Abstract
The complete mitochondrial DNA (mtDNA) of Gracilariopsis lemaneiformis was sequenced (25883 bp) and mapped to a circular model. The A+T composition was 72.5%. Forty six genes and two potentially functional open reading frames were identified. They include 24 protein-coding genes, 2 rRNA genes, 20 tRNA genes and 2 ORFs (orf60, orf142). There is considerable sequence synteny across the five red algal mtDNAs falling into Florideophyceae including Gr. lemaneiformis in this study and previously sequenced species. A long stem-loop and a hairpin structure were identified in intergenic regions of mt genome of Gr. lemaneiformis, which are believed to be involved with transcription and replication. In addition, the mtDNAs of two mutagenic cultivated breeds (“981” and “07-2”) were also sequenced. Compared with the mtDNA of wild Gr. lemaneiformis, the genome size and gene length and order of three strains were completely identical except nine base mutations including eight in the protein-coding genes and one in the tRNA gene. None of the base mutations caused frameshift or a premature stop codon in the mtDNA genes. Phylogenetic analyses based on mitochondrial protein-coding genes and rRNA genes demonstrated Gracilariopsis andersonii had closer phylogenetic relationship with its parasite Gracilariophila oryzoides than Gracilariopsis lemaneiformis which was from the same genus of Gracilariopsis.
Introduction
Gracilariopsis is an important economical marine red algae and widely used in food industry, agar extraction, etc [1], [2]. Gracilariopsis lemaneiformis has been cultivated on large scales in both the southern and the northern parts of China for food and agar production, playing an effective role against coastal eutrophication [3]. For the past few years, Gracilariopsis cultivation has become the third largest seaweed cultivation industry only after Laminaria and Porphyra in China. Among Gracilariopsis seaweeds, the Gr. lemaneiformis is one of the most important cultivated breeds, because of its high yields and commercially valuable extracts.
Mutation is one of the important means of breeding new varieties of Gr. lemaneiformis. Chemical mutagen treatments (EMS: ethyl methanesulphonate and N-methyl-N-nitrosoguanidine) could increase the frequency of mutations in Gracilaria species [4]. In China, two important commercial cultivated breeds of Gr. lemaneiformis (“981” and “07-2”) are both mutant stains. The strain “981” was bred from the wild Gr. lemaneiformis after mutation by N-methyl-N′-nitro-N-ni-trosoguanidine (MNNG) and selected with hydroxyproline (HYP) protocol. The strain “981” has the characteristics of high temperature tolerance, fast growth rate and high levels of content and quality of agar [5]. “981” has been extensively cultivated as a source of commercial agar in the coastal areas of Fujian and Guangdong Province of China. The strain “07-2” was obtained from the original “981” by the use of MNNG and selected with HYP protocol. The new strain (“07-2″) has excellent features of fast growing and strong resistance to bad environment [6].
Mitochondria are thought to be derived from eubacterial endosymbionts [7], because they have biochemical machineries to replicate and transcribe of their own genomes. Mitochondrial DNA has the features of compact size, mostly maternal inheritance and fast evolutionary rate, it is a good molecular marker for evolutionary and population studies [8], [9]. The cox1 and cox2-3 genes were good mtDNA taxonomic barcodes often used for species identification [10]. The whole mitochondrial genome makes it possible for the combination of multiple protein-coding genes, providing strong and precise phylogenetic analysis. However, limited information of complete mt genome is available. Since the first report on the complete mt genome of Chondrus crispus [11], there are only 6 red algal complete mt genomes deposited in the GenBank [12]–[14]. But for the seaweeds of brown algae (Phaeophyceae) and green algae (Chlorophyta), the complete mitochondrial data has reached 21 and 14 species respectively, more than that of red algal species. Here, we sequenced the complete mitochondrial genomes of wild Gr. lemaneiformis and two mutation strains. The comparison of the genome sequence and architecture reveals the influence of mutation to mtDNA. This work will contribute to further study about molecular phylogenetic analysis of red algae and germplasm improvement for the economic Gracilariopsis species.
Results
Genome Organization and Comparison
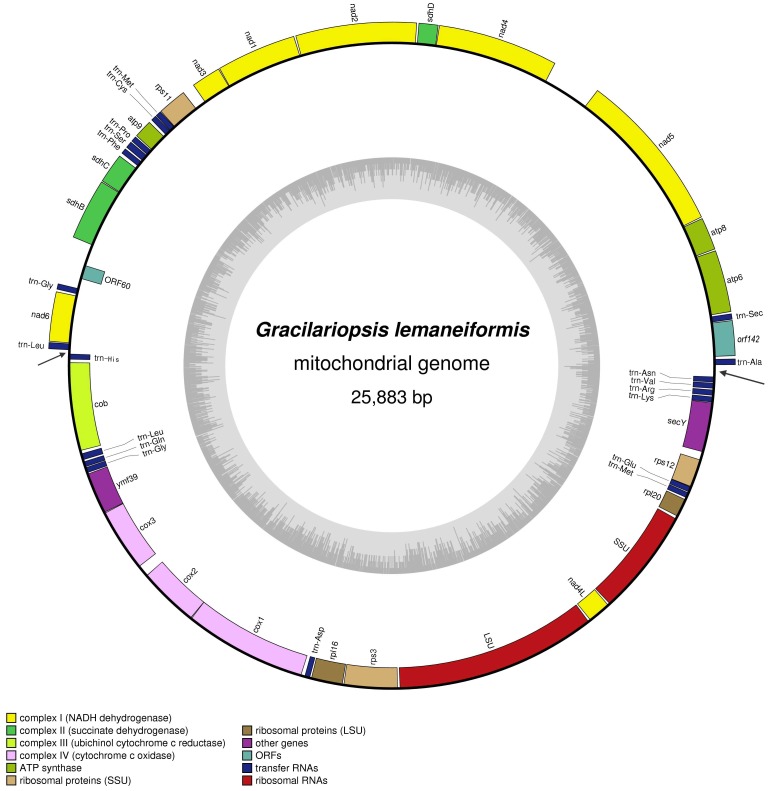
The mitochondrial DNA of Gr. lemaneiformis is a circular molecule of 25883 nucleotides (GenBank accession number: JQ071938), with an overall A+T content of 72.5%. This base composition is consistent with previously reports of red algal mitochondrial genomes [11]–[14]. Forty six genes including 24 protein-coding genes, 2 ribosomal RNA (rRNA) genes and 20 transfer RNA (tRNA) genes were identified. No intron is inserted in any of these genes and all genes are encoded on both heavy strand (H-strand) and light strand (L-strand) with approximately the same encoding proportion on each strand. The genes are encoded in two opposite major transcriptional directions, suggesting the existence of two main transcriptional units (Figure 1). Compared to the six known red algal mitochondrial genomes, the protein-coding gene content and order in all seven mt genomes are virtually identical. Besides, there is considerable sequence synteny across the five mitochondrial genomes which fall in Florideophyceae including Gr. lemaneiformis in this study and previously sequenced: Gracilariopsis andersonii, Gracilariophila oryzoides, Chondrus crispus and Plocamiocolax pulvinata [14] (Table 1).
Figure 1. Gene map of Gracilariopsis lemaneiformis mitochondrial genome.
Genes outside the map are transcribed counter clockwise and those inside the map are transcribed clockwise. Genes belonging to different functional groups are color coded. The black arrows indicate the positions of stem-loop and hairpin structures.
Table 1. The comparisons of protein-coding genes and rRNA genes of five mitochondrial genomes from Florideophyceae.
| Genes | Chondrus crispus | Plocamiocolax pulvinata | Gracilariopsis andersonii | Gracilariophila oryzoides | Gracilariopsis lemaneiformis |
| Protein-coding genes | |||||
| atp6 | + | + | + | + | + |
| atp8 | + | − | + | − | + |
| atp9 | + | + | + | + | + |
| cob | + | + | + | + | + |
| cox1 | + | + | + | + | + |
| cox2 | + | + | + | + | + |
| cox3 | + | + | + | + | + |
| nad1 | + | + | + | + | + |
| nad2 | + | + | + | + | + |
| nad3 | + | + | + | + | + |
| nad4 | + | + | + | + | + |
| nad4L | + | + | + | + | + |
| nad5 | + | + | + | + | + |
| nad6 | + | + | + | + | + |
| sdhB | + | + | + | + | + |
| sdhC | + | + | + | − | + |
| sdhD | + | + | + | + | + |
| secY | + | + | + | + | + |
| ymf39 | + | + | − | + | + |
| rpl16 | + | + | + | + | + |
| rpl20 | + | + | + | + | + |
| rps3 | + | + | + | + | + |
| rps11 | + | + | + | + | + |
| rps12 | + | + | − | + | + |
| Ribosomal RNA genes | |||||
| rRNA SSU | + | + | + | + | + |
| rRNA LSU | + | + | + | + | + |
Compared to the mt genome of Gr. andersonii that from the same genus of Gracilariopsis, both genomes code for 26S rRNA gene (LSU) and 16S rRNA gene (SSU). The protein-coding gene content and order are almost identical with the following exceptions: the ymf39 gene is present in the Gr. lemaneiformis genome between cox3 and cob gene; a fragment of nearly 2000 bp contains three predict ORFs are characteristic in intergenic region of Gr. andersonii mt genome; the gene rps11 appears in both mt genomes but in opposite directions; the gene length of rps12 in Gr. andersonii is 129 bp shorter than that of Gr. lemaneiformis for a premature stop codon. In addition, in mt genome of Gr. lemaneiformis, a fragment of ∼600 bp containing a predicted ORF (orf60) is inserted into intergenic region between sdhB and nad6 gene; another fragment of ∼150 bp containing a stem-loop structure is present between atp6 and secY gene (trn-Ala and trn-Asn, Figure 1).
Gene Content and Codon Usage
The mtDNA of Gr. lemaneiformis codes for three subunits of the cytochrome oxidase (cox1-3), apocytochrome b (cob), seven subunits of the NADH dehydrogenase complex (nad1-6, nad4L), four ATPase subunits (atp6, atp8, atp9, ymf39), five ribosomal proteins (rps3, rps11, rps12, rpl16, rpl20) and three succinate dehydrogenase complex (sdhB, sdhC, sdhD). Start and stop codons of all protein-coding genes were determined based on alignments with the corresponding genes and proteins of other Florideophyceae species. As shown in Table 2, the codon ATG was used as start codon for all genes, the stop codons include two types: TAA and TAG.
Table 2. Characteristics of the mitochondrial protein-coding genes of Gracilariopsis lemaneiformis.
| Codon | |||||
| Gene | Size (bp) | Amino acid | Start | Stop | Strand |
| apt6 | 762 | 253 | ATG | TAA | H |
| atp8 | 405 | 134 | ATG | TAA | H |
| nad5 | 1992 | 663 | ATG | TAA | H |
| nad4 | 1476 | 491 | ATG | TAG | H |
| sdhD | 240 | 79 | ATG | TAA | H |
| nad2 | 1476 | 491 | ATG | TAA | H |
| nad1 | 981 | 326 | ATG | TAA | H |
| nad3 | 366 | 121 | ATG | TAA | H |
| rps11 | 360 | 119 | ATG | TAA | H |
| atp9 | 231 | 76 | ATG | TAA | H |
| sdhC | 372 | 123 | ATG | TAA | H |
| sdhB | 753 | 250 | ATG | TAA | H |
| nad6 | 609 | 202 | ATG | TAG | H |
| cob | 1143 | 380 | ATG | TAA | L |
| ymf-39 | 543 | 180 | ATG | TAA | L |
| cox3 | 819 | 272 | ATG | TAA | L |
| cox2 | 792 | 263 | ATG | TAA | L |
| cox1 | 1596 | 531 | ATG | TAA | L |
| rpl16 | 411 | 136 | ATG | TAA | L |
| rps3 | 696 | 231 | ATG | TAA | L |
| nad4L | 306 | 101 | ATG | TAA | L |
| rpl20 | 243 | 80 | ATG | TAG | L |
| rps12 | 369 | 122 | ATG | TAG | L |
| secY | 735 | 244 | ATG | TAA | L |
Twenty tRNA genes were determined and show standard cloverleaf secondary structures with the length ranging from 70–88 bp. All tRNA genes are dispersed throughout the Gr. lemaneiformis on both strands (Figure 1). Like all the other algal mt genomes published, the genome did not contain all the tRNAs needed to complete translation alone.
Secondary Structures
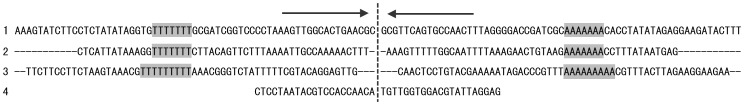
At the demarcation point of the two opposite transcriptional units, a long and stable stem-loop (63 nt) was identified in intergenic region of mt genome of Gr. lemaneiformis. Nearly diametrally opposite on the circular mt genome, another transcriptional demarcation point between trn-Leu and trn-His (Figure 1), a hairpin structure (21 nt) was found. Both secondary structures were complete inverted repeat sequences. The stem-loop and hairpin structures are likely to cause the termination of transcription, moreover, inverted repeat sequences provide the same binding site of enzyme at different directions. So, these structures could be involved in DNA transcription. Such strict stem-loop structures are also present in intergenic regions of Chondrus crispus (58 nt) and Plocamiocolax pulvinata (51 nt) mt genomes [11], [14], and also locate at the demarcation point of different transcriptional units. There was no visible sequence homology between these secondary structures even with similar sequence length. However, all of these three inverted repeat sequences contain polymers of A and T (n = 6, 8, Figure 2). The polymers with low free energy tend to unlink easily and may be a recognition site of some enzymes. So the stem-loop structure is putative origin of replication of mtDNA.
Figure 2. The comparison of sequences of stem-loop and hairpin structure from different mt genomes.
1: Gracilariopsis lemaneiformis (126 bp). 2: Plocamiocolax pulvinata (102 bp). 3: Chondrus crispus (116 bp). 4: the hairpin structure of Gr. lemaneiformis (42 bp). The dotted line indicates the middle point from where both sides are complete inverted repeat sequences. The shadow indicates polymers of A and T (n = 6, 8).
Comparison of mtDNA of Wild and Two Mutation Strains
The complete mt genomes of two mutation strains (“981”, “07-2”) were sequenced using the same method as the wild Gr. lemaneiformis. The mitochondrial genome size and gene order of the two mutation strains were the same as the wild Gr. lemaneiformis. Only nine base mutations were detected and all base mutations located at gene coding regions. Of which, eight were present in the protein-coding genes and one in the tRNA gene. The protein-coding genes contained base mutations were atp6, nad5, rps11, sdhC and cob genes. However, none of these base mutations caused frameshifts or a premature stop codon in the mtDNA genes. The only one mutation present in tRNA gene did not make any change to the structure and function of tRNAPro. Among the eight base mutations present in the protein-coding genes, five of them are samesense mutations and the other three are nonsynonymous mutations (Table 3).
Table 3. Comparison of mtDNA base mutations between wild Gr. lemaneiformis and two mutation strains.
| Gene | Position | wild | “981” | “07-2″ | Strand |
| apt6 | 732 | ATT (Ile) | ATT (Ile) | ATC (Ile) | H |
| nad5 | 1092 | CAG (Gln) | CAA (Gln) | CAG (Gln) | H |
| rps11 | 188 | TGT (Cys) | TGT (Cys) | TAT (Tyr) | H |
| tRNAPro | 27 | A (trn-Pro) | G (trn-Pro) | A (trn-Pro) | H |
| sdhC | 93 | TTC (Phe) | TTC (Phe) | TTT (Phe) | H |
| cob | 756 | CAT (His) | CAT (His) | CAC (His) | L |
| cob | 30 | TCT (Ser) | TCC (Ser) | TCT (Ser) | L |
| cob | 25–26 | CTT (Leu) | ACT (Thr) | CTT (Leu) | L |
Phylogenetic Analyses
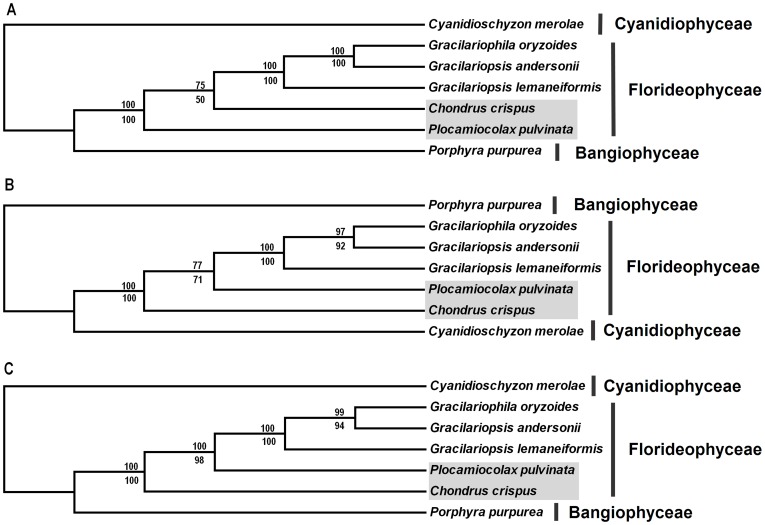
Three data partitions (19 protein-coding genes, LSU and SSU) from seven red algal mt genomes were used to construct phylogenetic trees. The aligned sequence length of protein-coding genes, LSU and SSU were 15915 bp, 2882 bp and 1573 bp respectively. Phylogenetic trees with bootstrap values are present in Figure 3. Both MP and ML analyses yielded the same trees for each data partition and phylogenetic trees of three data partitions were similar with each other, in which Cyanidioschyzon merolae and Porphyra purpurea formed single clade as they belong to Cyanidiophyceae and Bangiophyceae respectively; the remaining five species falling into the same class (Florideophyceae) clustered together (Figure 3). Among three Gracilariaceae species, phylogenetic analyses demonstrated Gr. andersonii had closer relationship with Gracilariophila oryzoides, even though Gr. andersonii and Gr. lemaneiformis belong to the same genus of Gracilariopsis. The topological differences were mainly occurred within Florideophyceae clade. Within this clade, the branching order of Plocamiocolax pulvinata and Chondrus crispus were different among the phylogenetic trees based on different data partitions of mtDNA: Plocamiocolax pulvinata clustered out ahead of Chondrus crispus when using the data partition of protein-coding genes, whereas the branch order was opposite when using the data partition of LSU and SSU (Figure 3).
Figure 3. ML and MP trees of seven red algae based on different data partitions of mtDNA.
A: 19 protein-coding genes. B: LSU. C: SSU. The ML and MP trees have the same topology and only one is shown. Numbers above and below nodes are ML and MP bootstrap support values respectively. The shadow indicates topological difference between trees based on different data partitions.
Discussion
To date, the complete mitochondrial genomes of seven red algae (including this work) have been reported. According to the results of previous studies and our research, the mtDNA sizes of red algae are ranging from 25.1 kb to 36.7 kb, and the A+T content of these mitochondrial genomes are 72.0–76.1% [11]–[14]. However, for the seaweeds, the mtDNA sizes of brown and green algae are in the range of 31.6–58.5 kb and 12.9–95.8 kb respectively. It is obvious that there exists great variance between mt genomes of seaweeds, including the kinds of protein-coding genes, species of tRNAs and rRNA genes. Moreover, the mtDNAs of green algae have special model of linear molecule and circular molecule [15], [16]. In addition, the mtDNAs of brown and red algae were both circular molecule and encoded on H-strand and L-strand, but the encoding proportion of two strands are very different. For red algae, the encoding proportion of each strand is nearly the same; while the L-strand of brown algae just encodes several ribosomal protein genes, the other protein-coding genes and all tRNAs genes are encoded on H-strand, indicating a clear strand-specific bias in encoding genes. As noted by Gray, the mitochondrial genomes of red algae are incredibly well conserved in both content and genome architecture [17]. In despite of the discrepant mt genome size of red algae, the A+T content remains at a relatively stable level. Especially for the five species from Florideophyceae, the number and order of mtDNA genes are almost the same (Table 1).
According to previous reports, the mitochondrial genome size had undergone a substantial expansion from the green algae Chara vulgaris to land plants [18], which was mainly accounted for the enlargement of intergenic regions and duplications, and also get DNA from the chloroplast and the nucleus [19]–[22]. However, such phenomenon has not been observed in the mt genomes of brown and red seaweeds except for few species have few introns [14]. For the seven known mt genomes of red algae, compared to the primitive red alga Cyanidioschyzon merolae from Cyanidiophyceae, the genome sizes of other six species from Bangiophyceae and Florideophyceae show a process of decreasing [11]–[14], the mitochondrial genomes of red algae have a trend to become smaller and more stable.
Codon Usage
For the 24 protein-coding genes of mt genome of Gr. lemaneiformis, there are just two types of stop codon, TAA and TAG. As reported before, special in the mitochondrial genome, the modified genetic code TGA specifies tryptophan instead of the stop codon [23]. These modifications are considered to be correlated to an A+T pressure [24]. Like the green-plant Acanthamoeba castellanii which displays a mitochrondrial A+T content of 71% use a modified genetic code, whereas another green alga Prototheca wickerhamii has the similar A+T content of 74% but with no usage of TGA as stop codon nor tryptophan [25], [26]. This situation of a deviant genetic code was observed in our research, and the codon TGA distributed over nearly all the genes of complete mt genome of Gr. lemaneiformis. For the known mt genomes of green and brown algae, we chose the gene cox1 for a simple search, the results indicated no modified genetic code TGA was used in brown algae and only one was present in green algae. However, all the seven red algal mt genomes contain different numbers of modified genetic code TGA, it can be said this modified genetic code has the highest frequency in red algae. Moreover, such phenomenon was also observed in cox1 genes of other red algal genera of Eucheuma and Kappaphycus [10].
Influence of Mutation
Colour mutants are relatively widespread in red algae [27]. Moreover, chemical mutagen treatments can increase the frequency of mutations [4]. For Gr. lemaneiformis, spontaneous colour mutants have been observed in the nature environment. Early in 2002, Sui found that the absorption spectra of phycoerythrins (PE) changed significantly between Gracilaria lemaneiformis Greville and its pigmental mutants [28]. The two mutants (“981” and “07-2″) used in this study were the products mutated by chemical mutagenesis and selected the strains with excellent characteristics. We sequenced the complete mt genomes of the two mutants, the comparison of mtDNA between the mutants and wild Gr. lemaneiformis revealed that there was no obvious variance except several amino acids changing caused by base mutations. Among the eight base mutations present in the protein-coding genes, five of them are samesense mutations and cause no impact to the gene structure and gene function. The other three are nonsynonymous mutations, but there is no significant clue for the influence on the protein-coding genes except an amino acid change. These results suggest that the mutation does not cause the large-scale rearrangements and obvious insertion or deletion to Gr. lemaneiformis mt genome. To further understand the exact gene in relevant to the new characteristics particular for mutants, more efforts need to be done.
Origin of Replication
As reported before, the mitochondrial genome of mammalian has the displacement-loop (D-loop) which also known as the control region, including transcriptional promoters for both strands and the heavy strand replication origin [29], [30]. This noncoding region attracted many studies for its supposedly rapid rate of evolution [31]. In our research, a length of 63 nt complete inverted repeat sequence was detected in intergenic region of mt genome of Gr. lemaneiformis, and the stem-loop structure located at the demarcation point of two transcriptional units. This inverted repeat sequence seems like the D-loop structure of animal mt genome that can cause the termination of transcription, so it was presumed to be the origin of replication and in relevant with transcription. Similar structures were found in C. crispus and P. pulvinata mt genomes, and in P. pulvinata it was considered to be the origin of replication [14]. However, these strict secondary structures were only found in three mt genomes of red algae, and no similar sequence was found throughout the all known mt genomes of brown algae. Interestingly, even Gr. andersonii and Gr. lemaneiformis both belong to the genus of Gracilaropsis, no such structure was found in the mt genome of Gr. andersonii.
In our research, the complete mitochondrial sequence was obtained by PCR strategy and sequenced by dideoxy chain termination method, the instability of PCR reaction may cause the deletion or base mutation of DNA fragments, special for the region include secondary structure. Frequently, normal rTaq enzyme can easily cross the folded hairpin structure and result in the loss of partial PCR fragment. Therefore, the accuracy and integrity of sequence must be considered when choosing the strategy of PCR to obtain the complete mitochondrial genome.
Materials and Methods
Ethics Statement
As a normal kind of economical red algae, no specific permits are required for the studies on Gr. lemaneiformis so far. The wild Gr. lemaneiformis was collected from Shandong Province of China in May 2011, the two mutation strains (“981”, “07-2”) were collected from Fujian Province of China in April 2011. All materials were collected in the coastal area and the location is neither privately-owned nor protected places.
DNA Isolation, Genome Sequencing
Total DNA was extracted from fresh thalli with modified CTAB method [32]. mtDNA of Gr. lemaneiformis was amplified by polymerase chain reaction (PCR) using primers (Table S1) designed in Primer Premier 5.0 from the conserved regions of the known red algal mitochondrial genomes [11]–[14]. The PCR reaction conditions include an initial denaturation cycle of 94°C for 3 min followed by 30 cycles of 94°C for 30 s, annealing temperature depending on primer sequences for 45 s, and 72°C for 1 min. A final extension step was performed at 72°C for 15 min followed by a 4°C hold. For large fragments, the extension time depend on the sequence length (approximately 1 min for 1,000 bp). PCR products were purified and sent for sequencing on an ABI 3730 automated sequencer (Applied Biosystems). Individual sequences were edited and assembled into contigs using DNAStar (DNASTAR, Inc., Madison, USA).
Genome Annotation
Genes were identified using the BlastN and BlastX algorithms to compare the predicted ORFs to the NCBI GenBank database [33]. Ribosomal RNA (rRNA) genes and coding regions were determined by sequence alignment with the known mitochondrial genes of Gr. andersonii and Gracilariophila oryzoides [14]. Transfer RNA (tRNA) genes were identified with tRNAscan-SE 1.21 software [34]. Mitochondrial genome map of Gr. lemaneiformis was produced by using OGDRAW software [35]. Sequence alignment and base composition were conducted using MEGA 4.0 software [36].
Phylogenetic Analyses
Phylogenetic analyses of the red algal species were performed based on the following data set: 19 protein-coding genes (atp6, atp9, cob, cox1, cox2, cox3, nad1, nad2, nad3, nad4L, nad4, nad5, nad6, rpl16, rps11, rps12, rps3, sdhB, sdhD), 26S rRNA gene (LSU) and 16S rRNA gene (SSU). All sequences are commonly present in all seven mt genomes publicly available in the GenBank database. Sequences were aligned using MEGA 4.0 [36] software and edited manually. Maximum parsimony (MP) analyses were performed with PAUP*4.0b10 [37]. Heuristic searches were conducted with 1000 random addition replicates and tree bisection reconnection (TBR) branch swapping with “multrees” option. For maximum likelihood (ML) analyses, RA×ML version 7.0 [38] was used with searches relied on the general time-reversible (GTR) and gamma models. The local bootstrap probability of each branch was calculated by 1000 replicates. MEGA 4.0 was used for displaying and printing phylogenetic trees.
Supporting Information
Primers sequences designed to sequence three strains of Gracilariopsis lemaneiformis.
(DOC)
Acknowledgments
We would like to thank Tianxiang Gao for critical review of the manuscript. We are indebted to Yan Sun for assistance with experiments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Province Science and Technology in the Guangdong Project (2010B060200010, 2010B020201015) and the National Public Benefit Research Foundation of China (201203063). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Santelices B, Doty MS. A review of Gracilaria faming. Aquaculture. 1989;78:59–133. [Google Scholar]
- 2.Xia BM, Zhang JF. Flora Algarum Marinarum Sinicarum: Rhodophyta. Science Press. 1999. pp. 55–58.
- 3.Xu JT, Gao KS. Growth, pigments, UV-absorbing compounds and agar yield of the economic red seaweed Gracilaria lemaneiformis (Rhodophyta) grown at different depths in the coastal waters of the South China Sea. J Appl Phycol. 2007;20:231–236. [Google Scholar]
- 4.Zhang X, Van Der Meer JP. A study on heterosis in diploid gametophytes of the marine red alga Gracilaria tikvahiae. Bot Mar. 1987;30:309–314. [Google Scholar]
- 5.Meng L, Xu D, Chen WZ, Zhang XC. Selection and characterization of a new strain of Gracilaria lemaneiformis. Periodical of Ocean University of China. 2009;39:94–98. [Google Scholar]
- 6.Chen WZ, Xu D, Wang LG, Men L, Du H, et al. Preliminary Study on economic characteristic s and agar characteristics of two new strains of Gracilaria lemaneiformis. Periodical of Ocean University of China. 2009;39:437–442. [Google Scholar]
- 7.Gray MW. The endosymbiont hypothesis revisited. Int Rev Cytol. 1992;141:233–357. doi: 10.1016/s0074-7696(08)62068-9. [DOI] [PubMed] [Google Scholar]
- 8.Wilson AC, Cann RL, Carr SM, George M, Gyllensten UB, et al. Mitochondrial DNA and two perspectives on evolutionary genetics. Biol J Linn Soci. 1985;26:375–400. [Google Scholar]
- 9.Brown WM, George MJR, Wilson AC. Rapid evolution of animal mitochondrial DNA. Pro Nat Acad Sci U S A. 1979;76:1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conklin KY, Kurihara A, Sherwood AR. A molecular method for identification of the morphologically plastic invasive algal genera Eucheuma and Kappaphycus (Rhodophyta, Gigartinales) in Hawaii. J Appl Phycol. 2009;21:691–699. [Google Scholar]
- 11.Leblanc C, Boyen C, Richard O, Bonnard G, Grienenberger JM, et al. Complete sequence of the mitochondrial DNA of the rhodophyte Chondrus crispus (Gigartinales): Gene content and genome organization. J Mol Biol. 1995;250:484–495. doi: 10.1006/jmbi.1995.0392. [DOI] [PubMed] [Google Scholar]
- 12.Burger G, Saint-Louis D, Gray MW, Lang BF. Complete sequence of the mitochondrial DNA of the red alga Porphyra purpurea: Cyanobacterial introns and shared ancestry of red and green algae. Plant Cell. 1999;11:1675–1694. doi: 10.1105/tpc.11.9.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohta N, Sato N, Kuroiwa T. Structure and organization of the mitochondrial genome of the unicellular red alga Cyanidioschyzon merolae deduced from the complete nucleotide sequence. Nucleic Acids Research. 1998;26:5190–5198. doi: 10.1093/nar/26.22.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock L, Goff L, Lane C. Red algae lose key mitochondrial genes in response to becoming parasitic. Genome Biol Evol. 2010;2:897–910. doi: 10.1093/gbe/evq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nosek J, Tomáska L, Kucejová B. The chromosome end replication: lessons from mitochondrial genetics. J Appl Biomed. 2004;2:71–79. [Google Scholar]
- 16.Smith DR, Hua J, Lee RW. Evolution of linear mitochondrial DNA in three known lineages of Polytomella. Curr Genet. 2010;56:427–438. doi: 10.1007/s00294-010-0311-5. [DOI] [PubMed] [Google Scholar]
- 17.Gray MW, Lang BF, Burger G. Mitochondria of protists. Annu Rev Genet. 2004;38:477–524. doi: 10.1146/annurev.genet.37.110801.142526. [DOI] [PubMed] [Google Scholar]
- 18.Turmel M, Otis C, Lemieux C. The mitochondrial genome of Chara vulgaris: Insights into the mitochondrial DNA architecture of the last common ancestor of green algae and land plants. The Plant Cell. 2003;15:1888–1903. doi: 10.1105/tpc.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turmel M, Otis C, Lemieux C. The chloroplast and mitochondrial genome sequences of the charophyte Chaetosphaeridium globosum: Insights into the timing of the events that restructured organelle DNAs within the green algal lineage that led to land plants. Proc Natl Acad Sci USA. 2002;99:11275–11280. doi: 10.1073/pnas.162203299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unseld M, Marienfeld JR, Brandt P, Brennicke A. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat Genet. 1997;15:57–61. doi: 10.1038/ng0197-57. [DOI] [PubMed] [Google Scholar]
- 21.Kubo T, Nishizawa S, Sugawara A, Itchoda N, Estiati A, et al. The complete nucleotide sequence of the mitochondrial genome of sugar beet (Beta vulgaris L.) reveals a novel gene for tRNA(Cys)(GCA). Nucleic Acids Res. 2000;28:2571–2576. doi: 10.1093/nar/28.13.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Notsu Y, Masood S, Nishikawa T, Kubo N, Akiduki G, et al. The complete sequence of the rice (Oryza sativa L.) mitochondrial genome: Frequent DNA sequence acquisition and loss during the evolution of flowering plants. Mol Genet Genomics. 2002;268:434–445. doi: 10.1007/s00438-002-0767-1. [DOI] [PubMed] [Google Scholar]
- 23.Boyen C, Leblanc C, Bonnard G, Grienenberger JM, Kloareg B. Nucleotide sequence of the cox3 gene from Chondrus crispus: evidence that UGA encodes tryptophan and evolutionary implications. Nucl Acids Res. 1994;22:1400–1403. doi: 10.1093/nar/22.8.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osawa S, Jukes TH, Watanabe K, Muto A. Recent evidence for evolution of genetic code. Microbiol Rev. 1992;56:229–264. doi: 10.1128/mr.56.1.229-264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burger G, Plante I, Lonergan KM, Gray MW. The mitochondrial DNA of the amoeboid protozoan, Acanthamoeba castellanii: complete sequence, gene content, and genome organization. J Mol Biol. 1995;245:522–537. doi: 10.1006/jmbi.1994.0043. [DOI] [PubMed] [Google Scholar]
- 26.Wolff G, Plante I, Lang BF, Kück U, Burger G. Complete sequence of the mitochondrial DNA of the chlorophyte alga Prototheca wickerhamii. . J Mol Biol. 1994;237:75–86. doi: 10.1006/jmbi.1994.1210. [DOI] [PubMed] [Google Scholar]
- 27.Van Der Meer JP. Cole KM, Sheath RG, editors. Genetics. 1990. pp. 103–119. Biology of the Red Algae. Cambridge. University Press.
- 28.Sui ZH, Zhang XC, Cheng XJ. Comparison of phycobiliproteins from Gracilaria lemaneiformis (Rhodophyceae) and its pigment mutants in spectral and molecular respects. Acta Botanica Sinica. 2002;44:557–561. [Google Scholar]
- 29.Clayton DA. Replication of animal mitochondrial DNA. Cell. 1982;28:693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- 30.Chang DD, Clayton DA. Identification of primary transcription start sites of mouse mitochondrial DNA: accurate in vitro initiation of both heavy- and light-strand transcriptions. Mol Cell Biol. 1986;6:1446–1453. doi: 10.1128/mcb.6.5.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoelzel AR, Hancock JM, Dover GA. Evolution of the cetacean mitochondrial D-Loop region. Mol Biol Evol. 1991;8:475–493. doi: 10.1093/oxfordjournals.molbev.a040662. [DOI] [PubMed] [Google Scholar]
- 32.Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- 33.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lohse M, Dreshsil O, Bock R. OrganellarGenomeDRAW (OGDRAW): a tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr Genet. 2007;52:267–264. doi: 10.1007/s00294-007-0161-y. [DOI] [PubMed] [Google Scholar]
- 36.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 37.Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts. 1998.
- 38.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers sequences designed to sequence three strains of Gracilariopsis lemaneiformis.
(DOC)