent more than twice that of the more anterior rhs 2 to 6 (Fig. 3A). Experimental mapping of VMN relative to highly conserved neuronal landmarks in vertebrates showed, for example, rostral VMN coincident with the caudal pole of the vagal motor column and the caudal pole of the precerebellar inferior olive, both of which originate from rh8 in tetrapods (10). Transneuronal neurobiotin labeling in larvae also showed vocal premotor neurons positioned in caudal rh8 immediately lateral and rostral to VMN (Fig. 3 B and C), matching the locations of VPN and VPP, respectively, in adults (e.g., Fig. 3D) (11).
Fig. 3.
Map of developing vocal pattern generator in rh8-spinal compartment. (A) Fluorescently labeled neurons in plainfin midshipman fish larvae visualized with laser scanning confocal microscopy (horizontal plane). Simultaneous visualization of reticulospinal neurons labeled via retrograde transport from the spinal cord (Alexa 546 dextran-amine, red) and VMN (Alexa 488 dextran-amine, green) labeled via the developing vocal muscle. Yellow is composite overlap and does not indicate double labeling. Inset: Clusters of reticulospinal neurons (Alexa biocytin 488, green) in each rh, from 1 to 8. (Scale bars: 0.2 mm.) (B and C) Mapping in horizontal plane of VPP, VPN, and VMN neurons (black) in Gulf toadfish larvae; labeling via transneuronal transport of neurobiotin from developing vocal muscle. Cresyl violet counterstain reveals segmental, reticulospinal clusters. (Scale bar: 0.2 mm.) (D) Transverse section in caudal hindbrain of toadfish showing transneuronal neurobiotin labeling (brown) of paired midline VMN and adjacent VPNs; VMNs and VPNs have extensive dendritic and axonal branching. VMN axons exit via occipital vocal nerve root (OVN; cresyl violet counterstain). (Scale bar: 100 μm.) (E) Sagittal view summarizing relative positions of hindbrain vocal premotor-motor networks in rh8-spinal compartment of fish, birds, frogs, and mammals including primates, based on early-stage and adult phenotypes (see ref. 11 for details). Most laryngeal motor neurons that shape the temporal envelope of mammalian calls originate from caudal nucleus ambiguus (Amb). Drt, dorsal reticular nucleus; PAm, nucleus parambigualis; RAb, nucleus retroambiguus; RAm, nucleus retroambigualis; Ri, inferior reticular formation; XIIts, tracheosyringeal division of hypoglossal motor nucleus; XMNc, caudal XMN. (Adapted from ref. 11.)
Taxonomic analysis, based on developing and adult hindbrain organization, next showed vocal premotor-motor circuitry (including sites of vocal–respiratory coupling, as detailed earlier) in amphibians, birds, and mammals mapping to the same rh8-spinal compartment as the developing VPP–VPN–VMN network of fish (reviewed in ref. 11; also see refs. 44–51; Fig. 3E). Together, the evidence led to the proposal that a rh8-spinal compartment is the developmental and evolutionary origin of hindbrain vocal pattern generating circuitry among all the major lineages of vocal vertebrates.
Shared Origins of Sonic–Vocal Musculature and Central Mechanisms
Peripheral sonic mechanisms vary between and even within fish lineages (20–22, 52). For example, sculpin (Scorpaeniformes, Cottidae) lack a swim bladder and instead vibrate a single pair of muscles attached to the pectoral girdle (25). Closely related sea robins (Scorpaeniformes, Triglidae), like distantly related midshipman and other toadfishes, have a pair of vocal muscles that are completely attached to the swim bladder (25). Important to the current discussion is that sound-generating muscles in sculpin and sea robins, like toadfishes and other families of sonic fishes, are innervated by occipital nerve roots (25). This suggests that vocal muscles among fishes share developmental origins from occipital somites (53, 54), irrespective of skeletal mechanics and degree of taxonomic relatedness.
Occipital innervation of vocal muscles originating from a VMN at the same hindbrain (rh8)-spinal level is now documented for nine families of closely and distantly related teleost taxa (20, 25, 55, 56). Piranhas (Characiformes) are an exception to the pattern, with spinal-only innervation and a spinal-positioned VMN (refs. 55, 56; ref. 56 describes other likely examples). However, brain stimulation indicates vocal premotor, pattern-generating circuitry in piranhas in the same caudal hindbrain region as the VPP–VPN circuit in toadfishes (57).
Like the sonic organs of fishes, the nonavian larynx and avian syrinx have lineage-specific skeletal characters (e.g., ref. 19), but share vocal muscle origins from occipital somites (see refs. 7, 58 for tetrapods). Laryngeal and syringeal premotor-motor networks are also located in the same rh8-spinal compartment as the vocal network in fishes (Fig. 3E). Hence, vocal premotor-motor circuitry, like vocal muscles, shares developmental and evolutionary origins among vertebrates. Studies of frogs further show that laryngeal nerve output resembles occipital nerve activity in fishes. Like the occipital motor volley in vocal fish, the laryngeal motor volley of frogs matches the temporal properties of natural calls (59, 60). These findings, together with those discussed earlier for piranhas, direct our attention to the conserved nature of vocal premotor mechanisms, regardless of motoneuron targets (also see ref. 61).
Among fishes, acoustic communication is widespread and best known for the highly speciose teleosts (e.g., refs. 52, 62–64). There is also well documented behavioral evidence for acoustic signaling in more basal actinopterygians (see ref. 3 for phylogeny) including sturgeon (Acipenseriformes) (65), bichir (Polypteriformes) (66), and bowfin (Amiiformes) (67). Among basal sarcopterygians, sound production (“grunting”) is noted for lungfish (Dipnoi) (68, 69). A critical test of the hypothesis that occipital somite-derived vocal muscle and rh8/occipital-spinal derived vocal networks are ancestral characters for both major clades of bony vertebrates awaits the demonstration of these characters in one of the more basal (i.e., nonteleost) actinopterygians and a basal sarcopterygian (i.e., nontetrapod). If evidence from one of these more basal groups does not support the hypothesis, we would conclude that the observed vocal characters have independently evolved among actinopterygian and sarcopterygian lineages.
Evolutionary Development of Pectoral Appendage Circuitry
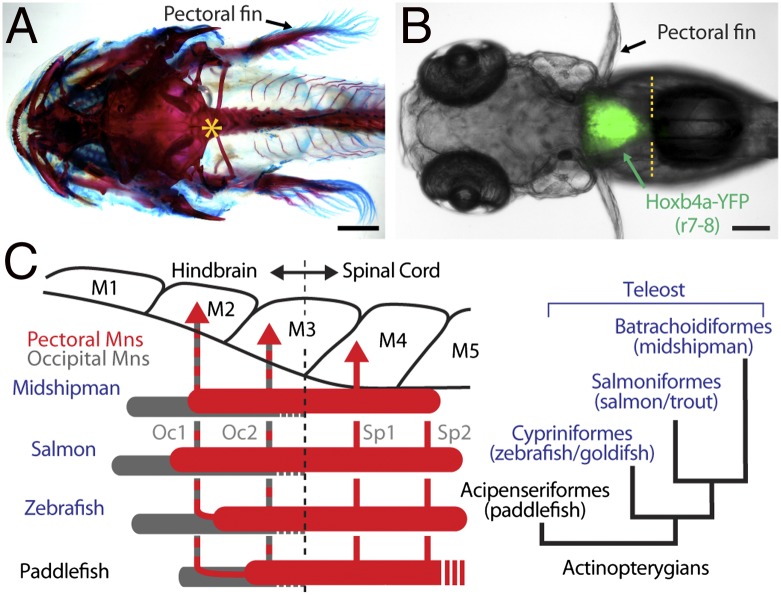
Developmental mapping of the VMN in toadfishes relative to other hindbrain landmarks showed VMN coextensive with the rostral pole of a pectoral motor nucleus (11). This finding led to the suggestion that the rh8-spinal compartment may be the source of neuronal innovations in the central patterning of nonvocal, pectoral-dependent function such as forelimb movement (figure S1 in ref. 11). Since that time, a pectoral motor nucleus in basal and derived groups of bony vertebrates has been shown conclusively to develop in the same rh8-spinal compartment as the vocal system. By using multiple neuronal markers and alignment of the neuroepithelium with myotomes during the pectoral fin bud stage, Ma et al. (13) precisely mapped the entire extent of the pectoral column along with the cranial-vertebral and rh8-spinal boundaries in representative species from three orders of teleosts used extensively as neurobehavioral models: midshipman (Batrachoidiformes), salmon (Salmoniformes), and zebrafish (Cypriniformes). The results for teleosts were compared with those for paddlefish (Acipenseriformes), a more basal order of actinopterygians (Fig. 4 A–C).
Fig. 4.
Map of developing pectoral motor nucleus in rh8-spinal compartment of basal and derived groups of actinopterygian fish (A and B, dorsal views). (A) Craniovertebral junction (asterisk) in postlarval, juvenile midshipman fish cleared and stained with alcian blue and alizarin red. (B) Demarcation of rh8-spinal boundary (yellow hatching) in zebrafish hoxb4a enhancer trap line. (C) Alignment of myotomes (“M”), occipital (Oc1, Oc2), and spinal (Sp1, Sp2) nerves and pectoral (red) and occipital (gray) motoneurons. Phylogeny of study species is also shown (Right). (Adapted from ref. 13.)
Pectoral muscles were innervated by paired occipital (Oc1, Oc2) and anterior spinal (Sp1, Sp2) nerves (Fig. 4C, Left). Pectoral motoneurons, identified following retrograde transport of fluorescent dye from fin buds, were concurrently mapped with other neuronal landmarks including (i) foramina where occipital nerve roots exit the embryonic skull, (ii) genetic markers (hoxb4 expression) for rh8-spinal boundary (70), (iii) nonpectoral motor nuclei including an islet1-GFP line labeling cranial motor nuclei (71), and (iv) cerebellar input from the rh8-derived inferior olive nucleus (10). Pectoral motoneurons extended between myotomes 2–3 and 5–6, with axons exiting via paired occipital roots (Oc1, Oc2) through a single foramen rostral to the cranial-vertebral boundary; axons also exited via the first one or two spinal roots (Sp1, Sp2) (13) (Oc1 and Oc2 also innervate vocal muscles; Fig. 4C, Left). Given the genetic mapping of cranial-vertebral (Fig. 4A) (72) and hindbrain-spinal (70) (Fig. 4B) boundaries between myotomes 3 and 4, the results demonstrated an rh8/occipital-spinal column innervating pectoral muscles in basal and derived actinopterygian species.
To extend the conclusions more broadly, the actinopterygian innervation pattern was compared with that of more basal cartilaginous fishes (Chondrichthyes, Chimaeriformes/ratfish), and representative fish species in the other major clade of bony vertebrates, Sarcopterygii (Dipnoi, lungfish), that includes tetrapods (Fig. 1B). Together with published accounts for a more basal sarcopterygian, the coelacanth (Latimeria) (73, 74) (Fig. 1B), the results showed that occipital and spinal nerve innervation of pectoral muscles was a consistent character across all the investigated lineages of vertebrates.
In sum, precise mapping in pre- and postlarval stages of development showed that the ancestral pattern for pectoral appendage innervation in bony vertebrates is from the rh8/occipital-spinal compartment (Fig. 1C). Pectoral fins, considered more ancient than pelvic fins (75), were previously assumed to receive innervation only from the spinal cord, like the pectoral forelimbs of tetrapods (reviewed in ref. 13). However, the new results in fishes indicate that spinal-only pectoral innervation is a shared derived character (i.e., synapomorphy), along with decoupling of pectoral appendages from the skull and evolution of a neck (76), only for tetrapod forelimbs (Fig. 1C). Despite the change in motoneuron location, premotor pectoral circuitry may be present in the caudal hindbrain of tetrapods, as it is in fishes (77, 78). Although direct evidence is lacking, brain stimulation suggests that caudal hindbrain circuits configure pectoral/forelimb motoneuron activity in mammals (e.g., ref. 79); single neuron recordings like those in fishes (77, 78) are needed to more rigorously test this hypothesis.
Shared Origins of Vocal and Pectoral Circuitry
Additional evidence from the studies reviewed here of pectoral motor development suggests that each functional segment of a myotome, e.g., vocal or pectoral, has an rh8/occipitospinal complement. Fluorescent dye labeling of occipital myotomes in midshipman, salmon, and zebrafish embryos showed an occipital motor column, inclusive of pectoral motoneurons, extending approximately one myotome anterior to the rostral pole of the pectoral column with axons exiting via Oc1 and Oc2 (Fig. 4C, Left). As vocal muscle develops from myotome 2 (53, 54), this more complete labeling of the developing occipital motor column would also include the vocal motor complement. Vocal motoneurons likely come from a vocal “segment” of the occipitospinal column, separate from a pectoral segment innervating pectoral muscle that is also derived, in part, from myotome 2 (53, 54). Together, the results indicate that vocal and pectoral motor systems in fishes share developmental origins from the rh8/occipitospinal compartment. As discussed later, this would also include pectoral-dependent mechanisms of acoustic signaling.
Sonic mechanisms engaging the pectoral skeleton range from tendon snapping in croaking gouramis (23) to pectoral spine vibration in catfish (Fig. 1A) (24) and pectoral girdle vibration in sculpin that lack a swim bladder (25). Despite these divergent mechanisms, sonic motoneurons are positioned in the same hindbrain-spinal region of the pectoral motor column in sculpin (25, 55), catfish (80, 81), and gouramis (82). Sonic neurons map to the same location in sea robins, close relatives of sculpin that have sonic muscles completely attached to the swim bladder as in distantly related toadfishes (25). Some species of catfish exhibit both swim bladder and pectoral-dependent sonic phenotypes (81). These results highlight the developmental and evolutionary coupling of pectoral motor systems that are multifunctional (locomotion and sound production) with vocal systems that are dedicated to acoustic signaling (e.g., in toadfish and sea robins; Fig. 1C), a character that is observed among tetrapods as well (as detailed later).
Shared Intrinsic and Network Properties for rh8
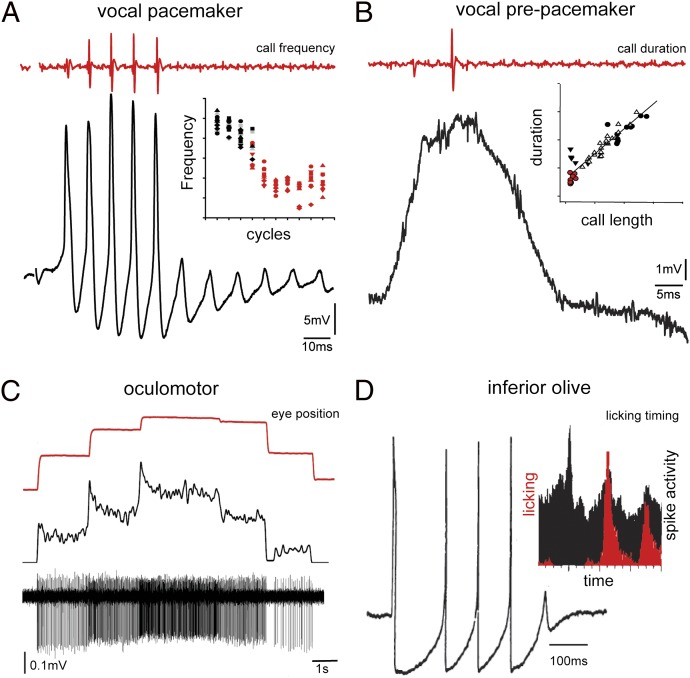
Precise temporal patterning of motor output and hence behavior, like that exemplified by vocalization, requires a suite of intrinsic and network properties to synchronize population level activity (e.g., refs. 83–85) including (i) repolarization conductances underlying oscillatory activity of premotor neurons; (ii) electrotonic coupling, within and between premotor and motor populations; (iii) widespread premotor excitatory input to target neurons; (iv) rhythmic firing of premotor and target population; (v) synchronous premotor firing; and (vi) inhibitory input to premotor neurons. Rhombomere 8/occipital premotor populations showing combinations of these characters include the VPP–VPN network (37, 38), pacemaker neurons in electromotor systems (86), area I neurons of the occulomotor system (87), T-reticular neurons (77, 88) driving pectoral motoneurons during the escape response (78), and the inferior olive (e.g., refs. 89, 90) (Table S1).
Each rh8 premotor population has a distinct electroresponsive “signature” coding for an equally distinct behavioral attribute. Pacemaker (i.e., VPN) membrane oscillations directly code vocal frequency, whereas VPP-sustained depolarizations code vocal duration (Fig. 5 A and B) (37). Oscillatory pacemaker neurons in weakly electric fish, a VPN analogue, directly set electric organ discharge frequency (a VPP analogue is likely missing given the constant electric organ discharge) (86). Area I in fish codes for eye position (Fig. 5C) (87, 91, 92). Like the vocal system, inhibitory coupling and synchronous firing, in this case shown by paired recordings, shape area I firing patterns (92, 93). Like VPN, rh8-derived inferior olive neurons (10) tend to fire synchronously in an oscillatory fashion (Fig. 5D) (94, 95). In addition to voltage-dependent conductances underlying this rhythmicity, inferior olive neurons show strong gap junctional coupling that synchronizes functional groups or patches of neurons involved in complex tasks such as tongue licking (Fig. 5D) (89, 96, 97).
Fig. 5.
Spatiotemporal coding of behavioral attributes by rh8 premotor nuclei. Shown are single traces of neuronal activity (black) of midshipman fish vocal pacemaker (A) and VPP (B), goldfish oculomotor (C) and guinea pig inferior olive (D), and corresponding behavioral readout (red). (A) Inset: Dependency of membrane oscillations (i.e., cycles) and pulse repetition rate/frequency of vocal output. (B) Inset: Dependency of duration of membrane-sustained depolarization and call duration (i.e., length). (D) Inset: Correlation in rats between tongue licking behavior (red) and cerebellar complex spike activity (black) that directly reflects levels of inferior olive activity. [A and B adapted from ref. 37; C reprinted by permission from Macmillan Publishers Ltd: Nature Neuroscience (ref. 93), copyright 2001; D reproduced with permission from John Wiley & Sons (ref. 95); D (Inset) reprinted by permission from Macmillan Publishers Ltd: Nature (ref. 96), copyright 1995.]
Although comparable investigations of intrinsic and network properties are currently lacking for the pectoral motor system, the available neurophysiological data on the neural basis of escape behavior in hatchetfish (Table S1) (78) are consistent with the view that rh8 premotor populations provide a coherent timing signal synchronizing the activity of one or more neuromuscular compartments determining a behavior (sensu ref. 97). Recent studies of hindbrain circuitry in zebrafish, including the pectoral network, have begun to identify developmental events establishing the neuronal complement of rh- and neurobehavioral-specific nuclei (77, 98).
Coupling of Vocal and Pectoral–Gestural Circuitry
There has been much discussion regarding the vocal vs. gestural origins of speech and language (e.g refs. 99, 100; also see refs. 101, 102). The comparison may, however, be a false dichotomy when we consider the shared developmental origins and social signaling functions of vocal and pectoral systems. Birds and mammals, like fishes (as detailed earlier; also see ref. 103), exhibit vocal and pectoral-dependent mechanisms of acoustic communication. For example, various bird species that use the syrinx to vocalize also use pectoral wings innervated by forelimb spinal motoneurons (Fig. 1C) to generate nonvocal, sonic signals important for communication (e.g., manakin; Fig. 1A) (104–108). Examples of nonvocal, sonic pectoral signaling among mammals that use the larynx to vocalize include drumming by macaque monkeys and gorillas and acoustic gesturing by humans (109, 110). More generally, temporal coupling between vocalization and pectoral forelimb movement in humans has led to the hypothesis that “tasks requiring precisely timed movements of the vocal tract and hands and arms appear to share common brain mechanisms” (ref. 111; also see ref. 100). Vocal–gestural coupling is largely considered to depend on forebrain (e.g., premotor/motor cortex, Broca area) and cerebellar (111, 112) mechanisms, with essentially no consideration of the potential role of hindbrain premotor circuitry. Collectively, the available developmental and behavioral evidence discussed here and in previous sections suggests that the neural basis for vocal and pectoral coupling observed among tetrapods, including nonvocal sonic and gestural signaling, has ancient origins among fishes at the most fundamental level of hindbrain pattern generators (Fig. 1C).
Concluding Comments
Pattern-generating circuitry underlying the vocal basis for acoustic communication in fishes and tetrapods evolved from an ancestrally shared hindbrain, rh8-spinal compartment. This compartment also gave rise to premotor-motor circuitry for pectoral appendages that serve locomotion and nonvocal, sonic–acoustic signaling functions in fishes. These shared developmental origins suggest that the functional coupling between more highly derived vocal and pectoral mechanisms that have evolved for acoustic and gestural signaling in tetrapods originated in fishes.
More broadly, we propose that, among vertebrates in general, rh8-spinal networks include anatomically separate premotor nuclei, each of which has a distinct suite of intrinsic and network properties determining specific behavioral attributes (Fig. 5). Each network’s ensemble of premotor nuclei configures the spatiotemporal activity of one or more neuromuscular systems underlying entire behaviors such as vocalization (also see ref. 97). By comparing rh8-spinal networks across vertebrate lineages, we can identify ancestral characters contributing to evolutionarily derived networks, e.g., the anatomical and neurophysiological properties of sonic–vocal networks in fishes found in the sonic–vocal networks of birds and mammals. This includes phylogenetically deep homologies, i.e., “molecular and cellular components… contributing to phenotypic novelties” that “enable us to reconstruct how a phenotype was built over evolutionary time” (113).
Supplementary Material
Acknowledgments
We thank R. Baker, D. Deitcher, L.-H. Ma, R. G. Northcutt, K. Rohmann, N. Segil, and the reviewers for helpful discussions and comments on earlier versions of the manuscript; R. Baker, E. Gilland, and L.-H. Ma for collaborations on research reviewed here; M. Nelson and M. Marchaterre for drawings in Fig. 1; M. Marchaterre for movies used in analysis; E. Adkins-Regan, K. Bostwick, F. Ladich, U. Jürgens, and M. Marchaterre for sound recordings; John Avise, Francisco Ayala, and Georg Striedter for the invitation to participate in the Sackler Colloquium. This work was supported by National Institutes of Health Grant DC00092 and National Science Foundation Grant IOS1120925.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VI: Brain and Behavior,” held January 19–21, 2012, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/evolution_vi.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201886109/-/DCSupplemental.
References
- 1.Gilland E, Baker R. Evolutionary patterns of cranial nerve efferent nuclei in vertebrates. Brain Behav Evol. 2005;66:234–254. doi: 10.1159/000088128. [DOI] [PubMed] [Google Scholar]
- 2.Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- 3.Nelson JS. Fishes of the World. 4th Ed. New York: Wiley; 2006. [Google Scholar]
- 4.Forey P, Janvier P. Agnathans and the origin of jawed vertebrates. Nature. 1993;361:129–134. [Google Scholar]
- 5.Northcutt RG. The brain and sense organs of the earliest vertebrates: Reconstruction of a morphotype. In: Foreman RE, Gorbaman A, Dodd JM, Olssen R, editors. Evolutionary Biology of Primitive Fishes. New York: Plenum; 1985. [Google Scholar]
- 6.Nieuwenhuys R, Donkelaar HJ, Nicholson C. The Central Nervous System of Vertebrates. Berlin: Springer; 1998. [Google Scholar]
- 7.Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- 8.Murakami Y, et al. Segmental development of reticulospinal and branchiomotor neurons in lamprey: insights into the evolution of the vertebrate hindbrain. Development. 2004;131:983–995. doi: 10.1242/dev.00986. [DOI] [PubMed] [Google Scholar]
- 9.Hanneman E, Trevarrow B, Metcalfe WK, Kimmel CB, Westerfield M. Segmental pattern of development of the hindbrain and spinal cord of the zebrafish embryo. Development. 1988;103:49–58. doi: 10.1242/dev.103.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Cambronero F, Puelles L. Rostrocaudal nuclear relationships in the avian medulla oblongata: A fate map with quail chick chimeras. J Comp Neurol. 2000;427:522–545. doi: 10.1002/1096-9861(20001127)427:4<522::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 11.Bass AH, Gilland EH, Baker R. Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science. 2008;321:417–421. doi: 10.1126/science.1157632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prince VE, Moens CB, Kimmel CB, Ho RK. Zebrafish hox genes: Expression in the hindbrain region of wild-type and mutants of the segmentation gene, valentino. Development. 1998;125:393–406. doi: 10.1242/dev.125.3.393. [DOI] [PubMed] [Google Scholar]
- 13.Ma L-H, Gilland E, Bass AH, Baker R. Ancestry of motor innervation to pectoral fin and forelimb. Nat Commun. 2010;1:49. doi: 10.1038/ncomms1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tümpel S, Wiedemann LM, Krumlauf R. hox genes and segmentation of the vertebrate hindbrain. In: Pourquié O, editor. HOX Genes. Vol 88. New York: Academic; 2009. pp. 103–137. [DOI] [PubMed] [Google Scholar]
- 15.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 16.Bass AH, Baker R. Phenotypic specification of hindbrain rhombomeres and the origins of rhythmic circuits in vertebrates. Brain Behav Evol. 1997;50(suppl 1):3–16. doi: 10.1159/000113351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gans C, Northcutt RG. Neural crest and the origin of vertebrates: A new head. Science. 1983;220:268–273. doi: 10.1126/science.220.4594.268. [DOI] [PubMed] [Google Scholar]
- 18.Northcutt RG, Gans C. The genesis of neural crest and epidermal placodes: A reinterpretation of vertebrate origins. Q Rev Biol. 1983;58:1–28. doi: 10.1086/413055. [DOI] [PubMed] [Google Scholar]
- 19.Bradbury J, Vehrencamp S. Principles of Animal Communication. Sunderland, MA: Sinauer; 2011. [Google Scholar]
- 20.Bass AH, Ladich F. Vocal-acoustic communication: From neurons to behavior. In: Webb JF, Fay RR, Popper AN, editors. Fish Bioacoustics. Berlin: Springer; 2008. pp. 253–278. [Google Scholar]
- 21.Fine ML, Ladich F. Sound production, spine locking, and related adaptations. In: Arratia G, Kapoor BG, Chardon M, Diogo R, editors. Catfishes. Vol 1. Enfield, NH: Science Publishers; 2003. pp. 249–290. [Google Scholar]
- 22.Ladich F, Fine ML. Sound-generating mechanisms in fishes: A unique diversity in vertebrates. In: Ladich F, Collin SP, Moller P, Kapoor BG, editors. Communication in Fishes. Vol 1. Enfield, NH: Science Publishers; 2006. pp. 3–43. [Google Scholar]
- 23.Kratochvil H. Der Bau des Lautapparates vom knurrenden Gurami (Trichopsis vittatus; Cuvier & Valenciennes) (Anabantidae, Belontiidae) Zoomorphology. 1978;91:91–99. [Google Scholar]
- 24.Fine ML, et al. Pectoral spine locking and sound production in the channel catfish Ictalurus punctatus. Copeia. 1997;4:777–790. [Google Scholar]
- 25.Bass AH, Baker R. Evolution of homologous vocal control traits. Brain Behav Evol. 1991;38:240–254. doi: 10.1159/000114391. [DOI] [PubMed] [Google Scholar]
- 26.Greenfield DW, Winterbottom R, Collette BB. Review of the toadfish genera (teleostei:Batrachoididae) Proc Calif Acad Sci. 2008;59:665–710. [Google Scholar]
- 27.Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog Neurobiol. 2003;69:1–26. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 28.Bass AH, Remage-Healey L. Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm Behav. 2008;53:659–672. doi: 10.1016/j.yhbeh.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bass AH, Baker R. Sexual dimorphisms in the vocal control system of a teleost fish: Morphology of physiologically identified neurons. J Neurobiol. 1990;21:1155–1168. doi: 10.1002/neu.480210802. [DOI] [PubMed] [Google Scholar]
- 30.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remage-Healey LH, Bass AH. From social behavior to neurons: Rapid modulation of advertisement calling and vocal pattern generators by steroid hormones. Horm Behav. 2006;50:432–441. doi: 10.1016/j.yhbeh.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Rubow TK, Bass AH. Reproductive and diurnal rhythms regulate vocal motor plasticity in a teleost fish. J Exp Biol. 2009;212:3252–3262. doi: 10.1242/jeb.032748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen MJ, Winn HE. Electrophysiological observations on hearing and sound production in the fish, Porichthys notatus. J Exp Zool. 1967;165:355–369. doi: 10.1002/jez.1401650305. [DOI] [PubMed] [Google Scholar]
- 34.Bass AH, Marchaterre MA, Baker R. Vocal-acoustic pathways in a teleost fish. J Neurosci. 1994;14:4025–4039. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448:298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- 36.Kittelberger JM, Land BR, Bass AH. Midbrain periaqueductal gray and vocal patterning in a teleost fish. J Neurophysiol. 2006;96:71–85. doi: 10.1152/jn.00067.2006. [DOI] [PubMed] [Google Scholar]
- 37.Chagnaud BP, Baker R, Bass AH. Vocalization frequency and duration are coded in separate hindbrain nuclei. Nat Commun. 2011;2:346. doi: 10.1038/ncomms1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chagnaud BP, Zee MC, Baker R, Bass AH. Innovations in motoneuron synchrony drive rapid temporal modulations in vertebrate acoustic signaling. J Neurophysiol. 2012 doi: 10.1152/jn.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weeg MS, Land BR, Bass AH. Vocal pathways modulate efferent neurons to the inner ear and lateral line. J Neurosci. 2005;25:5967–5974. doi: 10.1523/JNEUROSCI.0019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gans C. Sound production in the salientia: Mechanism and evolution of the emitter. Am Zool. 1973;13:1179–1194. [Google Scholar]
- 41.Gans C, Maderson PFA. Sound producing mechanisms in recent reptiles: Review and comment. Am Zool. 1973;13:1195–1203. [Google Scholar]
- 42.Brantley RK, Bass AH. Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish, Porichthys notatus (Teleostei, Batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- 43.Peterson RH. Pectoral fin and opercular movements of atlantic salmon (Salmo salar) alevins. J Fish Res Board Can. 1975;32:643–647. [Google Scholar]
- 44.Zornik E, Kelley DB. Breathing and calling: Neuronal networks in the Xenopus laevis hindbrain. J Comp Neurol. 2007;501:303–315. doi: 10.1002/cne.21145. [DOI] [PubMed] [Google Scholar]
- 45.Wild JM, Kubke MF, Mooney R. Avian nucleus retroambigualis: Cell types and projections to other respiratory-vocal nuclei in the brain of the zebra finch (Taeniopygia guttata) J Comp Neurol. 2009;512:768–783. doi: 10.1002/cne.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang SP, Bandler R, Davis PJ. Brain stem integration of vocalization: Role of the nucleus retroambigualis. J Neurophysiol. 1995;74:2500–2512. doi: 10.1152/jn.1995.74.6.2500. [DOI] [PubMed] [Google Scholar]
- 47.Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt MF, McLean J, Goller F. Breathing and vocal control: The respiratory system as both a driver and a target of telencephalic vocal motor circuits in songbirds. Exp Physiol. 2012;97:455–461. doi: 10.1113/expphysiol.2011.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 50.Straka H, Baker R, Gilland E. Preservation of segmental hindbrain organization in adult frogs. J Comp Neurol. 2006;494:228–245. doi: 10.1002/cne.20801. [DOI] [PubMed] [Google Scholar]
- 51.Jürgens U, Hage SR. On the role of the reticular formation in vocal pattern generation. Behav Brain Res. 2007;182:308–314. doi: 10.1016/j.bbr.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 52.Parmentier E, Vandewalle P, Brié C, Dinraths L, Lecchini D. Comparative study on sound production in different Holocentridae species. Front Zool. 2011;8:12. doi: 10.1186/1742-9994-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tracy HC. Stages in the development of the anatomy of motility of the toadfish (Opsanus tau) J Comp Neurol. 1959;111:27–81. [Google Scholar]
- 54.Tracy HC. Development of the spinal neural crest, nerves, and muscles in the toadfish (Opsanus tau) J Comp Neurol. 1961;116:291–315. doi: 10.1002/cne.901160304. [DOI] [PubMed] [Google Scholar]
- 55.Ladich F, Bass AH. Sonic motor pathways in piranhas with a reassessment of phylogenetic patterns of sonic mechanisms among teleosts. Brain Behav Evol. 2005;66:167–176. doi: 10.1159/000087157. [DOI] [PubMed] [Google Scholar]
- 56.Onuki A, Somiya H. Innervation of sonic muscles in teleosts: Occipital vs. spinal nerves. Brain Behav Evol. 2007;69:132–141. doi: 10.1159/000095202. [DOI] [PubMed] [Google Scholar]
- 57.Kastberger G. Economy of sound production in piranhas (Serrasalminae, Characidae): II. functional properties of sound emitter. Zool Jb Physiol. 1981;85:393–411. [Google Scholar]
- 58.Huang R, Zhi Q, Izpisua-Belmonte JC, Christ B, Patel K. Origin and development of the avian tongue muscles. Anat Embryol (Berl) 1999;200:137–152. doi: 10.1007/s004290050268. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt RS. Neural correlates of frog calling: Production by two semi-independent generators. Behav Brain Res. 1992;50:17–30. doi: 10.1016/s0166-4328(05)80284-0. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi A, Kelley DB. Generating sexually differentiated vocal patterns: Laryngeal nerve and EMG recordings from vocalizing male and female african clawed frogs (Xenopus laevis) J Neurosci. 2000;20:1559–1567. doi: 10.1523/JNEUROSCI.20-04-01559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zornik E, Katzen AW, Rhodes HJ, Yamaguchi A. NMDAR-dependent control of call duration in Xenopus laevis. J Neurophysiol. 2010;103:3501–3515. doi: 10.1152/jn.00155.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ladich F, Collin S, Moller P, Kapoor BG. Communication in Fishes. Enfield, NH: Science Publishers; 2006. [Google Scholar]
- 63.Lobel PS, Kaatz IM, Rice AN. Acoustical behavior of coral reef fishes. In: Cole KS, editor. Reproduction and Sexuality in Marine Fishes: Evolutionary Patterns and Innovations. San Diego: Elsevier; 2010. pp. 307–386. [Google Scholar]
- 64.Malavasi S, Collatuzzo S, Torricelli P. Interspecific variation of acoustic signals in Mediterranean gobies (Perciformes, Gobiidae): Comparative analysis and evolutionary outlook. Biol J Linn Soc Lond. 2008;93:763–778. [Google Scholar]
- 65.Johnston CE, Phillips CT. Sound production in sturgeon Scaphirhynchus albust and S. platorynchus (Acipenseridae) Environ Biol Fishes. 2003;68:59–64. [Google Scholar]
- 66.Ladich F, Tadler A. Sound production in polypterus (Osteichthyes: Polypteridae) Copeia. 1988;(4):1076–1077. [Google Scholar]
- 67.Fülleborn F. Bericht über eine zur Untersuchung der Entwickelung von Amia, Lepidosteus und Necturus unternommene Reise nach Nord-America. Berlin: Sitzungsberichte der Preussischen Akademie der Wissenschaften; 1894. [Google Scholar]
- 68.Günther A. Description of ceratodus, a genus of ganoid fishes, recently discovered in rivers of Queensland, Australia. Proc R Soc Lond. 1870;19:377–379. [Google Scholar]
- 69.Thomson KS. Lung ventilation in dipnoan fishes. Postila. 1968;122:1–6. [Google Scholar]
- 70.Ma L-H, Punnamoottil B, Rinkwitz S, Baker R. Mosaic hoxb4a neuronal pleiotropism in zebrafish caudal hindbrain. PLoS ONE. 2009;4:e5944. doi: 10.1371/journal.pone.0005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000;20:206–218. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morin-Kensicki EM, Melancon E, Eisen JS. Segmental relationship between somites and vertebral column in zebrafish. Development. 2002;129:3851–3860. doi: 10.1242/dev.129.16.3851. [DOI] [PubMed] [Google Scholar]
- 73.Northcutt RG, Bemis WE. Cranial nerves of the coelacanth, Latimeria chalumnae [Osteichthyes: Sarcopterygii: Actinistia], and comparisons with other craniata. Brain Behav Evol. 1993;42(suppl 1):1–76. doi: 10.1159/000114175. [DOI] [PubMed] [Google Scholar]
- 74.Millot J, Anthony J. Anatomie de Latimeria chalumnae. System Nerveux et Organes des Sens. Paris: Centre National de Recherche Scientifique; 1965. [Google Scholar]
- 75.Coates M. The evolution of paired fins. Theory Biosci. 2003;122:266–287. [Google Scholar]
- 76.Daeschler EB, Shubin NH, Jenkins FA., Jr A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature. 2006;440:757–763. doi: 10.1038/nature04639. [DOI] [PubMed] [Google Scholar]
- 77.Koyama M, Kinkhabwala A, Satou C, Higashijima S, Fetcho J. Mapping a sensory-motor network onto a structural and functional ground plan in the hindbrain. Proc Natl Acad Sci USA. 2011;108:1170–1175. doi: 10.1073/pnas.1012189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Auerbach AA, Bennett MVL. A rectifying electrotonic synapse in the central nervous system of a vertebrate. J Gen Physiol. 1969;53:211–237. doi: 10.1085/jgp.53.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Drew T, Dubuc R, Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol. 1986;55:375–401. doi: 10.1152/jn.1986.55.2.375. [DOI] [PubMed] [Google Scholar]
- 80.Ladich F, Bass AH. Sonic/vocal motor pathways in catfishes: Comparisons with other teleosts. Brain Behav Evol. 1998;51:315–330. doi: 10.1159/000006545. [DOI] [PubMed] [Google Scholar]
- 81.Ladich F, Fine ML. Localization of swimbladder and pectoral motoneurons involved in sound production in pimelodid catfish. Brain Behav Evol. 1994;44:86–100. doi: 10.1159/000113572. [DOI] [PubMed] [Google Scholar]
- 82.Ladich F, Fine ML. Localization of pectoral fin motoneurons (sonic and hovering) in the croaking gourami Trichopsis vittatus. Brain Behav Evol. 1992;39:1–7. doi: 10.1159/000114099. [DOI] [PubMed] [Google Scholar]
- 83.Perez Velazquez JL, Carlen PL. Gap junctions, synchrony and seizures. Trends Neurosci. 2000;23:68–74. doi: 10.1016/s0166-2236(99)01497-6. [DOI] [PubMed] [Google Scholar]
- 84.Van Vreeswijk C, Abbott LF, Ermentrout GB. When inhibition not excitation synchronizes neural firing. J Comput Neurosci. 1994;1:313–321. doi: 10.1007/BF00961879. [DOI] [PubMed] [Google Scholar]
- 85.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 86.Bennett MVL. Electric organs. In: Hoar WS, Randall DJ, editors. Fish Physiology. Vol V. New York: Academic; 1971. pp. 347–491. [Google Scholar]
- 87.Pastor AM, De la Cruz RR, Baker R. Eye position and eye velocity integrators reside in separate brainstem nuclei. Proc Natl Acad Sci USA. 1994;91:807–811. doi: 10.1073/pnas.91.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kimmel CB, Metcalfe WK, Schabtach E. T reticular interneurons: A class of serially repeating cells in the zebrafish hindbrain. J Comp Neurol. 1985;233:365–376. doi: 10.1002/cne.902330306. [DOI] [PubMed] [Google Scholar]
- 89.Urbano FJ, Simpson JI, Llinás RR. Somatomotor and oculomotor inferior olivary neurons have distinct electrophysiological phenotypes. Proc Natl Acad Sci USA. 2006;103:16550–16555. doi: 10.1073/pnas.0607888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aksay E, Baker R, Seung HS, Tank DW. Anatomy and discharge properties of pre-motor neurons in the goldfish medulla that have eye-position signals during fixations. J Neurophysiol. 2000;84:1035–1049. doi: 10.1152/jn.2000.84.2.1035. [DOI] [PubMed] [Google Scholar]
- 92.Aksay E, Baker R, Seung HS, Tank DW. Correlated discharge among cell pairs within the oculomotor horizontal velocity-to-position integrator. J Neurosci. 2003;23:10852–10858. doi: 10.1523/JNEUROSCI.23-34-10852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW. In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat Neurosci. 2001;4:184–193. doi: 10.1038/84023. [DOI] [PubMed] [Google Scholar]
- 94.Llinás R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- 95.Llinás R, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: An in vitro study. J Physiol. 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Welsh JP, Lang EJ, Sugihara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 97.Llinás R, Paré D. Role of intrinsic neuronal oscillations and network ensembles in the genesis of normal and pathological tremors. In: Findley LJ, Koller WC, editors. Handbook of Tremor Disorders. New York: Marcel Dekker; 1994. pp. 7–36. [Google Scholar]
- 98.Kinkhabwala A, et al. A structural and functional ground plan for neurons in the hindbrain of zebrafish. Proc Natl Acad Sci USA. 2011;108:1164–1169. doi: 10.1073/pnas.1012185108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liebal K, Call J. The origins of non-human primates’ manual gestures. Philos Trans R Soc Lond B Biol Sci. 2012;367:118–128. doi: 10.1098/rstb.2011.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gentilucci M, Dalla Volta R, Gianelli C. When the hands speak. J Physiol Paris. 2008;102:21–30. doi: 10.1016/j.jphysparis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 101.MacNeilage P. The Origin of Speech. New York: Oxford Univ Press; 2008. [Google Scholar]
- 102.Lieberman P. Toward an Evolutionary Biology of Language. Cambridge, MA: Harvard Univ Press; 2006. [Google Scholar]
- 103.Ladich F, Brittinger W, Kratochvil H. Significance of agonistic vocalization in the croaking gourami (Trichopsis vittatus, teleostei) Ethology. 1992;90:307–314. [Google Scholar]
- 104.Prum RO. Sexual selection and the evolution of mechanical sound production in manakins (Aves: Pipridae) Anim Behav. 1998;55:977–994. doi: 10.1006/anbe.1997.0647. [DOI] [PubMed] [Google Scholar]
- 105.Barske J, Schlinger BA, Wikelski M, Fusani L. Female choice for male motor skills. Proc R Soc B. 2011;278:3523–3528. doi: 10.1098/rspb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bostwick KS, Elias DO, Mason A, Montealegre-Z F. Resonating feathers produce courtship song. Proc R Soc B. 2010;277:835–841. doi: 10.1098/rspb.2009.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hingee M, Magrath RD. Flights of fear: A mechanical wing whistle sounds the alarm in a flocking bird. Proc R Soc B. 2009;276:4173–4179. doi: 10.1098/rspb.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miller EH, Baker AJ. Antiquity of shorebird acoustic displays. Auk. 2009;126:454–459. [Google Scholar]
- 109.Remedios R, Logothetis NK, Kayser C. Monkey drumming reveals common networks for perceiving vocal and nonvocal communication sounds. Proc Natl Acad Sci USA. 2009;106:18010–18015. doi: 10.1073/pnas.0909756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reynolds V. Some behavioral comparisons between the chimpanzee and the mountain gorilla in the wild. Am Anthropol. 1965;67:691–706. [Google Scholar]
- 111.Iverson JM, Thelen E. Hand, mouth and brain. The dynamic emergence of speech and gesture. J Conscious Stud. 1999;6:19–40. [Google Scholar]
- 112.Iverson JM, Fagan MK. Infant vocal-motor coordination: Precursor to the gesture-speech system? Child Dev. 2004;75:1053–1066. doi: 10.1111/j.1467-8624.2004.00725.x. [DOI] [PubMed] [Google Scholar]
- 113.McCune AR, Schimenti JC. Using genetic networks and homology to understand the evolution of phenotypic traits. Curr Genomics. 2012;13:74–84. doi: 10.2174/138920212799034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.