Abstract
Streptococcus sanguinis is one of the most common agents of infective endocarditis. Spx proteins are a group of global regulators that negatively or positively control global transcription initiation. In this study, we characterized the spxA1 gene in S. sanguinis SK36. The spxA1 null mutant displayed opaque colony morphology, reduced hydrogen peroxide (H2O2) production, and reduced antagonistic activity against Streptococcus mutans UA159 relative to the wild type strain. The ΔspxA1 mutant also demonstrated decreased tolerance to high temperature, acidic and oxidative stresses. Further analysis revealed that ΔspxA1 also exhibited a ∼5-fold reduction in competitiveness in an animal model of endocarditis. Microarray studies indicated that expression of several oxidative stress genes was downregulated in the ΔspxA1 mutant. The expression of spxB and nox was significantly decreased in the ΔspxA1 mutant compared with the wild type. These results indicate that spxA1 plays a major role in H2O2 production, stress tolerance and endocarditis virulence in S. sanguinis SK36. The second spx gene, spxA2, was also found in S. sanguinis SK36. The spxA2 null mutant was found to be defective for growth under normal conditions and showed sensitivity to high temperature, acidic and oxidative stresses.
Introduction
Streptococcus sanguinis is a member of the human indigenous oral microbiota and is known as a pioneering colonizer in the formation of dental plaque [1]–[4]. S. sanguinis is also one of the most common agents of infective endocarditis (IE) among the viridans streptococci [5]–[7]. IE is a serious endovascular infection that carries a high risk of morbidity and mortality and is the fourth leading cause of life-threatening infectious disease syndromes [8]. In cases of IE, it is thought that damage to the heart results in the formation of sterile cardiac “vegetations” composed of platelets and fibrin. These sterile vegetations can then be colonized by certain bacteria during periods of bacteremia [9]. This view is supported by animal studies in which formation of sterile vegetation by cardiac catheterization is required for the efficient establishment of streptococcal endocarditis [10].
On the other hand, in the oral cavity, S. sanguinis is antagonistic against S. mutans, a facultative anaerobic bacterium that is a significant contributor to tooth decay [11], [12]. It has been reported that relatively high proportions of S. sanguinis are generally found in dental plaque in association with lower levels of S. mutans [12], [13]. This antagonistic activity against S. mutans is reported to be via hydrogen peroxide (H2O2) production by S. sanguinis [14]. So the production of H2O2 is considered an important property of S. sanguinis concerning its protective role in the oral community.
Spx proteins are a group of global regulators that interact directly with the α-subunit of the RNA polymerase (RNAP) and thereby, negatively or positively control global transcription initiation [15], [16]. The Spx global regulator is highly conserved among low-GC Gram-positive bacteria [16]. It has been well studied in Bacillus subtilis [17]–[19] and global analysis has shown that it regulates the expression of different subsets of genes involved in oxidative stress, developmental programs and energy-consuming growth-related functions [15]. It is reported that Spx is a substrate of ClpXP proteolysis [19], which is critical for maintaining cellular homeostasis as well as expression of virulence properties, and that its accumulation is responsible for the pleiotropic phenotypes associated with clpXP mutations [20]. The interactions between ClpXP and Spx are suggested to be relatively conserved among Gram-positive bacteria [21]. To date, Spx regulators have been studied in many species, including Lactococcus lactis [22], B. subtilis [15], [17], [20], S. mutans [23] and Streptococcus pneumoniae [24], where they fulfill important roles in general stress protection. Concerning the mechanism of regulation, Spx has been shown to be involved in transcriptional repression by interacting with the C-terminal domain of the RNAP α-subunit (α-CTD), which may prevent interaction with specific activator proteins [15]. Furthermore, activation of transcription requires contact between the Spx/RNA polymerase complex and upstream promoter DNA, thereby allowing Spx-induced engagement of RNA polymerase subunits with the core promoter [25].
Due to its important regulatory role, Spx is involved in various physiological functions. In Staphylococcus aureus, Spx was shown a global effector impacting stress tolerance and biofilm formation [26]. SpxA1 was shown to be involved in X-state (competence) development in S. pneumoniae [24] and two Spx proteins were identified with the ability to modulate stress tolerance, survival and virulence in dental caries in S. mutans [23].
Here we report on the identification of a spxA1 gene in S. sanguinis SK36. Subsequent functional characterization of spxA1 revealed that spxA1 is involved in H2O2 production, stress tolerance and IE virulence in S. sanguinis SK36. Preliminary characterization of spxA2, which encodes the second Spx protein, is also reported.
Results
ΔspxA1 Demonstrated Opaque Colony Morphology and a Reduced Rate of H2O2 Production
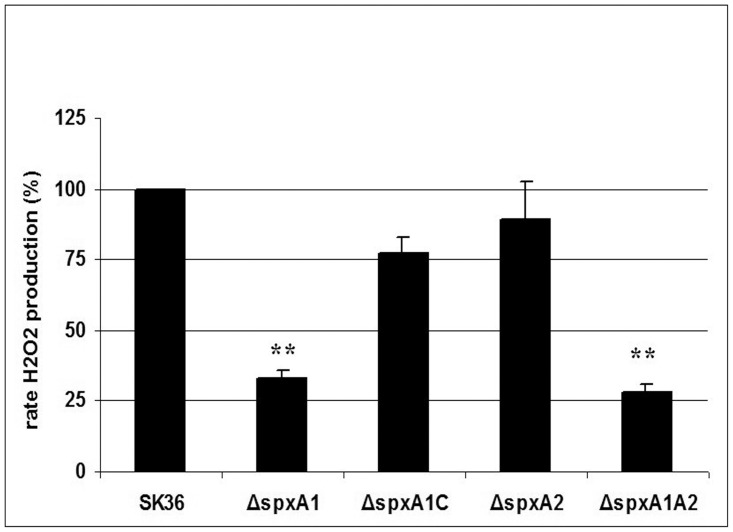
During genome-wide gene deletion studies in S. sanguinis SK36 [27], we identified a mutant of SSA_0937, (denoted as ΔspxA1) that demonstrated opaque colonies when cultured on BHI plates. Opaque colony morphology was previously found to correlate with decreased H2O2 production in S. sanguinis SK36 [28]. Subsequent quantification of H2O2 production in this mutant showed that when compared with the SK36 wild type strain, H2O2 levels in ΔspxA1 were significantly reduced. Specifically, the mutant produced only ∼33% of the H2O2 levels observed in the wild type (Fig. 1). These results suggest that SxpA1 is involved in colony morphology and H2O2 production in S. sanguinis SK36.
Figure 1. H2O2 production in S. sanguinis strains.
H2O2 production normalized to culture densities was determined relative to that produced by the wild type strain SK36. Results are expressed as the mean of percentage values relative to the wild-type strain SK36 from three biological repeats. Statistical significance is indicated (**P<0.01).
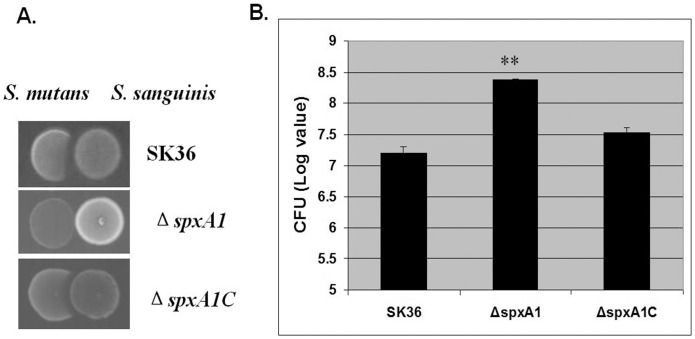
Since the formation of H2O2 in S. sanguinis plays an important role in interspecies interactions within the oral microflora [14], we performed competition assays [28] to examine whether ΔspxA1 showed any difference from the parent strain, SK36, with regard to S. sanguinis’ capacity for antagonism against S. mutans. The results indicated that ΔspxA1 showed reduced antagonistic activity against S. mutans UA159 both on plates (Fig. 2A) and in broth media (Fig. 2B), suggesting SpxA1 is an important protein that confers a competitive advantage for S. sanguinis.
Figure 2. Inhibitory effect of S. sanguinis strains on S. mutans .
(A) Inhibition assay on plates. Overnight cultures of S. sanguinis SK36, ΔspxA1 or ΔspxA1C were inoculated on BHI plates, which were incubated for 16 h at 37°C under microaerobic conditions. S. mutans UA159 was then inoculated next to the pioneer colonizer, and the plates were further incubated overnight and photographed. (B) Inhibition assay in liquid media. Overnight cultures of S. sanguinis SK36, ΔspxA1 or ΔspxA1C were adjusted to the same optical density and mixed with the S. mutans smx_42 in fresh BHI medium. After overnight growth, the cells were serially diluted and plated on BHI plates supplemented with chloramphenicol. The CFUs of S. mutans are indicated in logarithmic values, with standard deviations calculated from triplicate mixtures (**P<0.01 relative to the values obtained for the SK36 mixture).
To ensure the involvement of spxA1 in H2O2 production, we next constructed a complemented strain whereby spxA1 was re-introduced back into the mutant (ΔspxA1). For this purpose, a chloramphenicol resistance cassette was placed downstream of spxA1 for selection. The resulting complemented strain, denoted as ΔspxA1C, was examined for colony morphology and H2O2 production. The results indicated that the morphology of ΔspxA1C was restored to semi-transparent similar to the wild type and that H2O2 levels were also partially restored (∼77% that of the wild type; Fig. 1). These data confirm the involvement of spxA1 in H2O2 production, demonstrating that the observed phenotypes associated with ΔspxA1 were not the result of polar effects.
Sequence Analysis of SpxA1 and Identification of a Second Spx in S. sanguinis
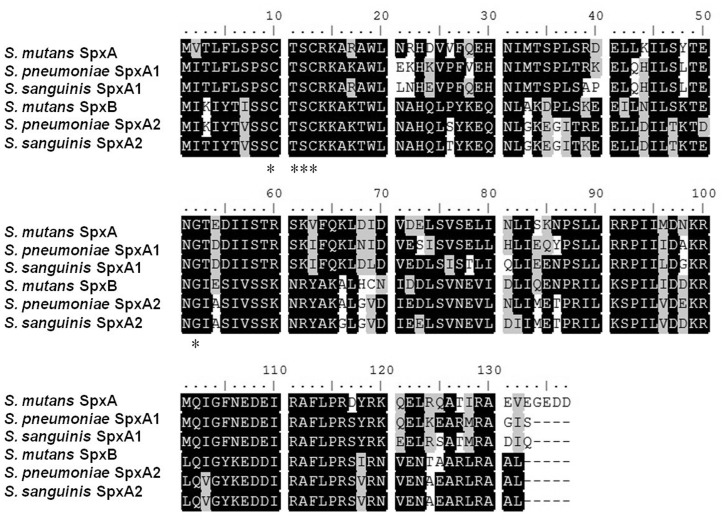
SpxA1 consists of 133 amino acids and is a member of the arsenate reductase family, which includes true arsenate reductases (ArsC) and Spx proteins as revealed by in silico analyses. Spx proteins are a group of global regulators that interact directly with the RNAP and have been well studied in B. subtilis [17]–[19] and streptococci species including S. pneumoniae [24] and S. mutans [23]. Motif analysis indicated that SpxA1 possesses the conserved amino terminus motif Cys10-X-X-Cys13 (CXXC) (Fig. 3) that has previously been shown to sense the intracellular redox state via disulfide bond formation [29], [30]. Moreover, the Gly52 residue that is essential for the interaction of the B. subtilis SpxA with the RNAP α-CTD [30]–[32] is also conserved in SpxA1 (Fig. 3). These findings suggest that SpxA1 in S. sanguinis may also share important physiological functions common to other Spx proteins.
Figure 3. Alignment of amino acid sequences of the two Spx proteins from S. mutans, S. pneumonia and S. sanguinis.
Identical residues are shaded in black and similar ones in gray. The conserved CXXC motif and Gly52 residue discussed in the text are stars (*) labeled. The GenBank accession numbers: S. mutans SpxA, SMU.1142c; S. pneumonia SpxA1, SPR1262; S. sanguinis SpxA1, SSA_0937; S. mutans SpxB, SMU.2084c; S. pneumonia SpxA2, SPR0173; S. sanguinis SpxA2, SSA_2244.
Two Spx paralogs have previously been identified in S. pneumoniae and S. mutans [23], [24], therefore, we performed a BLASTP search against proteins annotated in the genome of S. sanguinis SK36 [4] to determine if paralogs were also present in this strain. Using the SpxA (SMU1142) and SpxB (SMU2084) of S. mutans UA159 [23], two significant hits were identified; one of which was SSA_0937 (SpxA) and the other was SSA_2244 (Fig. 3). Since SSA_2244 is approximately the same size (132 AA) as SpxA1 and also contains the conserved amino terminus motif CXXC and the Gly52 residue, we named this second protein, SpxA2.
Next, SpxA2 was inactivated by replacing the ORF (SSA_2244) with the kanamycin resistance cassette (aphA-3) to determine its function in S. sanguinis SK36 [33]. We also constructed a simultaneous deletion of spxA1 and spxA2 to determine its function in relation to spxA1. In S. pneumoniae R6, it was reported that spxA1 and spxA2 were essential since simultaneous inactivation of both genes was lethal [24]. While in S. mutans UA159, the double mutant of the two spx genes was viable [23]. We were able to successfully obtain the simultaneous double mutant (ΔspxA1A2), indicating that, similar to S. mutans UA159, spxA1 and spxA2 are not essential in S. sanguinis SK36. Phenotypic analysis showed that the rate of H2O2 production of ΔspxA2 was not significantly different from wild type (Fig. 1), consistent with its normal semi-transparent colony morphology. However, the double mutant ΔspxA1A2 showed reduced rates of H2O2 production (Fig. 1).
SpxA1 Involved in Tolerance to High Temperature, Reduced pH and Oxidative Stresses
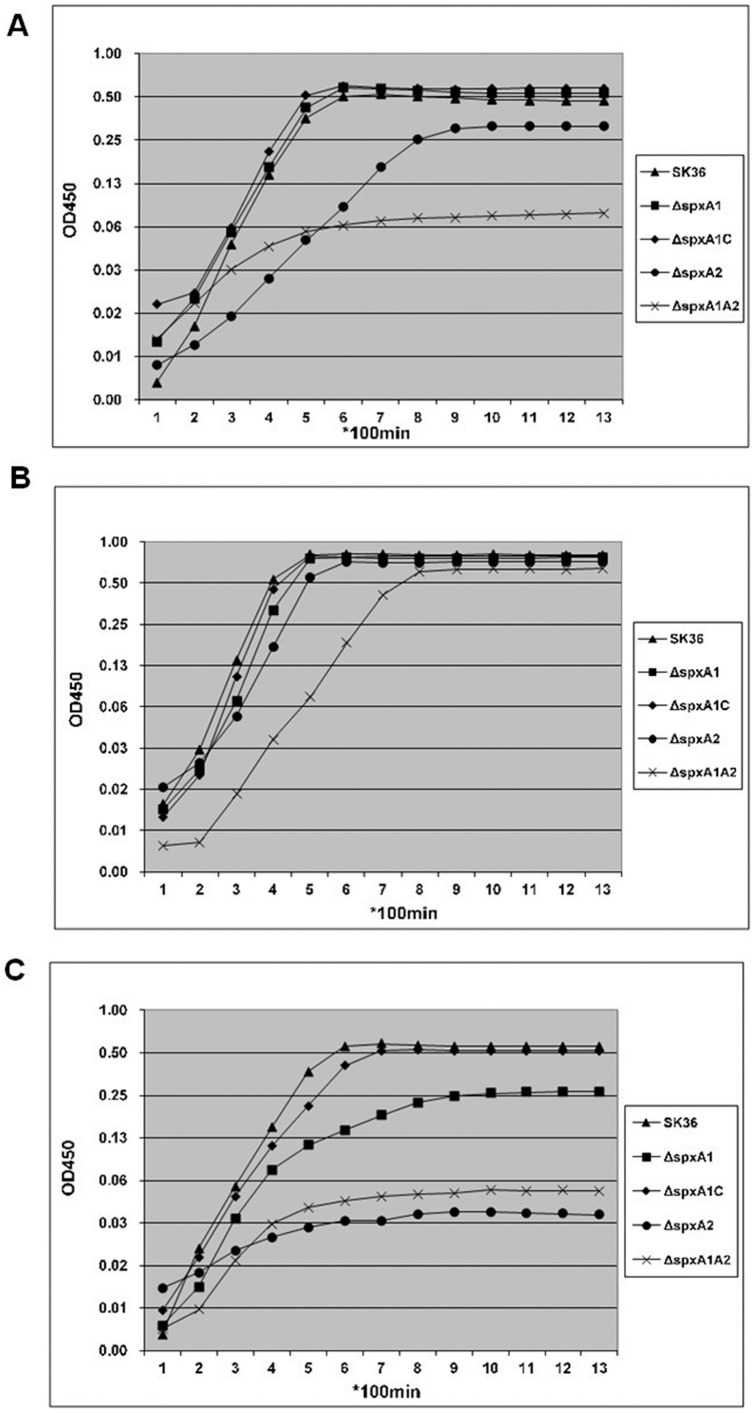
Since Spx proteins were found to play important roles in general stress protection in many species [23], [24], [26], we wondered if this too, was the case in S. sanguinis SK36. First, the impact of Spx proteins was analyzed in response to high temperature stress. Wild type and mutant strains were both cultured under a normal growth temperature of 37°C or a higher growth temperature of 40°C (Fig. 4). The wild type strain was able to grow under both temperature conditions (37°C and 40°C). While ΔspxA1 did not show any significant difference in growth compared to the wild type when cultivated under the normal growth temperature (37°C) either aerobically (Fig. 4A) or anaerobically (Fig. 4B), when cultivated at a higher temperature (40°C) (Fig. 4C), the growth of the ΔspxA1 mutant was significantly compromised compared to the wild type, as well as compared to the growth at 37°C (Fig. 4A). As expected, the growth of the complemented strain (ΔspxA1C) under a higher temperature was restored to that of the wild type (Fig. 4C). This indicated that spxA1 was involved in tolerance to high temperature stress in S. sanguinis SK36. At the same time, another spx mutant, ΔspxA2 was defective in growth both when cultivated under aerobic (Fig. 4A) or higher temperature conditions (Fig. 4C). The double mutant ΔspxA1A2 was much slower in growth than ΔspxA2 under aerobic (Fig. 4A) or higher temperature conditions (Fig. 4C).
Figure 4. Growth curves of S. sanguinis strains.
Overnight cultured bacteria were trans-inoculated into wells in a 96-well plate containing 200 µl BHI media with 1% inoculation and OD450 of each well was determined with a FLUOstar plate reader every 100 minutes at 37°C (A) or 40°C (C) under aerobic condition. For anaerobic conditions (B), an overlay of 50 µl of sterile mineral oil was included in each well of the plate to maintain an anaerobic environment. The growth curves were obtained from average OD450 of at least three repeated wells.
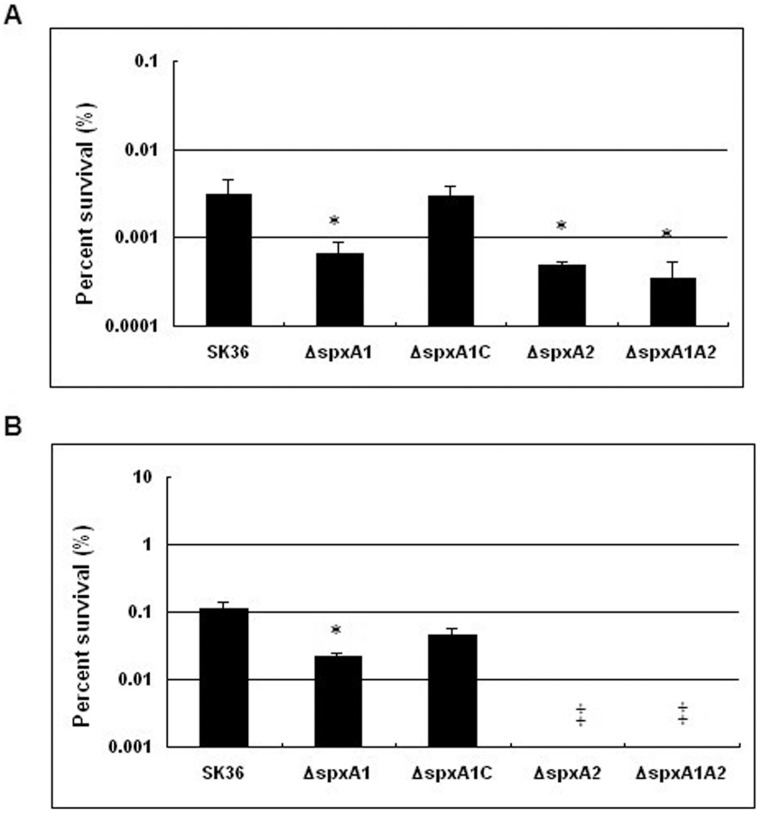
Next, acid tolerance assays were performed. Results demonstrated that ΔspxA1 showed a reduced ability to survive under acidic stress compared with the wild type SK36 strain (Fig. 5A). The acidic tolerance of the complemented strain ΔspxA1C was also analyzed and results demonstrated that its tolerance to acid was restored (Fig. 5A). These results suggest that SpxA1 plays a role in acidic stress tolerance. In the case of the ΔspxA2 mutant and the double mutant ΔspxA1A2, both mutants demonstrated reductions in their ability to survive acidic stress (Fig. 5A). In addition to spxA1, these data indicate that spxA2 may also play an important role in acidic stress tolerance.
Figure 5. Acid tolerance (A) and H2O2 sensitivity (B) assays of S. sanguinis strains.
The survival percentage after treatment was presented. Data from three biological replicates were averaged and the statistical significance differences relative to SK36 were determined. (*P<0.05). ‡: Bacteria did not survive.
Finally, we investigated the role of spx genes in H2O2 protection using H2O2 sensitivity assays. The results showed that as expected, ΔspxA1 was sensitive to H2O2 killing (Fig. 5B) relative to the wild type SK36 strain; while H2O2 sensitivity was partially restored in the complemented strain ΔspxA1C (Fig. 5B). To further address the possible polar effect, we inactivated two of the spxA1 downstream genes, ssa_0938 and ssa_0939, respectively and checked their H2O2 sensitivity under the same condition. Both mutants showed no significant difference from the wild type, which further supported the H2O2 sensitivity role of spxA1 (data not shown). The ΔspxA2 mutant and the double mutant ΔspxA1A2 also demonstrated an increase in sensitivity to oxidative stress (Fig. 5B); these strains were unable to survive at concentrations as low as 20 mM H2O2 condition for 60 min (Fig. 5B). Taken together, these results confirm that spxA1 and spxA2 play significant roles in oxidative stress tolerance of S. sanguinis SK36.
ΔspxA1 Demonstrated Reduced Competitiveness in CI Assays in the Animal Model of IE
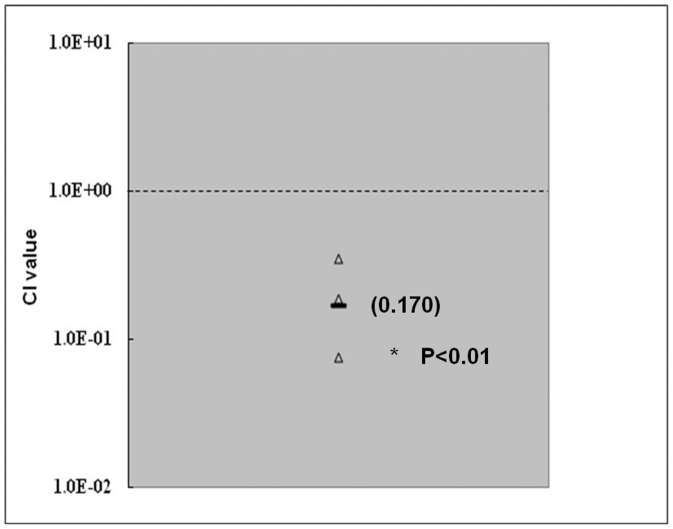
Since ΔspxA1 was sensitive to a number of stresses (Fig. 4 and 5) and was defective in H2O2 production (Fig. 1), we examined the in vivo competitiveness of ΔspxA1 by CI assays in the rabbit model of IE. Equivalent amounts of exponentially grown cells (∼108 CFUs) of ΔspxA1 and JFP36, an erythromycin resistant derivative of the wild type SK36 which demonstrated the same virulence as the wild type [33] was used in this study. Strains were mixed and inoculated into rabbits previously catheterized to create sterile vegetations. Experiments were performed in triplicate. The recovery of bacterial cells in vegetations was accounted to give the competitive index. The results showed that the in vivo CI value for ΔspxA1 was significantly less than 1 (with a geometric mean of 0.170) (Fig. 6). This suggested that SpxA1 was involved in IE virulence as mutagenesis of spxA1 caused a ∼5-fold reduction in competitiveness in vivo.
Figure 6. In vivo competitive index analyses of ΔspxA1.
The dashed line denotes a CI value of 1, indicating equal competitiveness. Each symbol indicates the CI value derived from a single animal; solid horizontal lines indicate geometric mean values. Mean CI values from 3 rabbits tested are indicated in parentheses. Paired t tests were used to determine whether CI values were significantly different from 1, with α = 0.05. *P<0.01. Open triangle: in vivo CI analysis from 3 rabbits.
Genes Involved in Oxidative Stress Responses are Positively Regulated by SpxA1
To identity the SpxA1 regulon, we performed microarray studies by comparing gene expression in ΔspxA1 and SK36. The results revealed 21 down-regulated and 39 up-regulated genes by 2-fold cutoff. Several genes involved in defense against oxidative stress (sodA, superoxide dismutase; tpx, thiol peroxidase; nox, water-forming NADH oxidase; trxA, thioredoxin and csbD, a general stress response protein) [23] were identified to be down-regulated in the SpxA1 null mutant (Table 1), suggesting the positive regulation of these genes by SpxA1. The expression ratios of these genes in ΔspxA1 compared to SK36 were also validated by qPCR analysis (Table 1). The down-regulation of these stress response genes in the spxA1 null mutant may be partially responsible for the stress sensitive phenotype of this mutant.
Table 1. Expression ratios of genes in S. sanguinis ΔspxA1 compared with SK36 by microarray and qPCR analyses.
| Gene | Name | Function | Arraya | qPCRb |
| ssa_2052 | trxA | Thioredoxin | 0.240* | 0.743 |
| ssa_1745 | csbD | General stressresponse protein | 0.327* | 0.533* |
| ssa_0721 | sodA | Mn/Fe-dependentsuperoxide dismutase | 0.387* | 0.586* |
| ssa_0259 | tpx | Thiol peroxidase | 0.543 | 0.492* |
| ssa_0391 | spxB | Pyruvate oxidase | ND | 0.372* |
| ssa_1127 | nox | H2O-forming NADHOxidase | 0.490* | 0.501* |
Array data are relative average levels of expression compared to expression in S. sanguinis SK36 from three microarray slides.
qPCR data are ratios of relative gene copy numbers normalized by that of the house keeping gene gyrA relative to those of S. sanguinis SK36.
ND, not determined by microarray analysis.
P<0.05.
Genes Involved in H2O2 Production are Positively Regulated by SpxA1
We previously identified four genes (ackA (ssa_0192), spxB (ssa_0391), spxR (ssa_1492) and tpk (ssa_2118)) involved in H2O2 production in S. sanguinis by screening mutants for opaque colony appearance [28]. Recently another gene, nox, was also shown to be involved in H2O2 production in S. sanguinis SK36 (Ge et al., manuscript in preparation). To examine whether these other H2O2-production related genes are under the control of SxpA1, in addition to microarray studies, we also determined the transcriptional level of these H2O2-production related genes by real-time quantitative PCR (qPCR) in the wild type and ΔspxA1. The results showed that the expression of spxB and nox, which encode two oxidases pyruvate oxidase and NADH oxidase respectively, decreased significantly in ΔspxA1 in comparison with SK36 (Table 1). The expressions of spxB and nox are directly responsible for H2O2 release. This suggested that the involvement of SpxA1 in H2O2 production may be via a mechanism affecting expressions of spxB and nox.
Discussion
In this study, we report on the characterization of the spxA1 gene and study another spx gene, spxA2, in S. sanguinis. The Spx global regulator is highly conserved among low-GC Gram-positive bacteria [16]. Two Spx homologs were identified in S. pneumoniae [24], a double mutation of both homologs resulted in S. pneumoniae lethality, supporting the idea of a potential overlap in the roles of the Spx proteins [24]. Furthermore, transcriptional repression by SpxA1 also had a negative effect in the development of the X-state (competence) [24]. S. mutans was also reported to harbor two Spx proteins which modulate stress tolerance, survival, and virulence [23]. Our study showed that the Spx proteins were also conserved in S. sanguinis SK36, though its GC content is higher than other streptococci (43.40% compared with 35.62 to 39.72%) [4]. In S. sanguinis, SpxA1 and SpxA2 share high homology (44% identity in amino acids), however independent inactivation of spxA1 and spxA2 led to different phenotypes, which suggest that spxA1 and spxA2 may have different functions. The H2O2 production is an obvious phenotype of the differing phenotypes controlled by SpxA1 or SpxA2. SpxA1 controls the expressions of spxB and nox (Table 1), resulting decreased H2O2 production in the mutant (Fig. 1). While in the ΔspxA2 mutant, though further experimental data are needed, it can be supposed that no significant difference for the expression of spxB and nox will be found because of its normal H2O2 production (Fig. 1). Because ΔspxA2 and the double mutant ΔspxA1A2 were significantly defective in growth (Fig. 4) and cannot be studied in the S. mutans inhibition experiment or our IE animal model which require equivalent growth of the studied objectives, we therefore focused on the ΔspxA1 mutant only in subsequent studies. However, we also performed the complementation experiment for the spxA2 mutant employing the same strategy as spxA1, and the results indicated that the growth and stress tolerance were restored (data not shown). We also further inactivated two of the spxA2 downstream genes, ssa_2243 and ssa_2242, respectively and examined their phenotypes. Both mutants showed no significant difference from the wild type strain concerning growth and stress tolerance (data not shown).
The spxA2 mutant produced normal level of H2O2 (Fig. 1) that indicated the spxA2 was not associated with production of H2O2 in S. sanguinis. However, it is interesting that the spxA2 mutant was extremely sensitive to the presence of oxygen (Fig. 2A). In addition, cell survival rate of the spxA2 mutant decreased dramatically in H2O2 treatment (Fig. 5B). H2O2 is one major contributor to oxidative damage. Our data suggested that the oxidative stress defense and repair systems may be impaired in spxA2 mutant. The oxidative stress from the normal H2O2 production of the spxA2 mutant may cause weak growth in the presence of oxygen.
Although inactivation of spxA1 had no impact on the growth of ΔspxA1 under normal temperature conditions, it did cause a significant reduction in the rate of H2O2 production (Fig. 1) and, consequently, negatively affected the competitive advantage of S. sanguinis towards S. mutans (Fig. 2). Previous reports have indicated that S. pneumoniae can also produce a certain amount of H2O2 [34], therefore, it would be of particular interest to determine if an ortholog of spxA1 (spr1262) in S. pneumoniae is also involved in H2O2 production and virulence. In S. pneumoniae, it is thought that virulence is likely related with H2O2 production [34]. In S. sanguinis, ΔspxA1 was defective in H2O2 production (Fig. 1) and at the same time, was more sensitive to exogenous H2O2 (Fig. 5B). It was reported that factors contributing to H2O2 resistance in S. pneumoniae include pyruvate oxidase (SpxB) [35]. In S. sanguinis, expression of spxB (ssa_0391) in ΔspxA1 decreased (Table 1), suggesting that exogenous H2O2 sensitivity in ΔspxA1 may also be related with pyruvate oxidase (SpxB). Our results also showed that inactivation of spxA1 affected the expression of nox (Table 1), encoding a NADH oxidase which was related with H2O2 production (data not shown). In S. mutans, consistent with our results, the ortholog of nox in S. mutans which is involved in oxidative stress response, was also positively regulated by SpxA [23]. This suggested that the regulation genes of Spx protein may also be conserved to some extent.
The increasing frequency of antibiotic resistance of viridans streptococci [36], coupled with the inability to administer antibiotics in every condition underlying bacteremia, highlights the need for the advancement of preventative measures against IE, for which no vaccine exists. Previous studies have evaluated putative virulence factors for endocarditis. For example, binding to laminin, fibrin, a complex extracellular matrix preparation, as well as platelet aggregation by S. sanguinis are all implicated as important in causing endocarditis [9], [37]. In this study, the transcriptional regulator SpxA1, was shown to be involved in IE virulence. To our knowledge, this is the first example of spx gene being involved in virulence of an invasive disease such as IE. It is possible that the downstream regulatory gene, nox, may contribute to the involvement of SpxA1 in IE virulence because nox was also shown to be involved in IE virulence (Ge et al, manuscript in preparation); however, it is also possible that other genes under the control of SpxA1 may also contribute to the virulence in S. sanguinis SK36. The involvement of SpxA1 in stresses (high temperature, acidity, H2O2) tolerance may also be responsible for its involvement of IE, because the pathogenesis of IE is a multi-step process and various stresses involve during its development such as the oxidative stress in the blood. We believe there is connection between stress survival and virulence of IE. Further identification of the SpxA1 regulon that is responsible for the reduced competitiveness will provide valuable information for understanding the pathogenicity of S. sanguinis.
In conclusion, in this study individual gene deletions of two spx genes revealed their role in important phenotypes concerning growth, H2O2 production, stresses (high temperature, acidity, H2O2) tolerance and IE virulence. Further investigations on Spx proteins will provide vital information required to better understand bacterial regulatory mechanisms involved in not only IE virulence but also stress tolerance.
Materials and Methods
Ethics Statement
Animals were treated humanely and in compliance with all applicable federal guidelines and institutional policies. All of the procedures were approved by Virginia Commonwealth University Institutional Animal Care and Use Committee.
Bacterial Strains and Medium
The strains used in this study are described in Table 2. S. sanguinis strain SK36, obtained from Dr. Mogens Kilian (University of Aarhus, Denmark), was isolated from human dental plaque [38]. S. mutans UA159, S. sanguinis SK36 and their derivatives were routinely grown in brain heart infusion broth (BHI; Difco Inc., Detroit, MI) supplemented with 1.5% (wt/vol) agar under microaerobic conditions (7.2% H2, 7.2% CO2, 79.6% N2, and 6% O2) at 37°C as previously described [13], [28], [39]. When needed, medium was supplemented with kanamycin (Sigma-Aldrich, CA) (500 µg/ml) or chloramphenicol (Sigma-Aldrich, CA) (5 µg/ml). For growth curve studies, overnight cultured bacteria were diluted 100-fold into wells of a 96-well plate containing 200 µl BHI media and the OD450 of each well was determined with a FLUOstar plate reader (BMG LABTECH, Offenburg, Germany) every 100 minutes under aerobic conditions. When assays were performed anaerobically, an overlay of 50 µl of sterile mineral oil was added to each well to create anaerobic conditions [23].
Table 2. Bacterial strains used in this study.
| Strain | Phenotype or description | Source |
| S. sanguinis strains | ||
| SK36 | Human plaque isolate | (38) |
| ΔspxA1 | Kmr; ΔspxA1::aphA-3 | This study |
| ΔspxA1C | Cmr; spxA1+:: magellan2 | This study |
| ΔspxA1’ | Cmr; ΔspxA1:: magellan2 | This study |
| ΔspxA2 | Kmr; ΔspxA2::aphA-3 | This study |
| ΔspxA1A2 | Kmr;Cmr;ΔspxA1::magellan2;ΔspxA2::aphA-3 | This study |
| S. mutans strains | ||
| UA159 | Wild-type, serotype c | ATCC 700610 |
| smx_42 | Cmr; Δsmu.42:: magellan2 | (28) |
Cm, chloramphenicol; Km, kanamycin.
Mutant Construction and Complementation
For the construction of S. sanguinis ΔspxA1 and ΔspxA2, a PCR-based recombination method was employed as described previously [27]. Briefly, for each targeted gene, three sets of primers were designed to amplify a linear DNA fragment containing the kanamycin resistance cassette (aphA-3) [33] with two flanking arms of DNA upstream and downstream of the targeted gene. The linear recombinant PCR amplicon was directly transformed into S. sanguinis SK36 competent cells as previously described [13]. For the construction of the double mutant strain ΔspxA1A2, a ΔspxA1’ strain was first constructed by replacing the spxA1 ORF with the chloramphenicol resistance cassette from the magellan2 mini-transposon [39]. Then using the competent cells of ΔspxA1’ strain, the spxA2 ORF in this strain was replaced with the kanamycin resistance cassette as described above. The mutants were confirmed by PCR and DNA sequencing analysis.
To construct the complemented strain ΔspxA1C, the DNA fragment containing the spxA1 ORF followed by the chloramphenicol resistance cassette [33] was integrated via double homologous recombination into ΔspxA1 (Table 2) to replace the kanamycin resistance cassette. A chloramphenicol-resistant and kanamycin-sensitive transformant was selected and confirmed by PCR analysis.
H2O2 Release Assays
H2O2 production was quantified using an amplex red hydrogen peroxide/peroxidase kit (Invitrogen, CA) as previously described [28]. Final values are shown relative to that of the wild-type strain, SK36. Paired t-test was used for statistical analysis.
Competition Assays
To determine the inhibitory effect of S. sanguinis SK36 or mutants against S. mutans, a competition assay on agar plates was employed as described previously [28]. Growth inhibition was evaluated based on the distance of the inhibition zone between the edges of both colonies.
Competition assays in liquid media was performed as described previously [28]. Cells of S. sanguinis SK36 or ΔspxA1 and S. mutans smx_42, a chloramphenicol-resistant derivative of S. mutans (Table 2), were grown in BHI medium overnight and adjusted to the same OD660. S. sanguinis or ΔspxA1 (3 µl of each) and S. mutans smx_42 (3 µl) were mixed with 200 µl fresh BHI medium in 96-well microtiter plate in triplicate. The cells were incubated overnight and then dispersed by vigorous pipetting and serial dilutions were plated on BHI agar plates supplemented with chloramphenicol. Assays were performed in triplicate and CFU counts were determined. Paired t-test was used for statistical analysis.
Acid Tolerance Assays
Acid tolerance experiments were performed as described previously [23] with the following modifications. Briefly, overnight cultures of S. sanguinis SK36 or mutants were diluted 100-fold into fresh BHI medium and cultured for 5 h under microaerobic conditions at 37°C. For each strain, three biological replicates were included. The cells were then harvested and washed once with one culture volume of 0.1 M glycine buffer (pH 7.0) and resuspended to give an OD660 of ∼2.0. Samples were centrifuged again and resuspended in the same volume of 0.1 M glycine buffer (pH 3.0) for 60 min. Aliquots were then serially diluted, plated on BHI agar, and incubated for 48 h before colonies were counted. Survival after treatment was determined as the percentage of the wild type, and the statistical significance of differences with SK36 were determined by paired t-test analysis.
H2O2 Sensitivity Assays
Exponentially growing cultures of S. sanguinis strains were prepared, washed and adjusted to OD660∼2.0 as described above. Triplicate 100 µl aliquots of each culture were centrifuged and resuspended in fresh BHI medium containing 20 mM H2O2 that had been diluted from a 30% (9.8 M) stock solution (Invitrogen), followed by incubation at 37°C for 60 min [35]. Serial dilutions from each sample were then plated onto BHI agar, and colonies were counted after 48 h incubation. The percentage survival was calculated by dividing the CFU of cultures after exposure to H2O2 by the CFU of a control tube without H2O2.
Competitive Index (CI) Assays
A CI assay for a rabbit model of IE was used to evaluate the competitiveness of ΔspxA1 as previously described [40], [41]. Briefly, for the preparation of the CI inoculum, overnight cultures of JFP36 [33], an erythromycin-resistant derivative of the wild type SK36 which demonstrated the same virulence as the wild type [33], and the mutant ΔspxA1 grown in BHI were diluted 10-fold into 14 ml pre-warmed BHI for an additional 3 h of growth at 37°C. Cells were then washed and suspended in phosphate-buffered saline (PBS) to give an OD660 of 0.8, corresponding to ∼108 bacteria/0.5 ml. Equal volumes of ΔspxA1 and JFP36 cells were then combined to make an inoculum of 1.0 ml. This inoculum was sonicated at 50% power for 1.5 min in a titanium cup adaptor (BioLogics Inc., Manassas, VA). An inoculum of 0.5 ml was then inoculated into each rabbit into which cardiac catheters had previously been inserted to create sterile vegetations. Experiments were performed in triplicate. The day after inoculation, rabbits were sacrificed and heart valve vegetations were collected. The bacterial cells in vegetations were homogenized, sonicated as per the inoculum, serially diluted and spread-plated onto BHI plates supplemented with erythromycin or kanamycin for enumeration. The CI was determined as the ΔspxA1:JFP36 ratio of the output CFUs divided by the ΔspxA1:JFP36 ratio of the inoculum. Paired t-test was used to determine whether CI values were significantly different from 1, with α = 0.05.
Microarray Analysis
Total RNA from each of three independent samples of S. sanguinis SK36 and ΔspxA1 was prepared from cells growing exponentially in BHI medium under microaerobic conditions for 5 h (OD660 ∼0.8). Cells were lysed after lysozyme treatment and mechanical disruption using FastPrep® lysing matrix B (Qbiogene, CA). RNA was isolated with an RNeasy mini kit (Qiagen, Hilden, Germany). DNA was removed from the RNeasy mini kit column by DNase I treatment. Total RNA was quantified using a NanoDrop® ND 1000 Spectrophotometer. Spotted microarray slides were obtained from the Pathogen Functional Genomics Resource Center at J. Craig Venter Institute (JCVI, MD). The microarray was performed according to the manufacturer’s protocol (http://pfgrc.jcvi.org/index.php/microarray/protocols.html). Hybridization was performed at 42°C for 16–20 h. Microarray slides were scanned on a GenePix 4000A scanner. Images were analyzed using the program Spotfinder (v321win). Data were LOWESS normalized and background subtracted using Midas (v2.20). Processed red signal/processed green signal ratios were calculated. Microarray data has been deposited into the NCBI Gene Expression Omnibus (GEO) with access number GSE34203.
qRT-PCR Analysis
Quantitative RT-PCR (qRT-PCR) was performed as described previously [28]. Total RNA was prepared as described above. First-strand cDNA synthesis was performed using SuperScript® III Reverse Transcriptase (200 U/µl, Invitrogen). Reactions lacking reverse transcriptase were prepared in parallel as negative controls for possible DNA contamination. First-strand cDNA from each reaction was subjected to eighty-fold dilutions, and 2 µl of each dilution was used as template for each PCR reaction. PCR was performed in reactions containing 5 µl of SYBR® Green PCR Master Mix (Applied Biosystems), and 1 µl of each PCR primer at 2 mM using the ABI 7500 Fast Real-Time PCR system. Data was collected and statistically analyzed from triplicate biological samples. The amount of relative gene transcript was normalized with that of gyrA in each sample. Data are reported as the percentage of the amount of normalized transcript from the wild type SK36.
Acknowledgments
We thank Drs. Todd Kitten and Cindy Munro and Mrs. Nicai Zollar for assisting with the endocarditis animal experiments. We also thank the Pathogen Functional Genomics Resource Center at JCVI for providing S. sanguinis spotted microarray slides.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants R01DE018138 from the National Institutes of Health (PX) and in part, by Virginia Commonwealth University Presidential Research Incentive Program (PRIP) 144602-3 (PX). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kuramitsu HK, He X, Lux R, Anderson MH, Shi W. Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev. 2007;71:653–670. doi: 10.1128/MMBR.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nyvad B, Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987;95:369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2:1599–1607. doi: 10.1016/s1286-4579(00)01316-2. [DOI] [PubMed] [Google Scholar]
- 4.Xu P, Alves JM, Kitten T, Brown A, Chen Z, et al. Genome of the opportunistic pathogen Streptococcus sanguinis. J Bacteriol. 2007;189:3166–3175. doi: 10.1128/JB.01808-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas CW, Heath J, Hampton KK, Preston FE. Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol. 1993;39:179–182. doi: 10.1099/00222615-39-3-179. [DOI] [PubMed] [Google Scholar]
- 6.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–1330. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 7.Tleyjeh IM, Steckelberg JM, Murad HS, Anavekar NS, Ghomrawi HM, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. 2005;293:3022–3028. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 8.Bayer AS, Bolger AF, Taubert KA, Wilson W, Steckelberg J, et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98:2936–2948. doi: 10.1161/01.cir.98.25.2936. [DOI] [PubMed] [Google Scholar]
- 9.Moreillon P, Que YA. Infective endocarditis. Lancet. 2004;363:139–149. doi: 10.1016/S0140-6736(03)15266-X. [DOI] [PubMed] [Google Scholar]
- 10.Durack DT, Beeson PB, Petersdorf RG. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br. J. Exp. Pathol. 1973;54:142–151. [PMC free article] [PubMed] [Google Scholar]
- 11.Becker MR, Paster BJ, Leys EJ, Moeschberger ML, Kenyon SG, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, et al. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun. 2000;68:4018–4023. doi: 10.1128/iai.68.7.4018-4023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge X, Kitten T, Chen Z, Lee SP, Munro CL, et al. Identification of Streptococcus sanguinis genes required for biofilm formation and examination of their role in endocarditis virulence. Infect Immun. 2008;76:2551–2559. doi: 10.1128/IAI.00338-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreth J, Merritt J, Shi W, Qi F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol. 2005;187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano S, Kuster-Schock E, Grossman AD, Zuber P. Spx-dependent global transcriptional control is induced by thiol-specific oxidative stress in Bacillus subtilis. Proc Natl Acad Sci U S A. 2003;100:13603–13608. doi: 10.1073/pnas.2235180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuber P. Spx-RNA polymerase interaction and global transcriptional control during oxidative stress. J Bacteriol. 2004;186:1911–1918. doi: 10.1128/JB.186.7.1911-1918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Zuber P. The ClpX protein of Bacillus subtilis indirectly influences RNA polymerase holoenzyme composition and directly stimulates sigma-dependent transcription. Mol Microbiol. 2000;37:885–897. doi: 10.1046/j.1365-2958.2000.02053.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakano MM, Nakano S, Zuber P. Spx (YjbD), a negative effector of competence in Bacillus subtilis, enhances ClpC-MecA-ComK interaction. Mol Microbiol. 2002;44:1341–1349. doi: 10.1046/j.1365-2958.2002.02963.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakano S, Zheng G, Nakano MM, Zuber P. Multiple pathways of Spx (YjbD) proteolysis in Bacillus subtilis. J Bacteriol. 2002;184:3664–3670. doi: 10.1128/JB.184.13.3664-3670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano MM, Hajarizadeh F, Zhu Y, Zuber P. Loss-of-function mutations in yjbD result in ClpX- and ClpP-independent competence development of Bacillus subtilis. Mol Microbiol. 2001;42:383–394. doi: 10.1046/j.1365-2958.2001.02639.x. [DOI] [PubMed] [Google Scholar]
- 21.Frees D, Varmanen P, Ingmer H. Inactivation of a gene that is highly conserved in Gram-positive bacteria stimulates degradation of non-native proteins and concomitantly increases stress tolerance in Lactococcus lactis. Mol Microbiol. 2001;41:93–103. doi: 10.1046/j.1365-2958.2001.02503.x. [DOI] [PubMed] [Google Scholar]
- 22.Veiga P, Bulbarela-Sampieri C, Furlan S, Maisons A, Chapot-Chartier MP, et al. SpxB regulates O-acetylation-dependent resistance of Lactococcus lactis peptidoglycan to hydrolysis. J Biol Chem. 2007;282:19342–19354. doi: 10.1074/jbc.M611308200. [DOI] [PubMed] [Google Scholar]
- 23.Kajfasz JK, Rivera-Ramos I, Abranches J, Martinez AR, Rosalen PL, et al. Two Spx proteins modulate stress tolerance, survival, and virulence in Streptococcus mutans. J Bacteriol. 2010;192:2546–2556. doi: 10.1128/JB.00028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turlan C, Prudhomme M, Fichant G, Martin B, Gutierrez C. SpxA1, a novel transcriptional regulator involved in X-state (competence) development in Streptococcus pneumoniae. Mol Microbiol. 2009;73:492–506. doi: 10.1111/j.1365-2958.2009.06789.x. [DOI] [PubMed] [Google Scholar]
- 25.Reyes DY, Zuber P. Activation of transcription initiation by Spx: formation of transcription complex and identification of a Cis-acting element required for transcriptional activation. Mol Microbiol. 2008;69:765–779. doi: 10.1111/j.1365-2958.2008.06330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pamp SJ, Frees D, Engelmann S, Hecker M, Ingmer H. Spx is a global effector impacting stress tolerance and biofilm formation in Staphylococcus aureus. J Bacteriol. 2006;188:4861–4870. doi: 10.1128/JB.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu P, Ge X, Chen L, Wang X, Dou Y, et al. Genome-wide essential gene identification in Streptococcus sanguinis. Sci Rep. 2011;1:125. doi: 10.1038/srep00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Ge X, Dou Y, Wang X, Patel JR, et al. Identification of hydrogen peroxide production-related genes in Streptococcus sanguinis and their functional relationship with pyruvate oxidase. Microbiology. 2011;157:13–20. doi: 10.1099/mic.0.039669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakano S, Erwin KN, Ralle M, Zuber P. Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol Microbiol. 2005;55:498–510. doi: 10.1111/j.1365-2958.2004.04395.x. [DOI] [PubMed] [Google Scholar]
- 30.Newberry KJ, Nakano S, Zuber P, Brennan RG. Crystal structure of the Bacillus subtilis anti-alpha, global transcriptional regulator, Spx, in complex with the alpha C-terminal domain of RNA polymerase. Proc Natl Acad Sci U S A. 2005;102:15839–15844. doi: 10.1073/pnas.0506592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano MM, Zhu Y, Liu J, Reyes DY, Yoshikawa H, et al. Mutations conferring amino acid residue substitutions in the carboxy-terminal domain of RNA polymerase alpha can suppress clpX and clpP with respect to developmentally regulated transcription in Bacillus subtilis. Mol Microbiol. 2000;37:869–884. doi: 10.1046/j.1365-2958.2000.02052.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakano S, Nakano MM, Zhang Y, Leelakriangsak M, Zuber P. A regulatory protein that interferes with activator-stimulated transcription in bacteria. Proc Natl Acad Sci U S A. 2003;100:4233–4238. doi: 10.1073/pnas.0637648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner LS, Das S, Kanamoto T, Munro CL, Kitten T. Development of genetic tools for in vivo virulence analysis of Streptococcus sanguinis. Microbiology. 2009;155:2573–2582. doi: 10.1099/mic.0.024513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos-Montanez S, Tsui HC, Wayne KJ, Morris JL, Peters LE, et al. Polymorphism and regulation of the spxB (pyruvate oxidase) virulence factor gene by a CBS-HotDog domain protein (SpxR) in serotype 2 Streptococcus pneumoniae. Mol Microbiol. 2008;67:729–746. doi: 10.1111/j.1365-2958.2007.06082.x. [DOI] [PubMed] [Google Scholar]
- 35.Pericone CD, Park S, Imlay JA, Weiser JN. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the fenton reaction. J Bacteriol. 2003;185:6815–6825. doi: 10.1128/JB.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prabhu RM, Piper KE, Litzow MR, Steckelberg JM, Patel R. Emergence of quinolone resistance among viridans group streptococci isolated from the oropharynx of neutropenic peripheral blood stem cell transplant patients receiving quinolone antimicrobial prophylaxis. Eur J Clin Microbiol Infect Dis. 2005;24:832–838. doi: 10.1007/s10096-005-0037-3. [DOI] [PubMed] [Google Scholar]
- 37.Herzberg MC, MacFarlane GD, Gong K, Armstrong NN, Witt AR, et al. The platelet interactivity phenotype of Streptococcus sanguis influences the course of experimental endocarditis. Infect Immun. 1992;60:4809–4818. doi: 10.1128/iai.60.11.4809-4818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilian M, Holmgren K. Ecology and nature of immunoglobulin A1 protease-producing streptococci in the human oral cavity and pharynx. Infect Immun. 1981;31:868–873. doi: 10.1128/iai.31.3.868-873.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paik S, Senty L, Das S, Noe JC, Munro CL, et al. Identification of virulence determinants for endocarditis in Streptococcus sanguinis by signature-tagged mutagenesis. Infect Immun. 2005;73:6064–6074. doi: 10.1128/IAI.73.9.6064-6074.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das S, Kanamoto T, Ge X, Xu P, Unoki T, et al. 2009. Contribution of lipoproteins and lipoprotein processing to endocarditis virulence in Streptococcus sanguinis. J Bacteriol. 191:4166–4179. doi: 10.1128/JB.01739-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turner LS, Kanamoto T, Unoki T, Munro CL, Wu H, et al. Comprehensive evaluation of Streptococcus sanguinis cell wall-anchored proteins in early infective endocarditis. Infect Immun. 2009;77:4966–4975. doi: 10.1128/IAI.00760-09. [DOI] [PMC free article] [PubMed] [Google Scholar]